Fabrication and Properties of Zn-Containing Intermetallic Compounds as Sacrificial Anodes of Zn-Based Implants
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Intermetallic Compounds
2.2. Phase Structure and Microstructure Characterizations of the Intermetallic Compounds
2.3. Microhardness Tests of the Intermetallic Compounds
2.4. Thermal Stability Tests of the Intermetallic Compounds
2.5. Electrochemical Tests of the Intermetallic Compounds
2.6. Immersion Tests of the Intermetallic Compounds
2.7. Cytotoxicity Tests of the Intermetallic Compounds
3. Results and Discussion
3.1. Phase Structures of the Intermetallic Compounds
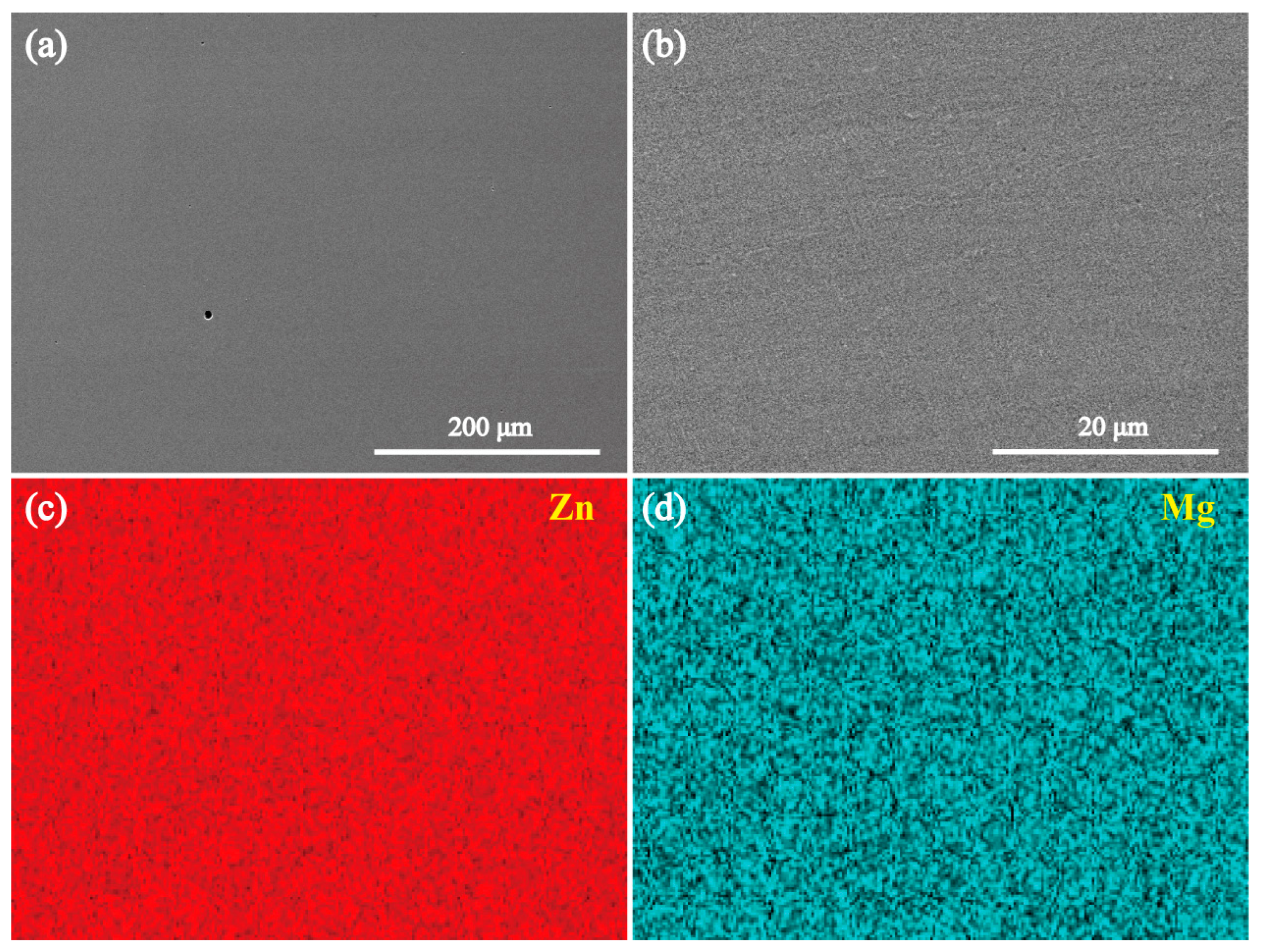
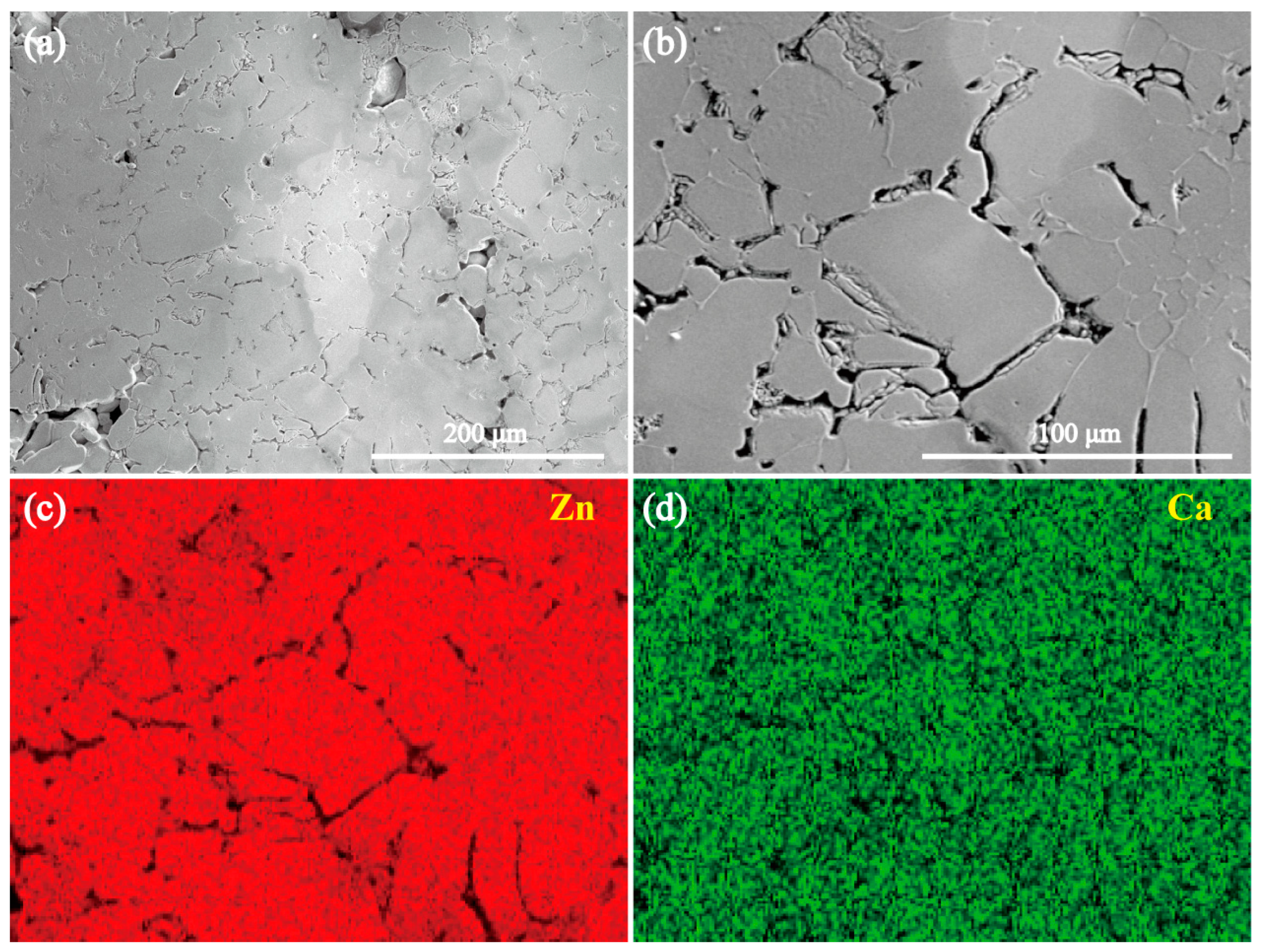
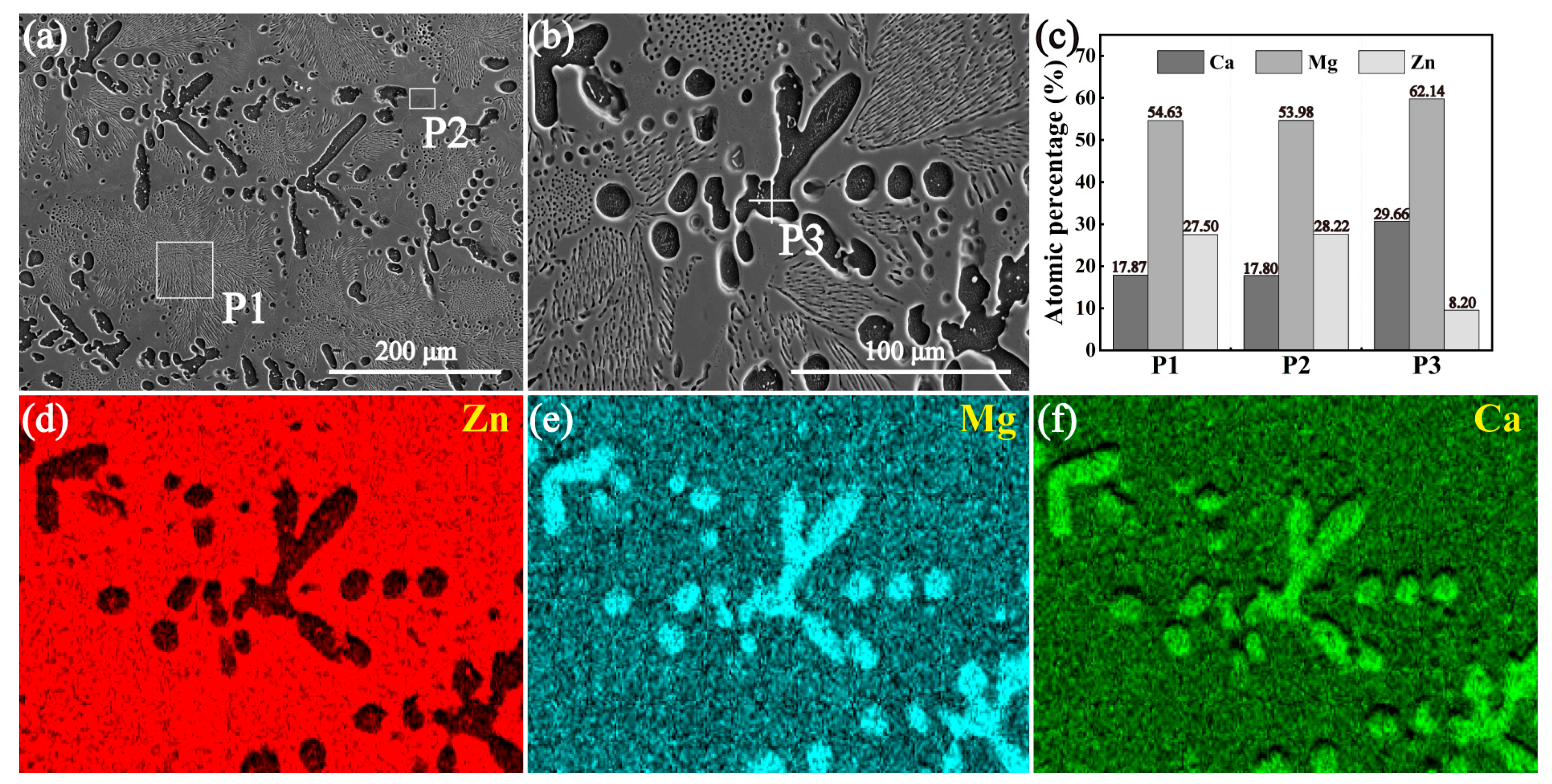
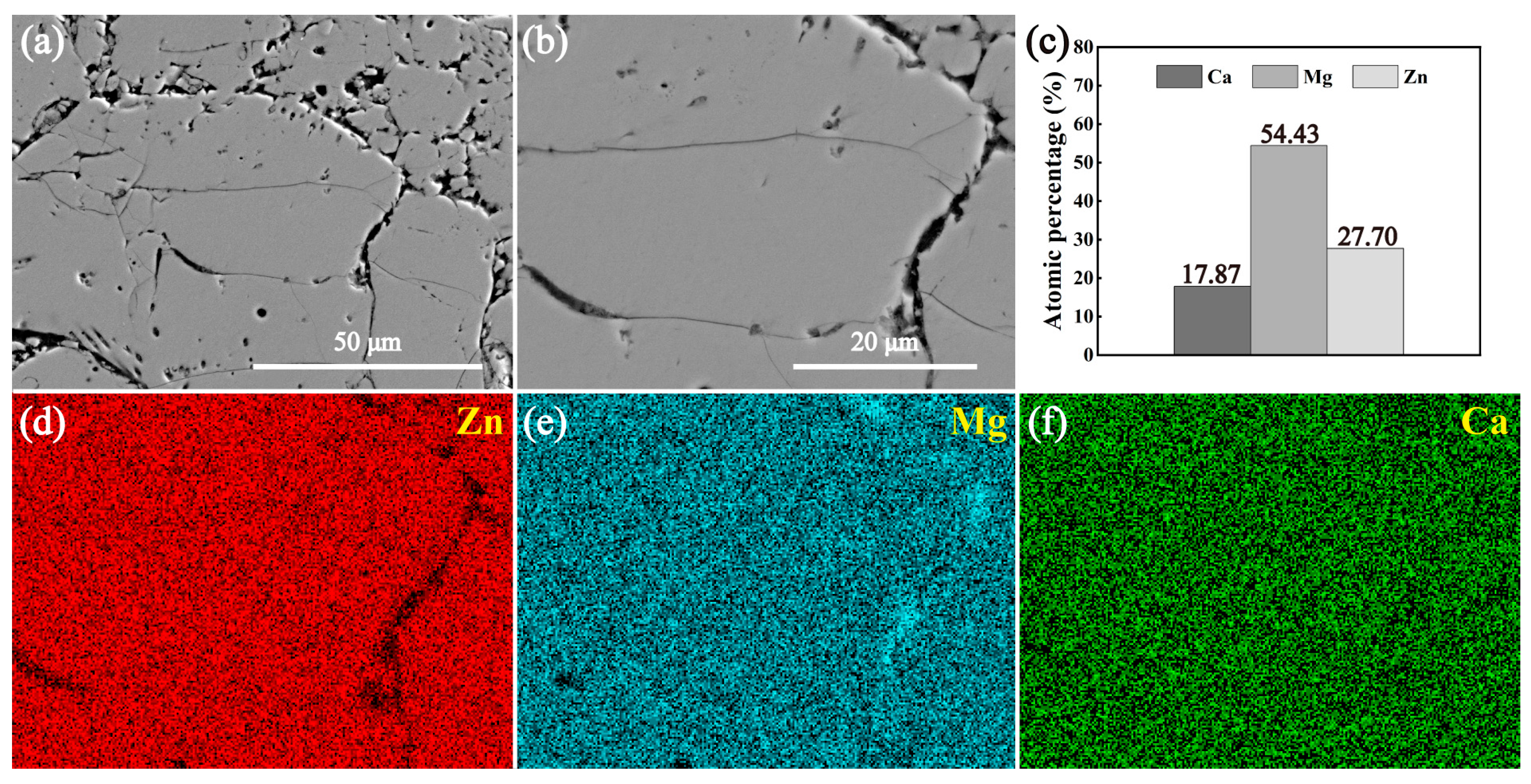
3.2. Microstructures of the Intermetallic Compounds
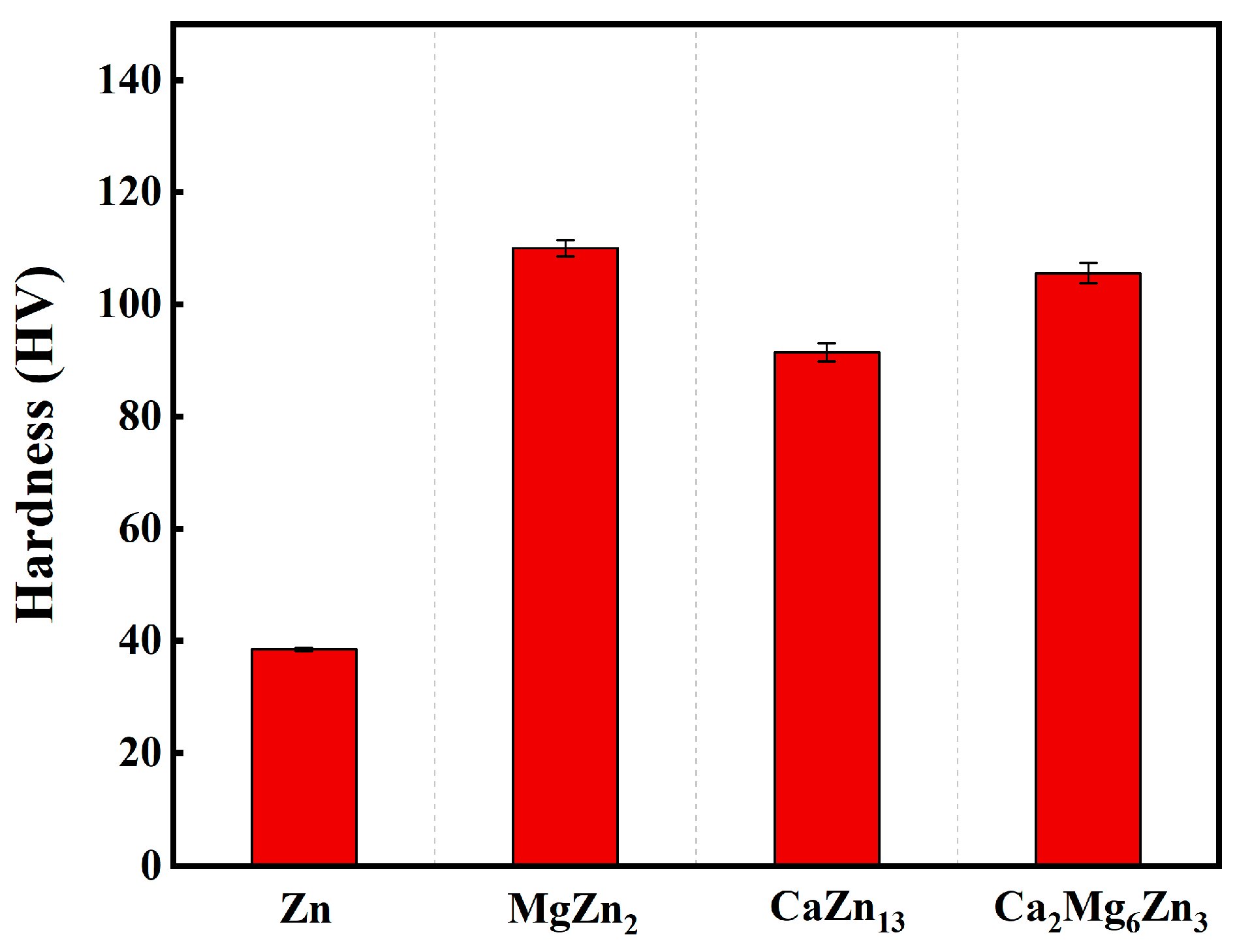
3.3. Microhardness of the Intermetallic Compounds
3.4. DSC/TG Curves of the Intermetallic Compounds
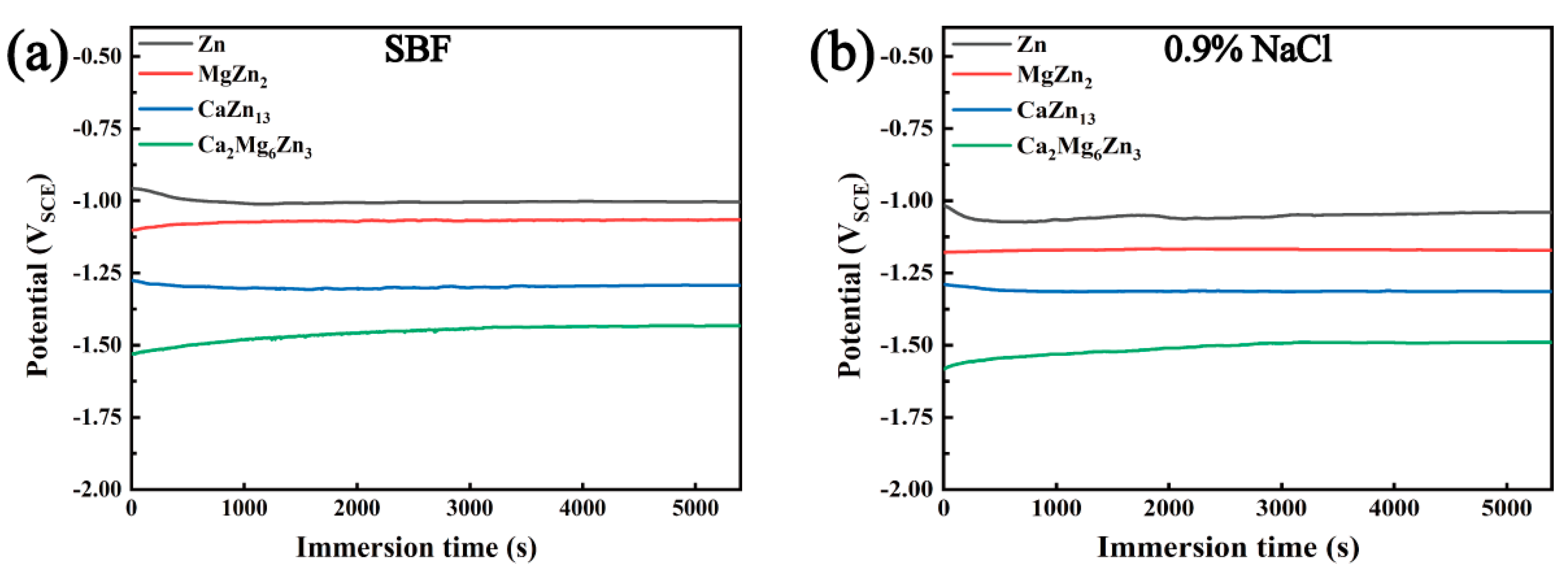
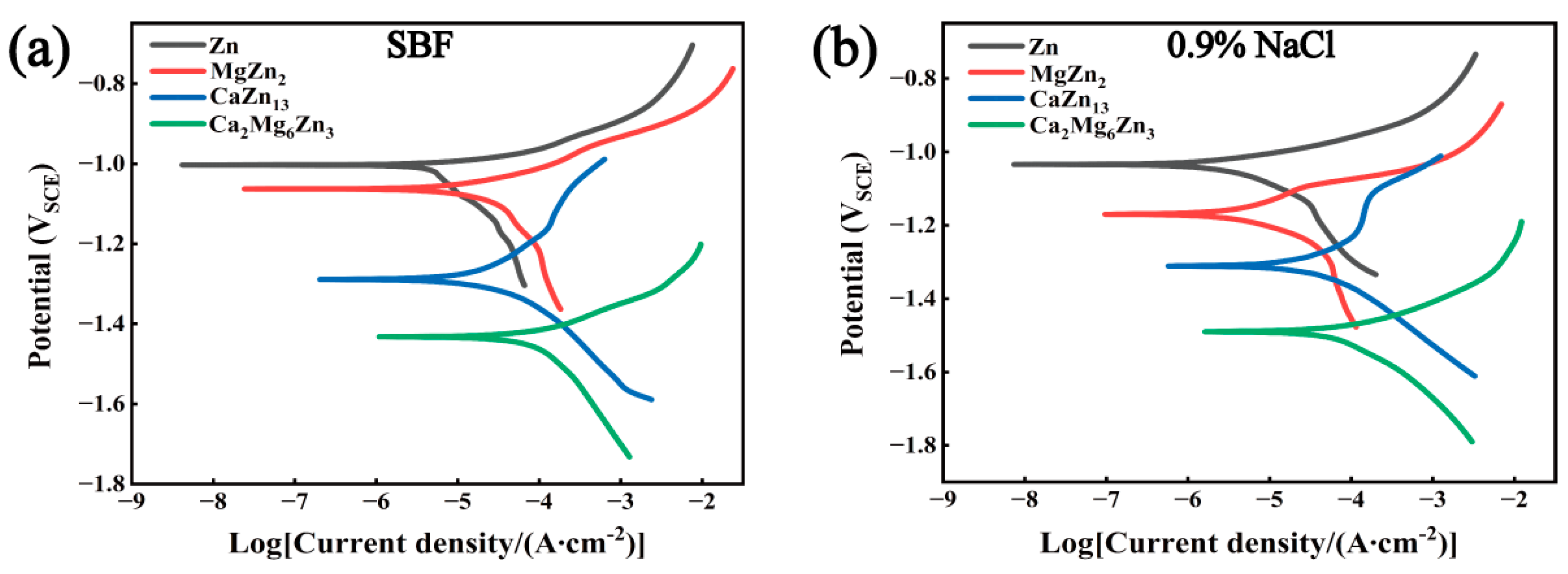
3.5. Polarization Curves of the Intermetallic Compounds
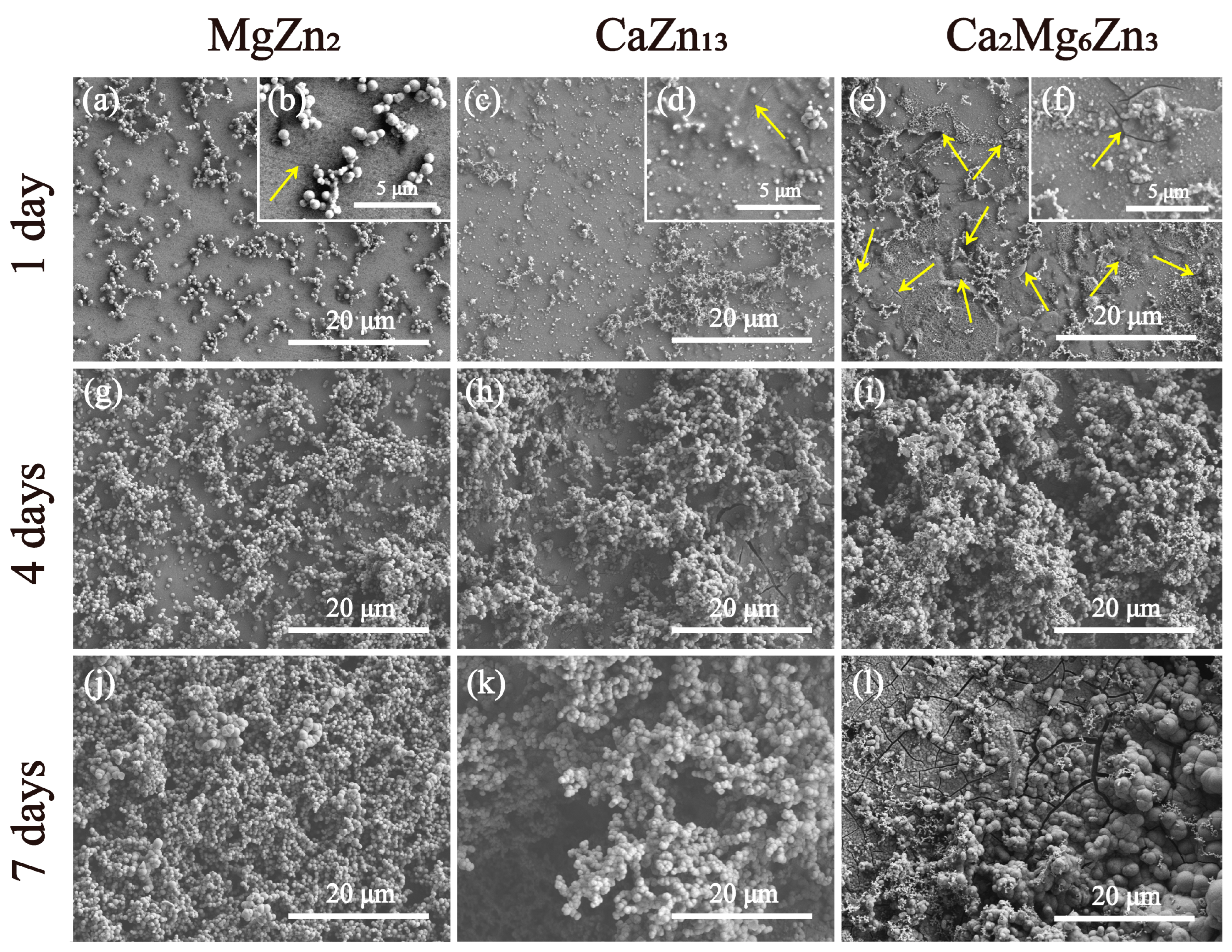
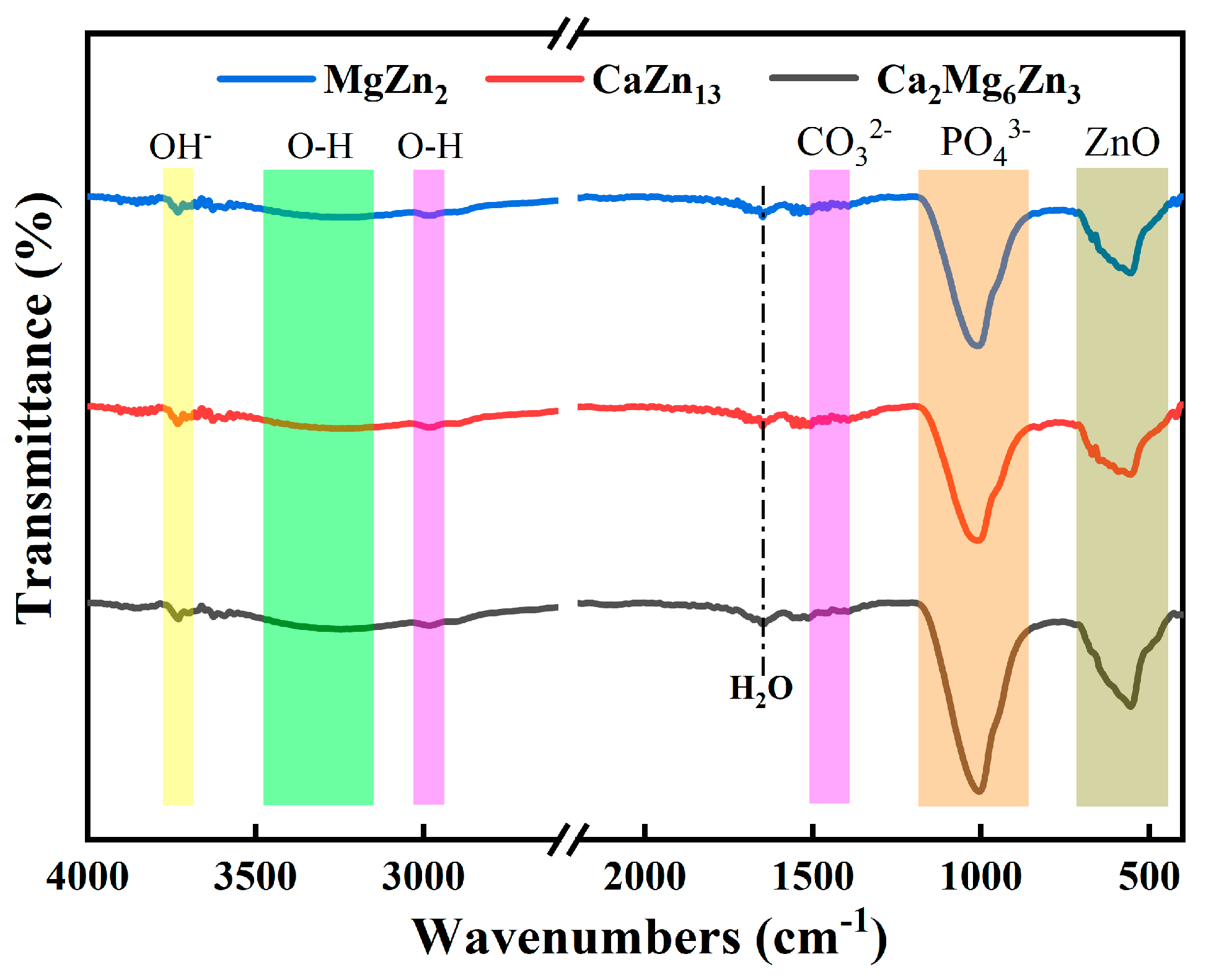
3.6. Degradation Behaviors of the Intermetallic Compounds in Corrosive Solutions
3.7. Cytotoxicity of the Intermetallic Compounds
4. Conclusions
- Both the annealed MgZn2 and CaZn13 specimens have high purity, while the annealed Ca2Mg6Zn3 specimen contains not only Ca2Mg6Zn3 phase but also Mg2Ca phase. After purifying the annealed Ca2Mg6Zn3 specimen (soaking in NaCl solution for some time), high purity Ca2Mg6Zn3 phase was obtained.
- The MgZn2 and CaZn13 specimens exhibited good thermal stability below 773 K. However, the Ca2Mg6Zn3 specimen melted between 719.7 and 750.9 K.
- The three intermetallic compounds were degraded according to the atomic ratio of the elements in each compound in corrosive media.
- The corrosion potentials of the three intermetallic compounds are all lower than that of pure Zn, that is, the three compounds can act as sacrificial anodes in Zn-matrix composites. Among the three compounds, MgZn2 has the highest corrosion potential (−1.063 VSCE in SBF or −1.170 VSCE in 0.9% NaCl solution), and Ca2Mg6Zn3 exhibits the lowest corrosion potential (−1.432 VSCE in SBF or −1.490 VSCE in 0.9% NaCl solution).
- The degradation rates of the three intermetallic compounds in corrosive media are all greater than that of pure Zn. Among the three compounds, MgZn2 has the lowest corrosion current density (12.03 µA/cm2 in SBF or 4.37 µA/cm2 in 0.9% NaCl solution), and Ca2Mg6Zn3 exhibits the highest corrosion current density (66.23 µA/cm2 in SBF or 69.49 µA/cm2 in 0.9% NaCl solution). After immersion in SBF for 7 days, localized corrosion occurred on the MgZn2. CaZn13 and Ca2Mg6Zn3 experienced severe corrosion during 7 days of immersion tests.
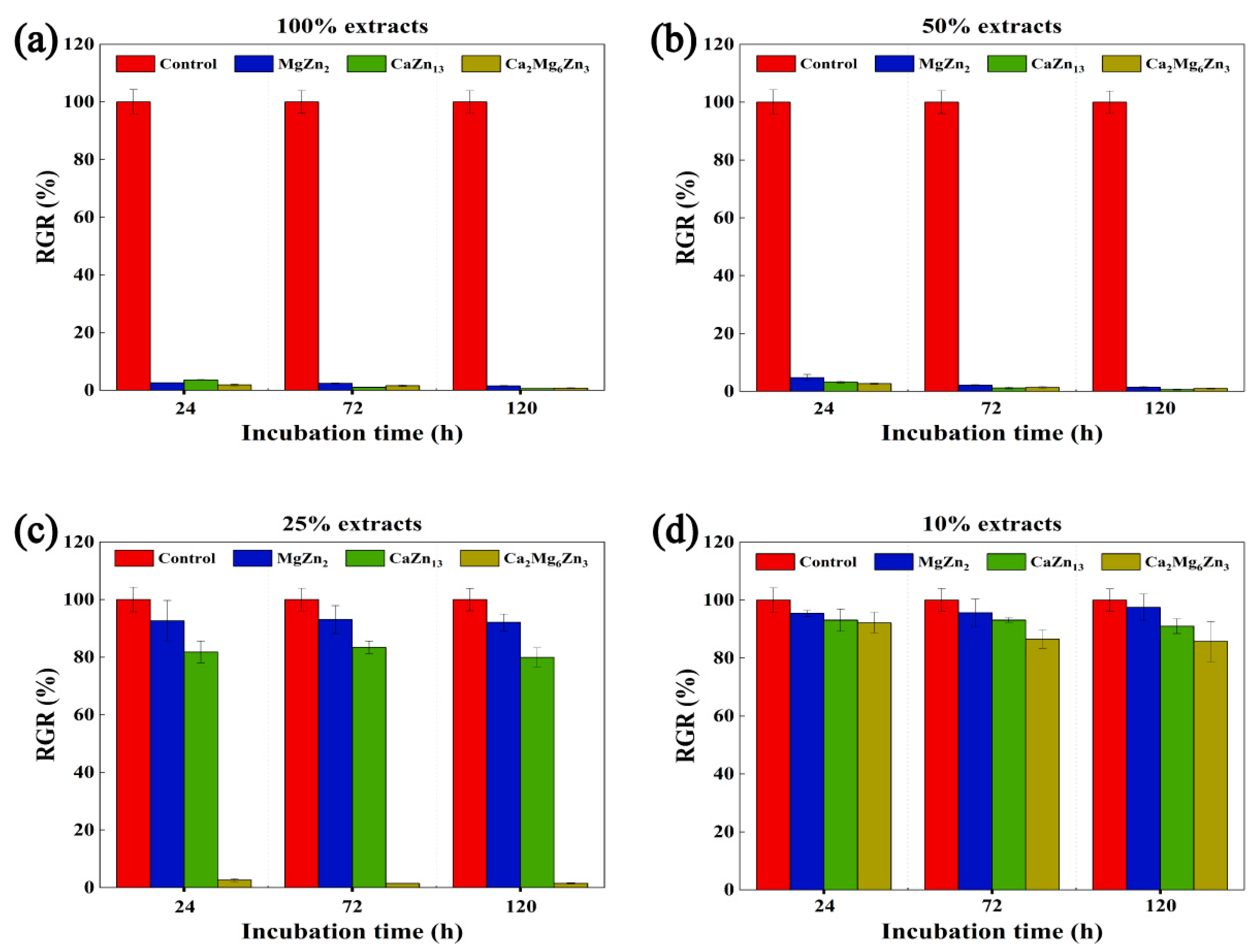
- The rapid degradation of the three compounds led to greater Zn2+ dissolution, resulting in severe cytotoxicity. But when the extracts were diluted to 10%, all extracts exhibited good cell activity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc exhibits ideal physiological corrosion behavior for bioabsorbable stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef] [PubMed]
- Fosmire, G.J. Zinc toxicity. Am. J. Clin. Nutr. 1990, 51, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.S.; Li, K.L.; Wang, T.B.; Wang, X.; Qi, Y.M.; Zhao, L.C.; Cui, C.X. Preparation and properties of biodegradable porous Zn-Mg-Y alloy scaffolds. J. Mater. Sci. 2024, 59, 8441–8464. [Google Scholar] [CrossRef]
- Shi, Z.Z.; Li, X.M.; Yao, S.L.; Tang, Y.Z.; Ji, X.J.; Wang, Q.; Gao, X.X.; Wang, L.N. 300 MPa grade biodegradable high-strength ductile low-alloy (BHSDLA) Zn-Mn-Mg alloys: An in vitro study. J. Mater. Sci. Technol. 2023, 138, 233–244. [Google Scholar] [CrossRef]
- Niu, K.N.; Zhang, D.C.; Qi, F.G.; Lin, J.G.; Dai, Y.L. Achieving high strength and antibacterial Zn-4Ag-Mn alloy with homogenous corrosion behavior via high-pressure solid solution. Trans. Nonferrous Met. Soc. China 2024, 34, 2231–2244. [Google Scholar] [CrossRef]
- Ji, H.Z.; Shen, G.; Liu, H.H.; Liu, Y.; Qian, J.Y.; Wan, G.J.; Luo, E. Biodegradable Zn-2Cu-0.5Zr alloy promotes the bone repair of senile osteoporotic fractures via the immune-modulation of macrophages. Bioact. Mater. 2024, 38, 422–437. [Google Scholar] [CrossRef]
- Liu, A.B.; Qin, Y.; Dai, J.B.; Song, F.; Tian, Y.; Zheng, Y.F.; Wen, P. Fabrication and performance of zinc-based biodegradable metals: From conventional processes to laser powder bed fusion. Bioact. Mater. 2024, 41, 312–335. [Google Scholar] [CrossRef]
- Liu, A.B.; Lu, Y.P.; Dai, J.B.; Wen, P.; Xia, D.D.; Zheng, Y.F. Mechanical properties, in vitro biodegradable behavior, biocompatibility and osteogenic ability of additively manufactured Zn-0.8Li-0.1Mg alloy scaffolds. Biomater. Adv. 2023, 153, 213571. [Google Scholar] [CrossRef]
- Lu, G.; Chen, C.; Zhang, D.C.; Guo, L.; Lin, J.G.; Dai, Y.L. Optimization of mechanical, corrosion properties and cytotoxicity of biodegradable Zn-Mn alloys by synergy of high-pressure solidification and cold rolling process. J. Alloys Comped. 2024, 1005, 175988. [Google Scholar] [CrossRef]
- Guan, Z.P.; Qian, J.Y.; Qin, H.T.; Hou, J.M.; Zhou, Y.K.; Xie, Z.H.; Yu, F.; Chen, Y.Q.; Wan, G.J.; Zeng, H. Osteogenerative and corrosion-decelerating teriparatide-mediated strontium-zinc phosphate hybrid coating on biodegradable zinc-copper alloy for orthopaedic applications. Mater. Today Commun. 2024, 39, 109010. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, H.T.; Liu, A.B.; Dai, J.B.; Wen, P.; Zheng, Y.F.; Tian, Y.; Li, S.; Wang, X.G. Processing optimization, mechanical properties, corrosion behavior and cytocompatibility of additively manufactured Zn-0.7Li biodegradable metals. Acta Biomater. 2022, 142, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.W.; Ma, A.B.; Jiang, J.H.; Song, D.; Liu, H.; Guo, S.S. Research status and future prospects of biodegradable Zn-Mg alloys. J. Alloys Comped. 2024, 993, 174669. [Google Scholar] [CrossRef]
- Niu, K.N.; Zhang, D.C.; Qi, F.G.; Lin, J.G.; Dai, Y.L. The effects of Cu and Mn on the microstructure, mechanical, corrosion properties and biocompatibility of Zn-4Ag alloy. J. Mater. Res. Technol. 2022, 21, 4969–4981. [Google Scholar] [CrossRef]
- Li, P.; Dai, J.T.; Li, Y.G.; Alexander, D.; Čapek, J.; Geis-Gerstorfer, J.; Wan, G.J.; Han, J.M.; Yu, Z.; Li, A. Zinc based biodegradable metals for bone repair and regeneration: Bioactivity and molecular mechanisms. Mater. Today Bio 2024, 25, 100932. [Google Scholar] [CrossRef]
- Li, Y.; Pavanram, P.; Zhou, J.K.; Lietaert, P.; Li, W.; San, H.; Leeflang, M.A.; Mol, J.M.C.; Jahr, H.; Zadpoor, A.A. Additively manufactured biodegradable porous zinc. Acta Biomater. 2020, 101, 609–623. [Google Scholar] [CrossRef]
- Yuan, W.; Xia, D.D.; Wu, S.L.; Zheng, Y.F.; Guan, Z.P.; Rau, J.V. A review on current research status of the surface modification of Zn-based biodegradable metals. Bioact. Mater. 2022, 7, 192–216. [Google Scholar] [CrossRef]
- Venezuela, J.; Dargusch, M.S. The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: A comprehensive review. Acta Biomater. 2019, 87, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, H.; Wang, L.S.; Li, Y.H.; Agbedor, S.O.; Bai, J.; Xue, F.; Jiang, J.H. A high-strength and biodegradable Zn-Mg alloy with refined ternary eutectic structure processed by ECAP. Acta Met. Sin. Engl. 2020, 33, 1191–1200. [Google Scholar] [CrossRef]
- Liu, X.W.; Sun, J.K.; Zhou, F.Y.; Yang, Y.H.; Chang, R.C.; Qiu, K.J.; Pu, Z.J.; Li, L.; Zheng, Y. Micro-alloying with Mn in Zn-Mg alloy for future biodegradable metals application. Mater. Des. 2016, 94, 95–104. [Google Scholar] [CrossRef]
- Xia, D.D.; Qin, Y.; Guo, H.; Wen, P.; Lin, H.; Voshage, M.; Schleifenbaum, J.H.; Cheng, Y.; Zheng, Y.F. Additively manufactured pure zinc porous scaffolds for critical-sized bone defects of rabbit femur. Bioact. Mater. 2023, 19, 12–23. [Google Scholar] [CrossRef]
- Shuai, C.J.; Li, S.; Peng, S.P.; Feng, P.; Lai, Y.X.; Gao, C.D. Biodegradable metallic bone implants. Mater. Chem. Front. 2019, 3, 544–562. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Zhao, L.C.; Yuan, P.K.; Zhang, M.S.; Wang, X.; Qi, Y.M.; Wang, T.B.; Cao, B.; Cui, C.X. Preparation and properties of porous Zn-based scaffolds as biodegradable implants: A review. J. Mater. Sci. 2023, 58, 8275–8316. [Google Scholar] [CrossRef]
- Chen, Y.J.; Xu, Z.G.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.A.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.Z.; Pan, C.; Liu, Y.C.; Liu, D.B.; He, X.H.; Li, X.H.; Sun, X.H. Fabrication, in vitro and in vivo properties of porous Zn-Cu alloy scaffolds for bone tissue engineering. Mater. Chem. Phys. 2022, 289, 126458. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.F.; Chen, X.H.; Yang, J.A.; Pan, H.B.; Chen, D.F.; Wang, L.N.; Zhang, J.L.; Zhu, D.H.; Wu, S.L.; et al. Fundamental theory of biodegradable metals-definition, criteria, and design. Adv. Funct. Mater. 2019, 29, 1805402. [Google Scholar] [CrossRef]
- Yang, H.T.; Qu, X.H.; Lin, W.J.; Chen, D.F.; Zhu, D.H.; Dai, K.R.; Zheng, Y.F. Enhanced osseointegration of Zn-Mg composites by tuning the release of Zn ions with sacrificial Mg-rich anode design. ACS Biomater. Sci. Eng. 2018, 5, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Shi, Z.M.; Xu, L.C.; Lin, J.X.; Zhang, D.C.; Wang, K.; Li, Y.C.; Wen, C.E. Degradation behavior, cytotoxicity, hemolysis, and antibacterial properties of electro-deposited Zn-Cu metal foams as potential biodegradable bone implants. Acta Biomater. 2020, 102, 481–492. [Google Scholar] [CrossRef]
- Zhao, L.C.; Xie, Y.; Zhang, Z.; Wang, X.; Qi, Y.M.; Wang, T.B.; Wang, R.; Cui, C.X. Fabrication and properties of biodegradable ZnO nano-rods/porous Zn scaffolds. Mater. Charact. 2018, 144, 227–238. [Google Scholar] [CrossRef]
- Yao, C.Z.; Wang, Z.C.; Tay, S.L.; Zhu, T.P.; Gao, W. Effects of Mg on microstructure and corrosion properties of Zn-Mg alloy. J. Alloys Comped. 2014, 602, 101–107. [Google Scholar] [CrossRef]
- Byun, J.M.; Yu, J.M.; Kim, D.K.; Kim, T.Y.; Jung, W.S.; Kim, Y.D. Corrosion behavior of Mg2Zn11 and MgZn2 single phases. Korean J. Met. Mater. 2013, 51, 413–419. [Google Scholar] [CrossRef]
- Hagihara, K.; Shakudo, S.; Fujii, K.; Nakano, T. Degradation behavior of Ca-Mg-Zn intermetallic compounds for use as biodegradable implant materials. Mater. Sci. Eng. C.-Mater. 2014, 44, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Wasiur-Rahman, S.; Medraj, M. Critical assessment and thermodynamic modeling of the binary Mg-Zn, Ca-Zn and ternary Mg-Ca-Zn systems. Intermetallics 2009, 17, 847–864. [Google Scholar] [CrossRef]
- Zhao, L.C.; Zhang, Z.; Song, Y.T.; Liu, S.J.; Qi, Y.M.; Wang, X.; Wang, Q.Z.; Cui, C.X. Mechanical properties and in vitro biodegradation of newly developed porous Zn scaffolds for biomedical applications. Mater. Des. 2016, 108, 136–144. [Google Scholar] [CrossRef]
- Yuan, P.K.; Zhang, M.S.; Wang, X.; Qi, Y.M.; Wang, T.B.; Zhao, L.C.; Cui, C.X. Effects of polylactic acid coating on properties of porous Zn scaffolds as degradable materials. Mater. Charact. 2023, 199, 112852. [Google Scholar] [CrossRef]
- Spencer, P.J.; Pelton, A.D.; Kang, Y.B.; Chartrand, P.; Fuerst, C.D. Thermodynamic assessment of the Ca-Zn, Sr-Zn, Y-Zn and Ce-Zn systems. Calphad 2008, 32, 423–431. [Google Scholar] [CrossRef]
- Dong, J.H.; Lin, T.; Shao, H.P.; Wang, H.; Dong, Y.X.; Bai, T.S.; Fan, M. Degradation behavior of MgZn2 and Mg2Zn3 intermetallic compounds in simulated body fluid. Mater. Lett. 2023, 347, 134637. [Google Scholar] [CrossRef]
- Okido, M.; Kuroda, K.; Ishikawa, M.; Ichino, R.; Takai, O. Hydroxyapatite coating on titanium by means of thermal substrate method in aqueous solutions. Solid State Ion. 2002, 151, 47–52. [Google Scholar] [CrossRef]
- Liu, X.; Yang, W.; Shen, D.N.; Cheng, Y.; Chen, D.F.; Zheng, Y.F. Exploring the biodegradation of pure Zn under simulated inflammatory condition. Corros. Sci. 2021, 189, 109606. [Google Scholar] [CrossRef]
- Guo, H.; Xia, D.D.; Zheng, Y.F.; Zhu, Y.; Liu, Y.S.; Zhou, Y.S. A pure zinc membrane with degradability and osteogenesis promotion for guided bone regeneration: In vitro and in vivo studies. Acta Biomater. 2020, 106, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.X.; Wang, X.; Xu, F.F.; Dai, T.Q.; Zhou, J.G.; Liu, J.; Song, K.; Tian, L.; Liu, B.; Liu, Y.P. In vivo biocompatibility and degradability of a Zn-Mg-Fe alloy osteosynthesis system. Bioact. Mater. 2022, 7, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Young, J.; Reddy, R.G. Synthesis, mechanical properties, and in vitro corrosion behavior of biodegradable Zn-Li-Cu alloys. J. Alloys Compd. 2020, 844, 156257. [Google Scholar] [CrossRef]
- Jamesh, M.; Kumar, S.; Sankara, T.S.N. Electrodeposition of hydroxyapatite coating on magnesium for biomedical applications. J. Coat. Technol. Res. 2012, 9, 495–502. [Google Scholar] [CrossRef]
- Seyedraoufi, Z.S.; Mirdamadi, S. Effects of pulse electrodeposition parameters and alkali treatment on the properties of nano hydroxyapatite coating on porous Mg-Zn scaffold for bone tissue engineering application. Mater. Chem. Phys. 2014, 148, 519–527. [Google Scholar] [CrossRef]
- Zhao, L.C.; Wang, X.; Wang, T.B.; Xia, Y.H.; Cui, C.X. Mechanical properties and biodegradation of porous Zn-1Al alloy scaffolds. Mater. Lett. 2019, 247, 75–78. [Google Scholar] [CrossRef]
- Su, Y.C.; Yang, H.T.; Gao, J.L.; Qin, Y.X.; Zheng, Y.F.; Zhu, D.H. Interfacial zinc phosphate is the key to controlling biocompatibility of metallic zinc implants. Adv. Sci. 2019, 6, 1900112. [Google Scholar] [CrossRef]
- Yang, H.T.; Wang, C.; Liu, C.Q.; Chen, H.W.; Wu, Y.F.; Han, J.N.; Jia, Z.C.; Lin, W.J.; Zhang, D.Y.; Li, W.T.; et al. Evolution of the degradation mechanism of pure zinc stent in the one-year study of rabbit abdominal aorta model. Biomaterials 2017, 145, 92–105. [Google Scholar] [CrossRef]
- Drelich, A.J.; Zhao, S.; Guillory, R.J.; Drelich, J.W.; Goldman, J. Long-term surveillance of zinc implant in murine artery: Surprisingly steady biocorrosion rate. Acta Biomater. 2017, 58, 539–549. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, P.K.; Wang, X.; Wang, T.B.; Zhao, L.C.; Cui, C.X. Fabrication and properties of Zn-3Mg-1Ti alloy as a potential biodegradable implant material. Materials 2022, 15, 940. [Google Scholar] [CrossRef]






| Number | Reagent | Concentration (g/L) |
|---|---|---|
| 1 | NaCl | 8.035 |
| 2 | NaHCO3 | 0.355 |
| 3 | KCl | 0.225 |
| 4 | K2HPO4∙3H2O | 0.231 |
| 5 | MgCl2∙6H2O | 0.311 |
| 6 | CaCl2 | 0.292 |
| 7 | Na2SO4 | 0.072 |
| Specimens | SBF | 0.9% NaCl | ||
|---|---|---|---|---|
| Ecorr (VSCE) | Icorr (µA/cm2) | Ecorr (VSCE) | Icorr (µA/cm2) | |
| Pure Zn | −1.003 | 5.84 | −1.034 | 3.53 |
| MgZn2 | −1.063 | 12.03 | −1.170 | 4.37 |
| CaZn13 | −1.289 | 12.93 | −1.311 | 31.66 |
| Ca2Mg6Zn3 | −1.432 | 66.23 | −1.490 | 69.49 |
| Specimens | Metal Ion Concentration (mmol/L) | Concentration | ||
|---|---|---|---|---|
| Ca2+ | Mg2+ | Zn2+ | Ratio | |
| MgZn2 | 34.81 ± 1.52 | 65.19 ± 1.50 | Mg2+/Zn2+ = 1/1.87 | |
| CaZn13 | 7.00 ± 1.67 | 93.00 ± 1.67 | Ca2+/Zn2+ = 1/13.29 | |
| Ca2Mg6Zn3 | 17.63 ± 1.02 | 54.08 ± 1.24 | 28.29 ± 0.26 | Ca2+/Mg2+/Zn2+ = 1/3.07/1.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Li, J.; Wang, T.; Wang, X.; Qi, Y.; Zhao, L.; Cui, C. Fabrication and Properties of Zn-Containing Intermetallic Compounds as Sacrificial Anodes of Zn-Based Implants. Materials 2025, 18, 2057. https://doi.org/10.3390/ma18092057
Li K, Li J, Wang T, Wang X, Qi Y, Zhao L, Cui C. Fabrication and Properties of Zn-Containing Intermetallic Compounds as Sacrificial Anodes of Zn-Based Implants. Materials. 2025; 18(9):2057. https://doi.org/10.3390/ma18092057
Chicago/Turabian StyleLi, Kelei, Junwei Li, Tiebao Wang, Xin Wang, Yumin Qi, Lichen Zhao, and Chunxiang Cui. 2025. "Fabrication and Properties of Zn-Containing Intermetallic Compounds as Sacrificial Anodes of Zn-Based Implants" Materials 18, no. 9: 2057. https://doi.org/10.3390/ma18092057
APA StyleLi, K., Li, J., Wang, T., Wang, X., Qi, Y., Zhao, L., & Cui, C. (2025). Fabrication and Properties of Zn-Containing Intermetallic Compounds as Sacrificial Anodes of Zn-Based Implants. Materials, 18(9), 2057. https://doi.org/10.3390/ma18092057








