Preparation of Diamond Nanofluids and Study of Lubrication Properties
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
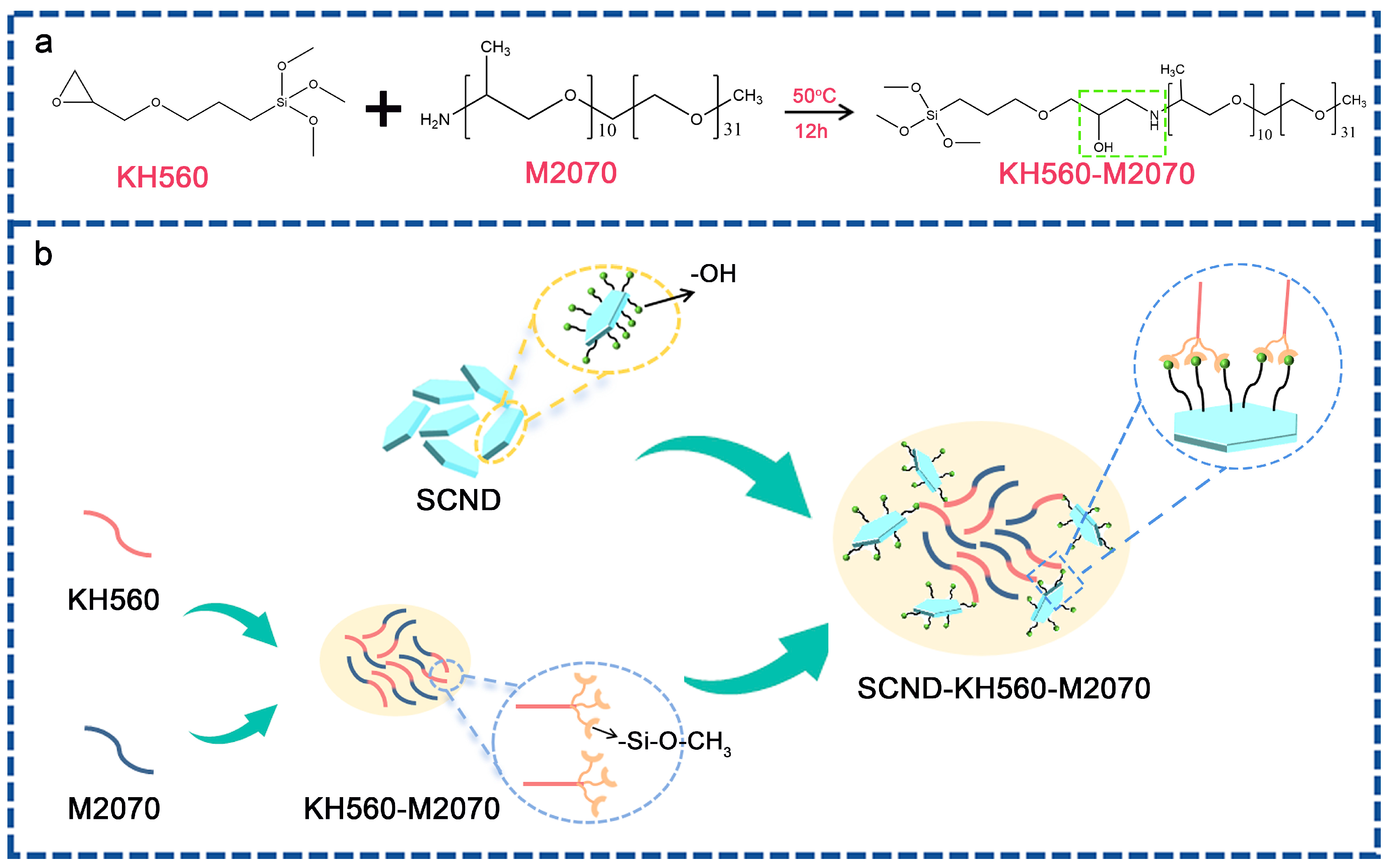
2.2. Preparation of the Solvent-Free 2D Diamond Nanofluid
2.3. Materials Characterization
2.4. Tribological Test
3. Results and Discussion
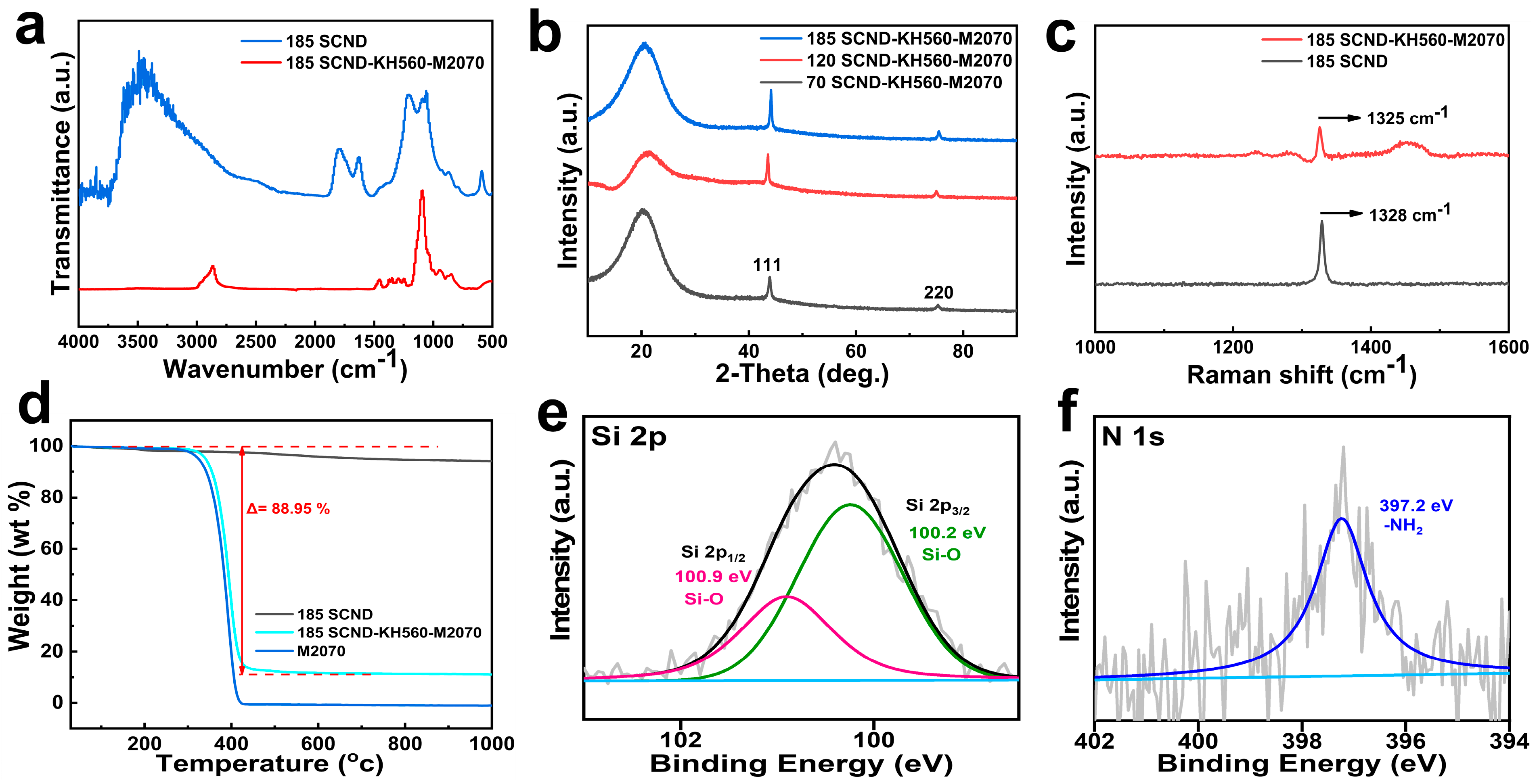
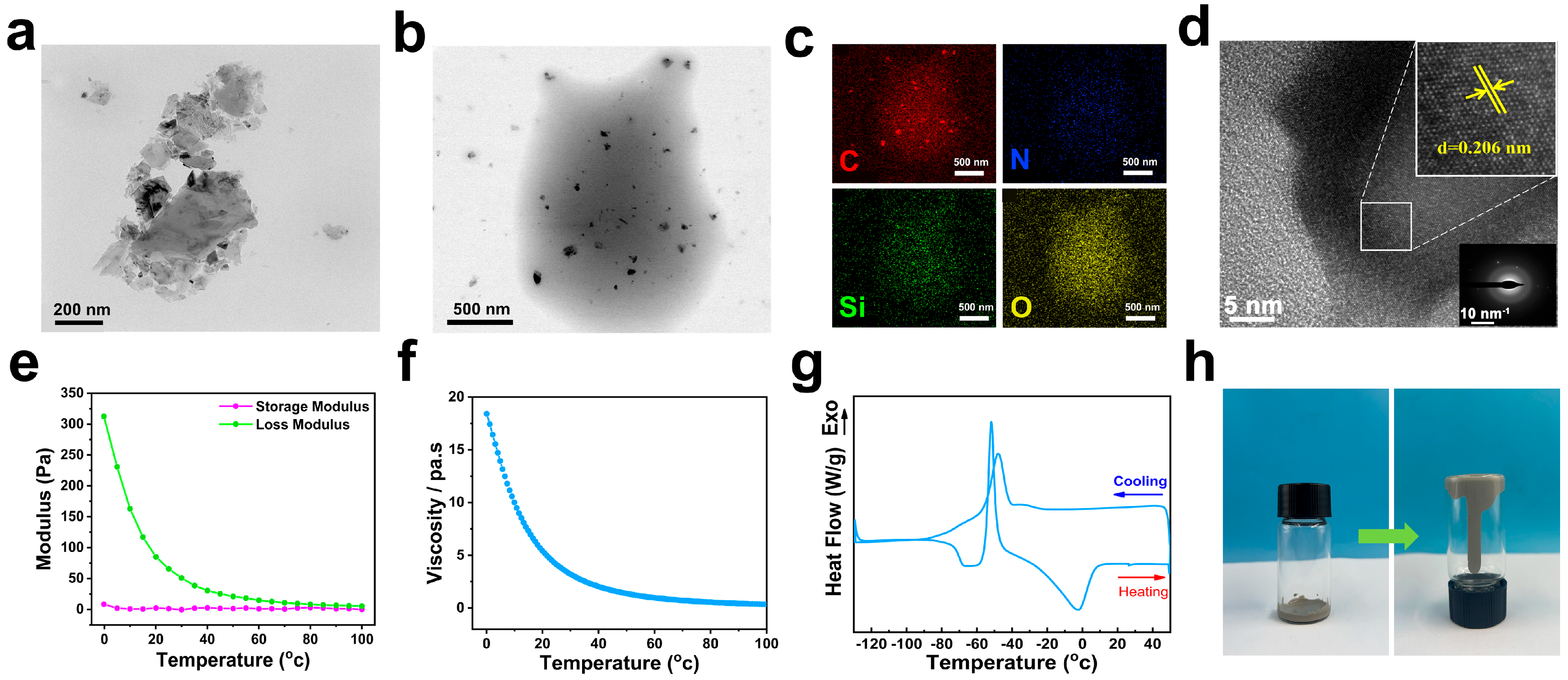
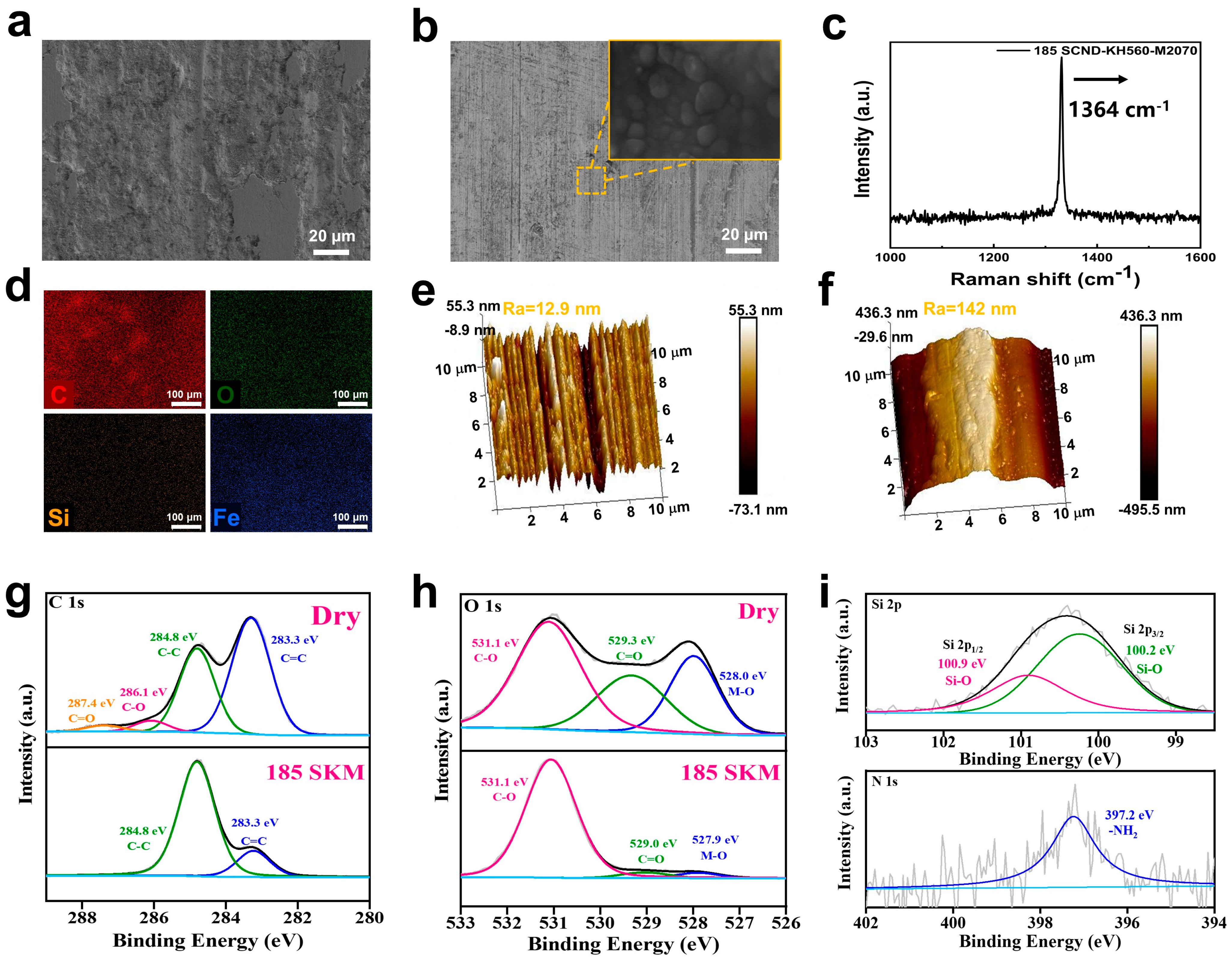
3.1. Characterization of the Solvent-Free 2D Diamond Nanofluids
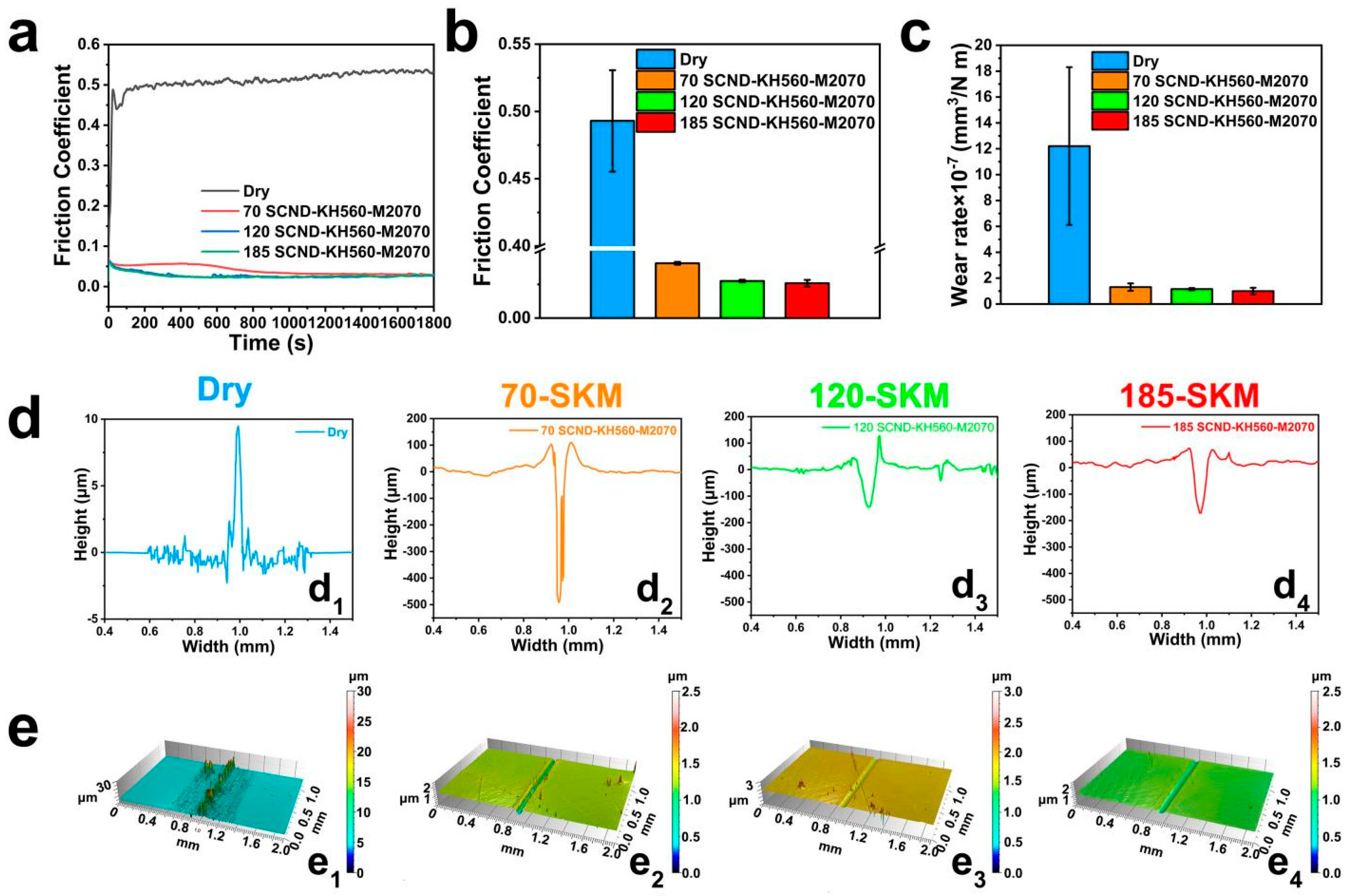
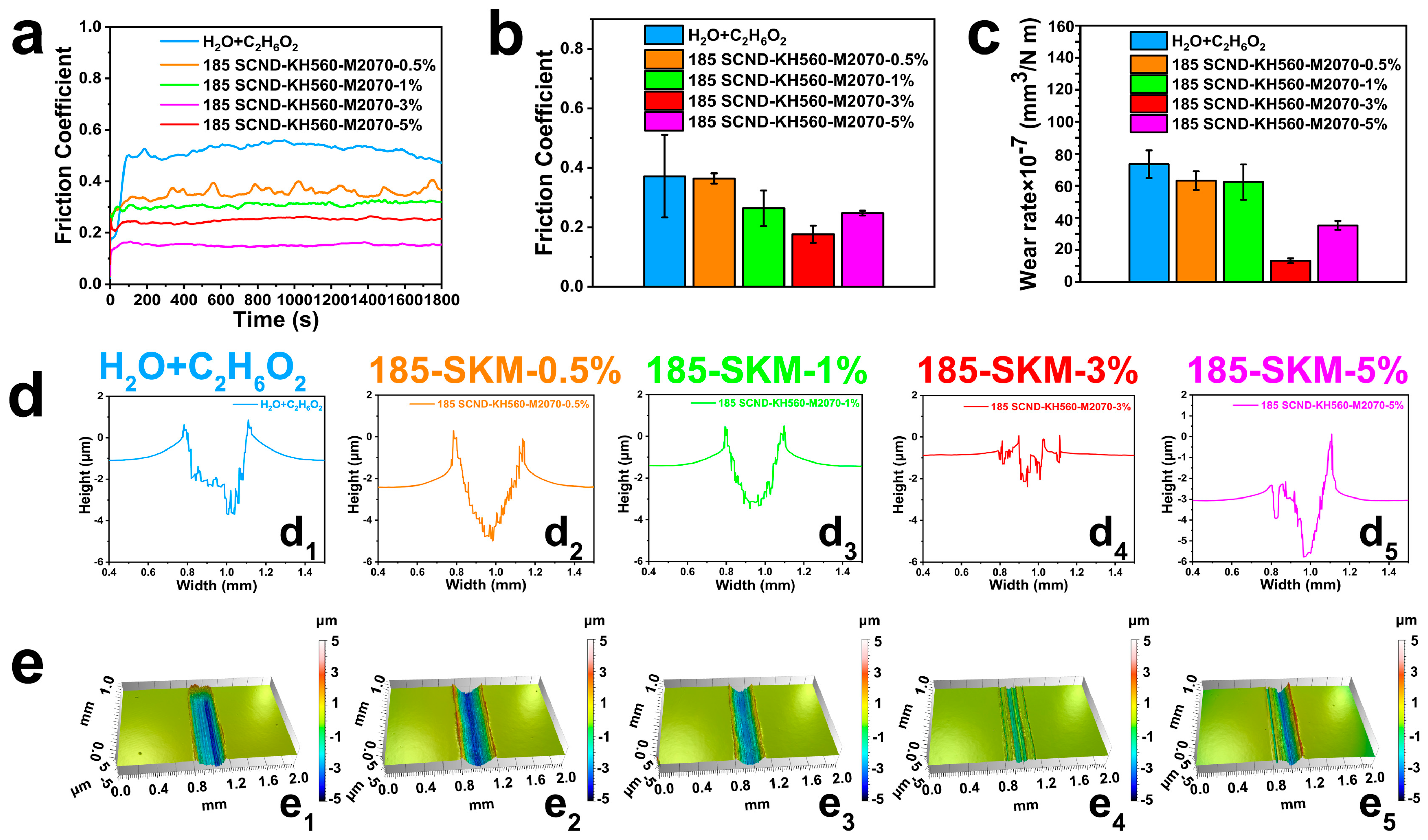
3.2. Tribological Properties
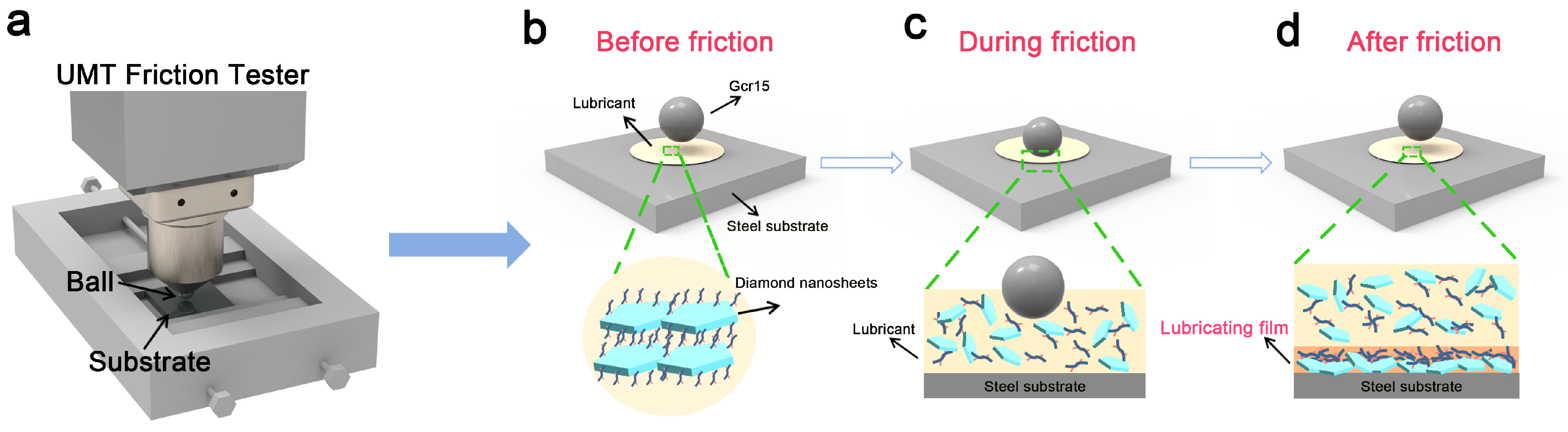
3.3. Lubrication Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, T.; Erdemir, A.; Li, Q. Tribology of two-dimensional materials: From mechanisms to modulating strategies. Mater. Today 2019, 26, 67–86. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, S.; Wang, W. Nanodiamond plates as macroscale solid lubricant: A “non-layered” two-dimension material. Carbon 2022, 198, 119–131. [Google Scholar] [CrossRef]
- Wang, W.; He, Y.; Zhao, J.; Mao, J.; Hu, Y.; Luo, J. Optimization of groove texture profile to improve hydrodynamic lubrication performance: Theory and experiments. Friction 2020, 8, 83–94. [Google Scholar] [CrossRef]
- Nyholm, N.; Espallargas, N. Functionalized carbon nanostructures as lubricant additives—A review. Carbon 2023, 201, 1200–1228. [Google Scholar] [CrossRef]
- Kole, M.; Dey, T.K. Effect of aggregation on the viscosity of copper oxide–gear oil nanofluids. Int. J. Therm. Sci. 2011, 50, 1741–1747. [Google Scholar] [CrossRef]
- Nascimento, A.R.C.; Chromik, R.R.; Schulz, R. Mechanical properties and wear resistance of industrial bearing liners in concentrated boundary-lubricated sliding. Wear 2021, 477, 203806. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Zhang, H.; Jiang, Z.; Sun, K.; Fan, J.; Tang, Y. The tribological properties of nano-lubricants and their application on bearings: Recent research progress. Int. J. Adv. Manuf. Technol. 2024, 134, 3051–3082. [Google Scholar] [CrossRef]
- Stephan, S.; Schmitt, S.H.; Hasse, H.; Urbassek, H.M. Molecular dynamics simulation of the Stribeck curve: Boundary lubrication, mixed lubrication, and hydrodynamic lubrication on the atomistic level. Friction 2023, 11, 2342–2366. [Google Scholar] [CrossRef]
- Dang, R.K.; Goyal, D.; Chauhan, A.; Dhami, S.S. Numerical and Experimental Studies on Performance Enhancement of Journal Bearings Using Nanoparticles Based Lubricants. Arch. Comput. Methods Eng. 2021, 28, 3887–3915. [Google Scholar] [CrossRef]
- Nadooshan, A.A.; Esfe, M.H.; Afrand, M. Evaluation of rheological behavior of 10W40 lubricant containing hybrid nano-material by measuring dynamic viscosity. Phys. Low-Dimens. Syst. Nanostructures 2017, 92, 47–54. [Google Scholar] [CrossRef]
- Chen, L.; Li, N.; Yang, B.; Zhang, J. Constructing high load-carrying capacity dual-layer film assembled from alkylsilane and semi-spherical molecules. Microelectron. Eng. 2016, 152, 1–5. [Google Scholar] [CrossRef]
- Snapp, P.; Kim, J.M.; Cho, C.; Leem, J.; Haque, M.F.; Nam, S. Interaction of 2D materials with liquids: Wettability, electrochemical properties, friction, and emerging directions. NPG Asia Mater. 2020, 12, 22. [Google Scholar] [CrossRef]
- Tapasztó, O.; Balko, J.; Puchý, V.; Kun, P.; Dobrik, G.; Fogarassy, Z.; Horváth, Z.E.; Dusza, J.; Balázsi, K.; Balázsi, C.; et al. Highly wear-resistant and low-friction Si3N4 composites by addition of graphene nanoplatelets approaching the 2D limit. Sci. Rep. 2017, 7, 10087. [Google Scholar] [CrossRef]
- Hao, L.; Hao, W.; Li, P.; Liu, G.; Li, H.; Aljabri, A.; Xie, Z. Friction and Wear Properties of a Nanoscale Ionic Liquid-like GO@SiO2 Hybrid as a Water-Based Lubricant Additive. Lubricants 2022, 10, 125. [Google Scholar] [CrossRef]
- Marian, M.; Berman, D.; Nečas, D.; Emami, N.; Ruggiero, A.; Rosenkranz, A. Roadmap for 2D materials in biotribological/biomedical applications—A review. Adv. Colloid Interface Sci. 2022, 307, 102747. [Google Scholar] [CrossRef]
- Wu, L.; Zhong, Y.; Yuan, H.; Liang, H.; Wang, F.; Gu, L. Ultra-dispersive sulfonated graphene as water-based lubricant additives for enhancing tribological performance. Tribol. Int. 2022, 174, 107759. [Google Scholar] [CrossRef]
- Cui, Y.; Xue, S.; Chen, X.; Bai, W.; Liu, S.; Ye, Q.; Zhou, F. Fabrication of two-dimensional MXene nanosheets loading Cu nanoparticles as lubricant additives for friction and wear reduction. Tribol. Int. 2022, 176, 107934. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, F.; Yang, K.; Xiong, Y.; Tang, J.; Chen, H.; Duan, M.; Li, Z.; Zhang, H.; Xiong, B. Review of two-dimensional nanomaterials in tribology: Recent developments, challenges and prospects. Adv. Colloid Interface Sci. 2023, 321, 103004. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Kedia, D.; Dilbaghi, N.; Tankeshwar, K.; Tankeshwar, K.; Kim, K.-H. Nanodiamonds: Emerging face of future nanotechnology. Carbon 2019, 143, 678–699. [Google Scholar] [CrossRef]
- Rasheed, A.K.; Khalid, M.; Rashmi, W.; Gupta, T.; Chan, A. Graphene based nanofluids and nanolubricants—Review of recent developments. Renew. Sustain. Energy Rev. 2016, 63, 346–362. [Google Scholar] [CrossRef]
- Kong, L.; Sun, J.; Bao, Y. Preparation, characterization and tribological mechanism of nanofluids. RSC Adv. 2017, 7, 12599–12609. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, L.; Zhang, G.; Wang, D.; Wang, T.; Wang, Q. High lubricity and electrical responsiveness of solvent-free ionic SiO2 nanofluids. J. Mater. Chem. A 2018, 6, 2817–2827. [Google Scholar] [CrossRef]
- Bao, Z.; Bing, N.; Zhu, X.; Xie, H.; Yu, W. Ti3C2Tx MXene contained nanofluids with high thermal conductivity, super colloidal stability and low viscosity. Chem. Eng. J. 2021, 406, 126390. [Google Scholar] [CrossRef]
- Mousavi, S.B.; Heris, S.Z.; Hosseini, M.G. Experimental investigation of MoS2/diesel oil nanofluid thermophysical and rheological properties. Int. Commun. Heat Mass Transf. 2019, 108, 104298. [Google Scholar] [CrossRef]
- Segu, D.Z.; Chae, Y.; Lee, S.-J.; Kim, C.-L. Synergistic influences of laser surface texturing and ZrO2-MoDTC hybrid nanofluids for enhanced tribological performance. Tribol. Int. 2023, 183, 108377. [Google Scholar] [CrossRef]
- Mishra, K.K.; Panda, K.; Kumar, N.; Malpani, D.; Ravindran, T.; Khatri, O.P. Nanofluid lubrication and high pressure Raman studies of oxygen functionalized graphene nanosheets. J. Ind. Eng. Chem. 2018, 61, 97–105. [Google Scholar] [CrossRef]
- Wang, D.; Xin, Y.; Wang, Y.; Li, X.; Wu, H.; Zhang, W.; Yao, D.; Wang, H.; Zheng, Y.; He, Z. A general way to transform Ti3C2Tx MXene into solvent-free fluids for filler phase applications. Chem. Eng. J. 2021, 409, 128082. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Luo, J. Superlubricity of nanodiamonds glycerol colloidal solution between steel surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 400–406. [Google Scholar] [CrossRef]
- Tang, J.; Liu, S.; Liu, W.; Wang, Y.; Li, L.; Li, Z.; Wang, J. Comparative study on tribological performance and mechanism of eco-friendly solvent-free covalent MXene nanofluids in glycerin and polyethylene glycol. Tribol. Int. 2023, 190, 109051. [Google Scholar] [CrossRef]
- Ilyas, S.U.; Shamsuddin, R.; Xiang, T.K.; Estellé, P.; Pendyala, R. Rheological profile of graphene-based nanofluids in thermal oil with hybrid additives of carbon nanotubes and nanofibers. J. Mol. Liq. 2023, 376, 121443. [Google Scholar] [CrossRef]
- Ghasemi, R.; Fazlali, A.; Mohammadi, A.H. Effects of TiO2 nanoparticles and oleic acid surfactant on the rheological behavior of engine lubricant oil. J. Mol. Liq. 2018, 268, 925–930. [Google Scholar] [CrossRef]
- Esfe, M.H.; Alidoust, S.; Ardeshiri, E.M.; Toghraie, D. Comparative rheological study on hybrid nanofluids with the same structure of MWCNT (50%)-ZnO (50%)/SAE XWX to select the best performance of nano-lubricants using response surface modeling. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128543. [Google Scholar] [CrossRef]
- Liu, S.; Gao, Q.; Hou, K.; Li, Z.; Wang, J.; Yang, S. Solvent-free covalent MXene nanofluid: A new lubricant combining the characteristics of solid and liquid lubricants. Chem. Eng. J. 2023, 462, 142238. [Google Scholar] [CrossRef]
- Esfe, M.H.; Esfandeh, S. Investigation of rheological behavior of hybrid oil based nanolubricant-coolant applied in car engines and cooling equipments. Appl. Therm. Eng. 2018, 131, 1026–1033. [Google Scholar] [CrossRef]
- Liu, X.; Xu, N.; Li, W.; Zhang, M.; Chen, L.; Lou, W.; Wang, X. Exploring the effect of nanoparticle size on the tribological properties of SiO2/polyalkylene glycol nanofluid under different lubrication conditions. Tribol. Int. 2017, 109, 467–472. [Google Scholar] [CrossRef]
- Esfe, M.H.; Mosaferi, M. Effect of MgO nanoparticles suspension on rheological behavior and a new correlation. J. Mol. Liq. 2020, 309, 112632. [Google Scholar] [CrossRef]
- Du, S.; Sun, J.; Wu, P. Preparation, characterization and lubrication performances of graphene oxide-TiO2 nanofluid in rolling strips. Carbon 2018, 140, 338–351. [Google Scholar] [CrossRef]
- Golchin, A.; Villain, A.; Emami, N. Tribological behaviour of nanodiamond reinforced UHMWPE in water-lubricated contacts. Tribol. Int. 2017, 110, 195–200. [Google Scholar] [CrossRef]
- Peng, R.; Zhu, X.; Zhou, M.; Zhao, L.; Xiao, X.; Chen, M. Preparation and tribological properties of hybrid nanofluid of BNNs and SiC modified by plasma. Tribol. Int. 2024, 191, 109168. [Google Scholar] [CrossRef]
- Mirzaamiri, R.; Akbarzadeh, S.; Ziaei-Rad, S.; Shin, D.-G.; Kim, D.-E. Molecular dynamics simulation and experimental investigation of tribological behavior of nanodiamonds in aqueous suspensions. Tribol. Int. 2021, 156, 106838. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R. Tribological characteristics of synthesized hybrid nanofluid composed of CuO and TiO2 nanoparticle additives. Wear 2023, 518, 204623. [Google Scholar] [CrossRef]
- He, J.; Sun, J.; Meng, Y.; Pei, Y. Superior lubrication performance of MoS2-Al2O3 composite nanofluid in strips hot rolling. J. Manuf. Process. 2020, 57, 312–323. [Google Scholar] [CrossRef]
- Singh, B.; Awasthi, S.; Mohan, A. Synergistic effects of melamine functionalized graphene oxide and imidazolium ionic liquid on the tribological performance and thermal stability of polyalphaolefin based hybrid nanolubricants. Colloids Surf. A Physicochem. Eng. Asp. 2024, 688, 133611. [Google Scholar] [CrossRef]
- Han, X.; Thrush, S.J.; Zhang, Z.; Barber, G.C.; Qu, H. Tribological characterization of ZnO nanofluids as fastener lubricants. Wear 2021, 468, 203592. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Jia, D.; Zhang, D.; Zhang, X. Experimental evaluation of the lubrication performance of MoS2/CNT nanofluid for minimal quantity lubrication in Ni-based alloy grinding. Int. J. Mach. Tools Manuf. 2015, 99, 19–33. [Google Scholar] [CrossRef]
- Jiao, C.; Cai, T.; Chen, H.; Ruan, X.; Wang, Y.; Gong, P.; Li, H.; Atkin, R.; Yang, F.; Zhao, H. A mucus-inspired solvent-free carbon dot-based nanofluid triggers significant tribological synergy for sulfonated h-BN reinforced epoxy composites. Nanoscale Adv. 2023, 5, 711–724. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, L.; Li, G.; Zhang, L.; Zhao, F.; Wang, C.; Zhang, G. Solvent-free ionic nanofluids based on graphene oxide-silica hybrid as high-performance lubricating additive. Appl. Surf. Sci. 2019, 471, 482–493. [Google Scholar] [CrossRef]
- Zhai, W.; Lu, W.; Liu, X.; Zhou, L. Nanodiamond as an effective additive in oil to dramatically reduce friction and wear for fretting steel/copper interfaces. Tribol. Int. 2019, 129, 75–81. [Google Scholar] [CrossRef]
- Gui, P.; Long, W.; Cai, X.; Yin, Y.; Wang, W.; Wang, P. Influence analysis of lubrication and friction reduction of graphene oxide lubricant at SiC interface. Colloids Surf. A Physicochem. Eng. Asp. 2024, 691, 133897. [Google Scholar] [CrossRef]
- Li, T.; He, Y.; Zhang, C.; Xue, H.; Zhong, Y.; Gu, L.; Wu, L. Remarkable lubricity of CNTs microspheres as additives in oil lubricant for ceramic components. Ceram. Int. 2024, 50, 1411–1418. [Google Scholar] [CrossRef]
| Parameter | Value | Unit | Description |
|---|---|---|---|
| Load | 5 | N | Constant load applied in the friction test |
| Frequency | 5 | Hz | Frequency of the friction test |
| Test duration | 30 | min | Duration of each friction test |
| Steel plate size | 2 × 2 × 1 | cm3 | Size of AISI 52100 steel plate |
| Steel ball diameter | 6 | mm | Diameter of GCr15 steel ball |
| Steel ball material | GCr15 | Material of the steel ball used in the friction test | |
| Steel plate material | AISI 52100 | Material of the steel plate used in the friction test | |
| Steel ball surface roughness | 6 | nm | Surface roughness of GCr15 steel ball |
| Steel plate surface roughness | 12.9 | nm | Surface roughness of AISI 52100 steel plate |
| Condition | Parameter | Value | Unit | Description |
|---|---|---|---|---|
| Nanofluid size | Diamond nanosheet size | 70, 120, 185 | nm | Three different sizes of diamond nanosheets |
| Nanofluid concentration | Nanofluid concentration | 0.5%, 1%, 3%, 5% | wt% | Different concentrations of diamond nanofluids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Wu, J.; Jiao, C.; Chen, H.; Ruan, X.; Li, W.; Gong, G.; Yu, J.; Nishimura, K.; Jiang, N.; et al. Preparation of Diamond Nanofluids and Study of Lubrication Properties. Materials 2025, 18, 2052. https://doi.org/10.3390/ma18092052
Yu J, Wu J, Jiao C, Chen H, Ruan X, Li W, Gong G, Yu J, Nishimura K, Jiang N, et al. Preparation of Diamond Nanofluids and Study of Lubrication Properties. Materials. 2025; 18(9):2052. https://doi.org/10.3390/ma18092052
Chicago/Turabian StyleYu, Jiamin, Junhao Wu, Chengcheng Jiao, Huanyi Chen, Xinxin Ruan, Wei Li, Genxiang Gong, Jinhong Yu, Kazuhito Nishimura, Nan Jiang, and et al. 2025. "Preparation of Diamond Nanofluids and Study of Lubrication Properties" Materials 18, no. 9: 2052. https://doi.org/10.3390/ma18092052
APA StyleYu, J., Wu, J., Jiao, C., Chen, H., Ruan, X., Li, W., Gong, G., Yu, J., Nishimura, K., Jiang, N., Cai, T., & Wu, Z. (2025). Preparation of Diamond Nanofluids and Study of Lubrication Properties. Materials, 18(9), 2052. https://doi.org/10.3390/ma18092052






