A Review of Joining Technologies for SiC Matrix Composites
Abstract
1. Introduction
2. Bonding Technology of SiCf/SiC Composites
2.1. Bonding Without Filling Materials
2.2. Metal Solid-State Diffusion Bonding
2.3. NITE Phase Bonding
2.4. MAX Phase Bonding
2.5. Glass Ceramic Bonding
2.6. Polymer Precursor Bonding
2.7. Metal-Brazed Bonding
2.8. Si-C Reaction Bonding
3. Conclusions and Outlook
- Extreme Environment Performance: Future research must prioritize bonding solutions capable of withstanding high radiation, thermal cycling, and corrosive environments, particularly for nuclear and aerospace applications.
- Process Optimization: Developing low-temperature and low-pressure bonding methods will be essential to minimize residual stresses and substrate degradation while maintaining joint reliability.
- Hybrid Bonding Strategies: Combining the strengths of different techniques—such as integrating NITE phase bonding with Si–C reaction bonding—could unlock new possibilities for high-performance joints.
- Computational and Data-Driven Approaches: Incorporating numerical simulations and machine learning could accelerate the optimization of bonding parameters, enabling the predictive modeling of joint behavior under operational stresses.
- Material and Interface Engineering: Refining filler materials, surface treatments, and interfacial designs will be crucial to enhance joint durability and compatibility with base materials.
- Real-World Validation: The systematic evaluation of bonded joints under practical operating conditions is needed to bridge the gap between laboratory research and industrial deployment.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| NITE | Nano-Infiltration and Transient Eutectic |
| CVl | Chemical Vapor Infiltration |
| CET | Coefficient of thermal expansion |
| XRD | X-ray diffraction |
| FEA | Finite element analysis |
| SPS | Spark Plasma Sintering |
| ECAJ | Electric-Current-Assisted Bonding |
| CVD | Chemical Vapor Deposition |
| PCS | Polycarbosilane |
| EDS | Energy-Dispersive Spectroscopy |
| SEM | Scanning electron microscopy |
References
- Liu, G.; Zhang, X.; Yang, J.; Qiao, G. Recent Advances in Bonding SiC-Based Materials (Monolithic SiC and SiCf/SiC Composites): Joining Processes, Joint Strength, and Interfacial Behavior. J. Adv. Ceram. 2019, 8, 19–38. [Google Scholar] [CrossRef]
- Magnani, G.; Brentari, A.; Burresi, E.; Raiteri, G. Pressureless Sintered Silicon Carbide with Enhanced Mechanical Properties Obtained by the Two-Step Sintering Method. Ceram. Int. 2014, 40, 1759–1763. [Google Scholar] [CrossRef]
- Justine, D.; Saiz, E.; Al Nasiri, N. Fracture behaviour of SiC/SiC ceramic matrix composite at room temperature. J. Eur. Ceram. Soc. 2022, 42, 3156–3167. [Google Scholar] [CrossRef]
- Bhatt, R.T.; Sola-Lopez, F.; Halbig, M.C.; Jaskowiak, M.H. Thermal stability of CVI and MI SiC/SiC composites with Hi-Nicalon™-S fibers. J. Eur. Ceram. Soc. 2022, 42, 3383–3394. [Google Scholar] [CrossRef]
- Xie, X.; Tang, X.; Yan, L.; Cheng, G.; Li, J.; Liao, J.; Zhang, Y. Morphological and microstructural evolutions of chemical vapor reaction-fabricated SiC under argon ion irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2023, 541, 151–160. [Google Scholar] [CrossRef]
- Koyanagi, T.; Katoh, Y.; Nozawa, T.; Snead, L.L.; Kondo, S.; Henager, C.H., Jr.; Ferraris, M.; Hinoki, T.; Huang, Q. Recent progress in developing SiC composites for nuclear fusion applications. J. Nucl. Mater. 2018, 511, 544–555. [Google Scholar] [CrossRef]
- Qin, Y.; Zheng, C.; Jin, H.; Xu, S.; Zhang, J.; Wei, C.; Zhang, C.; Li, X. The effects of vacancies and fission products on the structures and properties of β-SiC: A first-principles study. J. Mater. Res. Technol. 2023, 24, 5598–5612. [Google Scholar] [CrossRef]
- Allemand, A.; Guerin, C.; Besnard, C.; Billard, R.; Le Petitcorps, Y. A comparison between a new Ultra Fast Pressureless Sintering (UFPS) technology and Spark Plasma Sintering (SPS) for Barium AluminoSilicate metastable phase. J. Eur. Ceram. Soc. 2021, 41, 1524–1529. [Google Scholar] [CrossRef]
- Zhan, C.-T.; He, S.-J.; Guo, W.-M.; Chen, Y.-B.; Sun, S.-K.; Lin, H.-T. Joining of SiC ceramics by combining NITE-SiC interlayer and its thickness control. J. Eur. Ceram. Soc. 2023, 43, 3070–3076. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, Z.; Zhang, H.; Yang, J.; Sun, A.; Liu, X.; Huang, Z.; Liu, Y. Nano-infiltration and transient eutectic (NITE) bonding of SiC ceramics applied to harsh environments. J. Eur. Ceram. Soc. 2023, 43, 2366–2375. [Google Scholar] [CrossRef]
- Zhou, L.; Li, C.; Si, X.; Zhang, C.; Yang, B.; Qi, J.; Cao, J. Flash joining of SiC at ultra-low temperature. J. Eur. Ceram. Soc. 2023, 43, 2713–2717. [Google Scholar] [CrossRef]
- Fitriani, P.; Kwon, H.; Zhou, X.; Yoon, D.H. Joining of SiCf/SiC using a layered Ti3SiC2-SiCw and TiC gradient filler. J. Eur. Ceram. Soc. 2020, 40, 1043–1051. [Google Scholar] [CrossRef]
- Fitriani, P.; Septiadi, A.; Hyuk, J.D.; Yoon, D.-H. Joining of SiC monoliths using a thin MAX phase tape and the elimination of joining layer by solid-state diffusion. J. Eur. Ceram. Soc. 2018, 38, 3433–3440. [Google Scholar] [CrossRef]
- He, S.-J.; Su, L.-F.; Zhan, C.-T.; Guo, W.-M.; Sun, S.-K.; Lin, H.-T. Low-temperature joining of SiC ceramics with Y2O3-Al2O3 interlayer by SiO2-based liquid phase extrusion strategy. Ceram. Int. 2023, 49, 12285–12292. [Google Scholar] [CrossRef]
- Zhao, C.-C.; Lin, R.-L.; Dai, M.-M.; Xu, X.; Zhu, L.-L.; Xue, J.-X.; Zhai, J.-H.; Ma, H.-B.; Guo, W.-M.; Lin, H.-T.; et al. Facile joining of SiC ceramics with screen-printed polycarbosilane without pressure. J. Eur. Ceram. Soc. 2021, 41, 2157–2161. [Google Scholar] [CrossRef]
- Wu, L.-X.; Xue, J.-X.; Zhai, J.-H.; Ma, H.-B.; Liu, Y.; Ren, Q.-S.; Liao, Y.-H.; Sun, S.-K.; Guo, W.-M.; Zhu, L.-L.; et al. The improved SiC joints prepared by pressureless braze joining using Ti–Si interlayer with metallic infiltration. Ceram. Int. 2022, 48, 37049–37054. [Google Scholar] [CrossRef]
- Sung, H.-W.; Kim, Y.-H.; Kim, D.J. Joining of reaction bonded silicon carbide using self-infiltration of residual Si present in the RBSC. Ceram. Int. 2020, 46, 28800–28805. [Google Scholar] [CrossRef]
- Xia, X.; Deng, J.; Kou, S.; Luan, C.; Fan, S.; Wang, P.; Cheng, L.; Zhang, L. Microstructure and properties of pressure-less joining of SiCf/SiC composites by Ti–Si alloys. Ceram. Int. 2022, 48, 22387–22400. [Google Scholar] [CrossRef]
- Montanari, R.; Sili, A.; Costanza, G. Improvement of the fatigue behaviour of Al 6061/20% SiCp composites by means of titanium coatings. Compos. Sci. Technol. 2001, 61, 2047–2054. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.; He, R.; Wang, K.; Wei, K.; Zhang, B. Joining of Cf/SiC ceramic matrix composites: A review. Adv. Mater. Sci. Eng. 2018, 2018, 6176054. [Google Scholar] [CrossRef]
- Yoon, D.-H.; Reimanis, I.E. A review on the joining of SiC for high-temperature applications. J. Korean Ceram. Soc. 2020, 57, 246–270. [Google Scholar] [CrossRef]
- Sun, L.; Qi, Q.; Liu, C.; Wen, Y.; Shan, T.; Wang, S.; Zhang, X.; Zhang, J. Characterization of microstructure and hardening of SiC ceramic brazed joint using TiZrCuNi filler irradiated by He ions. Vacuum 2023, 208, 111720. [Google Scholar] [CrossRef]
- Sozhamannan, G.G.; Prabu, S.B. Influence of interface compounds on interface bonding characteristics of aluminum and silicon carbide. Mater. Charact. 2009, 60, 986–990. [Google Scholar] [CrossRef]
- Grasso, S.; Tatarko, P.; Rizzo, S.; Porwal, H.; Hu, C.; Katoh, Y.; Salvo, M.; Reece, M.J.; Ferraris, M. Joining of β-SiC by spark plasma sintering. J. Eur. Ceram. Soc. 2014, 34, 1681–1686. [Google Scholar] [CrossRef]
- Tatarko, P.; Grasso, S.; Saunders, T.G.; Casalegno, V.; Ferraris, M.; Reece, M.J. Flash bonding of CVD-SiC coated Cf/SiC composites with a Ti interlayer. J. Eur. Ceram. Soc. 2017, 37, 3841–3848. [Google Scholar] [CrossRef]
- Li, H.; Koyanagi, T.; Ang, C.; Katoh, Y. Electric current–assisted direct joining of silicon carbide. J. Eur. Ceram. Soc. 2021, 41, 3072–3081. [Google Scholar] [CrossRef]
- Aroshas, R.; Rosenthal, I.; Stern, A.; Shmul, Z.; Kalabukhov, S.; Frage, N. Silicon Carbide Diffusion Bonding by Spark Plasma Sintering. Mater. Manuf. Process. 2014, 30, 122–126. [Google Scholar] [CrossRef]
- Matsuo, G.; Shibayama, T.; Kishimoto, H.; Hamada, K.; Watanabe, S. Micro-chemical analysis of diffusion bonded W–SiC joint. J. Nucl. Mater. 2011, 417, 391–394. [Google Scholar] [CrossRef]
- Naka, M.; Saito, T.; Okamoto, I. Effect of a silicon sintering additive on solid-state bonding of SiC to Nb. J. Mater. Sci. 1991, 26, 1983–1987. [Google Scholar] [CrossRef]
- Parthasarathy, T.A.; Przybyla, C.P.; Hay, R.S.; Cinibulk, M.K. Modeling Environmental Degradation of SiC-Based Fibers. J. Am. Ceram. Soc. 2016, 99, 1725–1734. [Google Scholar] [CrossRef]
- Yang, X.; Huang, Q.; Su, Z.; Chai, L.; Wang, X.; Zhou, L. A double layer nanostructure SiC coating for anti-oxidation protection of carbon/carbon composites prepared by chemical vapor reaction and chemical vapor deposition. Ceram. Int. 2013, 39, 5053–5062. [Google Scholar] [CrossRef]
- Takeda, M.; Sakamoto, J.-I.; Imai, Y.; Ichikawa, H. Thermal stability of the low-oxygen-content silicon carbide fiber, Hi-NicalonTM. Compos. Sci. Technol. 1999, 59, 813–819. [Google Scholar] [CrossRef]
- Takeda, M.; Urano, A.; Sakamoto, J.-I.; Imai, Y. Microstructure and oxidative degradation behavior of silicon carbide fiber Hi-Nicalon type S. J. Nucl. Mater. 1998, 258, 1594–1599. [Google Scholar] [CrossRef]
- Yao, R.Q.; Wang, Y.Y.; Feng, Z.D. The effect of high-temperature annealing on tensile strength and its mechanism of Hi-Nicalon SiC fibres under inert atmosphere. Fatigue Fract. Eng. Mater. Struct. 2008, 31, 777–787. [Google Scholar] [CrossRef]
- Chollon, G.; Pailler, R.; Naslain, R.; Laanani, F.; Monthioux, M.; Olry, P. Thermal stability of a PCS-derived SiC fibre with a low oxygen content (Hi-Nicalon). J. Mater. Sci. 1997, 32, 327–347. [Google Scholar] [CrossRef]
- Sha, J.; Nozawa, T.; Park, J.; Katoh, Y.; Kohyama, A. Effect of heat treatment on the tensile strength and creep resistance of advanced SiC fibers. J. Nucl. Mater. 2004, 329, 592–596. [Google Scholar] [CrossRef]
- Silberglitt, R.; Ahmad, I.; Tian, Y.L. Microwave Joining of SiC. No. ORNL/TM-13399; Oak Ridge National Lab (ORNL): Oak Ridge, TN, USA, 1997. [Google Scholar] [CrossRef]
- Larker, R.; Nissen, A.; Pejryd, L.; Loberg, B. Diffusion bonding reactions between a SiC/SiC composite and two superalloys during joining by hot isostatic pressing. Acta Metall. Et Mater. 1992, 40, 3129–3139. [Google Scholar] [CrossRef]
- Shi, H.; Chai, Y.; Li, N.; Yan, J.; Zhu, X.; Chen, K.; Bai, D.; Liu, Z.; Wu, M.; Zhang, R.; et al. Interfacial reaction mechanism of SiC joints joined by pure nickel foil. J. Eur. Ceram. Soc. 2020, 40, 5162–5171. [Google Scholar] [CrossRef]
- Zhao, X.; Duan, L.; Wang, Y. Fast interdiffusion and Kirkendall effects of SiC-coated C/SiC composites joined by a Ti-Nb-Ti interlayer via spark plasma sintering. J. Eur. Ceram. Soc. 2019, 39, 1757–1765. [Google Scholar] [CrossRef]
- Li, H.; Shen, W.; He, Y.; Zhong, Z.; Zheng, W.; Ma, Y.; Yang, J.; Wu, Y. Microstructural evolution and characterization of interfacial phases in diffusion-bonded SiC/Ta–5W/SiC joints. Ceram. Int. 2020, 46, 22650–22660. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, D.S.; Lim, S.T.; Kim, D.K. Interfacial microstructure of diffusion-bonded SiC and Re with Ti interlayer. J. Alloys Compd. 2017, 701, 316–320. [Google Scholar] [CrossRef]
- Martinelli, A.E.; Drew, R.A.L.; Berriche, R. Correlation between the strength of SiC-Mo diffusion couples and the mechanical properties of the interfacial reaction products. J. Mater. Sci. Lett. 1996, 15, 307–310. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, X.; Shi, W.; Wang, J.; Li, P.; Chen, F.; Deng, Q.; Lee, J.; Han, Y.-H.; Huang, F.; et al. Thickness-dependent phase evolution and bonding strength of SiC ceramics joints with active Ti interlayer. J. Eur. Ceram. Soc. 2017, 37, 1233–1241. [Google Scholar] [CrossRef]
- Kishimoto, H.; Park, J.-S.; Nakazato, N.; Kohyama, A. Silicon dissolution and morphology modification of NITE SiC/SiC claddings in pressurized flowing water under neutron irradiation. J. Nucl. Mater. 2021, 557, 153253. [Google Scholar] [CrossRef]
- Zhao, X.; Duan, L.; Liu, W.; Wang, Y. Shear strength enhancement of SiC-coated 3D C/SiC composite joints with a Ni-Ti-Nb multi-interlayer by interfacial microstructure tailoring. J. Eur. Ceram. Soc. 2019, 39, 5473–5478. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Y.; Wang, M.; Yang, Y.; Zhao, Y.; He, R.; Tan, C.; Wang, W.; Zhou, X. Brazing of Ti-coated SiC using a CoFeCrNiCu high entropy alloy filler via electric field-assisted sintering. J. Mater. Res. Technol. 2023, 23, 5142–5151. [Google Scholar] [CrossRef]
- Li, H.; Zhong, Z.; Zhang, H.; Zhu, Z.; Hua, P.; Chen, C.; Wu, Y. Microstructure characteristic and its influence on the strength of SiC ceramic joints diffusion bonded by spark plasma sintering. Ceram. Int. 2018, 44, 3937–3946. [Google Scholar] [CrossRef]
- Jung, Y.-I.; Kim, S.-H.; Kim, H.-G.; Park, J.-Y.; Kim, W.-J. Microstructures of diffusion-bonded SiC ceramics using Ti and Mo interlayers. J. Nucl. Mater. 2013, 441, 510–513. [Google Scholar] [CrossRef]
- Qin, H.; Li, X.; Zheng, C.; Xu, S.; Cheng, L. Interfacial diffusion behavior and mechanical properties of SiC/Ta hybrid accident tolerance fuel cladding structure. Compos. Part A Appl. Sci. Manuf. 2023, 165, 107332. [Google Scholar] [CrossRef]
- Zhu, L.; Jian, Y.; Tan, X.; Chen, J.; Liu, H.; Zhang, G.; Zhao, C.; Lin, H.; Dai, M.; Xu, X. Joining SiC ceramics with ZrSi2–SiC filler at low temperatures. J. Am. Ceram. Soc. 2024, 107, 5954–5963. [Google Scholar] [CrossRef]
- Yu, Y.; Dong, H.; Ma, B.; Ren, Q.; Ma, W. Effect of different filler materials on the microstructure and mechanical properties of SiCSiC joints joined by spark plasma sintering. J. Alloys Compd. 2017, 708, 373–379. [Google Scholar] [CrossRef]
- Yu, T.; Xu, J.; Zhou, X.; Tatarko, P.; Li, Y.; Huang, Z.; Huang, Q. Near-seamless bonding of Cf/SiC composites using Y3Si2C2 via electric field-assisted sintering technique. J. Adv. Ceram. 2022, 11, 1196–1207. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, T.; Xu, J.; Li, Y.; Huang, Z.; Huang, Q. Ultrafast low-temperature near-seamless bonding of Cf/SiC using a sacrificial Pr3Si2C2 filler via electric current field-assisted sintering technique. J. Eur. Ceram. Soc. 2022, 42, 6865–6875. [Google Scholar] [CrossRef]
- Son, S.; Park, K.; Katoh, Y.; Kohyama, A. Interfacial reactions and mechanical properties of W–SiC in-situ joints for plasma facing components. J. Nucl. Mater. 2004, 329–333, 1549–1552. [Google Scholar] [CrossRef]
- Jung, H.-C.; Park, Y.-H.; Park, J.-S.; Hinoki, T.; Kohyama, A. R&D of joining technology for SiC components with channel. J. Nucl. Mater. 2009, 386, 847–851. [Google Scholar] [CrossRef]
- Niu, W.B.; Xue, J.X.; Wu, L.X.; Liao, Y.H.; Liu, T.; Ren, Q.S.; Guo, W.M.; Sun, S.K.; Lin, H.T. Low-temperature bonding of SiC ceramics using NITE phase with Al2O3-Ho2O3 additive. J. Am. Ceram. Soc. 2019, 103, 731–736. [Google Scholar] [CrossRef]
- Koyanagi, T.; Ozawa, K.; Hinoki, T.; Shimoda, K.; Katoh, Y. Effects of neutron irradiation on mechanical properties of silicon carbide composites fabricated by nano-infiltration and transient eutectic-phase process. J. Nucl. Mater. 2014, 448, 478–486. [Google Scholar] [CrossRef]
- Parish, C.M.; Terrani, K.A.; Kim, Y.J.; Koyanagi, T.; Katoh, Y. Microstructure and hydrothermal corrosion behavior of NITE-SiC with various sintering additives in LWR coolant environments. J. Eur. Ceram. Soc. 2017, 37, 1261–1279. [Google Scholar] [CrossRef]
- Wu, L.-X.; Lin, R.-L.; Niu, W.-B.; Zhu, L.-L.; Xue, J.-X.; Liao, Y.-H.; Liu, T.; Guo, W.-M.; Sun, S.-K.; Lin, H.-T. Nano-infiltration and transient eutectic (NITE) phase bonding SiC ceramics at 1500 °C. Ceram. Int. 2019, 45, 24927–24931. [Google Scholar] [CrossRef]
- Dong, H.; Li, S.; Teng, Y.; Ma, W. Joining of SiC ceramic-based materials with ternary carbide Ti3SiC2. Mater. Sci. Eng. B 2011, 176, 60–64. [Google Scholar] [CrossRef]
- Tatarko, P.; Casalegno, V.; Hu, C.; Salvo, M.; Ferraris, M.; Reece, M.J. Joining of CVD-SiC coated and uncoated fiber-reinforced ceramic matrix composites with pre-sintered Ti3SiC2 MAX phase using Spark Plasma Sintering. J. Eur. Ceram. Soc. 2016, 36, 3957–3967. [Google Scholar] [CrossRef]
- Tatarko, P.; Chlup, Z.; Mahajan, A.; Casalegno, V.; Saunders, T.G.; Dlouhý, I.; Reece, M.J. High-temperature properties of the monolithic CVD β-SiC materials joined with a pre-sintered MAX phase Ti3SiC2 interlayer via solid-state diffusion bonding. J. Eur. Ceram. Soc. 2017, 37, 1205–1216. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, X.; Mao, Y.; Zhang, L.X.; Feng, J.C. Joining of SiC ceramics using CaO-Al2O3-SiO2 (CAS) glass-ceramics. J. Eur. Ceram. Soc. 2020, 40, 267–275. [Google Scholar] [CrossRef]
- Zhan, C.-T.; Wu, J.-Y.; Huang, K.-H.; Li, Y.; Wu, L.-X.; He, S.-J.; Liu, Y.-Q.; Zhang, Z.-X.; Guo, W.-M.; Xue, J.-X.; et al. Joining of textured PLS-SiC ceramic with CaO-Al2O3-MgO-TiO2-SiO2 glass filler. Ceram. Int. 2024, 50, 495–502. [Google Scholar] [CrossRef]
- Fang, J.; Sun, L.; Guo, S.; Shan, T.; Wen, Y.; Liu, C.; Zhang, J. Wetting and bonding of surface-oxidized SiC ceramic with calcium lithium aluminosilicate glass filler. Appl. Surf. Sci. 2021, 568, 150951. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, J.; Liu, C.; Sun, L. A novel glass-ceramic with high crystallinity for bonding of SiC ceramic. Mater. Lett. 2020, 277, 128318. [Google Scholar] [CrossRef]
- Casalegno, V.; Kondo, S.; Hinoki, T.; Salvo, M.; Czyrska-Filemonowicz, A.; Moskalewicz, T.; Katoh, Y.; Ferraris, M. CaO-Al2O3 glass-ceramic as a bonding material for SiC-based components: A microstructural study of the effect of Si-ion irradiation. J. Nucl. Mater. 2018, 501, 172–180. [Google Scholar] [CrossRef]
- Yang, S.; Meng, X.; Chen, B.; Ma, Y.; Kou, S.; Deng, J.; Fan, S. Encapsulation of SiC/SiC cladding tubes with excellent mechanical performance and air tightness using a CaO-modified Y2O3–Al2O3–SiO2 glass. Ceram. Int. 2024, 50, 29850–29858. [Google Scholar] [CrossRef]
- Malik, R.; Ryu, E.-H.; Kim, Y.-W. Pressureless bonding of SiC ceramics with magnesia-alumina-silica glass-ceramics. Int. J. Appl. Ceram. Technol. 2022, 19, 992–1000. [Google Scholar] [CrossRef]
- Malinverni, C.; Salvo, M.; Ziętara, M.; Cempura, G.; Kruk, A.; Maier, J.; Prentice, C.; Farnham, M.; Casalegno, V. A yttrium aluminosilicate glass ceramic to join SiC/SiC composites. J. Eur. Ceram. Soc. 2024, 44, 3579–3587. [Google Scholar] [CrossRef]
- Shan, T.; Sun, L.; Liu, C.; Fang, J.; Li, Z.; Wen, Y.; Wang, B.; Guo, S.; Zhang, J. Microstructure and mechanical properties of SiC joint using anorthite-based glass ceramic and first principles calculation of joint interface. Ceram. Int. 2023, 49, 40149–40157. [Google Scholar] [CrossRef]
- Colombo, P.; Riccardi, B.; Donato, A.; Scarinci, G. Joining of SiC/SiCf ceramic matrix composites for fusion reactor blanket applications. J. Nucl. Mater. 2000, 278, 127–135. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, S.; Zhang, X.; Jin, T. Joining SiC ceramics with silicon resin YR3184. Ceram. Int. 2009, 35, 3241–3245. [Google Scholar] [CrossRef]
- Tang, B.; Wang, M.; Liu, R.; Liu, J.; Du, H.; Guo, A. A heat-resistant preceramic polymer with broad working temperature range for silicon carbide bonding. J. Eur. Ceram. Soc. 2018, 38, 67–74. [Google Scholar] [CrossRef]
- Tang, M.; Liu, Y.; Zhang, H.; Chen, W.; Liu, X.; Lin, T. Preparation and microstructure characterization of SiC/SiC joints reinforced by in-situ SiC nanowires. Ceram. Int. 2020, 46, 12559–12565. [Google Scholar] [CrossRef]
- Guyett, P.C.; Chew, D.; Azevedo, V.; Blennerhassett, L.C.; Rosca, C.; Tomlinson, E. Optimizing SEM-EDX for fast, high-quality and non-destructive elemental analysis of glass. J. Anal. At. Spectrom. 2024, 39, 2565–2579. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Wang, H. Joining of SiC ceramics via a novel liquid preceramic polymer (V-PMS). Ceram. Int. 2015, 41, 7283–7288. [Google Scholar] [CrossRef]
- Shi, H.; Peng, H.; Chai, Y.; Li, N.; Wen, Y.; Bai, D.; Liu, Z.; Yan, J.; Zhang, R.; Li, M.; et al. Effect of Zr addition on the interfacial reaction of the SiC joint brazed by Inconel 625 powder filler. J. Eur. Ceram. Soc. 2021, 41, 6238–6247. [Google Scholar] [CrossRef]
- Valenza, F.; Gambaro, S.; Muolo, M.L.; Salvo, M.; Casalegno, V. Wetting of SiC by Al-Ti alloys and bonding by in-situ formation of interfacial Ti3Si(Al)C2. J. Eur. Ceram. Soc. 2018, 38, 3727–3734. [Google Scholar] [CrossRef]
- Lin, D.; Hu, J.; Song, X.; Tang, Z.; Wang, Y.; Hu, S.; Bian, H.; Fu, W.; Song, Y. Vacuum brazing SiC to Mo using Nb0.74CoCrFeNi2 eutectic high-entropy alloy filler. Mater. Charact. 2023, 204, 113199. [Google Scholar] [CrossRef]
- Matthews, R.B. Irradiation damage in reaction-bonded silicon carbide. J. Nucl. Mater. 1974, 51, 203–208. [Google Scholar] [CrossRef]
- Liu, G.W.; Valenza, F.; Muolo, M.L.; Passerone, A. SiC/SiC and SiC/Kovar bonding by Ni–Si and Mo interlayers. J. Mater. Sci. 2010, 45, 4299–4307. [Google Scholar] [CrossRef]
- Xiong, H.-P.; Chen, B.; Pan, Y.; Zhao, H.-S.; Ye, L. Joining of Cf/SiC composite with a Cu–Au–Pd–V brazing filler and interfacial reactions. J. Eur. Ceram. Soc. 2014, 34, 1481–1486. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Zhang, H.; Jiang, J.; Lin, T.; Liu, X.; Huang, Z. Joining of SiC ceramics using the Ni-Mo filler alloy for heat exchanger applications. J. Eur. Ceram. Soc. 2021, 41, 7533–7542. [Google Scholar] [CrossRef]
- Ma, H.-B.; Xue, J.-X.; Zhai, J.-H.; Liu, T.; Ren, Q.-S.; Liao, Y.-H.; Wu, L.-X.; Guo, W.-M.; Sun, S.-K.; Lin, H.-T. Pressureless bonding of silicon carbide using Ti3SiC2 MAX phase at 1500 °C. Ceram. Int. 2020, 46, 14269–14272. [Google Scholar] [CrossRef]
- Fu, W.; Song, X.; Tian, R.; Lei, Y.; Long, W.; Zhong, S.; Feng, J. Wettability and bonding of SiC by Sn-Ti: Microstructure and mechanical properties. J. Mater. Sci. Technol. 2020, 40, 15–23. [Google Scholar] [CrossRef]
- Li, Z.; Wei, R.; Wen, Q.; Zhong, Z.; Song, K.; Wu, Y. Microstructure and mechanical properties of SiC ceramic joints vacuum brazed with in-situ formed SiC particulate reinforced Si–24Ti alloy. Vacuum 2020, 173, 109160. [Google Scholar] [CrossRef]
- Huang, C.-C.; Chen, J.; Zhu, M.; Li, F.-F.; Huang, Z.-R. Joining of SiC ceramics using high-silicon aluminum alloy fillers assisted by laser cladding. J. Mater. Res. Technol. 2023, 24, 5675–5686. [Google Scholar] [CrossRef]
- Gianchandani, P.K.; Casalegno, V.; Smeacetto, F.; Ferraris, M. Pressure-less bonding of C/SiC and SiC/SiC by a MoSi2/Si composite. Int. J. Appl. Ceram. Technol. 2017, 14, 305–312. [Google Scholar] [CrossRef]
- Liu, W.; Wang, P.; Shi, W.; Zhao, S.; Nai, X.; Song, X.; Chen, H.; Li, W. Investigating the effect of WC reinforcements in CuTi composite filler on the microstructural evolution and mechanical characteristics in brazing Cf/SiC and GH3536. J. Manuf. Process. 2024, 121, 312–322. [Google Scholar] [CrossRef]
- Yang, J.; Liu, W.; Fu, M.; Wen, J.; Li, K.; Lin, P.; Wang, C.; He, P.; Lin, T.; Mei, H.; et al. Joining SiCf/SiC composites to Al0.3CoCrFeNi high-entropy alloys with a Cu–Ti filler alloy: Interfacial reactions, high-entropy effects, and mechanical properties. Mater. Sci. Eng. A 2023, 881, 145390. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, H.; Nai, X.; Wang, P.; Deng, H.; Wen, G.; Liu, F.; Li, W. Microstructure and mechanical properties of SiCf/SiC composites/GH536 superalloy joints brazed with CoFeNiCrCu high-entropy alloy filler. Mater. Charact. 2022, 194, 112419. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, H.; Nai, X.; Wang, P.; Deng, H.; Wen, G.; Liu, F.; Li, W. Effect of Ti content on microstructure and mechanical properties of SiCf/SiC composites/GH536 superalloy joints brazed with CoFeCrNiCuTi high entropy filler. J. Manuf. Process. 2023, 85, 132–140. [Google Scholar] [CrossRef]
- Zhao, S.; Nai, X.; Chen, H.; Wang, P.; Liu, Y.; Wang, P.; Li, W.; Song, X. Improved the mechanical properties of SiCf/SiC-GH536 joints by in-situ introducing better toughness MoNiSi phase to the brazing seam. Mater. Charact. 2024, 209, 113773. [Google Scholar] [CrossRef]
- Zhao, S.; Nai, X.; Chen, H.; Wang, P.; Wang, Q.; Liu, Y.; Wang, P.; Li, W. Role of Nb elements in SiCf/SiC/(CoFeNiCrMn)100-xNbx/GH536 brazed joints: Joint residual stress transfer and pinning of dislocations. Mater. Sci. Eng. A 2024, 891, 145914. [Google Scholar] [CrossRef]
- Luo, Z.; Jiang, D.; Zhang, J.; Lin, Q.; Chen, Z.; Huang, Z. Development of SiC–SiC joint by reaction bonding method using SiC/C tapes as the interlayer. J. Eur. Ceram. Soc. 2012, 32, 3819–3824. [Google Scholar] [CrossRef]
- Wu, X.; Huang, Q.; Zhu, Y.; Huang, Z. Joining of SiC Ceramic by Si–C Reaction Bonding Using Organic Resin as Carbon Precursor. Materials 2022, 15, 4242. [Google Scholar] [CrossRef]
- Wu, X.; Pei, B.; Zhu, Y.; Huang, Z. Joining of the Cf/SiC composites by a one-step Si infiltration reaction bonding. Mater. Charact. 2019, 155, 109799. [Google Scholar] [CrossRef]
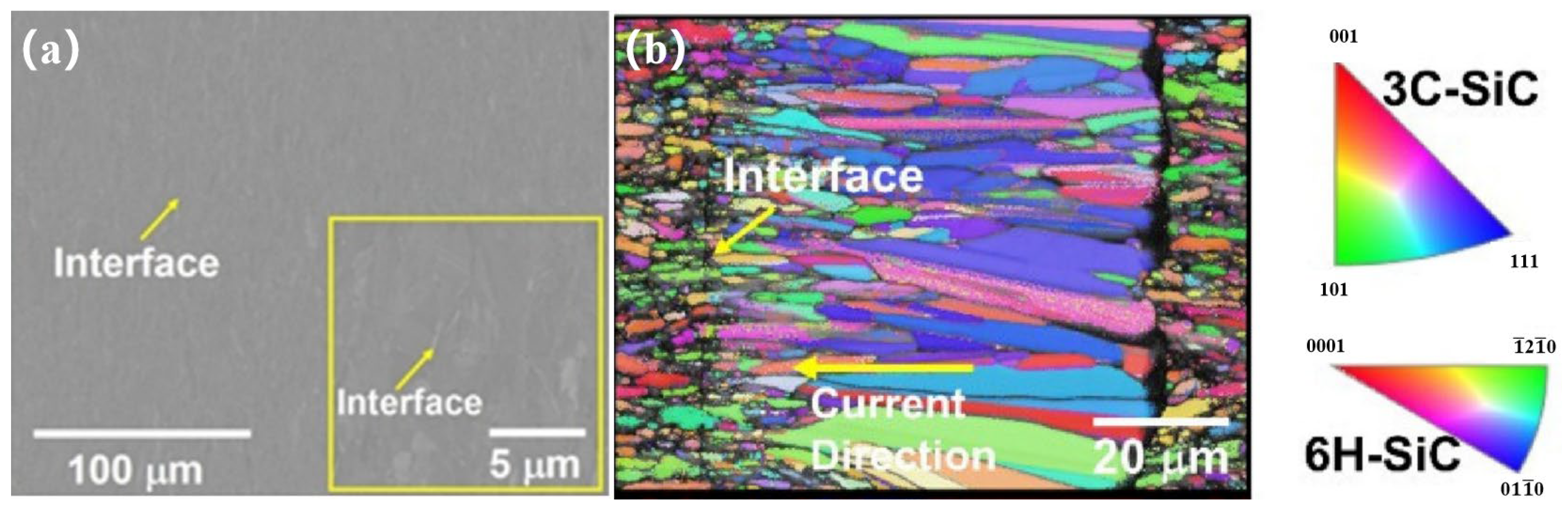
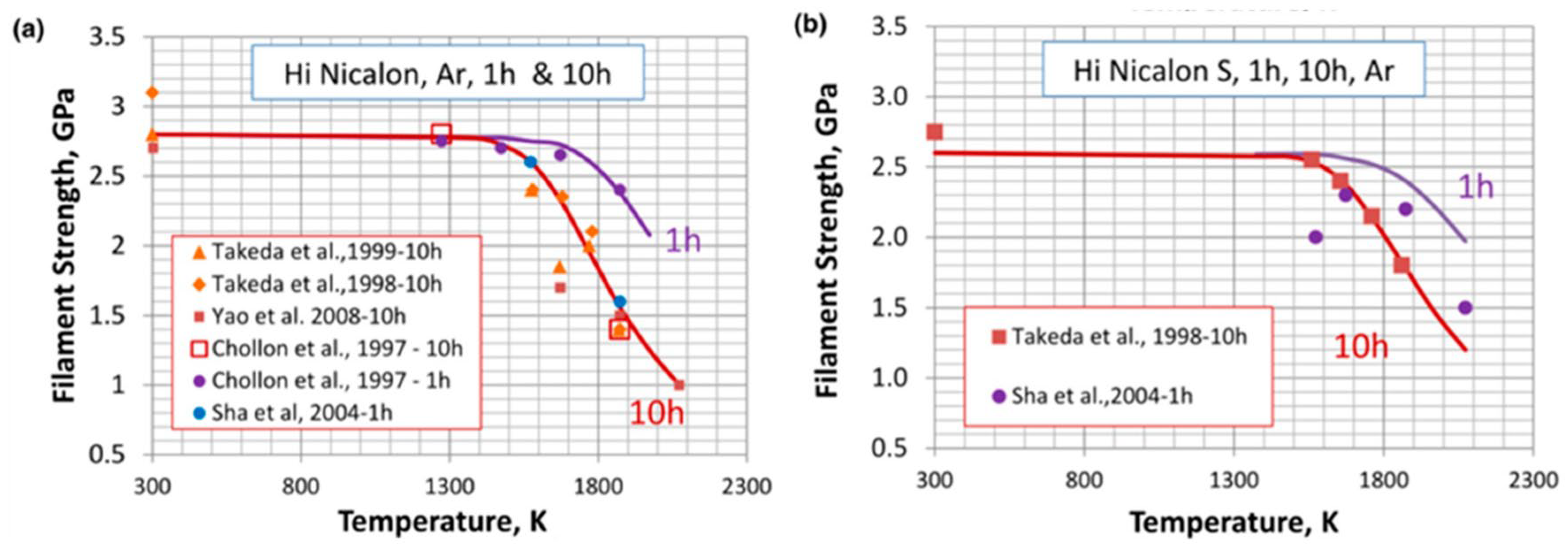
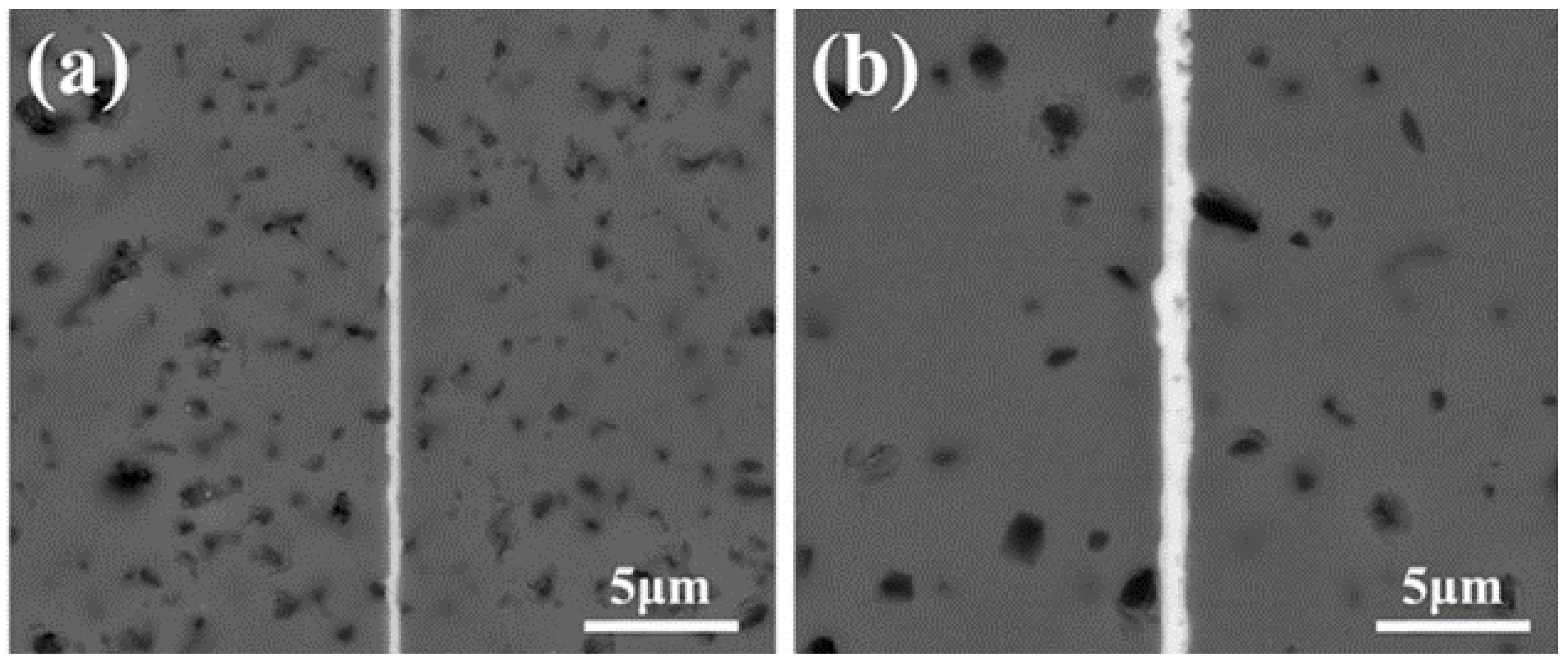
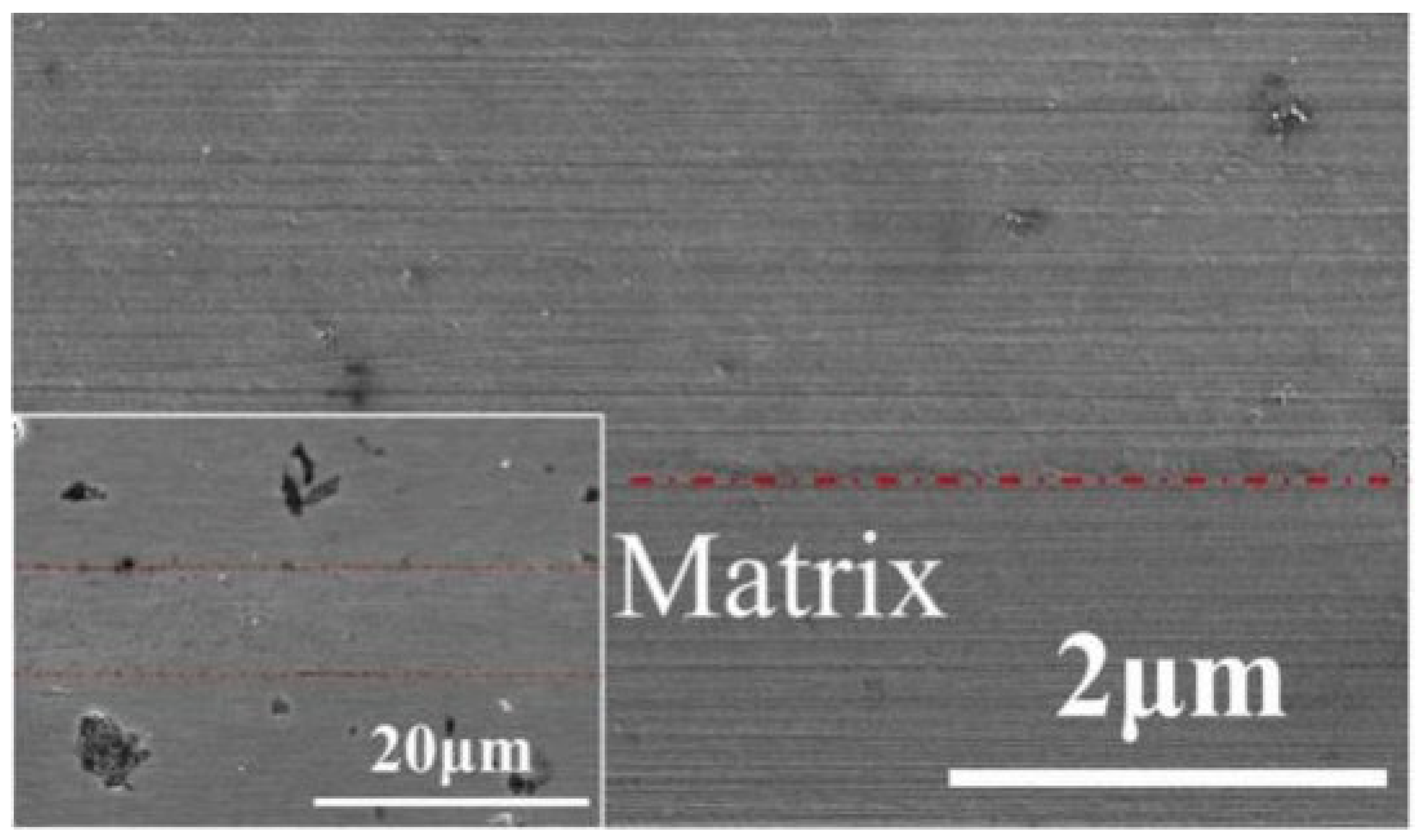
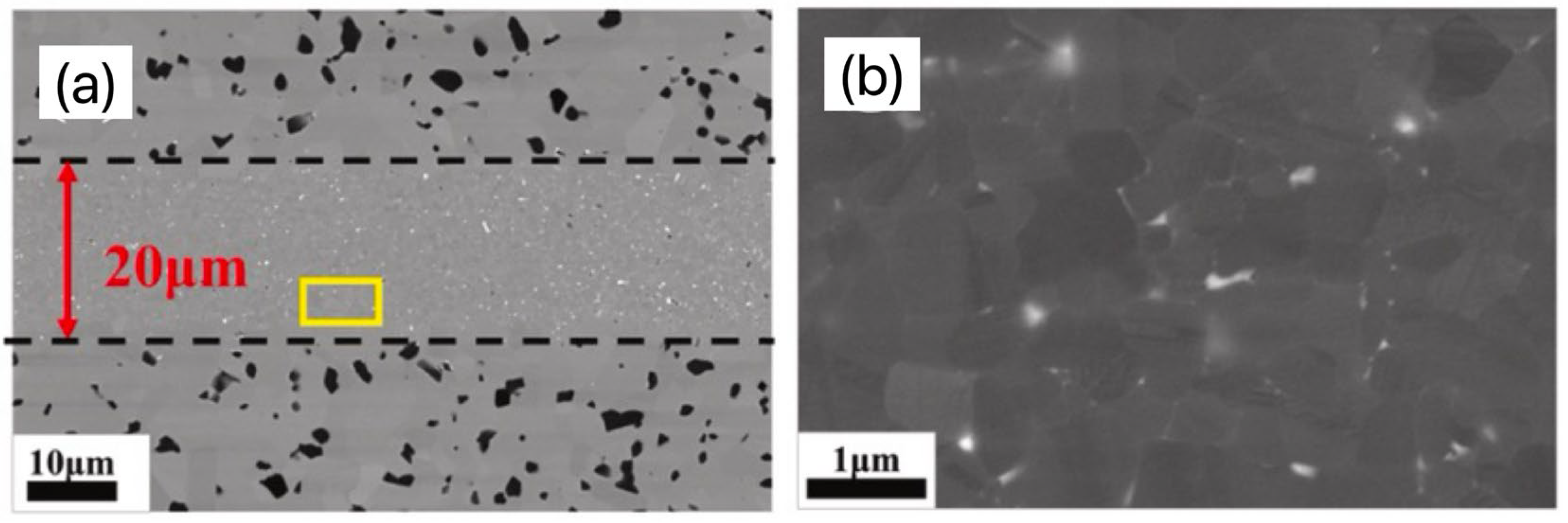
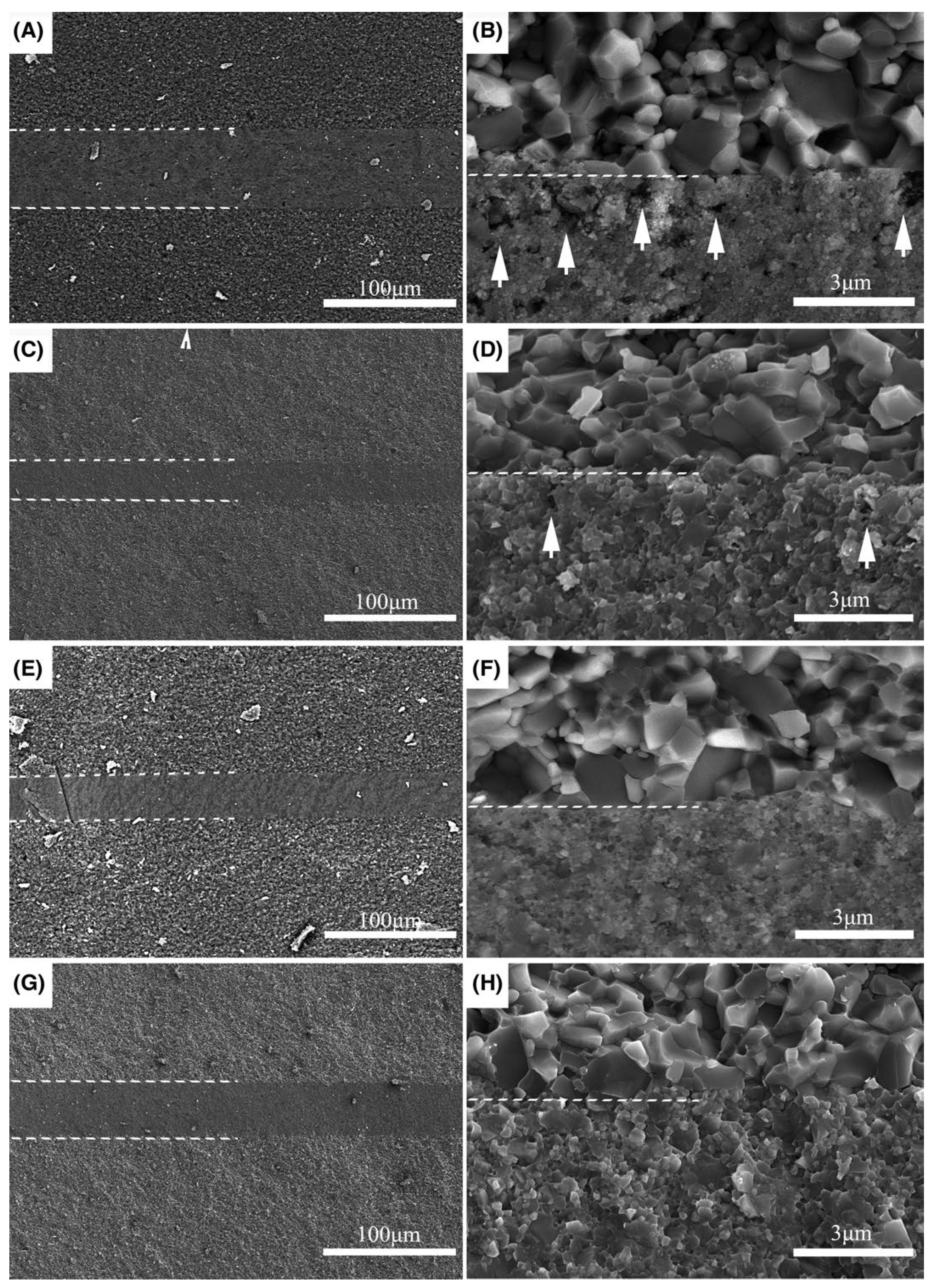
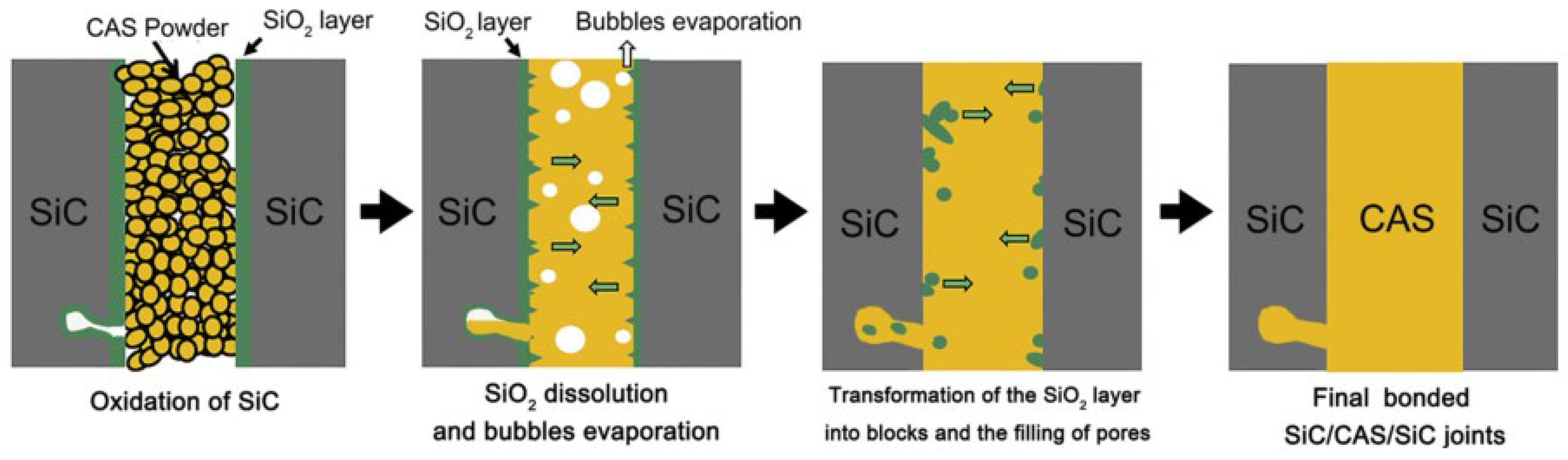
| Bonding Base Material | Bonding Method | Bonding Condition | Joint Strength | Source |
|---|---|---|---|---|
| SiC-SiC | Electric-Current-Assisted Bonding | 1750 °C, 10 min 2160 °C, 1 min | / | [26] |
| SiC-SiC | Spark Plasma Sintering (SPS) | 1900 °C, 5 min, 60 MPa, SiC Surface Polishing | Bending strength 193 ± 21 MPa | [24] |
| 2000 °C, 5 min, 60 MPa, SiC Unpolished | Bending strength 68 ± 9 MPa | |||
| SiC-SiC | DC-field-assisted Sintering | 400 °C, 30 min, 2 MPa, Air | / | [11] |
| SiC-SiC | Hot pressing Sintering | 1465 °C, 30–45 min, 0.23 MPa | / | [37] |
| SiCf/SiC (Hastelloy X) | Hot pressing Sintering | 900 °C/1000 °C, 1 h, 200 MPa | / | [38] |
| SiCf/SiC (Incoloy 909) | Hot pressing Sintering | 900 °C/1000 °C, 1 h, 200 MPa | / | [38] |
| Bonding Base Material | Bonding Layer | Bonding Condition | Bonding Method | Joint Strength | Source |
|---|---|---|---|---|---|
| Cf/SiC-Cf/SiC (3D) | Ni-Ti-Nb-Ti-Ni | Spark Plasma Sintering (SPS) | 1400 °C, 5 min, Vacuum, 50 MPa | Shear strength 108 ± 5 MPa | [46] |
| SiC-SiC | Ti-CoFeCrNiCu-Ti | Spark Plasma Sintering (SPS) | 1200 °C, 15 min, Ar, 50 MPa | Bend strength 72 ± 2 MPa | [47] |
| SiC-SiC | Ta-5W (100 μm) | Spark Plasma Sintering (SPS) | 1600 °C, 5 min, Ar, 30 MPa | Shear strength 122 ± 15 MPa | [48] |
| Cf/SiC-Cf/SiC (3D) | Ti-Nb-Ti | Spark Plasma Sintering (SPS) | 1200 °C, 5 min, 30 MPa | Shear strength 61 ± 6 MPa | [40] |
| SiC-SiC | Ti | Spark Plasma Sintering (SPS) | 1400 °C,10 min, Ar, 30 MPa | Shear strength 109.3 ± 4.5 MPa | [8] |
| SiC-SiC | Ti-Re-Ti | Hot Pressing Sintering | 1400–1600 °C, 2 h, Ar, 25 MPa | / | [42] |
| SiC-SiC | Ti | Hot Pressing Sintering | 1400 °C,1 h, Vacuum, 7 MPa | Shear strength 67 MPa | [49] |
| SiC-SiC | Mo | Hot Pressing Sintering | 1400 °C, 1 h, Vacuum, 7 MPa | Shear strength 76 MPa | [49] |
| SiCf/SiC-SiCf/SiC | Ta | Pressureless Sintering | 1500 °C, 5 h, vacuum | Shear strength 13.86 MPa | [50] |
| SiC-SiC | ZrSi2-SiC | Spark Plasma Sintering (SPS) | 1600 °C, 30 min, 30 MPa | Shear strength 168.1 ± 12.6 MPa | [51] |
| SiC-SiC | Y2O3-ZrO2-Al2O3 | Spark Plasma Sintering (SPS) | 1600–1800 °C, 40 MPa | Bend strength 107.3 MPa | [52] |
| Cf/SiC-Cf/SiC | Y3Si2C2 | Spark Plasma Sintering (SPS) | 1600 °C, 10 min | Shear strength 17.2 ± 2.9 MPa | [53] |
| Cf/SiC-Cf/SiC | Pr3Si2C2 | Spark Plasma Sintering (SPS) | 1000–1500 °C, 30 s, 30 MPa, Vacuum | Shear strength 17.6 ± 3 MPa | [54] |
| SiC-SiC | W | Hot Pressing Sintering | 1700–1900 °C, 10–120 min, Ar | Shear strength 90 MPa | [55] |
| Bonding Base Material | Bonding Layer | Bonding Method | Bonding Condition | Joint Strength | Source |
|---|---|---|---|---|---|
| SiC-SiC | Al2O3+CeO2+SiC | Spark Plasma Sintering (SPS) | 1700 °C, 10 min, 20 MPa, Ar | Shear strength 163.9 MPa | [60] |
| SiC-SiC | Al2O3+Y2O3+SiC | Spark Plasma Sintering (SPS) | 1700 °C, 10 min, 20 MPa, Ar | Shear strength 115 MPa | [60] |
| SiC-SiC | SiO2+Al2O3+Y2O3+SiC | Spark Plasma Sintering (SPS) | 1700 °C, 10 min, 20 MPa, Ar | Shear strength 104.2 MPa | [60] |
| SiC-SiC | Al2O3+Ho2O3+SiC | Spark Plasma Sintering (SPS) | 1700 °C, 10 min, 20 MPa, Ar | Shear strength 157.8 MPa | [57] |
| SiC-SiC | Al2O3+Y2O3+MgO+CaO+SiC | Spark Plasma Sintering (SPS) | 1650 °C, 30 MPa, Vac | Shear strength 69.5 ± 8.9 MPa | [9] |
| SiC-SiC | SiO2+Al2O3+Y2O3+SiC | Hot Pressing Sintering | 1800 °C, 20 MPa, 1 h | Tensile strength 249 MPa | [56] |
| SiC-SiC | SiC+AlN+Y2O3 | Vacuum Sinter | 1750 °C, 2 h, Ar | Bend strength 320.5 ± 37.6 MPa | [10] |
| Bonding Base Material | Bonding Layer | Bonding Method | Bonding Condition | Joint Strength | Source |
|---|---|---|---|---|---|
| SiC-SiC | Ti3SiC2 | Hot Pressing Sintering | 1900 °C, 3.5 MPa, 5 h, Ar | Bend strength 230.55 ± 18.96 MPa | [13] |
| SiC-SiC | Ti3AlC2 | Hot Pressing Sintering | 1900 °C, 3.5 MPa, 5 h, Ar | Bend strength 298.34 ± 9.38 MPa | [13] |
| Cf/SiC-Cf/SiC | Ti3SiC2 | Hot Pressing Sintering | 1200–1600 °C, 20–40 MPa, 30 min | Bend strength 110.4 MPa | [61] |
| SiCf/SiC-SiCf/SiC | Ti3SiC2 | Spark Plasma Sintering (SPS) | 1300 °C, 50 MPa, 5 min, Vac | Shear strength 18.3 ± 5.8 MPa | [62] |
| SiCf/SiC-SiCf/SiC | Ti3SiC2+SiC | Hot Pressing Sintering | 1400 °C/1500 °C, 3.5 MPa, 2 h, Ar | Bend strength 198 MPa | [12] |
| SiC-SiC | Ti3SiC2 | Spark Plasma Sintering (SPS) | 1300 °C, 50 MPa, 5 min, Vac | Bend strength 220.3 ± 3.2 MPa | [63] |
| Bonding Baes Material | Bonding Layer | Bonding Method | Bonding Condition | Joint Strength | Source |
|---|---|---|---|---|---|
| SiC-SiC | CaO-Al2O3-MgO-TiO2-SiO2 | Pressureless Sintering | 1400 °C, 15 min, Ar | Shear strength 69.3 ± 9.9 MPa | [65] |
| SiC-SiC | CaO-Li2O-Al2O3-SiO2 | Pressureless Sintering | 1240 °C, 10 min, Ar | Shear strength 127 MPa | [66] |
| SiC-SiC | CaO-Al2O3-SiO2-Li2O | Pressureless Sintering | 1260 °C, 10 min, Ar | / | [67] |
| SiC-SiC | CaO-Al2O3 | Pressureless Sintering | 1480 °C, 10 min, Ar | / | [68] |
| SiCf/SiC-SiCf/SiC | CaO-Y2O3-Al2O3-SiO2 | Pressureless Sintering | 1400 °C, 30 min, Ar | Shear strength 57.1 ± 6 MPa | [69] |
| SiC-SiC | MgO-Al2O3-SiO2 | Pressureless Sintering | 1450–1600 °C, 2 h, Ar | Four-point bending strength 286 ± 40 MPa | [70] |
| SiCf/SiC-SiCf/SiC | Y2O3-Al2O3-SiO2 | Pressureless Sintering | 1450 °C, 20 min, Ar | Shear strength 61 ± 12 MPa | [71] |
| SiC-SiC | CaO-Al2O3-SiO2 | Pressureless Sintering | 1450 °C, 10 min, Ar | Shear strength 86 MPa | [72] |
| Bonding Base Material | Bonding Layer | Bonding Method | Bonding Condition | Joint Strength | Source |
|---|---|---|---|---|---|
| SiC-SiC | (-SiHCH3-CH2-)n(PCS) | Pressureless Sintering | 1500 °C, 2 h, Ar | Shear strength 105.8 ± 10.4 MPa | [15] |
| SiC-SiC | Polysiloxane | Pressureless Sintering | 1200 °C, 1 h, N2 | Bend strength 197 MPa | [74] |
| SiC-SiC | Polymethylsiloxane, epoxy resin | Pressureless Sintering | 1100 °C, 1 h, air | Shear strength 37.28 ± 1.33 MPa | [75] |
| SiC-SiC | PMS, D4Vi | Pressureless Sintering | 1000 °C, 2 h, N2 | Shear strength 34.5 ± 4.6 MPa | [78] |
| SiC-SiC | Polycarbosilane (PCS), ferrocene | Pressureless Sintering | Step1: 1300 °C, 2 h, Ar; step2: 1600 °C, 1 h | / | [76] |
| Bonding Base Material | Bonding Layer | Bonding Method | Bonding Condition | Joint Strength | Source |
|---|---|---|---|---|---|
| SiC-SiC | (Ni-56Si)-Mo-(Ni-56Si) | Pressureless Sintering | 1350 °C, 10 min, vacuum | Shear strength 41 MPa | [83] |
| Cf/SiC-Cf/SiC (2D) | Cu-18Au-32Pd-(6–10) V | Pressureless Sintering | 1120 °C, 10 min, vacuum | Bend strength 135 MPa | [84] |
| SiC-SiC | Ni-28Mo | Pressureless Sintering | 1300 °C, 40 min, vacuum | Bend strength 174 ± 11 MPa | [85] |
| SiCf/SiC-SiCf/SiC | Ti16Si84 | Pressureless Sintering | 1410 °C, 10 min, Ar | Shear strength 42.5 MPa | [18] |
| SiC-SiC | Al3Ti-Ti-Al3Ti | Pressureless Sintering | 1500 °C, 10 min, Ar | Shear strength 89 MPa | [80] |
| SiC-SiC | Si(powder)- Ti (Foil)- Si(powder) | Pressureless Sintering | 1500 °C, 1 h, vacuum | Shear strength 76.2 ± 19.6 MPa | [86] |
| SiC-SiC | Sn-Ti | Pressureless Sintering | 950 °C, 10 min, vacuum | Shear strength 27–32 MPa | [87] |
| SiC-SiC | Si-24Ti-3 Carbon nanotube | Pressureless Sintering | 1380 °C, 20 min, Ar | Shear strength 88.5 MPa | [88] |
| SiC-SiC | Ni Foil | Pressureless Sintering | 1245 °C, 60 min, vacuum | Shear strength 29.43 MPa | [39] |
| SiC-SiC | Si-Al | Pressureless Sintering | 900 °C, 30 min, vacuum | Bend strength 279 ± 13 MPa | [89] |
| SiCf/SiC- SiCf/SiC | Mo-Si | Pressureless Sintering | 1450 °C, 1–10 min, Ar | Shear strength 7~10 MPa | [90] |
| Cf/SiC-GH3536 | Cu-Ti-WC | Pressureless Sintering | 1140 °C, 20 min, vacuum | Shear strength 74.3 MPa | [91] |
| SiCf/SiC-Al0.3CoCrFeNi | Cu-Ti | Pressureless Sintering | 1125 °C, 10 min, vacuum | Shear strength 59 MPa | [92] |
| SiCf/SiC-GH536 | CoFeNiCrCu | Pressureless Sintering | 1160 °C, 60 min, vacuum | Shear strength 86.07 ± 4.5 MPa | [93] |
| SiCf/SiC-GH536 | CoFeCrNiCuTi | Pressureless Sintering | 1160 °C, 60 min, vacuum | Shear strength 98.23 ± 5.12 MPa | [94] |
| SiCf/SiC-GH536 | CoFeNiCrMnNb-MoNiSi-CoFeNiCrMnNb | Pressureless Sintering | 1200 °C, 20 min, vacuum | Shear strength 98.1 MPa | [95] |
| SiCf/SiC-GH536 | CoFeNiCrMnNb | Pressureless Sintering | 1200 °C, 20 min, vacuum | Shear strength 89.7 MPa | [96] |
| SiC-Mo | Nb0.74CoCrFeNi2 | Pressureless Sintering | 1300 °C, 15 min, vacuum | Shear strength 62 MPa | [81] |
| Bonding Base Material | Bonding Layer | Bonding Method | Bonding Condition | Joint Strength | Source |
|---|---|---|---|---|---|
| SiC-SiC | SiC, C | Pressureless Sintering | 1450 °C, 30 min, vac | Bend strength 439 ± 31 MPa | [97] |
| SiC-SiC | SiC, C | Pressureless Sintering | 1600 °C, 30 min, vac | Bend strength 308 ± 27 MPa | [98] |
| Cf/SiC- Cf/SiC | SiC, C | Pressureless Sintering | 1600 °C, vac | Bend strength 203 ± 24 MPa | [99] |
| SiC-SiC | SiC, C | Pressureless Sintering | 1450 °C~1550 °C, 10~60 min, vac | Bend strength 243~246 MPa | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Zhang, J.; Li, G.; Wang, Z.; Wu, J.; Wei, C. A Review of Joining Technologies for SiC Matrix Composites. Materials 2025, 18, 2046. https://doi.org/10.3390/ma18092046
Lu Y, Zhang J, Li G, Wang Z, Wu J, Wei C. A Review of Joining Technologies for SiC Matrix Composites. Materials. 2025; 18(9):2046. https://doi.org/10.3390/ma18092046
Chicago/Turabian StyleLu, Yongheng, Jinzhuo Zhang, Guoquan Li, Zaihong Wang, Jing Wu, and Chong Wei. 2025. "A Review of Joining Technologies for SiC Matrix Composites" Materials 18, no. 9: 2046. https://doi.org/10.3390/ma18092046
APA StyleLu, Y., Zhang, J., Li, G., Wang, Z., Wu, J., & Wei, C. (2025). A Review of Joining Technologies for SiC Matrix Composites. Materials, 18(9), 2046. https://doi.org/10.3390/ma18092046







