Preparation, Thermal, and Optical Properties of D-A-Type Molecules Based on 1,3,5-Triazine for Violet-Blue Fluorescent Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
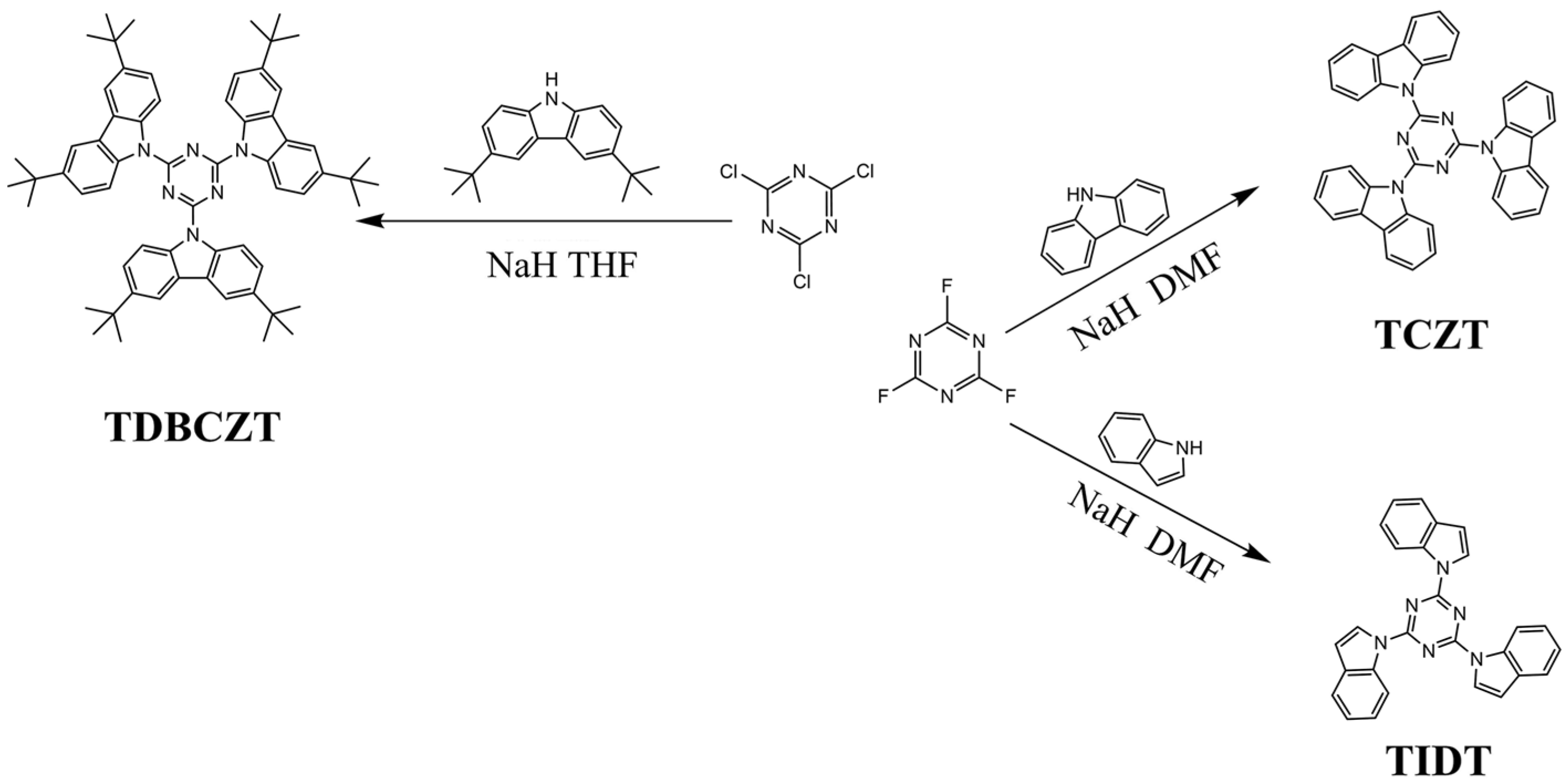
2.2. Synthesis and Characterization
3. Results and Discussion
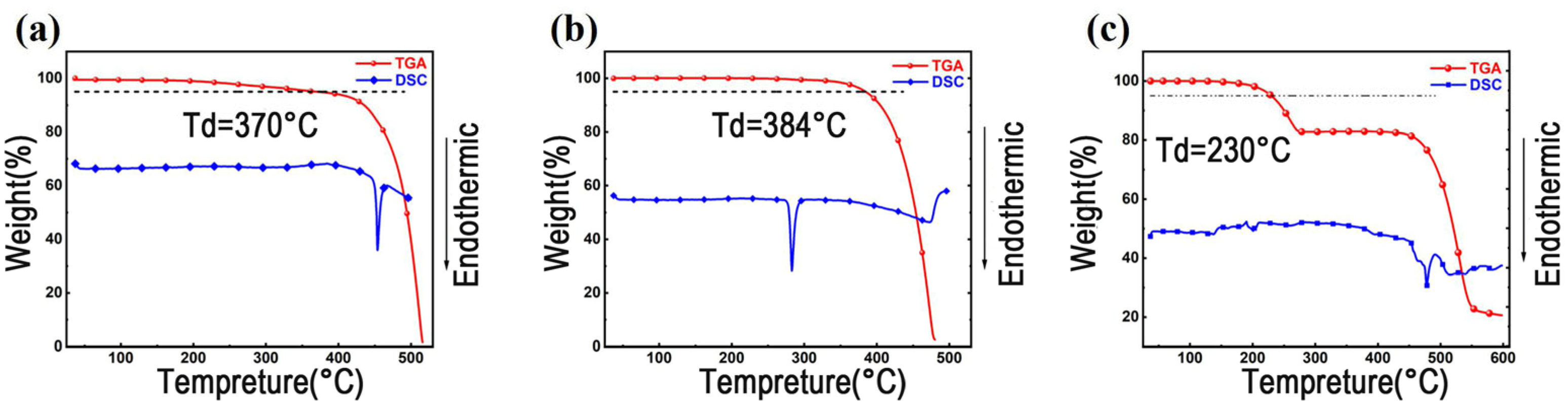
3.1. Thermal Properties
3.2. Electrochemical Properties
3.3. Photophysical Properties and Theoretical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| D-A type | Donor–acceptor type |
| TD-DFT | Time-dependent density functional theory |
| DFT | Density functional theory |
| PL | Photoluminescence |
| PLQYs | Photoluminescence quantum yields |
| CT | Charge transfer |
| ICT | Intramolecular charge transfer |
| RISC | Reverse intersystem crossing |
| CV | Cyclic voltammetry |
| HRMS | High-resolution mass spectra |
| TGA | Thermogravimetric analysis |
| DSC | Differential scanning calorimetry |
| TPEF | Two-photon excited fluorescence |
| TPA | Two-photon absorption |
| FWHM | Full Width at Half Maximum |
| HOMO | Highest occupied molecular orbital |
| LUMO | Lowest occupied molecular orbital |
| TADF | Thermally activated delayed fluorescence |
| DCM | Dichloromethane |
| THF | Tetrahydrofuran |
| DMF | Dimethylformamide |
References
- Lee, S.J.; Park, J.S.; Yoon, K.J.; Kim, Y.I.; Jin, S.H.; Kang, S.K.; Gal, Y.S.; Kang, S.; Lee, J.Y.; Kang, J.W.; et al. High-Efficiency Deep-Blue Light-Emitting Diodes Based on Phenylquinoline/Carbazole-Based Compounds. Adv. Funct. Mater. 2008, 18, 3922–3930. [Google Scholar] [CrossRef]
- Fisher, A.L.; Linton, K.E.; Kamtekar, K.T.; Pearson, C.; Bryce, M.R.; Petty, M.C. Efficient Deep-Blue Electroluminescence from an Ambipolar Fluorescent Emitter in a Single-Active-Layer Device. Chem. Mater. 2011, 23, 1640–1642. [Google Scholar] [CrossRef]
- Thirion, D.; Poriel, C.; Métivier, R.; Rault-Berthelot, J.; Barrière, F.; Jeannin, O. Violet-to-Blue Tunable Emission of Aryl-Substituted Dispirofluorene-Indenofluorene Isomers by Conformationally-Controllable Intramolecular Excimer Formation. Chem.–A Eur. J. 2011, 17, 10272–10287. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Bai, Q.; Yao, L.; Hu, D.H.; Tang, X.Y.; Shen, F.Z.; Zhang, H.H.; Gao, Y.; Lu, P.; Yang, B.; et al. Solution-Processable Hosts Constructed by Carbazole/PO Substituted Tetraphenylsilanes for Efficient Blue Electrophosphorescent Devices. Adv. Funct. Mater. 2014, 24, 5881–5888. [Google Scholar] [CrossRef]
- van Santen, H.; Neijzen, J.H.M. Deep-UV liquid immersion mastering of high density optical discs. Jpn. J. Appl. Phys. Part 1-Regul. Pap. Brief Commun. Rev. Pap. 2003, 42, 1110–1112. [Google Scholar] [CrossRef]
- Tomb, R.M.; White, T.A.; Coia, J.E.; Anderson, J.G.; MacGregor, S.J.; Maclean, M. Review of the Comparative Susceptibility of Microbial Species to Photoinactivation Using 380-480 nm Violet-Blue Light. Photochem. Photobiol. 2018, 94, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.P.; Liu, J.; Shi, F.P.; Zhao, G.X.; Tan, J.W.; Wang, G. Recent Advances in Silver Nanowires Electrodes for Flexible Organic/Perovskite Light-Emitting Diodes. Front. Chem. 2022, 10, 864186. [Google Scholar] [CrossRef]
- Li, J.-J.; Nie, X.-M.; Zhen, W.; Du, Y.-G. New developments and comparisons in display technology. Chin. J. Liq. Cryst. Disp. 2018, 33, 74–84. [Google Scholar]
- Zhang, D.D.; Huang, T.Y.; Duan, L. Emerging Self-Emissive Technologies for Flexible Displays. Adv. Mater. 2020, 32, 1902391. [Google Scholar] [CrossRef]
- Nakanotani, H.; Higuchi, T.; Furukawa, T.; Masui, K.; Morimoto, K.; Numata, M.; Tanaka, H.; Sagara, Y.; Yasuda, T.; Adachi, C. High-efficiency organic light-emitting diodes with fluorescent emitters. Nat. Commun. 2014, 5, 4016. [Google Scholar] [CrossRef]
- Nakanotani, H.; Masui, K.; Nishide, J.; Shibata, T.; Adachi, C. Promising operational stability of high-efficiency organic light-emitting diodes based on thermally activated delayed fluorescence. Sci. Rep. 2013, 3, 2127. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.-B.; Xu, W.; Zhang, Q.-L.; Zhang, X.-X.; Wen, S.-Y.; Yi, H.-B.; Yuan, L.; Zhang, X.-B. Enhancing the Anti-Solvatochromic Two-Photon Fluorescence for Cirrhosis Imaging by Forming a Hydrogen-Bond Network. Angew. Chem. Int. Ed. 2018, 57, 7473–7477. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Zhu, S.; Liu, Y.; Su, Y.; Feng, Z.; Zhang, W.; Niu, X. AIE properties of blue-light-emitting molecules based on triazine derivatives. Opt. Mater. 2023, 140, 113883. [Google Scholar] [CrossRef]
- Peng, L.; Huo, Y.; Hua, L.; Lv, J.; Liu, Y.; Ying, S.; Yan, S. A highly efficient violet-blue OLED with Rec.2020 CIEy based on an orthogonal phenanthroimidazole-substituted 1,2,4-triazole derivative. J. Mater. Chem. C 2022, 10, 9621–9627. [Google Scholar] [CrossRef]
- Nakamura, S.; Krames, M.R. History of Gallium-Nitride-Based Light-Emitting Diodes for Illumination. Proc. IEEE 2013, 101, 2211–2220. [Google Scholar] [CrossRef]
- DenBaars, S.P.; Feezell, D.; Kelchner, K.; Pimputkar, S.; Pan, C.C.; Yen, C.C.; Tanaka, S.; Zhao, Y.J.; Pfaff, N.; Farrell, R.; et al. Development of gallium-nitride-based light-emitting diodes (LEDs) and laser diodes for energy-efficient lighting and displays. Acta Mater. 2013, 61, 945–951. [Google Scholar] [CrossRef]
- Zhang, H.S.; Zhu, J.; Zhu, Z.D.; Jin, Y.H.; Li, Q.Q.; Jin, G.F. Surface-plasmon-enhanced GaN-LED based on a multilayered M-shaped nano-grating. Opt. Express 2013, 21, 13492–13501. [Google Scholar] [CrossRef]
- Yan, H.; Ku, P.C.; Gan, Z.Y.; Liu, S.; Li, P. Strain Effects in Gallium Nitride Adsorption on Defective and Doped Graphene: First-Principles Calculations. Crystals 2018, 8, 58. [Google Scholar] [CrossRef]
- Wu, Y.B.; Wang, R.X.; Lin, R.; Xu, X.E.; Zhang, X.Y.; Alsalman, O.; Qiu, Y.; Uddin, A.; Ouyang, X.H. Excited-state intramolecular proton transfer emitter for efficient violet-blue organic light-emitting diodes with hybridized local/charge transfer channel. Chem. Eng. J. 2023, 465, 142929. [Google Scholar] [CrossRef]
- Ding, L.P.; Shao, P.; Yin, Y.L.; Ding, F. Synthesis of 2D Phosphorene: Current Status and Challenges. Adv. Funct. Mater. 2024, 34, 2316612. [Google Scholar] [CrossRef]
- Li, G.J.; Xu, K.W.; Zheng, J.B.; Fang, X.L.; Lou, W.W.; Zhan, F.; Deng, C.; Yang, Y.F.; Zhang, Q.S.; She, Y.B. High-Performance Ultraviolet Organic Light-Emitting Diodes Enabled by Double Boron-Oxygen-Embedded Benzo[m]tetraphene Emitters. J. Am. Chem. Soc. 2024, 146, 1667–1680. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Li, W.; Du, S.Y.; Zhang, J.S.; Wang, Z.C.; Zhang, X.L.; Li, Y.; Ge, Z.Y. Purely Nitrogen-Based Multi-Resonance Deep-Blue Emitter with an Ultralow y Color Coordinate of <0.03 via Rationally Intramolecular Charge Transfer. Adv. Opt. Mater. 2023, 11, 2300491. [Google Scholar] [CrossRef]
- Tromayer, M.; Gruber, P.; Rosspeintner, A.; Ajami, A.; Husinsky, W.; Plasser, F.; González, L.; Vauthey, E.; Ovsianikov, A.; Liska, R. Wavelength-optimized Two-Photon Polymerization Using Initiators Based on Multipolar Aminostyryl-1,3,5-triazines. Sci. Rep. 2018, 8, 17273. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhou, H.P.; Xu, G.Y.; Yu, Z.P.; Yang, X.F.; Cheng, L.H.; Kong, L.; Feng, Y.; Wu, J.Y.; Tian, Y.P. Synthesis and two-photon absorption properties of multi-branched styryl derivatives containing π-bond and σ-electron pair as bridge based on 1,3,5-triazine. Tetrahedron 2012, 68, 6569–6574. [Google Scholar] [CrossRef]
- Michinobu, T.; Osako, H.; Shigehara, K. Synthesis and Properties of Conjugated Poly(1,8-carbazole)s. Macromolecules 2009, 42, 8172–8180. [Google Scholar] [CrossRef]
- Melhuish, W.H. Quantum Efficiencies of Fluorescence of Organic Substances: Effect of Solvent and Concentration of the Fluorescent Solute1. J. Phys. Chem. 1961, 65, 229–235. [Google Scholar] [CrossRef]
- Xu, C.; Webb, W.W. Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm. J. Opt. Soc. Am. B 1996, 13, 481–491. [Google Scholar] [CrossRef]
- Lee, D.R.; Kim, M.; Jeon, S.K.; Hwang, S.-H.; Lee, C.W.; Lee, J.Y. Design Strategy for 25% External Quantum Efficiency in Green and Blue Thermally Activated Delayed Fluorescent Devices. Adv. Mater. 2015, 27, 5861–5867. [Google Scholar] [CrossRef]
- Jiang, S.; Lin, J.; Li, D.; Li, M.; He, Y.; Xie, W.; Chen, J.; Gan, Y.; Yang, G.-X.; Yang, Z.; et al. Planar D-σ-A type thermally activated delayed fluorescence material with Intra- and intermolecular charge transfer characteristics. Chem. Eng. J. 2023, 452, 139201. [Google Scholar] [CrossRef]
- Li, G.; Zhao, Y.; Li, J.; Cao, J.; Zhu, J.; Sun, X.W.; Zhang, Q. Synthesis, Characterization, Physical Properties, and OLED Application of Single BN-Fused Perylene Diimide. J. Org. Chem. 2015, 80, 196–203. [Google Scholar] [CrossRef]
- Yuan, C.; Sun, Z.; Wang, Y. Study on the effect of different amounts of hydroxyl and tert-butyl substituted triphenylpyridine units on the properties of polyimide. J. Polym. Res. 2020, 27, 193. [Google Scholar] [CrossRef]
- Connelly, N.G.; Geiger, W.E. Chemical Redox Agents for Organometallic Chemistry. Chem. Rev. 1996, 96, 877–910. [Google Scholar] [CrossRef]
- Fang, X.; Wang, C.; Tian, Q.; Zhang, J.; Chen, Y.; Sun, Z.; Chu, W. Based on triphenylamine-imidazole skeleton electrofluorochromic small organic molecules: Synthesis and electrofluorochromic properties. Mater. Lett. 2023, 333, 133659. [Google Scholar] [CrossRef]
- Chen, Y.M.; Hung, W.Y.; You, H.W.; Chaskar, A.; Ting, H.C.; Chen, H.F.; Wong, K.T.; Liu, Y.H. Carbazole-benzimidazole hybrid bipolar host materials for highly efficient green and blue phosphorescent OLEDs. J. Mater. Chem. 2011, 21, 14971–14978. [Google Scholar] [CrossRef]
- Liu, Z.; Li, K.L.; Hu, Z.H.; Liu, Z.Q.; Cui, D.L. Single and two-photo excited violet-blue fluorescence from aza 6 helicenes and the plannar precursors based-on indole, carbazole, and fluorene. J. Lumin. 2023, 263, 120006. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, S.; Wang, R.; Li, W.; Shen, F.; Yang, B.; Ma, Y. Highly Efficient Near-Infrared Organic Light-Emitting Diode Based on a Butterfly-Shaped Donor–Acceptor Chromophore with Strong Solid-State Fluorescence and a Large Proportion of Radiative Excitons. Angew. Chem. Int. Ed. 2014, 53, 2119–2123. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, S.-L.; Tong, Q.-X.; Lo, M.-F.; Ng, T.-W.; Chan, M.-Y.; Wen, Z.-C.; He, J.; Jeff, K.-S.; Tang, X.-L.; et al. High Efficiency Nondoped Deep-Blue Organic Light Emitting Devices Based on Imidazole-π-triphenylamine Derivatives. Chem. Mater. 2012, 24, 61–70. [Google Scholar] [CrossRef]
- Zhang, Q.; Tsang, D.; Kuwabara, H.; Hatae, Y.; Li, B.; Takahashi, T.; Lee, S.Y.; Yasuda, T.; Adachi, C. Nearly 100% Internal Quantum Efficiency in Undoped Electroluminescent Devices Employing Pure Organic Emitters. Adv. Mater. 2015, 27, 2096–2100. [Google Scholar] [CrossRef]
- Maué, D.; Strebert, P.H.; Bernhard, D.; Rösel, S.; Schreiner, P.R.; Gerhards, M. Dispersion-Bound Isolated Dimers in the Gas Phase: Observation of the Shortest Intermolecular CH...H-C Distance via Stimulated Raman Spectroscopy. Angew. Chem.-Int. Ed. 2021, 60, 11305–11309. [Google Scholar] [CrossRef]
- Wang, S.F.; Su, B.K.; Wang, X.Q.; Wei, Y.C.; Kuo, K.H.; Wang, C.H.; Liu, S.H.; Liao, L.S.; Hung, W.Y.; Fu, L.W.; et al. Polyatomic molecules with emission quantum yields >20% enable efficient organic light-emitting diodes in the NIR(II) window. Nat. Photonics 2022, 16, 843–850. [Google Scholar] [CrossRef]
- Zou, J.; Fang, Y.; Shen, Y.; Xia, Y.; Wang, K.; Zhang, C.; Zhang, Y. Piezochromic Tetracoordinate Boron Complex: Blue-Shifted and Enhanced Luminescence. Angew. Chem. Int. Ed. 2022, 61, e202207426. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.X.; Guo, L.B.; Li, C.M.; Yang, X.Y.; Li, J.M.; Li, X.Y.; Zeng, X.Y.; Lu, Y.F. Investigation of the self-absorption effect using spatially resolved laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2016, 31, 961–967. [Google Scholar] [CrossRef]
- He, X.; Lou, J.L.; Li, B.X.; Dong, X.B.; Zhong, F.Y.; Liu, W.; Feng, X.; Yang, D.Z.; Ma, D.G.; Zhao, Z.J.; et al. Rational Medium-Range Charge Transfer Strategy Toward Highly Efficient Violet-Blue Organic Light-Emitting Diodes with Narrowed Emission. Adv. Mater. 2024, 36, 2310417. [Google Scholar] [CrossRef]
- Khan, A.; Tang, X.; Zhong, C.; Wang, Q.; Yang, S.Y.; Kong, F.C.; Yuan, S.; Sandanayaka, A.S.D.; Adachi, C.; Jiang, Z.Q.; et al. Intramolecular-Locked High Efficiency Ultrapure Violet-Blue (CIE-y < 0.046) Thermally Activated Delayed Fluorescence Emitters Exhibiting Amplified Spontaneous Emission. Adv. Funct. Mater. 2021, 31, 2009488. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Z.L.; Coropceanu, V.; Brédas, J.L.; Ginger, D.S. Lower limits for non-radiative recombination loss in organic donor/acceptor complexes. Mater. Horiz. 2022, 9, 325–333. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, C.; Su, L.W.; Wang, D.; Jiang, Y.S.; Tsuboi, T.; Zhang, Q.S. Efficient Intramolecular Charge-Transfer Fluorophores Based on Substituted Triphenylphosphine Donors. Angew. Chem.-Int. Ed. 2021, 60, 15049–15053. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.H.; Feng, X.J.; Zhou, M.S.; Qian, Y.; Yu, H.F.; Qiu, X.Q. Investigation of Aggregation and Assembly of Alkali Lignin Using Iodine as a Probe. Biomacromolecules 2011, 12, 1116–1125. [Google Scholar] [CrossRef]
- Ahn, D.H.; Kim, S.W.; Lee, H.; Ko, I.J.; Karthik, D.; Lee, J.Y.; Kwon, J.H. Highly efficient blue thermally activated delayed fluorescence emitters based on symmetrical and rigid oxygen-bridged boron acceptors. Nat. Photonics 2019, 13, 540–546. [Google Scholar] [CrossRef]
- Hung, W.-Y.; Chi, L.-C.; Chen, W.-J.; Chen, Y.-M.; Chou, S.-H.; Wong, K.-T. A new benzimidazole/carbazole hybrid bipolar material for highly efficient deep-blue electrofluorescence, yellow–green electrophosphorescence, and two-color-based white OLEDs. J. Mater. Chem. 2010, 20, 10113–10119. [Google Scholar] [CrossRef]
- Regan, C.K.; Craig, S.L.; Brauman, J.I. Steric effects and solvent effects in ionic reactions. Science 2002, 295, 2245–2247. [Google Scholar] [CrossRef]
- An, B.K.; Gierschner, J.; Park, S.Y. π-Conjugated Cyanostilbene Derivatives: A Unique Self-Assembly Motif for Molecular Nanostructures with Enhanced Emission and Transport. Acc. Chem. Res. 2012, 45, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Xu, Y.C.; Li, C.L.; Li, Z.Q.; Wang, J.X.; Xue, J.A.; Wang, Q.Y.; Cai, X.L.; Wang, Y. Highly Efficient Electroluminescent Materials with High Color Purity Based on Strong Acceptor Attachment onto B-N-Containing Multiple Resonance Frameworks. Ccs Chem. 2022, 4, 2065–2079. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, Y.; Liu, Z.Y.; Zhang, Y.W.; Wang, X.W.; Zhang, D.D.; Duan, L. A π-D and π-A Exciplex-Forming Host for High-Efficiency and Long-Lifetime Single-Emissive-Layer Fluorescent White Organic Light-Emitting Diodes. Adv. Mater. 2020, 32, 2004040. [Google Scholar] [CrossRef]
- Cao, W.; Abdurahman, A.; Zheng, P.; Zhang, M.; Li, F. High-performance non-doped blue OLEDs based on 1,2,4-triazole-phenanthroimidazole derivatives with negligible efficiency roll-off. J. Mater. Chem. C 2021, 9, 6873–6879. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Tang, B.Z.; Wong, K.S. Aggregation Enhancement on Two-Photon Optical Properties of AIE-Active D-TPE-A Molecules. J. Phys. Chem. C 2014, 118, 26981–26986. [Google Scholar] [CrossRef]
- Hua, W.; Liu, Z.; Duan, L.; Dong, G.; Qiu, Y.; Zhang, B.; Cui, D.; Tao, X.; Cheng, N.; Liu, Y. Deep-blue electroluminescence from nondoped and doped organic light-emitting diodes (OLEDs) based on a new monoaza[6]helicene. Rsc. Adv. 2015, 5, 75–84. [Google Scholar] [CrossRef]

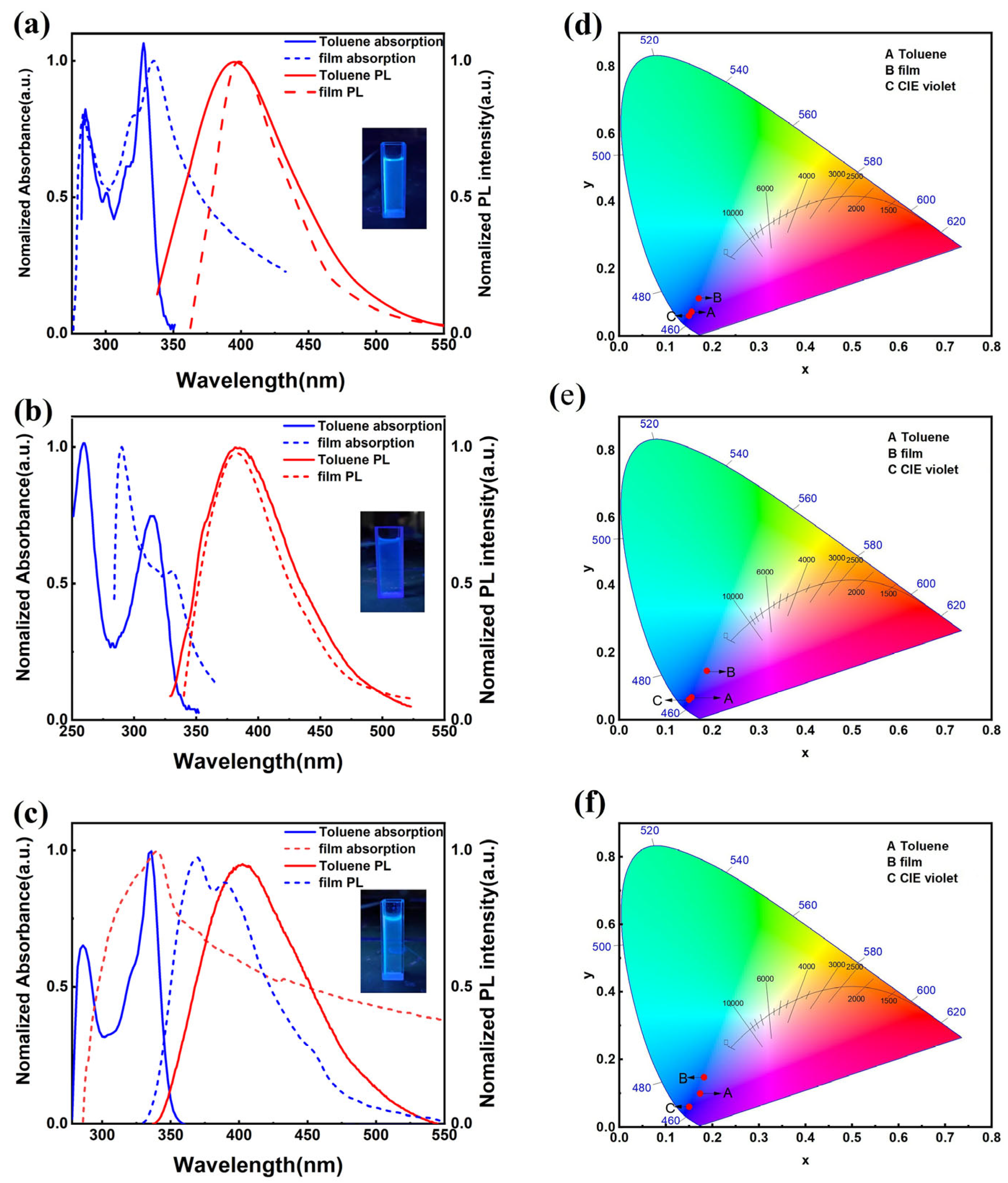

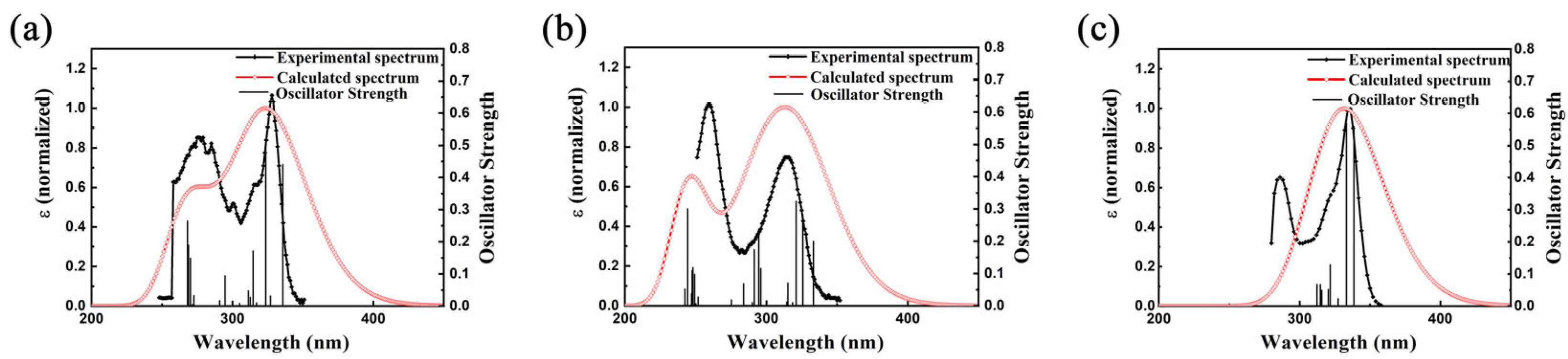
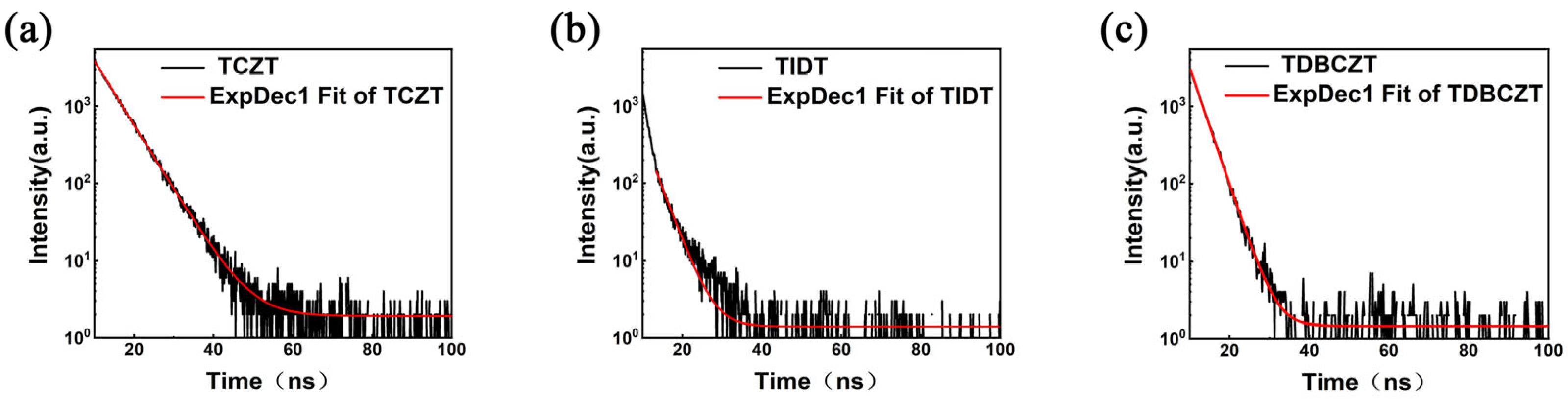
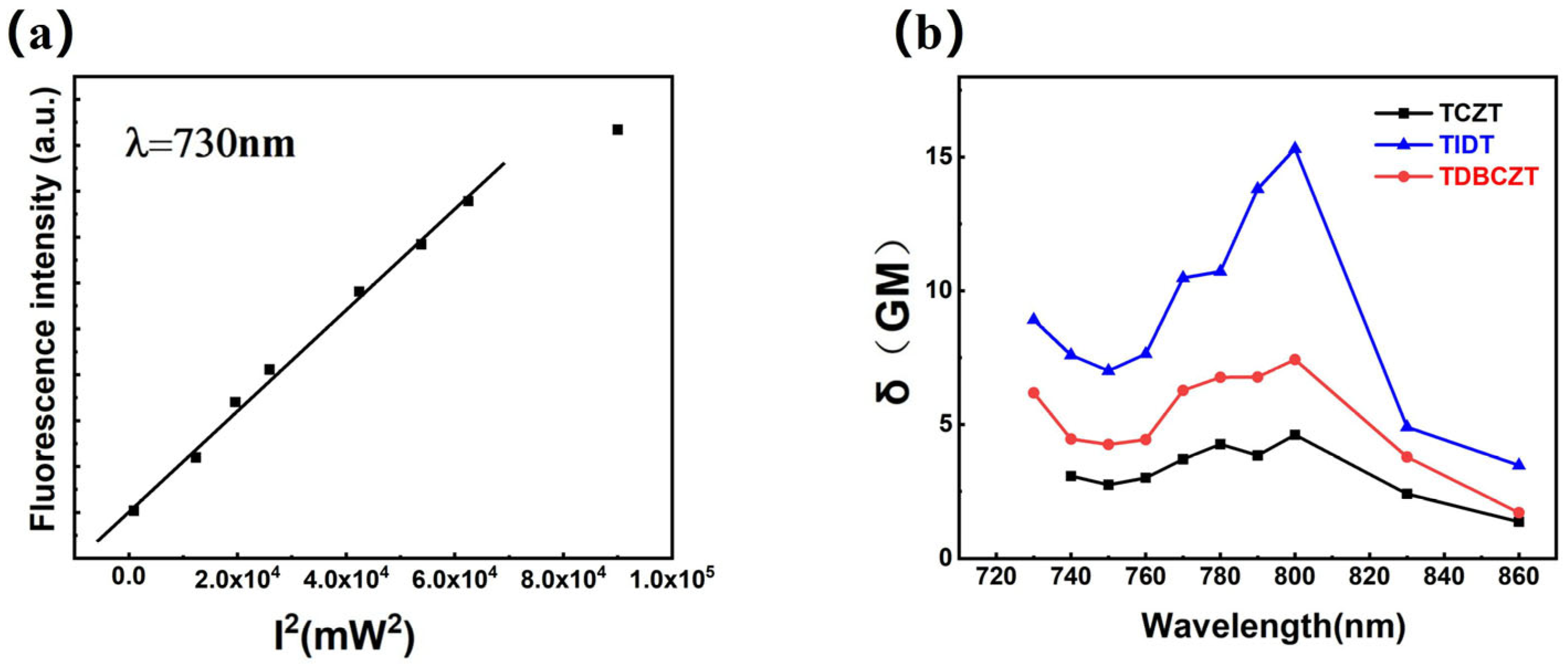
| Compounds | λabs (a) [nm] | λem (b) [nm] | FWHM (c) [nm] | CIE (x, y) (d) | Фf (e) [%] | δmax (f) [GM] |
|---|---|---|---|---|---|---|
| TCZT | 285, 328/284, 336 | 397/408 | 93/61 | (0.155, 0.072) | 46.0/1.43 | 4.6 |
| TIDT | 260, 317/291, 331 | 383/381 | 80/75 | (0.155, 0.067) | 18.0/2.67 | 15.3 |
| TDBCZT | 286, 336/288, 340 | 402/369 | 92/79 | (0.173, 0.995) | 31.4/3.16 | 7.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Du, E.; Liu, Z.; Liu, Z. Preparation, Thermal, and Optical Properties of D-A-Type Molecules Based on 1,3,5-Triazine for Violet-Blue Fluorescent Materials. Materials 2025, 18, 2043. https://doi.org/10.3390/ma18092043
Wang L, Du E, Liu Z, Liu Z. Preparation, Thermal, and Optical Properties of D-A-Type Molecules Based on 1,3,5-Triazine for Violet-Blue Fluorescent Materials. Materials. 2025; 18(9):2043. https://doi.org/10.3390/ma18092043
Chicago/Turabian StyleWang, Lu, Enwang Du, Zhi Liu, and Zhiqiang Liu. 2025. "Preparation, Thermal, and Optical Properties of D-A-Type Molecules Based on 1,3,5-Triazine for Violet-Blue Fluorescent Materials" Materials 18, no. 9: 2043. https://doi.org/10.3390/ma18092043
APA StyleWang, L., Du, E., Liu, Z., & Liu, Z. (2025). Preparation, Thermal, and Optical Properties of D-A-Type Molecules Based on 1,3,5-Triazine for Violet-Blue Fluorescent Materials. Materials, 18(9), 2043. https://doi.org/10.3390/ma18092043





