1. Introduction
Lithium is a critical element with high electrochemical activity, low density, and high capacity for energy storage and release, and is widely used across various fields, such as energy technology, transportation, nuclear energy, metallurgy, chemistry, pharmaceuticals, and aerospace [
1,
2,
3]. Lithium isotope properties enhance their value in future green technologies for clean nuclear energy production. With a natural abundance of only 7.5%,
6Li is a rare isotope that can absorb neutrons and produce tritium in nuclear reactions, causing it to be of interest in research, technology, and future technologies [
4]. On the other hand,
7Li, which has a natural abundance of 92.5%, has valuable importance for electric vehicle propulsion, electrical equipment, and heavy water reactors, and its properties help reduce neutron absorption in water [
5,
6,
7,
8,
9]. The separation of lithium isotopes, particularly
6Li and
7Li, remains a complex challenge in modern industry, with significant implications for the development of advanced technologies. Various separation techniques have been developed, each offering distinct advantages and limitations [
3,
10,
11,
12,
13]. Although
6Li and
7Li share similar chemical properties, their unique nuclear characteristics underscore the importance of their separation and enrichment [
14,
15,
16]. A notable approach was developed by Hoshino and Terai [
17], who introduced a technique for separating lithium isotopes using a highly porous Teflon foil, 1 to 3 mm thick, as an organic membrane [
17].
Electromigration is a relatively new technique for separating Li isotopes using ionic liquid systems. Using this approach, Hoshino et al. [
10] achieved Li isotope separation via electrodialysis with an ionic-liquid-impregnated organic membrane in an aqueous solution, obtaining a notable separation coefficient of 1.4. This efficiency was attributed to the different mobilities of
6Li and
7Li ions within the ionic liquid medium. The advantages of ionic liquid–crown ether systems are being intensively studied as promising approaches for Li isotope separation through extraction processes [
10,
13,
17,
18]. For instance, Zhang et al. [
19] used three different ionic liquids in electromigration, achieving striking results in isotope separation. They found notable diffusivity differences between
6Li and
7Li across various ionic liquids, thus enhancing separation efficiency. Sun et al. [
11] reported a maximum single-stage separation coefficient of 1.046 by substituting conventional molecular solvents with ionic liquids in liquid–liquid extraction. Zhou et al. [
20] developed a sustainable method for lithium isotope separation by doping mesoporous silicon with ionic liquids and benzo-15-crown-5. However, they observed that crown ether tended to leach from the doped silica material after immersion in ethanol for 36 h. In addition, 15-Crown-5 is a macrocyclic ether known to selectively bind lithium ions (Li⁺) due to its cavity size of about 1.7–2.2 Å. This precise fit makes the formation of stable complexes possible, and these have been utilized in Li isotope separation processes. In a separation system, 15-Crown-5 can preferentially interact with one Li isotope through selective binding, thus facilitating separation. Various studies reported that a separation system using 15-Crown-5 can transport Li
+ between aqueous and organic phases, enabling effective isotope fractionation [
21]. 15-crown-5-ether into dibutyl phthalate (DBP) in a Li
+ detection membrane depends on several factors, including ion selectivity, membrane stability, and sensor performance [
22].
The membrane-based process for Li separation is relatively novel and is easy to operate on a small scale, with low energy consumption, promising efficiency, and a low environmental footprint. The membranes on the market are widely available for Li harvesting technologies, such as recycling, which uses nanofilms, membrane electrodialysis, membrane absorption, and membrane solvent extraction. However, commercially available organic membranes used for separation are less accessible. They are subjected to various disadvantages, including availability and materials beyond the cost of purchase, which are affected by market fluctuations. One significant advantage of laboratory-made membranes is their customizability. Customization includes the integration of crown ethers for different periods in various combinations, which play a crucial role in selectively capturing lithium ions and can be produced on a small scale, allowing for rapid prototyping and testing of new designs without the need for large-scale manufacturing facilities. This also offers a cost-effective solution, which is especially valuable for niche applications such as Li isotope separation at an industrial scale [
18,
23,
24,
25]. Membranes impregnated with crown ethers help minimize cross-contamination between isotopes, ensuring higher purity levels of the desired isotope, which increases
6Li separation and enrichment efficiency.
In this study, we develop two groups of laboratory-made membranes, non-impregnated membranes with LiNTf2 before electromigration (AI-1) and impregnated membranes (AI-2), to evaluate their performance in lithium isotope separation. The membranes were characterized, tested, and assessed in the first stage to understand the mechanism behind isotope separation. The study aimed to test individual and combined interactions between several factors—such as membranes, voltage, migration time, and organic solutions—involved in the electromigration process. In addition, the experiments indicate the minimal thresholds of the parameters for efficient lithium ion migration in an electrolyte solution. The following hypotheses were adopted: (i) the membranes would exhibit varying degrees of performance, with potential failure, and (ii) by adjusting and combining different parameters, the impact of external factors on membrane properties and separation efficiency can be examined.
2. Materials and Methods
2.1. Chemical Reagents
Laboratory-made membranes for Li isotope separation require specialized materials that ensure selective permeability and durability. They are composed of polymers known for their stability and resistance to harsh chemical environments. In this study, polyvinyl chloride (PVC, Sigma-Aldrich, Munich, Germany), dibutyl phthalate (DBP, 99%, Sigma-Aldrich), 15-crown-5 (Sigma-Aldrich, Munich, Germany), and tetrahydrofuran (THF, Supelco, St. Louis, MO, USA) were selected as primary raw materials. Lithium carbonate (Li2CO3, ≥98%, IAEA, LSVEC, Vienna, Austria), anisol (99.8%), and ammonium chloride (NH4Cl, 99.999%) were used (Sigma-Aldrich, Munich, Germany). Additionally, ionic liquids, including 1-butyl-3-methylimidazolium bis (trifluoromethylsulfonyl)imide (Sigma-Aldrich, Buchs, Switzerland) and bis(trifluoromethane)sulfonimide lithium salt (LiNTf2, 99%), were obtained from Sigma-Aldrich, Buchs, Switzerland. All chemicals used in the electromigration experiments were of the highest purity. Other reagents included tetrabutylammonium perchlorate (Fluka Analytical, London, UK), perchloric acid (70–72%, Sigma-Aldrich, Germany), and nitric acid (Suprapur 65%, Sigma-Aldrich, Buchs, Switzerland). Tetrabutylammonium perchlorate and acetonitrile enhanced the electrochemical processes and provided high conductivity without interfering with the electrochemical reactions.
2.2. Experimental Apparatus
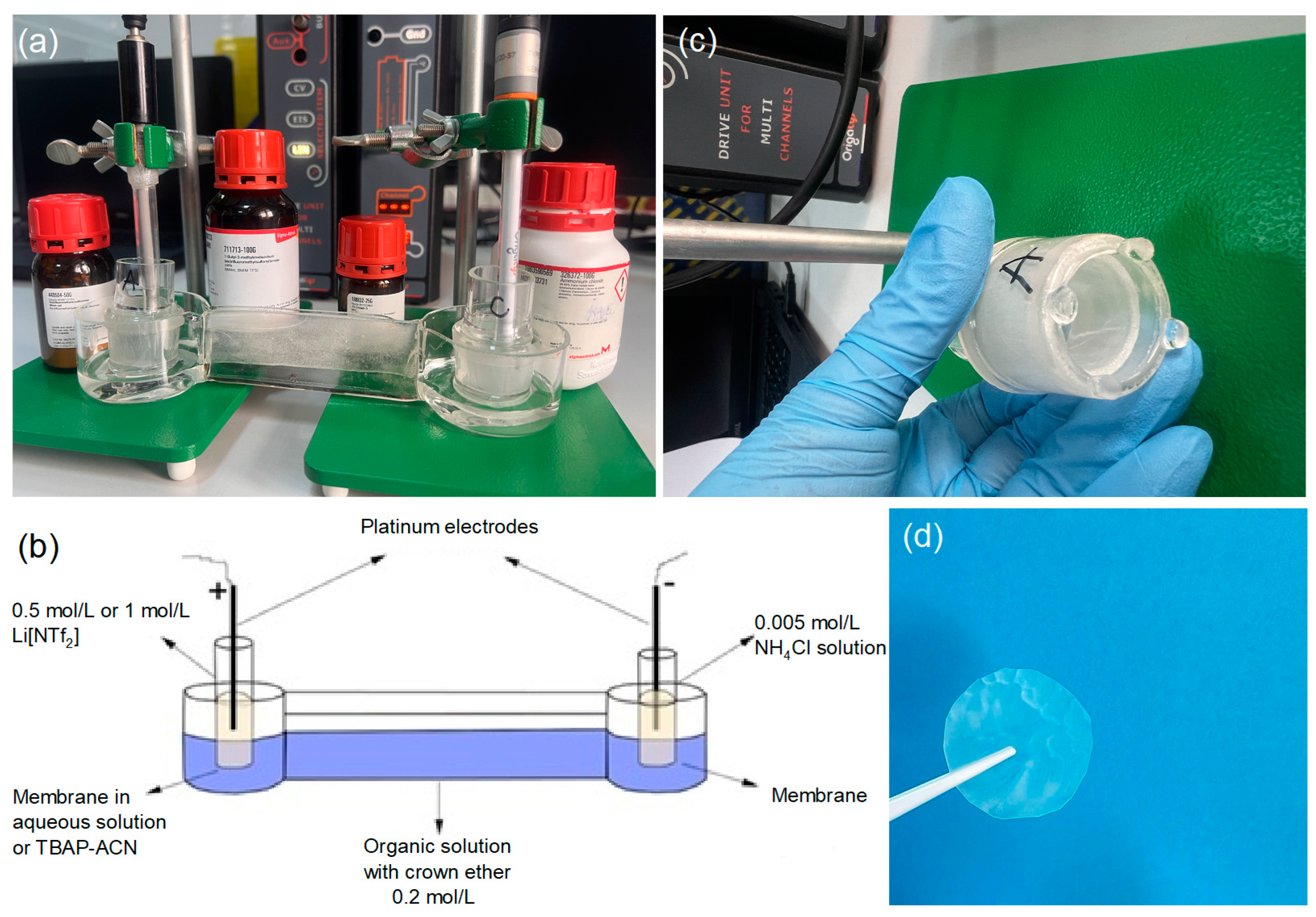
The concentrations of Li+ in the resulting samples were determined using a quadrupole inductively coupled plasma mass spectrometer (Q-ICP-MS, PlasmaQuantMS Elite, Analytik Jena, Jena, Germany) equipped with an autosampler (AIM 3300, A.I. Scientific, Clontarf, Australia) and collision reaction interface (iCRI) operating in the H2 and He modes for lithium isotope analysis. This setup was used to evaluate Li isotope separation effects and to measure isotope ratios (6Li/7Li) in the sample solutions. A multi-channel potentiostat with impedance (1A; model: Origalys, Rillieux-la-Pape, France), which was controlled with OrigaMaster 5 software (Evreux, France), was used to supply the electric field. Electrochemical cells with two platinum wire electrodes (1 mm diameter) were used, designated as the counter electrode (CE–Pt wire) and the working electrode (EL–Pt wire), for the experimental studies conducted in the ISO-Li project. Solution viscosity measurements were carried out with a Haake Viscotester iQ rheometer equipped with a Thermo Scientific Peltier temperature control system (Dreieich, Germany).
2.3. Membrane Synthesis
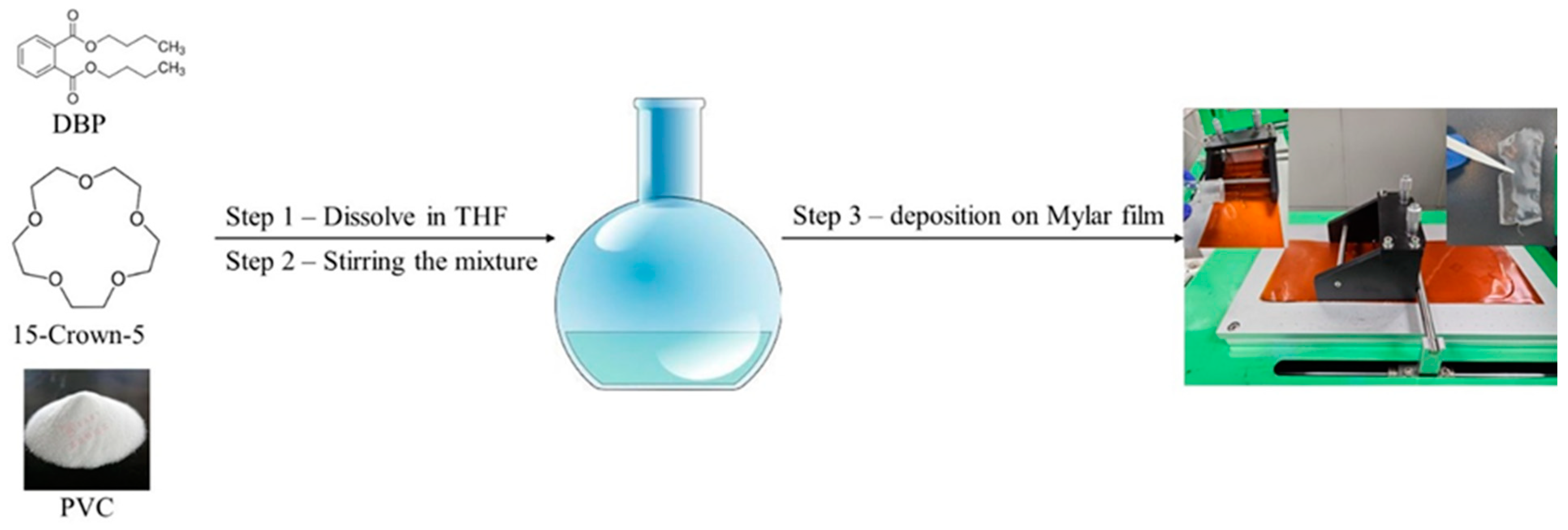
The Li
+ detection membrane was synthesized by mixing 15-crown-5, dibutyl phthalate, and polyvinyl chloride (PVC) at a molar ratio of 1:0.523:0.31 in tetrahydrofuran (THF) as a dispersing solvent. The mixture was stirred for 8 h at 55 °C and kept below the boiling point of THF to achieve a homogenous solution. The membrane was then formed using Doctor Blade deposition equipment and allowed to rest for 24 h at room temperature for THF evaporation (
Figure 1). Two membranes were prepared with different molar ratios of 15-crown-5 to DBP of 1:1 (AI-1) and 2:1 (AI-2). The resulting membranes had a thickness of 0.039 mm.
2.4. Laboratory-Made Membrane Characterization
This study developed and tested two groups of laboratory-made membranes, AI-1 and AI-2, which were exposed to ionic liquid and crown ether in synthesis (in the first manufacturing stage). The AI-2 membranes were subjected to double the amount of crown ether with a ratio of 2:1. The AI-1 was non-impregnated with LiNTf2 before electromigration (hereafter, non-impregnated), and AI-2 was impregnated with LiNTf2 before electromigration (hereafter, impregnated); they were further adjusted by varying the crown ether content to evaluate their efficacy in lithium isotope separation. A detailed description of the experimental setup for membrane production and electromigration is presented in the following.
The solution viscosity was measured using a Haake Viscotester iQ rheometer (Karlsruhe, Germany) with Thermo Scientific Peltier temperature control, employing cylindrical coaxial geometries with a 10 mL sample volume. Flow curves were recorded at 25 °C, with a logarithmic distribution over 120 s and an integration time of 5 s, covering a shear rate ranging from 10
−1 s
−1 to 1500 s
−1. Data were analyzed using the power law model according to Equation (1). Broadband dielectric measurements were conducted with a Novocontrol Concept 40 high-resolution Alpha dielectric analyzer (Montabaur, Germany), covering a frequency range from 10
−1 Hz to 10
7 Hz and a temperature range of −150 °C to 200 °C; this was managed by a Quattro Cryosystem (Montabaur, Germany) with a temperature stability of up to 0.3 K in a dry nitrogen atmosphere. The polyelectrolyte thin film was placed between two polished gold electrodes (25 and 15 mm) to create a parallel-plate capacitor cell. These sandwich samples were then positioned in the Novocontrol spectrometer, where the dielectric permittivity and conductivity were measured isothermally at temperature intervals of 10 K during cooling and 5 K during heating, ensuring reproducibility. An AC voltage amplitude of 0.05 V was applied across the whole temperature range and frequency spectrum. Additionally, these polymers could be modified with functional groups or additives, such as crown ethers, to enhance Li
+ affinity, particularly for the separation of
6Li and
7Li isotopes. The impregnation of laboratory-made membranes is essential in enriching the target isotope
6Li. Therefore, we tested two types of impregnation for all membranes during their creation and conducted hyper-impregnation on the second group of membranes before electromigration (
Table 1). After manufacturing the membranes, we tested their performance in the electromigration process, particularly in terms of the properties described in the section on membrane optimization.
where η (Pa·s) is the viscosity as a function of the shear velocity, ẏ (s
−1), K is the flow consistency index, and fb indicates the flow behavior index.
2.5. Electromigration and Analytical Experiments
The ionic liquid 1-butyl-3-methylimidazoliu bis((trifluoromethyl)sulfonyl)imide was mixed with anisole in a ratio of 7:3, and 0.2 mol/L of 15-crown-5 ether was added to create the organic solution, which was then placed in the intermediate tank. The anode solution was prepared using different concentrations (0.5 mol/L and 1 mol/L) of lithium bis(trifluoromethane)sulfonimide (LiNT
f2) in aqueous and tetrabutylammonium perchlorate (TBAP) solution and placed in the anode tank. The cathode solution consisted of a 0.005 mol/L aqueous solution of NH
4Cl and was placed in the cathode tank. Two laboratory-made membranes impregnated with 15-crown-5 and DBP in different molar ratios, 1:1 (AI-1) and 2:1 (AI-2), were used to separate the anode, organic, and cathode solutions. A platinum wire electrode with a 1 mm diameter was placed in both the anode and cathode solutions. An electric field was applied using a DC power supply, Origalys potentiostat (Rillieux-la-Pape, France), at different potentials (3–15 V). Samples from the anodic and cathodic solutions were collected at 2, 6, 20, 25, and 48 h intervals. In our experiment, electrochemical studies on the lithiation/delithiation process were conducted using the chronoamperometric method at a constant electric potential.
Table 1 provides the operating conditions under which optimization tests were performed for the electrochemical lithiation/delithiation process. The chronoamperometric method was applied over 48 h. The experiment evaluated each membrane with different factor variations, such as lithium salt concentration, membrane type, working environment, and applied electric potential, to optimize the electrochemical procedure. Additionally, experiments were conducted in aqueous and organic (0.1 M TBAP/CH
3CN) environments. The electromigration process was performed using a manufactured experimental setup (
Figure 2).
2.6. Sample Analysis Using Isotopic Ratio Measurements
Organic matter was collected through a five-step back-extraction from the organic phase into a hydrolytic acid solution to evaluate the Li
+ concentration. The samples were dry-extracted in a sand bath with concentrated nitric acid and perchloric acid in a 4:1 volume ratio at 195 °C with a heating power of 70 W. After dry digestion, the resulting solution was clear and transparent, allowing for the accurate testing of the Li
+ concentration and Li isotope values. Sample digestion was conducted at the Isotope Metals Laboratory within the ICSI Analytics Department at the ICSI Institute. Samples of 1 mL were taken from both the anode and cathode solutions at intervals of 2, 6, 20, 25, and 48 h. For precise Li isotope measurement, we focused on purifying lithium by eluting other elements (e.g., Na and K) that could interfere with the Li isotope ratios. The chromatography system was designed in two stages to ensure high sample loading and a fixed Li elution range. In this experiment, BioRadTM Econo-Pac columns (1.5 cm ID × 12 cm H, polypropylene) were used following the starting procedure of a volume of 1 mL of 0.7 mol/L HNO
3 that was eluted through the columns and cleaned with 50 mL of 6N HCl. In the recovered Li solutions, we analyzed the concentrations of
23Na,
39K,
6Li, and
7Li. Aliquots of each sample were prepared in 0.7 M HNO
3 (
v/
v) containing 1, 5, 25, 50, and 100 ppb of Li for quantitative Q-ICP-MS analyses, along with 10 ppb of LSVEC reference material for Li isotope ratio measurements. Then, 45 mL of Li fractions were collected after column separation and quantitatively analyzed for
6Li,
7Li,
23Na, and
39K using Q-ICP-MS Plasma-Quant MS Elite (Analytik Jena, Jena, Germany). A standard extraction ratio (
E) was employed to quantify the total ion content transferred into the organic phase, represented as the percentages of cation concentration in the aqueous phase before extraction (C
o) and after extraction (C
e), as detailed in Equation (2).
We used a quadrupole inductively coupled plasma mass spectrometer (Q-ICP-MS, PlasmaQuant MS Elite, Analytik Jena, Jena, Germany) and the sample–standard bracketing method. Each sample was bracketed with the L-SVEC Lithium Carbonate standard, and the resulting measurements were compared with the standard according to the calculation outlined in Equation (3). The optimized instrumental setup was used to conduct high-precision lithium isotope measurements (2RSD = ±0.30‰) for natural carbonate samples. The organic solution in the (
6Li/
7Li) standard, which reflects the lithium isotope abundance ratio, for the L-SVEC standard is 0.08215 ± 0.00023 (IAEA-LSVEC Lithium Carbonate, Vienna, Austria). The Li isotope ratio (δ
7Li) was calculated by standardizing the tested sample against the LSVEC standard, which was lithium carbonate (Li
2CO
3) manufactured by the National Institute of Standards and Technology [
26].
The efficiency of the isotopic enrichment was quantified using the separation factor (α), which reflects the distribution of the desired isotope between two phases: the enriched and depleted phases. The key parameter for characterizing this separation process is the single-stage separation coefficient, denoted as α, or the alpha factor (Equation (4)). The separation factor indicates the degree of fractionation achieved in each phase after an enrichment step [
27]. By definition, this equals the isotopic abundance ratio in each phase after an enrichment step.
where (
6Li/
7Li)
sample represents the Li isotope abundance ratio in samples extracted during the experiment, while (
6Li/
7Li)
standard L-
SVEC denotes the Li isotope abundance ratio in the L-SVEC standard. The resulting
δ6Li reflects the Li isotopic values from the experiment. The separation coefficient, α, was used to indicate the Li isotope separation effect during the electromigration process and was calculated using Equation (4).
where [
6Li]/[
7Li] represents the Li isotope abundance ratio at the cathode phase and the anode phase in the two liquid separation processes.
4. Results and Discussion
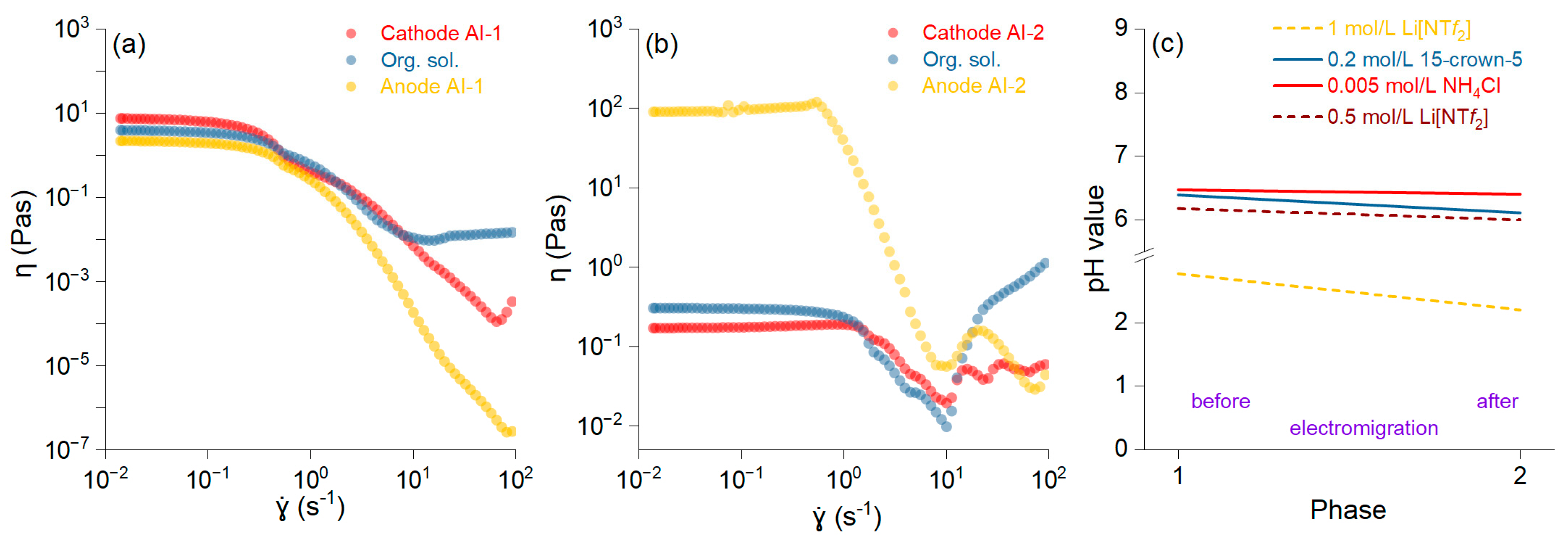
To conduct our experiment, an electromigration laboratory system was created to separate Li isotopes using solution–organic and solution–aqueous environments with the lithium salt solution in the anode chamber. The initial Li salt solution concentration was 7000 mg/L. After the experiment, it was measured at 6491 mg/L in the anode chamber, indicating that only 0.19% of the Li
+ migrated from the anode solution to the organic phase in the case of the AI-2 membranes. Higher Li
+ migration at the level of 48% was achieved in the case of non-impregnated membranes with LiNT
f2 before electromigration (AI-1). Our results show that the transfer of Li
+ from the aqueous anode solution to the organic phase was highly restricted, which was likely due to the bulk organic solution impeding migration, with only 10.20 mg/L being detected from the total concentration in the case of AI-2. Li
+ presence near the cathode chamber reached 16.51% at 48 h (AI-2), resulting in a low concentration in the cathode solution. The low Li
+ migration efficiency of the AI-2 membrane could be associated with the high viscosity induced by the pH, which renders the membrane susceptible to structural degradation at high shear rates. Similar studies show that Li
+ in the cathode solution depends on that from the organic solution, and the increasing time of electromigration promotes the increase in Li
+ in the cathode solution [
33]. An experiment evaluating the separation system with B12C4, B15C5, and B18C6 transfer catalysis in a LiCl solution showed that separation effectiveness was mainly influenced by the interface between the anolyte and organic solution; the electric field had no significant effect [
34]. Separation forces are dynamic, and diffusion increases with the concentration gradient, while chelation negatively correlates with increasing Li
+ concentrations in organic solutions [
35]. Another study using a three-stage system of “LiCl aqueous solution (anolyte)|B12C4-[EMIm][NTf
2] organic solution|NH
4Cl aqueous solution (catholyte)” found that voltage and migration time significantly affect isotope separation [
36].
Notably, results were achieved after modifying the system with a TBAP (tetra butyl ammonium perchlorate) solution in CH
3CN and a Li salt solution in the anode chamber. From 7000 mg/L, the stock salt solution concentration was measured at only 6850 mg/L in the anode chamber, indicating a 0.20% migration rate. The Li
+ in the organic solution near the cathode increased by 48.34% at 24 h, resulting in a higher concentration of Li
+ in the cathode solution. The AI-1.1 membrane had no fractionation effect in the anode liquid–liquid environment, with no observed
6Li enrichment, which diminished until 25 h (
Figure 5). For AI-1.2, the membrane showed the best performance with a positive enrichment factor at each evaluated interval, achieving the maximum at 25 h (
Figure 6b). In the case of
7Li, the transfer occurred until 25 h, after which enrichment reached 52.37%, suggesting different migration rates for the two isotopes. Using a 0.5 mol/L Li[NT
f2] (Bis-(trifluoromethane) sulfonimide lithium salt) solution with 0.1 M TBAP in CH
3CN significantly improved lithium isotope separation compared with that in an aqueous environment with higher salt concentrations. Following digestion, the TBAP solution was visually clear with brown particles and filaments, contrasting with the aqueous solution.
4.1. Driving Effects of the Migration Electric Field
The concentration of Li
+ in the organic solution fluctuated differently from that in the catholyte with intensifying voltage, but after 12 V, the trend was significantly increasing in both cases (
Figure 5a). Li
+ had an evident migration rate in the catholyte only after 9 V. The separation effect could be observed differently on each membrane, and the results need to be understood based on their peculiarities. It was noted that AI-1.2 had, from the very beginning, values higher than 1.
Figure 5b shows that for AI-1.2, the catholyte–organic solution interface was permanently positive, increasing to 9 V, followed by a constant decrease. The Δ
6Li value was constantly upbeat, indicating an enrichment in the organic solution.
Figure 5c shows that after 9 V, there was a jump, documenting a positive effect of growing voltage after this threshold, reaching values of α = 1.021 (9 V), α = 1.029 (12 V), and α = 1.0317 (15 V). When evaluating the performance of membrane AI-1.3, which also presented only positive Δ
6Li values, the decrease was slow from 3 V to 15 V. In this case, the effect of voltage was noted at 6 V. AI-1.2 and AI-1.3 were identical; only the organic solution in the anode chamber differed by, respectively, 0.5 mol/L and 1 mol/L LiNT
f2 in the TBAP solution. For AI-2.2, a membrane similar to AI-1.3 (differing in that tetra butyl ammonium perchlorate (TBAP) had a concentration of 0.1M in CH
3CN), it was noted that the α values were below 1, even if the trend constantly increased. For AI-2.2, the Δ
6Li values were, in each case, negative, slowly decreasing from 3 V to 15 V. Evaluating these three cases, it can be stated that an organic solution of 1 mol/L shows a low separation factor, and Li
7 is advantageous for enrichment.
The impregnated membranes before electromigration with double the amount of crown ether (ratio 2:1; AI-2) demonstrated lower enrichment than those with a single crown ether structure (ratio 1:1; AI-1). In all membranes, the crown ether concentration in the ionic liquid within the transfer tube from anode to cathode remained constant at 0.2 mol/L. Similar experiments have shown that crown ether improves
6Li ion migration performance [
23,
27,
32]. However, Li
+ migration from the anode solution to the organic solution remains limited, with negligible enrichment. Research on the separation of Li
+ from aqueous to organic solutions using ionic liquid electromigration indicated consistent outcomes. It highlighted that the migration efficiency of Li
+ in the organic phase is low, which is attributed to the increasing concentration gradient between the cathode and anode [
37]. The increased concentration difference at the cathode first raises the Li
+ concentration, followed by a decrease, though higher voltages still impact migration [
3]. The experimental results show that the separation coefficient is conditioned by cathode ion mobility and high electrical potential [
25]. In addition, a similar study found that 16 V caused a higher enrichment of
6Li in the catholyte due to the weakening of the stability of crown ether [
37].
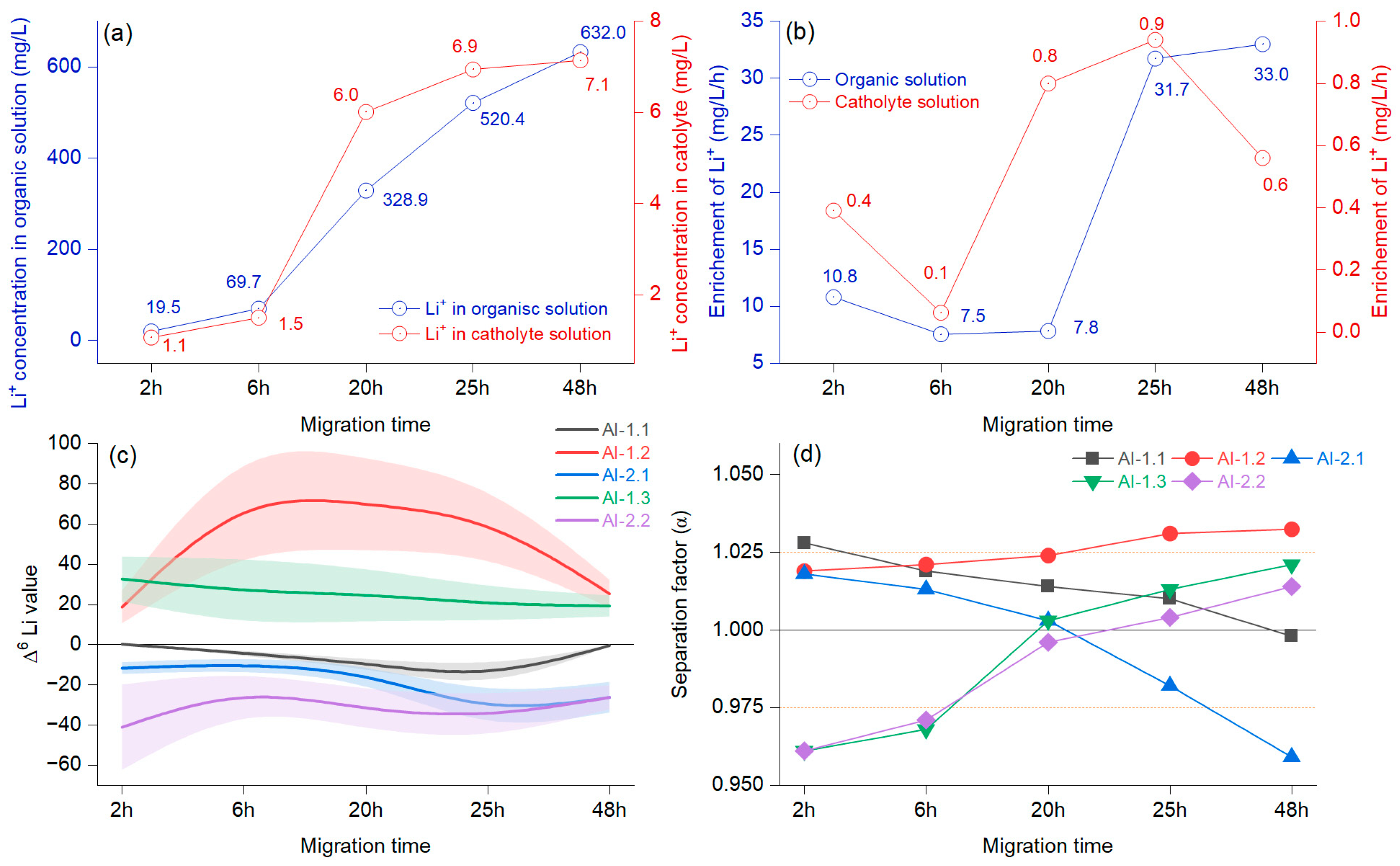
4.2. Driving Effects of Migration Time
Figure 6a illustrates the concentration of Li
+ in the organic solution and the changes at the cathode when considering the migration time. In the first 6 h, the growth was slow and became faster at 20 h, after which the trend grew but with reduced intensity. The Li
+ migration in the organic solution decreased in the first 6 h and increased by 20 h (
Figure 6b). The enrichment in Li
+ concentration in the catholyte decreased at 6 h, after which it increased strongly until 25 h, resulting in another reduction. After the first 6 h, the Δ
6Li values indicated an enrichment, which was evident until 48 h and was more apparent for AI-1.2 (
Figure 6c). The negative values in AI-1.1 document that the use of this system slowly increased Δ
7Li.
The separation factor was above 1, with slow, sustained growth only in the case of the AI-1.2 membrane (
Figure 6d). The experiment that included the AI-1.3 membrane showed that Δ
6Li had only positive values with a slight decrease, mainly until 20 h, indicating that the enrichment of
6Li occurred in the cathode chamber. The separation factor was initially α = 0961 at the 20 h overhead threshold of 1 (α = 1.003), and a maximum of α = 1.021 was achieved at 48 h. A different pattern was observed in the case of the AI-1.1 membrane, which mainly decreased, and at 48 h, the separation factor was below 1 (α = 0.998), documenting that the
6Li enrichment was weakening. According to the presented results, there was a change in the enrichment factor at around 20 h in the catholyte. A notable increase in the migration potential of
6Li was observed before 6 h, alongside the potential for accumulation in the catholyte, which was influenced by the molar concentration of the organic solution. Previous reports have indicated that Li
+ derived from the catholyte originates from an organic solution [
33]. Furthermore, variations are anticipated due to the combined effects of voltage, diffusion, chelation by the crown ether, and the migration duration, which collectively facilitate an increase in the concentration of Li
+ in the catholyte [
35]. When assessing the combined effect of voltage and migration time, it is worth noting that crown ether can be assigned comparable significance. This is particularly true for the highly impregnated AI-2 membrane, where the Li isotope ions were almost inseparable from the crown ether. In the literature, the opposite process was mentioned, where the crown ether was found to have minimal importance [
8]. Even so, regarding our preliminary findings after testing various impregnations, we can state that controlled membrane impregnation helps minimize cross-contamination between isotopes and increases enrichment efficiency.
5. Conclusions
In this study, we developed and tested two groups of laboratory-made membranes, non-impregnated membranes before electromigration with Li[NTf2 (AI-1) and impregnated membranes (AI-2). The membranes were characterized under electromigration conditions within a Li salt solution–organic and solution–inorganic system, and the results indicated a better performance for those that were non-impregnated. The optimization process documented that shear viscosity, which was influenced by temperature variations, insignificantly affected Li separation efficiency through electromigration. The main conclusions are as follows: (i) the voltage, migration time, and concentration of tetra butyl ammonium perchlorate (TBAP) with a concentration of 0.1 M in CH3CN highly improved the separation factor (α) of the catholyte organic solution system and the enrichment of Li+ in the catholyte; (ii) the migration of Li+ in the catholyte environment was evident after 9 V, with a migration time that increased from a minimum of 6 h and that differed with different concentrations of organic solution in the anode chamber; (iii) hyper impregnation of membranes with crown ethers (AI-2) increases the migration but not necessarily the separation, mainly in the first hours and when applying high voltage; (iv) an equilibrium between voltage and migration time must be considered and developed dynamically during separation. Since the testing was performed together with several organic and electromigration solutions, it was evident that the separation effect varied significantly. Based on our results, we can state that (i) the membranes exhibited varying performance degrees under different experimental conditions as a result of significant interactions between all factors evaluated, and (ii) by adjusting and combining different parameters, as in this experimental design, the impact of external factors (voltage, migration time, organic solution) on membrane proprieties and separation efficiency must be adjusted in the laboratory to obtain the maximum performance. This approach shows promise for achieving higher levels of 6Li enrichment by optimizing impregnation methods and conditions, which is valuable for a range of scientific and industrial applications.