Anti-Freezing Conductive Ionic Hydrogel-Enabled Triboelectric Nanogenerators for Wearable Speech Recognition
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CS-PAM Hydrogel
2.3. Manufacture of the G-TENG
3. Results and Discussion
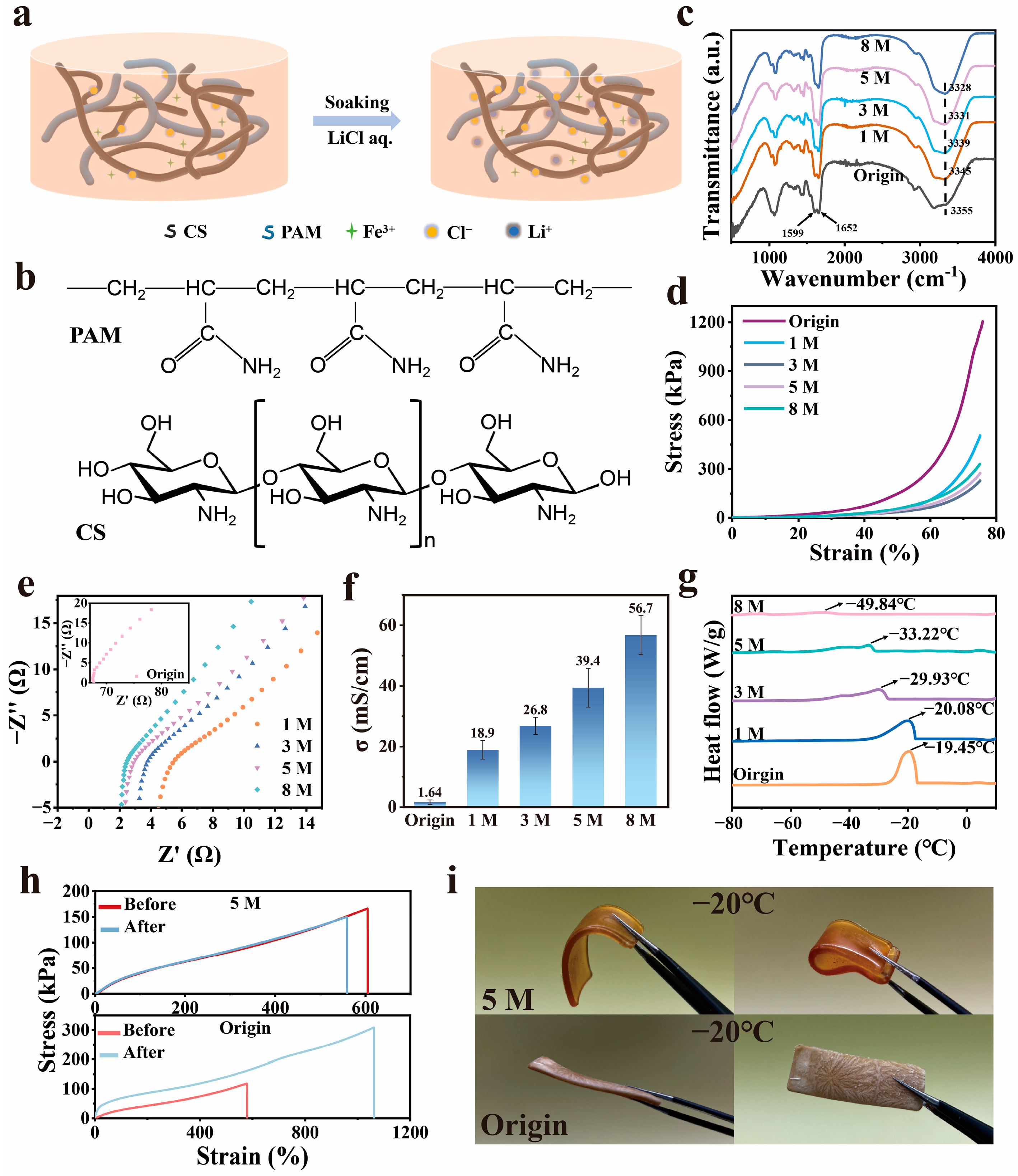
3.1. Preparation and Properties of the CS-PAM/LiCl Gel
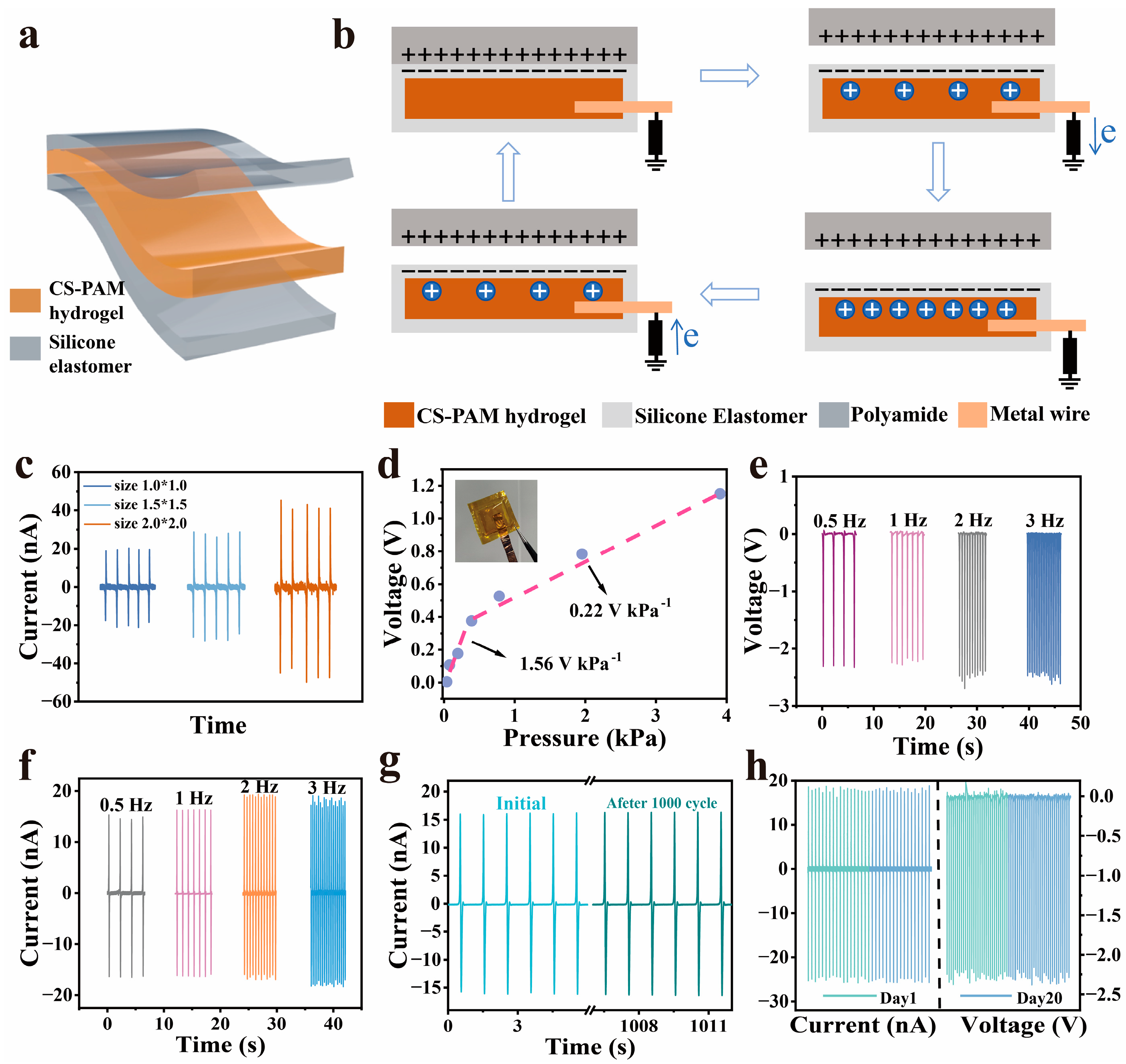
3.2. Design of the G-TENG
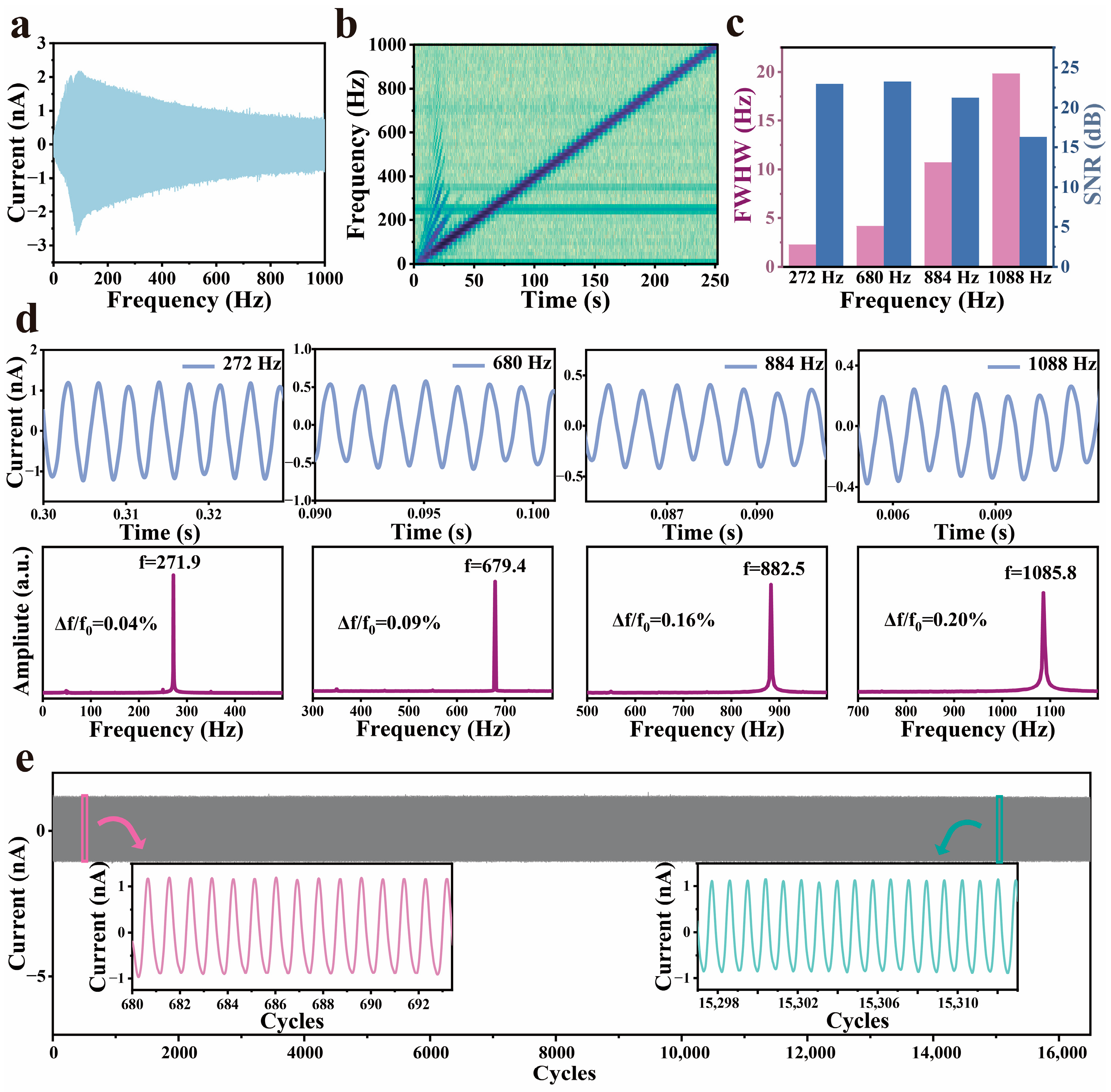
3.3. Performance of G-TENG Sensors
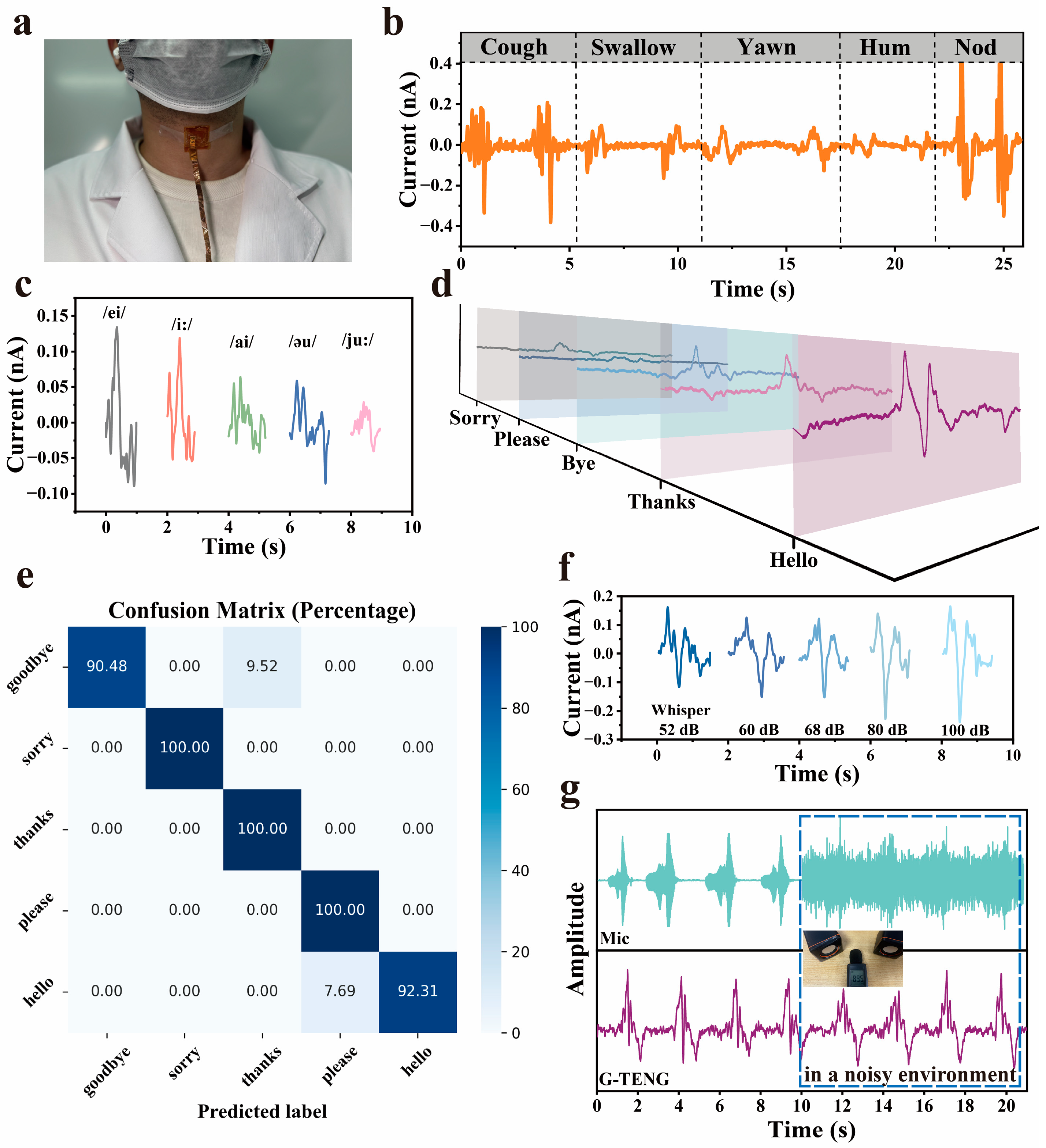
3.4. Speech Recognition Function of G-TENG
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, F.; Han, J.L.; Qi, J.; Zhang, Y.; Yu, J.L.; Li, W.P.; Lin, D.; Chen, L.X.; Li, B.W. Research and Application Progress of Intelligent Wearable Devices. Chin. J. Anal. Chem. 2021, 49, 159–171. [Google Scholar] [CrossRef]
- Lyu, Q.X.; Gong, S.; Yin, J.L.; Dyson, J.M.; Cheng, W.L. Soft Wearable Healthcare Materials and Devices. Adv. Healthc. Mater. 2021, 10, 2100577. [Google Scholar] [CrossRef]
- Wu, Y.H.; Li, Y.W.; Tao, Y.; Sun, L.Y.; Yu, C.Y. Recent advances in the material design for intelligent wearable devices. Mater. Chem. Front. 2023, 7, 3278–3297. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.; Park, M.K.; Ko, S.H. Recent developments in wearable breath sensors for healthcare monitoring. Commun. Mater. 2024, 5, 41. [Google Scholar] [CrossRef]
- Li, S.; Li, H.; Lu, Y.C.; Zhou, M.H.; Jiang, S.; Du, X.S.; Guo, C. Advanced Textile-Based Wearable Biosensors for Healthcare Monitoring. Biosensors 2023, 13, 909. [Google Scholar] [CrossRef]
- Ye, S.; Feng, S.L.; Huang, L.; Bian, S.T. Recent Progress in Wearable Biosensors: From Healthcare Monitoring to Sports Analytics. Biosensors 2020, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Abad, S.A.; Herzig, N.; Raitt, D.; Koltzenburg, M.; Wurdemann, H. Bioinspired adaptable multiplanar mechano-vibrotactile haptic system. Nat. Commun. 2024, 15, 7631. [Google Scholar] [CrossRef]
- Flavin, M.T.; Ha, K.H.; Guo, Z.R.; Li, S.P.; Kim, J.T.; Saxena, T.; Simatos, D.; Al-Najjar, F.; Mao, Y.X.; Bandapalli, S.; et al. Bioelastic state recovery for haptic sensory substitution. Nature 2024, 635, 345–352. [Google Scholar] [CrossRef]
- Jung, Y.H.; Yoo, J.Y.; Vázquez-Guardado, A.; Kim, J.H.; Kim, J.T.; Luan, H.W.; Park, M.; Lim, J.; Shin, H.S.; Su, C.J.; et al. A wireless haptic interface for programmable patterns of touch across large areas of the skin. Nat. Electron. 2022, 5, 374–385. [Google Scholar] [CrossRef]
- Li, T.L.; Wang, Q.A.; Cao, Z.C.; Zhu, J.L.; Wang, N.; Li, R.; Meng, W.; Liu, Q.; Yu, S.F.; Liao, X.Q.; et al. Nerve-Inspired Optical Waveguide Stretchable Sensor Fusing Wireless Transmission and AI Enabling Smart Tele-Healthcare. Adv. Sci. 2024, 12, 2410395. [Google Scholar] [CrossRef]
- Qu, J.; Xie, K.; Chen, S.; He, X.D.; Wang, Y.; Chamberlin, M.; Zhao, X.; Zhu, G.Y.; Xu, C.J.; Shi, P. Multifunctional hydrogel electronics for closed-loop antiepileptic treatment. Sci. Adv. 2024, 10, eadq9207. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Chen, R.; Li, M.Z.; Song, H.Z.; Zhao, X.Y.; Zhang, L.; Zhou, Y.Z.; Chen, J.; Li, J.L.; Chen, M. High Antimicrobial Electrotherapy and Wound Monitoring Hydrogel with Bimetal Phenolic Networks for Smart Healthcare. Adv. Funct. Mater. 2025, 35, 2413080. [Google Scholar] [CrossRef]
- Rahman, M.T.; Rana, S.M.S.; Salauddin, M.; Maharjan, P.; Bhatta, T.; Park, J.Y. Biomechanical Energy-Driven Hybridized Generator as a Universal Portable Power Source for Smart/Wearable Electronics. Adv. Energy Mater. 2020, 10, 1903663. [Google Scholar] [CrossRef]
- Wang, W.L.; Yang, D.F.; Yan, X.R.; Wang, L.C.; Hu, H.; Wang, K. Triboelectric nanogenerators: The beginning of blue dream. Front. Chem. Sci. Eng. 2023, 17, 635–678. [Google Scholar] [CrossRef]
- Li, K.S.; Zhang, D.Z.; Zhang, H.; Wang, D.Y.; Xu, Z.Y.; Cai, H.L.; Xia, H. Triboelectric Nanogenerators Based on Super-Stretchable Conductive Hydrogels with the Assistance of Deep-Learning for Handwriting Recognition. ACS Appl. Mater. Interfaces 2023, 15, 32993–33002. [Google Scholar] [CrossRef]
- Zhang, B.S.; Wang, R.G.; Wang, R.Z.; Chen, B.J.; Li, H.D.; Shen, A.; Mao, Y.C. Recent advances in stretchable hydrogel-based triboelectric nanogenerators for on-skin electronics. Mater. Chem. Front. 2024, 8, 4003–4028. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, D.Z.; Wang, Z.H.; Xi, G.S.; Mao, R.Y.; Ma, Y.H.; Wang, D.Y.; Tang, M.C.; Xu, Z.Y.; Luan, H.X. Ultrastretchable, Self-Healing Conductive Hydrogel-Based Triboelectric Nanogenerators for Human-Computer Interaction. ACS Appl. Mater. Interfaces 2023, 15, 5128–5138. [Google Scholar] [CrossRef]
- Tordi, P.; Tamayo, A.; Jeong, Y.; Bonini, M.; Samorì, P. Multiresponsive Ionic Conductive Alginate/Gelatin Organohydrogels with Tunable Functions. Adv. Funct. Mater. 2024, 34, 11. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X.Y.; Tan, R.J.; Zhang, S.; Zhang, K.; Hu, J.L. Hierarchically Structured Hydrogel Composites with Ultra-High Conductivity for Soft Electronics. Adv. Funct. Mater. 2024, 34, 13. [Google Scholar] [CrossRef]
- Patnam, H.; Graham, S.A.; Manchi, P.; Paranjape, M.V.; Yu, J.S. Single-Electrode Triboelectric Nanogenerators Based on Ionic Conductive Hydrogel for Mechanical Energy Harvester and Smart Touch Sensor Applications. ACS Appl. Mater. Interfaces 2023, 15, 16768–16777. [Google Scholar] [CrossRef]
- Wei, Y.H.; Sun, X.P.; Sun, Y.F.; Yuan, J.; Chen, H.Q.; Luo, L.X. Multibetwork-structured PAM-AG/CNF-MXene triboelectric hydrogels. Ind. Crops Prod. 2024, 216, 118695. [Google Scholar] [CrossRef]
- Hao, X.P.; Zhang, C.W.; Zhang, X.N.; Hou, L.X.; Hu, J.; Dickey, M.D.; Zheng, Q.; Wu, Z.L. Healable, Recyclable, and Multifunctional Soft Electronics Based on Biopolymer Hydrogel and Patterned Liquid Metal. Small 2022, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Chen, X.Y.; Wang, Z.L. Biopolymer and Biomimetic Techniques for Triboelectric Nanogenerators (TENGs). Adv. Mater. 2024, 21, 2409440. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Yang, Z.L.; Jiang, Z.C.; Ni, M.Y.; Xu, M. A self-healing and self-adhesive chitosan based ion-conducting hydrogel sensor by ultrafast polymerization. Int. J. Biol. Macromol. 2022, 209, 1975–1984. [Google Scholar] [CrossRef]
- Cui, C.; Shao, C.Y.; Meng, L.; Yang, J. High-Strength, Self-Adhesive, and Strain-Sensitive Chitosan/Poly(acrylic acid) Double-Network Nanocomposite Hydrogels Fabricated by Salt-Soaking Strategy for Flexible Sensors. ACS Appl. Mater. Interfaces 2019, 11, 39228–39237. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Pu, X.; Liu, M.M.; Chen, X.Y.; Sun, J.M.; Du, C.H.; Zhang, Y.; Zhai, J.Y.; Hu, W.G.; Wang, Z.L. Ultrastretchable, transparent triboelectric nanogenerator as electronic skin for biomechanical energy harvesting and tactile sensing. Sci. Adv. 2017, 3, e1700015. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Liu, A.; Lei, W.; Wu, G.; Xiang, J.; Dong, Y.; Chen, Y.; Chen, B.; Ye, M.; Zhao, J.; et al. Anti-Freezing Conductive Ionic Hydrogel-Enabled Triboelectric Nanogenerators for Wearable Speech Recognition. Materials 2025, 18, 2014. https://doi.org/10.3390/ma18092014
Chen T, Liu A, Lei W, Wu G, Xiang J, Dong Y, Chen Y, Chen B, Ye M, Zhao J, et al. Anti-Freezing Conductive Ionic Hydrogel-Enabled Triboelectric Nanogenerators for Wearable Speech Recognition. Materials. 2025; 18(9):2014. https://doi.org/10.3390/ma18092014
Chicago/Turabian StyleChen, Tao, Andeng Liu, Wentao Lei, Guoxu Wu, Jiajun Xiang, Yixin Dong, Yangyang Chen, Bingqi Chen, Meidan Ye, Jizhong Zhao, and et al. 2025. "Anti-Freezing Conductive Ionic Hydrogel-Enabled Triboelectric Nanogenerators for Wearable Speech Recognition" Materials 18, no. 9: 2014. https://doi.org/10.3390/ma18092014
APA StyleChen, T., Liu, A., Lei, W., Wu, G., Xiang, J., Dong, Y., Chen, Y., Chen, B., Ye, M., Zhao, J., & Guo, W. (2025). Anti-Freezing Conductive Ionic Hydrogel-Enabled Triboelectric Nanogenerators for Wearable Speech Recognition. Materials, 18(9), 2014. https://doi.org/10.3390/ma18092014




