Preparation and Characterization of Microencapsulated Phase Change Materials with Enhanced Thermal Performance for Cold Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
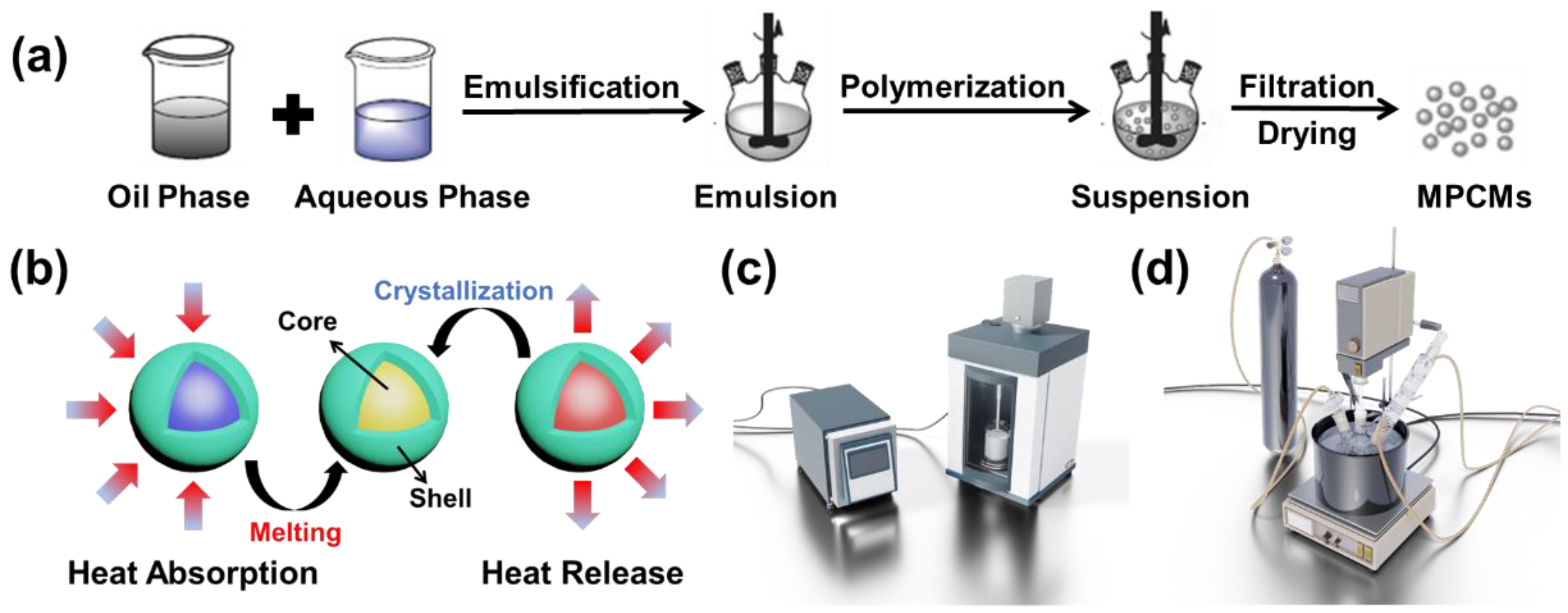
2.2. Synthesis Methods
2.3. Characterization
3. Results and Discussion
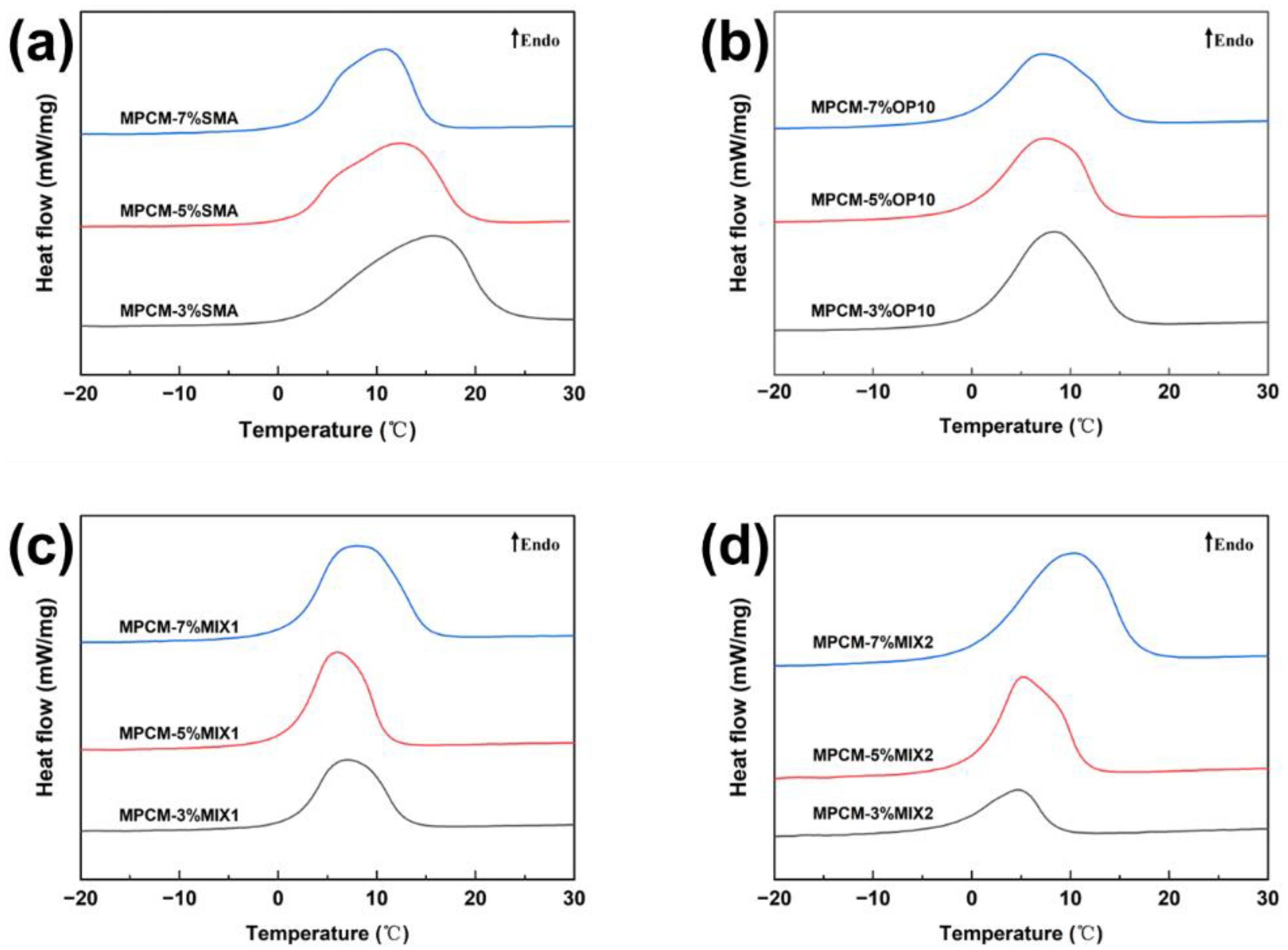
3.1. Effects of Emulsification Process on Thermal Properties
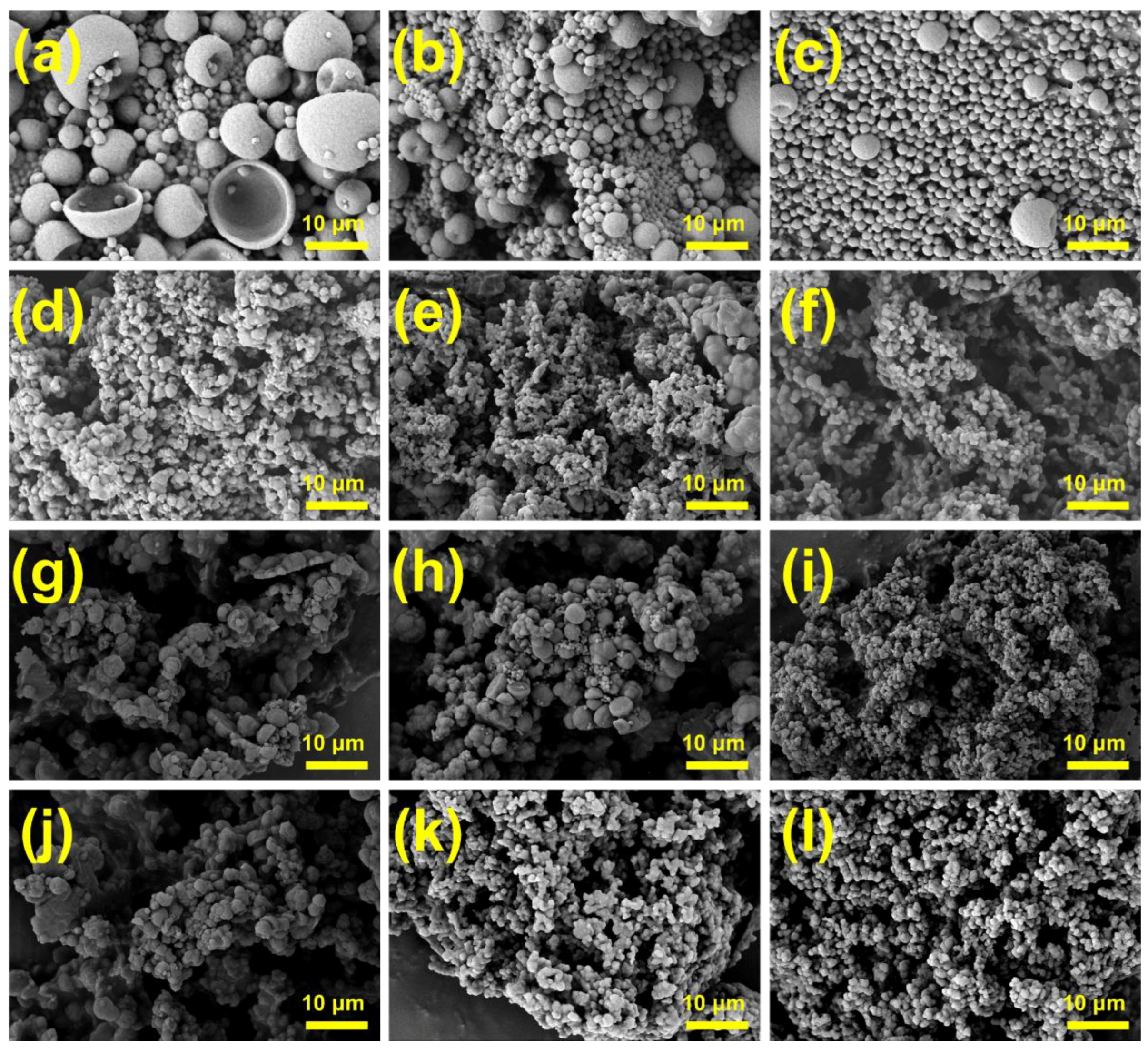
3.2. Effects of Emulsification Process on Micromorphology
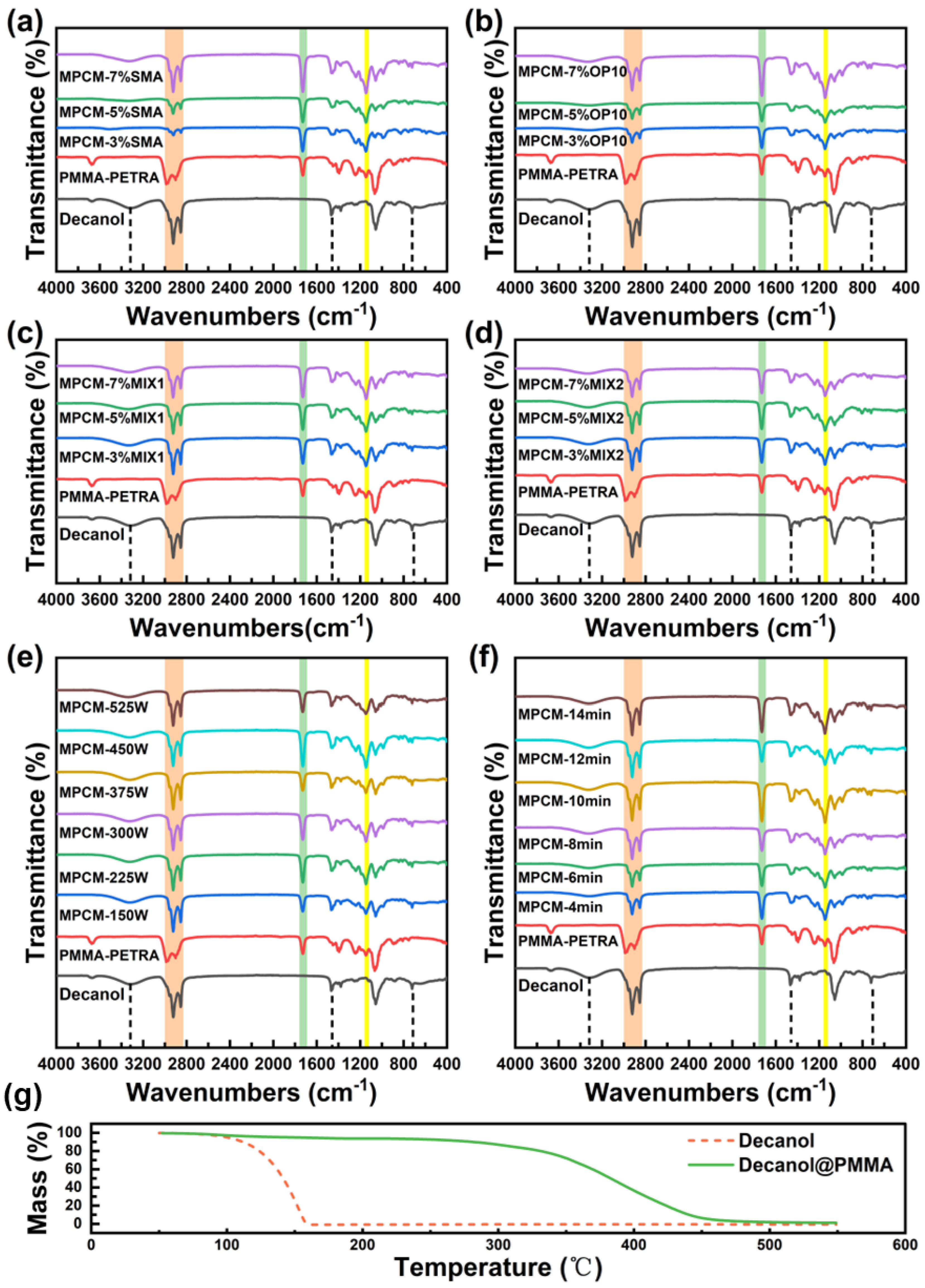
3.3. Composition and Structure of MPCMs
3.4. Enhancement of Thermal Properties of Microcapsules by Binary Eutectic Method
3.5. Properties of MPCMs Prepared by Binary Eutectic Cores
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, K.J.; Chen, Q.Y.; Qin, M.L.; Gao, S.; Wang, Q.; Gao, S.; Xiong, F.; Lv, Y.; Zou, R.Q. Micro/nano encapsulated phase change materials: Preparation, principle, and emerging advances in medical field. Adv. Funct. Mater. 2024, 34, 2314487. [Google Scholar] [CrossRef]
- Zheng, H.F.; Tian, G.J.; Yang, C.W.; Zhao, Y.H.; Cao, L.H.; Xin, X.; Zhou, J.; Zheng, Y.H. Experimental study on performance of phase change microcapsule cold storage solar composite refrigeration system. Renew. Energy 2022, 198, 1176–1185. [Google Scholar] [CrossRef]
- Cheng, H.T.; Zhou, X.Q.; Sun, J.; Ngombe, J.N.; Mzyece, A.; Feng, W.; Guo, S.Z.; Liu, B.D. Assessing the efficacy of agricultural cold chain facility expansion in China. J. Stored Prod. Res. 2024, 105, 102244. [Google Scholar] [CrossRef]
- Kong, J.; Li, W.X.; Hu, J.Y.; Zhao, S.X.; Yue, T.L.; Li, Z.H.; Xia, Y.Q. The Safety of Cold-Chain Food in Post-COVID-19 Pandemic: Precaution and Quarantine. Foods 2022, 11, 1540. [Google Scholar] [CrossRef]
- Ikutegbe, C.A.; Al-Shannaq, R.; Farid, M.M. Microencapsulation of low melting phase change materials for cold storage applications. Appl. Energy 2022, 321, 119347. [Google Scholar] [CrossRef]
- Zhang, X.H.; Shi, Q.L.; Luo, L.G.; Fan, Y.L.; Wang, Q.; Jia, G.G. Research Progress on the Phase Change Materials for Cold Thermal Energy Storage. Energies 2021, 14, 8233. [Google Scholar] [CrossRef]
- Dutkowski, K.; Kruzel, M.; Kochanowska, M. Transition Boundary from Laminar to Turbulent Flow of Microencapsulated Phase Change Material Slurry—Experimental Results. Materials 2024, 17, 6041. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, R.; Hu, W.X.; Faraj, Y.; Zhao, Q.; Fan, X.X.; Wang, W.; Ju, X.J.; Liu, Z.; Chu, L.Y. Microfluidic fabrication of core–sheath composite phase change microfibers with enhanced thermal conductive property. J. Mater. Sci. 2018, 53, 15769–15783. [Google Scholar] [CrossRef]
- Park, S.; Jo, B. Microencapsulated sodium nitrate with titanium-dioxide shell for high-temperature and high-density latent heat storage. Sol. Energy Mater. Sol. Cells 2024, 272, 112905. [Google Scholar] [CrossRef]
- Xue, F.; Qi, X.D.; Huang, T.; Tang, C.Y.; Zhang, N.; Wang, Y. Preparation and application of three-dimensional filler network towards organic phase change materials with high performance and multi-functions. Chem. Eng. J. 2021, 419, 129620. [Google Scholar] [CrossRef]
- Nalepa, B.; Dutkowski, K.; Kruzel, M.; Bialko, B.; Zajaczkowski, B. Experimental Investigation of the Viscosity and Density of Microencapsulated Phase Change Material Slurries for Enhanced Heat Capacity and Transfer. Energies 2024, 17, 2324. [Google Scholar] [CrossRef]
- Sha, Y.S.; Hua, W.S.; Cao, H.F.; Zhang, X.L. Properties and encapsulation forms of phase change material and various types of cold storage box for cold chain logistics: A review. J. Energy Storage 2022, 55, 105426. [Google Scholar] [CrossRef]
- Tafone, A.; Borri, E.; Cabeza, L.F.; Romagnoli, A. Innovative cryogenic Phase Change Material (PCM) based cold thermal energy storage for Liquid Air Energy Storage (LAES)–Numerical dynamic modelling and experimental study of a packed bed unit. Appl. Energy 2021, 301, 117417. [Google Scholar] [CrossRef]
- Li, H.; Yu, G.; Xu, H.; Han, X.; Liu, H.H. A review of the mathematical models for the flow and heat transfer of microencapsulated phase change slurry (MEPCS). Energies 2023, 16, 2914. [Google Scholar] [CrossRef]
- Zheng, H.F.; Zhang, C.C.; Tian, G.J.; Gao, H.Y.; Zhang, L.B.; Zhao, Y.H. Experimental study on the characteristics of phase change cold storage system based on solar ejector composite refrigeration. J. Energy Storage 2025, 114, 115758. [Google Scholar] [CrossRef]
- Li, Z.J.; Sha, Y.S.; Zhang, X.L. Research on Phase Change Cold Storage Materials and Innovative Applications in Air Conditioning Systems. Energies 2024, 17, 4365. [Google Scholar] [CrossRef]
- Lu, X.T.; Qian, R.D.; Xu, X.Y.; Liu, M.; Liu, Y.F.; Zou, D.Q. Modifications of microencapsulated phase change materials: Supercooling suppression, thermal conductivity enhancement and stability improvement. Nano Energy 2024, 124, 109520. [Google Scholar] [CrossRef]
- Weng, K.Y.; Xu, X.Y.; Chen, Y.Y.; Li, X.L.; Qing, C.Y.; Zou, D.Q. Development and applications of multifunctional microencapsulated PCMs: A comprehensive review. Nano Energy 2024, 122, 109308. [Google Scholar] [CrossRef]
- Sheikh, Y.; Hamdan, M.O.; Sakhi, S. A review on micro-encapsulated phase change materials (EPCM) used for thermal management and energy storage systems: Fundamentals, materials, synthesis and applications. J. Energy Storage 2023, 72, 108472. [Google Scholar] [CrossRef]
- Lu, W.; Yu, A.Q.; Dong, H.; He, Z.L.; Liang, Y.T.; Liu, W.T.; Sun, Y.; Song, S.L. High-performance palmityl palmitate phase change microcapsules for thermal energy storage and thermal regulation. Energy 2023, 274, 127336. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, Z.Y.; Hai, A.M.; Zhang, S.F.; Tang, B.T. Shape-stabilization micromechanisms of form-stable phase change materials-A review. Compos. Part A Appl. Sci. Manuf. 2022, 160, 107047. [Google Scholar] [CrossRef]
- Liu, C.Z.; Cao, H.X.; Jin, S.C.; Cheng, Q.J.; Rao, Z.H. Synthesis and characterization of microencapsulated phase change material with phenol-formaldehyde resin shell for thermal energy storage. Sol. Energy Mater. Sol. Cells 2022, 243, 111789. [Google Scholar] [CrossRef]
- Ding, J.; Chen, S.L.; Zhao, Y.L.; Wu, X.D.; Zhao, S.W.; Yuan, A.Q.; Lei, J.X.; Fu, X.W. Engineering polymeric phase-change sub-microcapsules with a high encapsulation fraction by facile, versatile suspension polymerization. ACS Sustain. Chem. Eng. 2023, 11, 14000–14009. [Google Scholar] [CrossRef]
- Zhang, W.H.; Zhang, H.; Liu, S.H.; Zhang, X.X.; Li, W. Preparation and crystallization behavior of sensitive thermochromic microencapsulated phase change materials. Appl. Energy 2024, 362, 122993. [Google Scholar] [CrossRef]
- Yan, L.C.; Wang, Y.; Lu, S.J.; Zhu, Z.P.; Xu, L.L. Preparation and Performance Study of n-Undecane Phase Change Cold Storage Material. Materials 2024, 17, 1570. [Google Scholar] [CrossRef]
- Liu, L.; Niu, J.; Wu, J.Y. Ultrasonication for preparing high-performance phase change material nano-emulsions: Optimization and characterization. J. Mol. Liq. 2023, 380, 121776. [Google Scholar] [CrossRef]
- Jiang, L.L.; Chen, G.Y.; Zhao, L.; Li, M.X.; Zhang, W.Q.; Ma, X.X. Research on the low-temperature waste heat storage performance of a novel microencapsulated phase change material with a double-shell encapsulation of hexadecylamine. J. Energy Storage 2024, 80, 110253. [Google Scholar] [CrossRef]
- Chen, H.B.; Zhao, R.; Wang, C.C.; Feng, L.Y.; Li, S.Q.; Gong, Y.T. Preparation and characterization of microencapsulated phase change materials for solar heat collection. Energies 2022, 15, 5354. [Google Scholar] [CrossRef]
- Feng, T.; Ji, J.; Zhang, X. Research progress of phase change cold energy storage materials used in cold chain logistics of aquatic products. J. Energy Storage 2023, 60, 106568. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, J.; Zhang, Z.B.; Gu, C.H.; Wang, X.L. Influencing factors of thermal performance of small-size vaccine cold storage: An experiment-based parametric study. J. Energy Storage 2022, 51, 104496. [Google Scholar] [CrossRef]
- Zotova, I.; Gendelis, S.; Kirilovs, E.; Stefanec, D.; Mongibello, L.; Morciano, M. Thermal Performance of Lignocellulose’s By-Product Wallboards with Bio-Based Microencapsulated Phase Change Materials. Energies 2024, 17, 257. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, S.L.; He, J.C.; Xu, D.J.; Abdou, S.N.; Ibrahim, M.M.; Sun, S.Q.; Chen, Y.J.; Li, H.D.; Xu, B.B.; et al. Experimental design of paraffin/methylated melamine-formaldehyde microencapsulated composite phase change material and the application in battery thermal management system. J. Mater. Sci. Technol. 2024, 169, 124–136. [Google Scholar] [CrossRef]
- Rakkappan, S.R.; Sivan, S.; Raza, M.A.; Relkar, A.; Mittal, H.; Adak, M. A facile and reliable approach to enhance the energy storage performance of 1-Decanol for sustainable and energy-efficient cold storage system. J. Energy Storage 2022, 52, 104933. [Google Scholar] [CrossRef]
- Wan, X.; Chen, C.; Tian, S.Y.; Guo, B.H. Thermal characterization of net-like and form-stable ML/SiO2 composite as novel PCM for cold energy storage. J. Energy Storage 2020, 28, 101276. [Google Scholar] [CrossRef]
- Rakkappan, S.R.; Sivan, S.; Pethurajan, V.; Aditya, A.; Mittal, H. Preparation and thermal properties of encapsulated 1-Decanol for low-temperature heat transfer fluid application. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126167. [Google Scholar] [CrossRef]
- Kalidasan, B.; Pandey, A.K.; Saidur, R.; Kothari, R.; Sharma, K.; Tyagi, V.V. Eco-friendly coconut shell biochar based nano-inclusion for sustainable energy storage of binary eutectic salt hydrate phase change materials. Sol. Energy Mater. Sol. Cells 2023, 262, 112534. [Google Scholar] [CrossRef]
- Wang, L.Y.; Huang, Y.Q.; Li, L.F.; Li, Y.Y.; Cheng, X.M. Binary nitrate molten salt magnetic microcapsules modified with Fe3O4-functionalized carbon nanotubes for accelerating thermal energy storage. J. Energy Storage 2023, 74, 109394. [Google Scholar] [CrossRef]
- Geng, L.; Huang, W.B.; Jiang, J.P.; Zhang, C.L.; Cui, J.P.; Yan, Y.B.; Liu, C.H. Cold energy storage enhancement and phase transition temperature regulation. J. Energy Storage 2024, 97, 112903. [Google Scholar] [CrossRef]
- Ma, F.; Hou, Y.J.; Fu, Z.; Qin, W.; Tang, Y.J.; Dai, J.S.; Wang, Y.H.; Peng, C. Microencapsulated binary eutectic phase change materials with high energy storage capabilities for asphalt binders. Constr. Build. Mater. 2023, 392, 131814. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.L.; Xu, X.F.; Lin, X.W.; Zhao, Y.; Zou, L.G.; Wu, Y.F.; Zheng, H.F. Development of low-temperature eutectic phase change material with expanded graphite for vaccine cold chain logistics. Renew. Energy 2021, 179, 2348–2358. [Google Scholar] [CrossRef]
- Li, S.L.; Dong, B.B.; Wang, J.H.; Li, J.; Shen, T.T.; Peng, H.; Ling, X. Synthesis and characterization of mixed alkanes microcapsules with phase change temperature below ice point for cryogenic thermal energy storage. Energy 2019, 187, 115898. [Google Scholar] [CrossRef]
- Yan, G.S.; Wang, S.R.; Li, Y.; He, L.Q.; Li, Y.; Zhang, L.B. Effect of emulsifier HLB on aerated emulsions: Stability, interfacial behavior, and aeration properties. J. Food Eng. 2023, 351, 111505. [Google Scholar] [CrossRef]
- Holmberg, K.; Jönsson, B.; Kronberg, B.; Lindman, B. Surfactants and Polymers in Aqueous Solution; John Wiley & Sons: Chichester, UK, 2003. [Google Scholar]
- Hu, Q.; Deng, Y.L.; Xiao, L.F.; Shu, L.C.; Zhou, L.W.; Yin, G.L. Study on modification of room temperature vulcanized silicone rubber by microencapsulated phase change material. J. Energy Storage 2021, 41, 102842. [Google Scholar] [CrossRef]
- Kang, Z.Q.; Li, X.; Zhou, L.; Li, D.; Wang, J.P. Mitigating supercooling in microencapsulated phase change materials by incorporating compatible polyethylene wax as a nucleating agent. Surf. Interfaces 2024, 44, 103646. [Google Scholar] [CrossRef]
- Gao, Y.; Geng, X.Y.; Wang, X.J.; Han, N.; Zhang, X.X.; Li, W. Synthesis and characterization of microencapsulated phase change materials with chitosan-based polyurethane shell. Carbohydr. Polym. 2021, 273, 118629. [Google Scholar] [CrossRef]
- Mustapha, A.; Wang, Y.; Ding, Y.L.; Li, Y.L. Supercooling elimination of cryogenic-temperature microencapsulated phase change materials (MPCMs) and the rheological behaviors of their suspension. J. Mater. Res. Technol. 2022, 21, 2277–2295. [Google Scholar] [CrossRef]


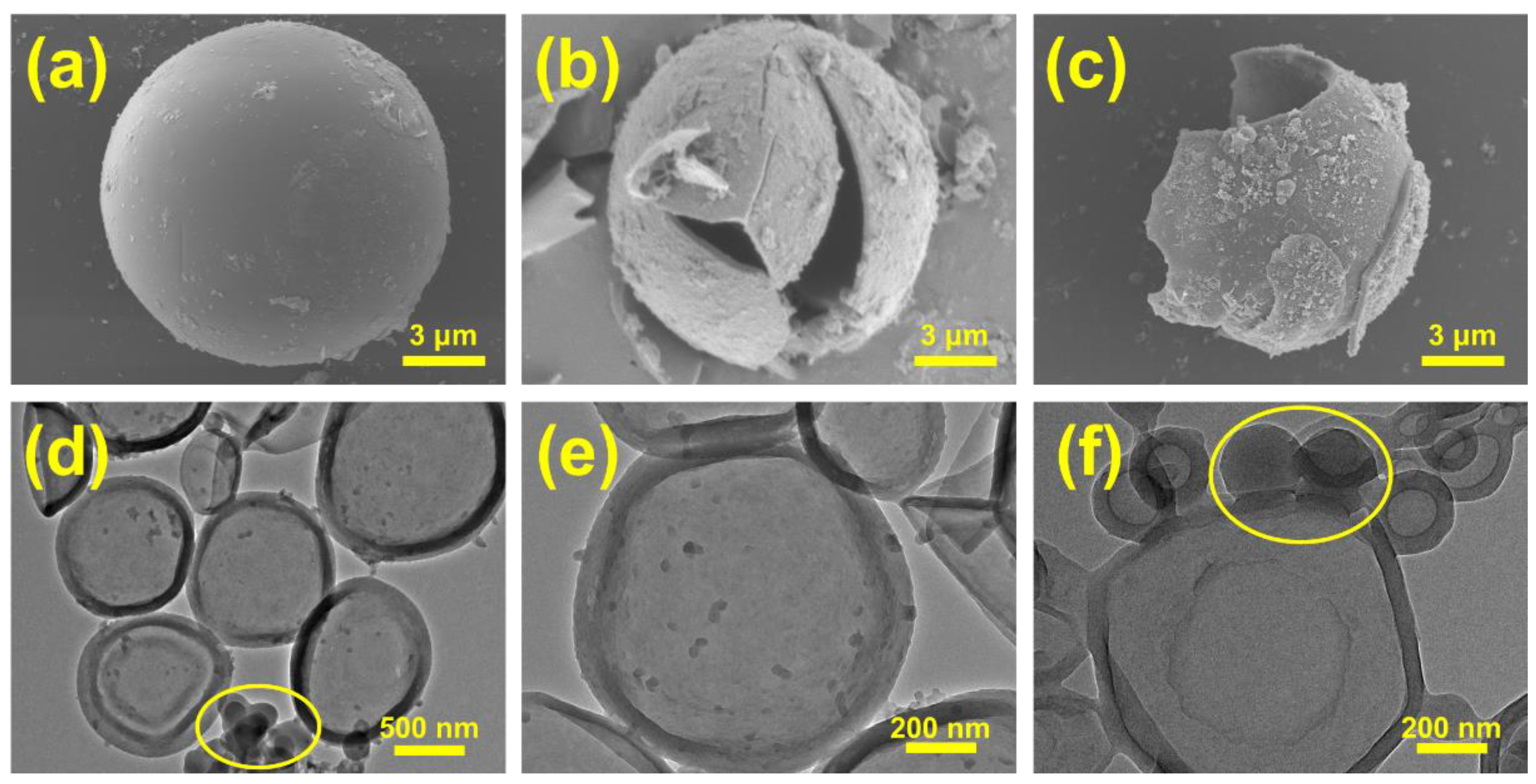

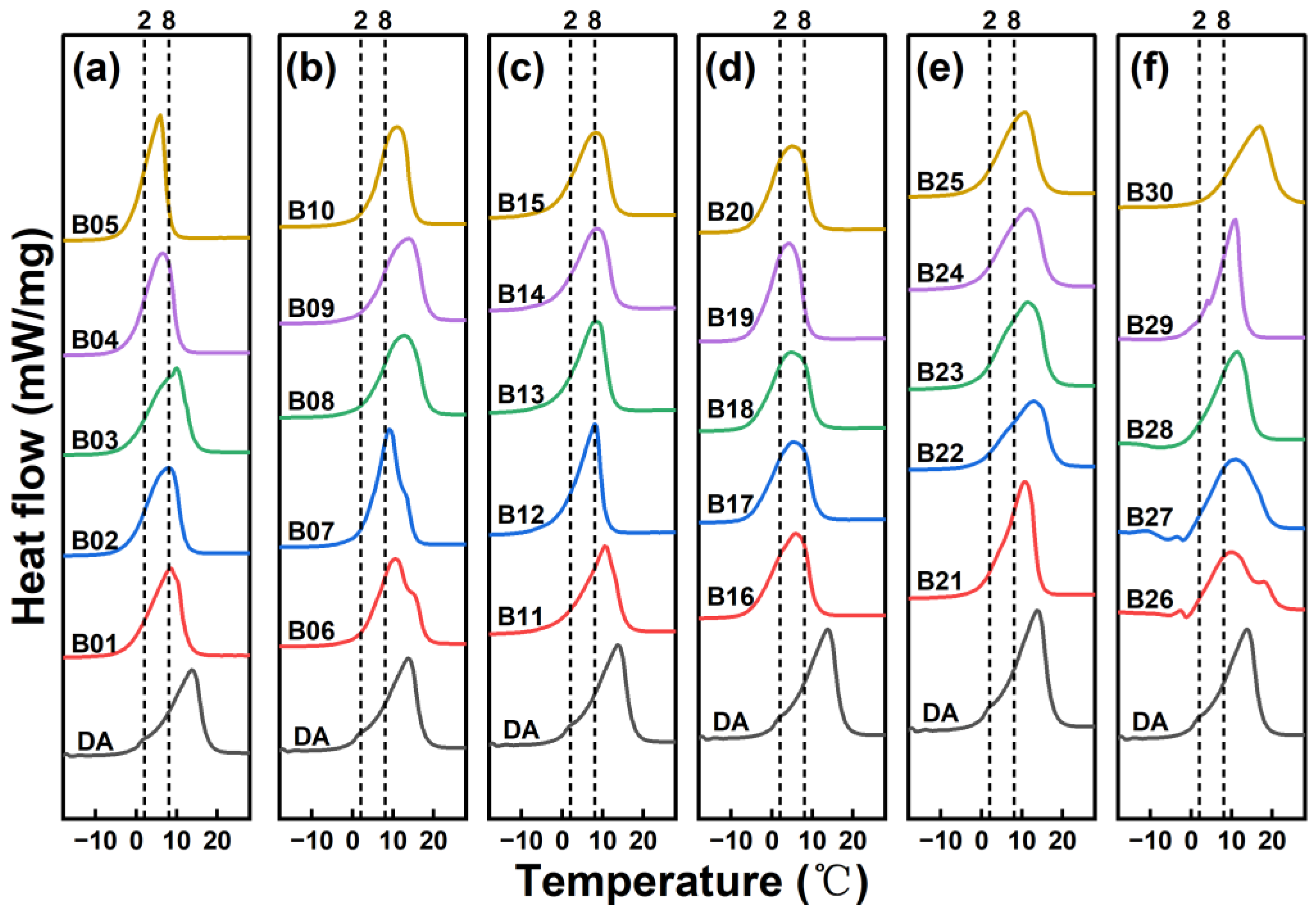
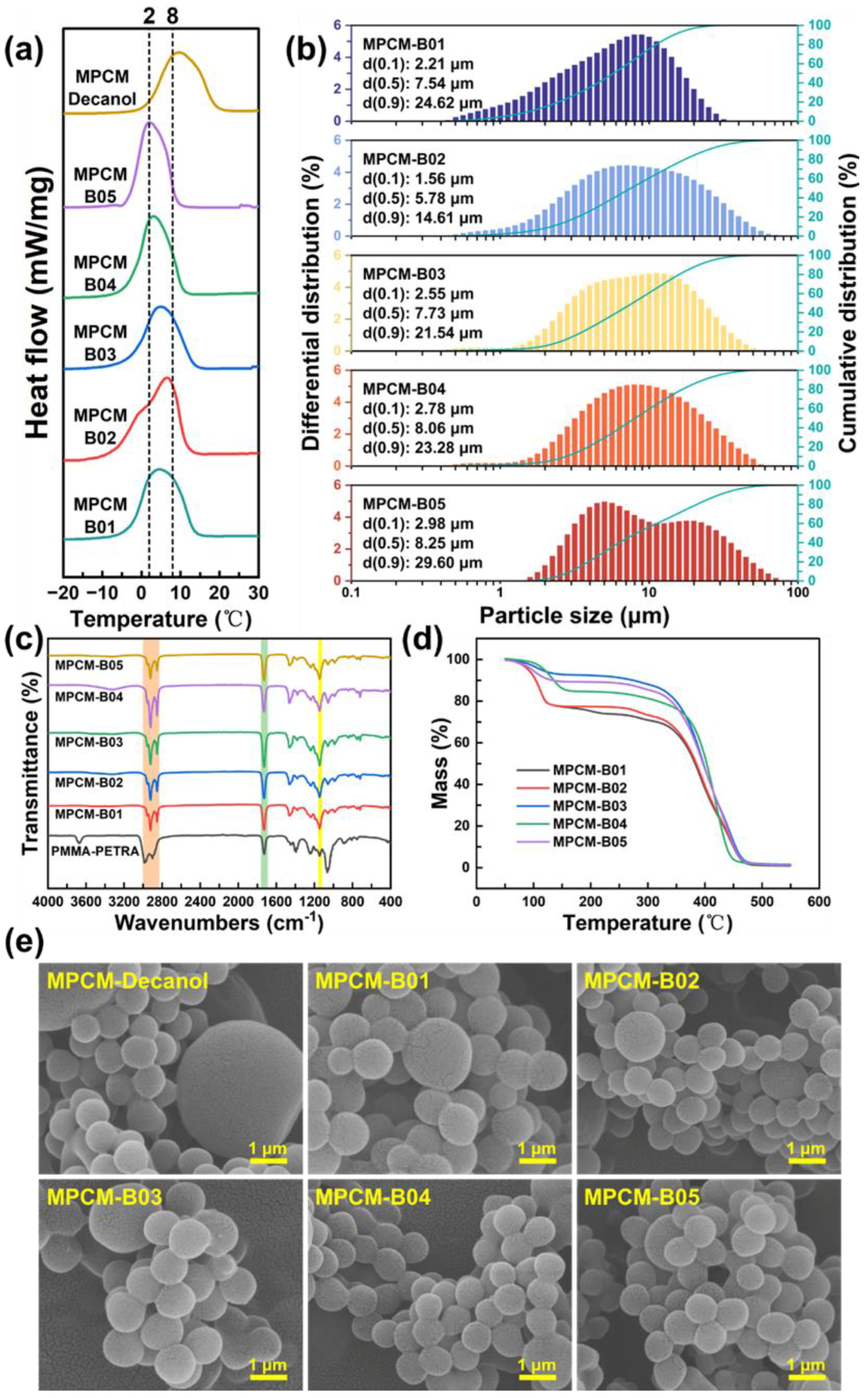
| Samples | Emulsifiers | Ionic Properties | HLB |
|---|---|---|---|
| SMA | 100 wt% SMA | Anionic | 15.4 |
| OP10 | 100 wt%OP10 | Nonionic | 13.3~14.0 |
| MIX1 | 15 wt% Span80/85 wt% Tween80 | Nonionic/Nonionic | 13.4 |
| MIX2 | 75 wt% Span80/25 wt% SDS | Nonionic/Anionic | 13.2 |
| Samples | Onset (°C) | Peak (°C) | Endset (°C) | ΔHm (kJ/kg) | E (%) |
|---|---|---|---|---|---|
| MPCM-3%SMA | 1.8 | 15.5 | 22.0 | 118.4 | 56.8 |
| MPCM-5%SMA | 2.3 | 12.9 | 19.5 | 95.1 | 45.6 |
| MPCM-7%SMA | 2.8 | 10.6 | 15.3 | 74.8 | 35.9 |
| MPCM-3%OP10 | 0.4 | 8.3 | 15.5 | 101.9 | 48.8 |
| MPCM-5%OP10 | −0.2 | 7.2 | 13.6 | 92.8 | 44.5 |
| MPCM-7%OP10 | −0.1 | 7.0 | 15.7 | 86.5 | 41.5 |
| MPCM-3%MIX1 | 1.7 | 7.1 | 12.9 | 58.9 | 28.2 |
| MPCM-5%MIX1 | 1.2 | 6.0 | 11.0 | 71.0 | 34.0 |
| MPCM-7%MIX1 | 1.6 | 7.8 | 15.2 | 95.8 | 45.9 |
| MPCM-3%MIX2 | −2.4 | 4.6 | 8.5 | 26.1 | 12.5 |
| MPCM-5%MIX2 | 0.5 | 5.1 | 11.5 | 60.8 | 29.1 |
| MPCM-7%MIX2 | 0.3 | 10.3 | 16.7 | 98.7 | 47.3 |
| Samples | Onset (°C) | Peak (°C) | Endset (°C) | ΔHm (kJ/kg) | E (%) |
|---|---|---|---|---|---|
| MPCM-150W | 2 | 6.5 | 11.6 | 53.4 | 25.7 |
| MPCM-225W | 2.5 | 8.7 | 14.9 | 103.6 | 49.8 |
| MPCM-300W | 2.1 | 10.3 | 18.1 | 116.6 | 56.1 |
| MPCM-375W | 3 | 10.6 | 17.6 | 113.9 | 54.8 |
| MPCM-450W | 2.9 | 9.7 | 18.2 | 110.1 | 52.9 |
| MPCM-525W | 2.9 | 7.8 | 12.6 | 81.8 | 39.3 |
| MPCM-4 min | 1.8 | 12.4 | 19 | 95.1 | 45.7 |
| MPCM-6 min | 1.2 | 10.2 | 18.5 | 104.4 | 50.2 |
| MPCM-8 min | 1.2 | 9 | 18.3 | 119.4 | 57.4 |
| MPCM-10 min | 1.4 | 9.7 | 19 | 126.1 | 60.6 |
| MPCM-12 min | 1.4 | 12.2 | 21.5 | 126.7 | 60.9 |
| MPCM-14 min | −0.5 | 1.4 | 2.7 | 13.6 | 6.5 |
| Component 1 | Mole Fraction (%) | Component 2 | Mole Fraction (%) | Eutectic Point (K) |
|---|---|---|---|---|
| 1-decanol | 57.1 | 1-tetradecane | 42.9 | 265.3 |
| 1-decanol | 79.4 | 1-hexadecane | 20.6 | 271.2 |
| 1-decanol | 61.3 | 1-undecanol | 38.7 | 266.6 |
| 1-decanol | 76.1 | 1-dodecanol | 23.9 | 270.5 |
| 1-decanol | 73.8 | Capric acid | 26.2 | 269.9 |
| 1-decanol | 90.1 | Lauric acid | 9.9 | 273.8 |
| Samples | Components | Mole Ratio | Onset (°C) | Peak (°C) | Endset (°C) | ΔHm (kJ/kg) |
|---|---|---|---|---|---|---|
| B01 | Decanol/Tetradecane | 51.1:48.9 | −1.8 | 8.5 | 12.4 | 215.9 |
| B02 | 54.1:45.9 | −2.1 | 8.0 | 12.0 | 221.0 | |
| B03 | 57.1:42.9 | −2.2 | 10.0 | 14.5 | 231.6 | |
| B04 | 60.1:39.9 | −1.9 | 6.5 | 10.5 | 225.0 | |
| B05 | 63.1:36.9 | −2.2 | 5.9 | 8.2 | 217.3 | |
| B06 | Decanol/Hexadecane | 73.4:26.6 | 2.0 | 10.6 | 18.7 | 214.8 |
| B07 | 76.4:23.6 | 2.6 | 9.3 | 13.3 | 223.7 | |
| B08 | 79.4:20.6 | 2.6 | 12.8 | 18.8 | 216.8 | |
| B09 | 82.4:17.6 | 2.9 | 13.8 | 18.7 | 221.2 | |
| B10 | 85.4:14.6 | 3.2 | 10.8 | 15.1 | 209.8 | |
| B11 | Decanol/Undecanol | 55.3:44.7 | 0.8 | 10.8 | 15.8 | 194.0 |
| B12 | 58.3:41.7 | −0.7 | 8.1 | 10.6 | 192.7 | |
| B13 | 61.3:38.7 | −1.1 | 8.1 | 12.4 | 195.3 | |
| B14 | 64.3:35.7 | −1.7 | 8.6 | 13.2 | 190.4 | |
| B15 | 67.3:32.7 | −2.0 | 8.3 | 13.0 | 196.3 | |
| B16 | Decanol/Dodecanol | 70.1:29.9 | −5.0 | 5.8 | 10.8 | 180.5 |
| B17 | 73.1:26.9 | −5.0 | 5.5 | 11.1 | 180.6 | |
| B18 | 76.1:23.9 | −4.7 | 4.8 | 11.0 | 176.8 | |
| B19 | 79.1:20.9 | −4.4 | 4.3 | 8.8 | 183.4 | |
| B20 | 82.1:17.9 | −4.3 | 5.1 | 10.4 | 183.5 | |
| B21 | Decanol/Capric acid | 67.8:32.2 | 1.8 | 10.8 | 14.2 | 186.3 |
| B22 | 70.8:29.2 | −1.1 | 12.8 | 18.2 | 150.0 | |
| B23 | 73.8:26.2 | −1.1 | 11.4 | 17.0 | 189.7 | |
| B24 | 76.8:23.2 | −1.0 | 11.4 | 16.7 | 174.2 | |
| B25 | 79.8:20.2 | −0.9 | 10.6 | 15.6 | 169.6 | |
| B26 | Decanol/Lauric acid | 84.1:15.9 | 0.4 | 22.7 | 22.7 | 149.1 |
| B27 | 87.1:12.9 | 0.1 | 20.2 | 20.2 | 165.2 | |
| B28 | 90.1:9.9 | 0.3 | 15.9 | 15.9 | 163.2 | |
| B29 | 93.1:6.9 | 0.5 | 13.3 | 13.3 | 172.7 | |
| B30 | 96.1:3.9 | 3.6 | 22.3 | 22.2 | 180.1 |
| Samples | Onset (°C) | Peak (°C) | Endset (°C) | ΔHm (kJ/kg) | E (%) |
|---|---|---|---|---|---|
| MPCM-B01 | −2.8 | 4.5 | 13.6 | 144.3 | 66.8 |
| MPCM-B02 | −3.7 | 6.5 | 12.8 | 136.8 | 61.9 |
| MPCM-B03 | −2.7 | 5.2 | 13.5 | 130.4 | 56.3 |
| MPCM-B04 | −2.8 | 3.0 | 10.7 | 135.6 | 60.3 |
| MPCM-B05 | −3.0 | 2.2 | 8.8 | 138.8 | 63.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xu, Y.; Zhao, H.; Cao, R.; Huang, B.; Xu, L. Preparation and Characterization of Microencapsulated Phase Change Materials with Enhanced Thermal Performance for Cold Storage. Materials 2025, 18, 2074. https://doi.org/10.3390/ma18092074
Wang Y, Xu Y, Zhao H, Cao R, Huang B, Xu L. Preparation and Characterization of Microencapsulated Phase Change Materials with Enhanced Thermal Performance for Cold Storage. Materials. 2025; 18(9):2074. https://doi.org/10.3390/ma18092074
Chicago/Turabian StyleWang, Yang, Yunchuan Xu, Haojie Zhao, Ruilin Cao, Bei Huang, and Lingling Xu. 2025. "Preparation and Characterization of Microencapsulated Phase Change Materials with Enhanced Thermal Performance for Cold Storage" Materials 18, no. 9: 2074. https://doi.org/10.3390/ma18092074
APA StyleWang, Y., Xu, Y., Zhao, H., Cao, R., Huang, B., & Xu, L. (2025). Preparation and Characterization of Microencapsulated Phase Change Materials with Enhanced Thermal Performance for Cold Storage. Materials, 18(9), 2074. https://doi.org/10.3390/ma18092074





