Multifunctional Sol–Gel Coatings for Both Anticorrosion and Electrical Conduction Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrates and Process
2.2. Precursors and Sol–Gel Formulations
2.3. Characterization Techniques
- Sol characterization
- Coating characterization
3. Results and Discussion
- (i)
- Firstly, proportions and/or concentrations of carbon fillers for a hydrolysis ratio equal to 50 associated to the corresponding sols characteristics and coatings morphologies and properties;
- (ii)
- Then, the hydrolysis ratio and the incorporation of organic additives in order to modify the viscosity and the flash point of the sols.
3.1. Carbon Filler’s Selection
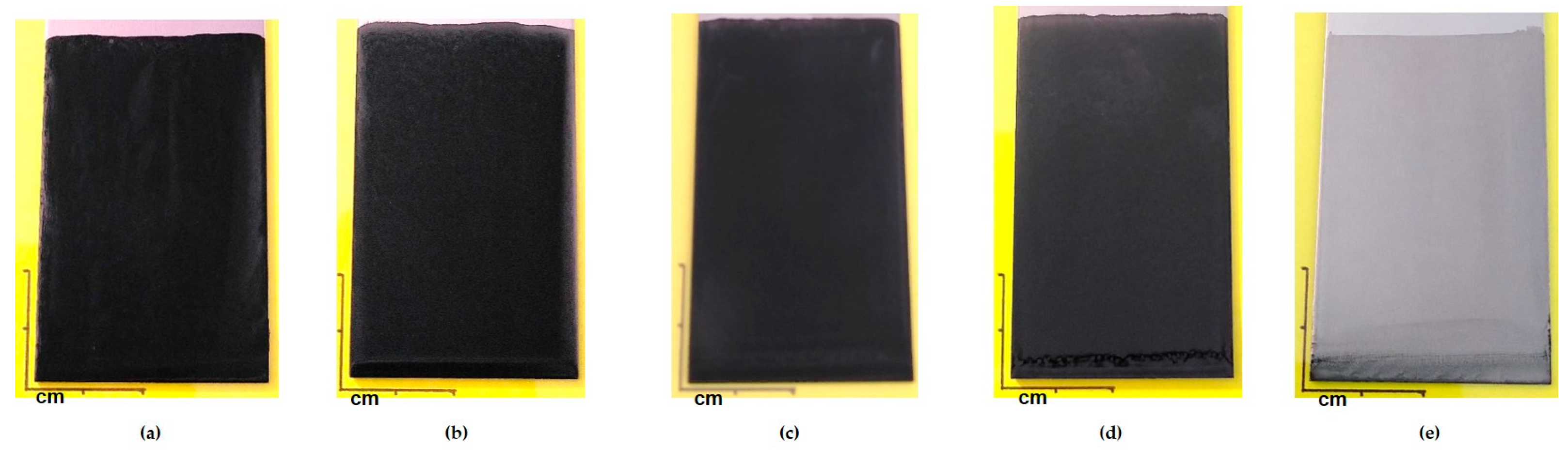
3.2. Filler’s Concentration Variation
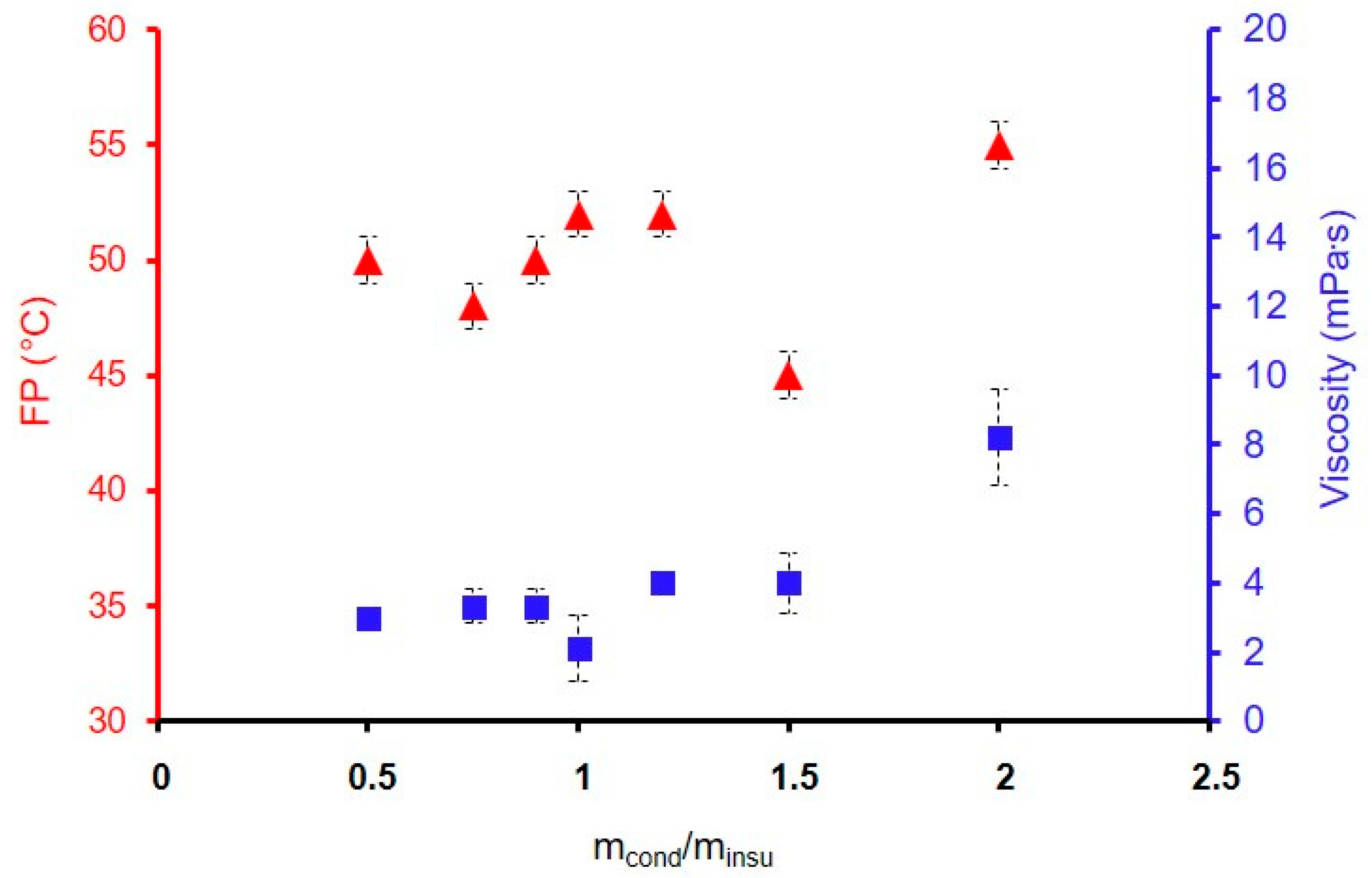
3.3. Properties’ Improvement and Potential Industrial Transfer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| mcond | Mass of the conductive filler |
| minsu | Mass of the sol–gel precursor constituting the insulating part of the coating |
| PVA | Polyvinylalcool |
| PEG | Polyethyleneglycol |
| PVP | Polyvinylpyrrolidone |
| AZ | Poly(2-ethyl-2-oxazoline) |
| HPMC | Hydroxymethylpropylcellulose |
| NSS | Neutral salt spray |
References
- ECHA-REACH Authorization List. Available online: https://echa.europa.eu/fr/authorisation-list (accessed on 23 March 2024).
- Minami, T. Advanced sol–gel coatings for practical applications. J. Sol-Gel Sci. Technol. 2013, 65, 4–11. [Google Scholar] [CrossRef]
- Mishra, N.K.; Chaudhary, P.; Kumar, A.; Singh, R. Thin film coating through sol-gel technique. Res. J. Chem. Sci. 2016, 6, 65–72. [Google Scholar]
- Soucek, M.D. Inorganic/Organic Hybrid Coatings. In Hybrid Materials, 1st ed.; Kickelbick, G., Ed.; Wiley: Hoboken, NJ, USA, 2006; Chapter 11; pp. 433–476. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Organic–inorganic hybrid sol–gel coatings for metal corrosion protection: A review of recent progress. J. Coat. Technol. Res. 2015, 12, 1–35. [Google Scholar] [CrossRef]
- Kesmez, Ö. Hydrophobic, organic–inorganic hybrid sol–gel coatings containing boehmite nanoparticles for metal corrosion protection. Chem. Pap. 2020, 74, 673–688. [Google Scholar] [CrossRef]
- Genet, C.; Azougaghe, H.; Gressier, M.; Ansart, F.; Gavard, O.; Menu, M.-J. State of the art and recent advances in electrical conductive coatings by sol-gel process. J. Mater. Sci. Eng. 2021, 10, 1–12. Available online: https://hal.science/hal-04970157 (accessed on 27 March 2025).
- Acquadro, J.; Noël, S.; Chrétien, P.; Houzé, F.; Testé, P.; Genet, C.; Azougaghe, H.; Ansart, F.; Gressier, M.; Menu, M.-J.; et al. Innovative sol-gel coatings for corrosion protection of connector housings. In Proceedings of the 2022 IEEE 67th Holm Conference on Electrical Contacts (HLM), Tampa, FL, USA, 26 October 2022; pp. 1–8. [Google Scholar] [CrossRef]
- Jespen, T. ATEX—Explosive Atmospheres: Risk Assessment, Control and Compliance, 1st ed.; Springer Series in Reliability Engineering; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Pütz, J.; Heusing, S.; Aegerter, M.A. Characterization of Electrical Properties. In Handbook of Sol-Gel Science and Technology; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer: Cham, Switzerland, 2016; pp. 1–30. [Google Scholar] [CrossRef]
- Genovese, D.B. Shear rheology of hard-sphere, dispersed, and aggregated suspensions, and filler-matrix composites. Adv. Colloid Interface Sci. 2012, 171–172, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Barnes, H.A. A Handbook of Elementary Rheology; Institute of Non-Newtonian Fluid Mechanics, University of Wales: Aberystwyth, UK, 2000. [Google Scholar]
- Mohamed, M.E.; Ezzat, A.; Abdel-Gaber, A.M. Fabrication of eco-friendly graphene-based superhydrophobic coating on steel substrate and its corrosion resistance chemical and mechanical stability. Sci. Rep. 2022, 12, 10530. [Google Scholar] [CrossRef] [PubMed]
- Ceylan, A.; Suvaci, E.; Mandal, H. Role of organic additives on non-aqueous tape casting of SiAlON ceramics. J. Eur. Ceram. Soc. 2011, 31, 167–173. [Google Scholar] [CrossRef]
- Martini, A.; Ramasamy, U.S.; Len, M. Review of viscosity modifier lubricant additives. Tribol. Lett. 2018, 66, 58. [Google Scholar] [CrossRef]
- Wang, W.; Hu, Y.; Li, L.; Zeng, J.; Yao, Y. Effect of various polymer additives on the rheology and thixotropy of organic vehicles. J. Mater. Sci. Mater. Electron. 2022, 33, 12002–12015. [Google Scholar] [CrossRef]
- Li, J.; Cui, J.; Yang, J.; Ma, Y.; Qiu, H.; Yang, J. Silanized graphene oxide reinforced organofunctional silane composite coatings for corrosion protection. Prog. Org. Coat. 2016, 99, 443–451. [Google Scholar] [CrossRef]





| Element | Al | Mg | Si | Fe | Cu | Zn | Mn | Cr | Ti |
|---|---|---|---|---|---|---|---|---|---|
| wt% | balance | 0.8–1.2 | 0.4–0.8 | 0.7 | 0.15–0.4 | 0.25 | 0.15 | 0.04–0.35 | 0.15 |
 |  |  |  |  |  | |
|---|---|---|---|---|---|---|
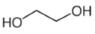
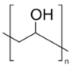
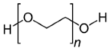
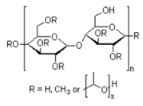
| Additives 1 | EG | PVA13000 | PEG1500 | PVP3500 | AZ500 | HPMC |
| Flash point (°C) | 111 | >113 | 171–235 | >215 | - | ≥93 |
| Boiling point (°C) | 197 | 228 | 250 | 217 | - | - |
| Formulation | Sol Characteristics | Coating Characteristics | ||||||
|---|---|---|---|---|---|---|---|---|
| mcond/minsu | H | Carbon Fillers | Viscosity (mPa·s) | Flash Point (±1 °C) | e (µm) | R□ (mΩ□) | NSS Resistance | |
| GZ50-90F1 | 0.9 | 50 | F1 | 4.0 ± 0.5 | 53 | / | 106 ± 8 | 500 h |
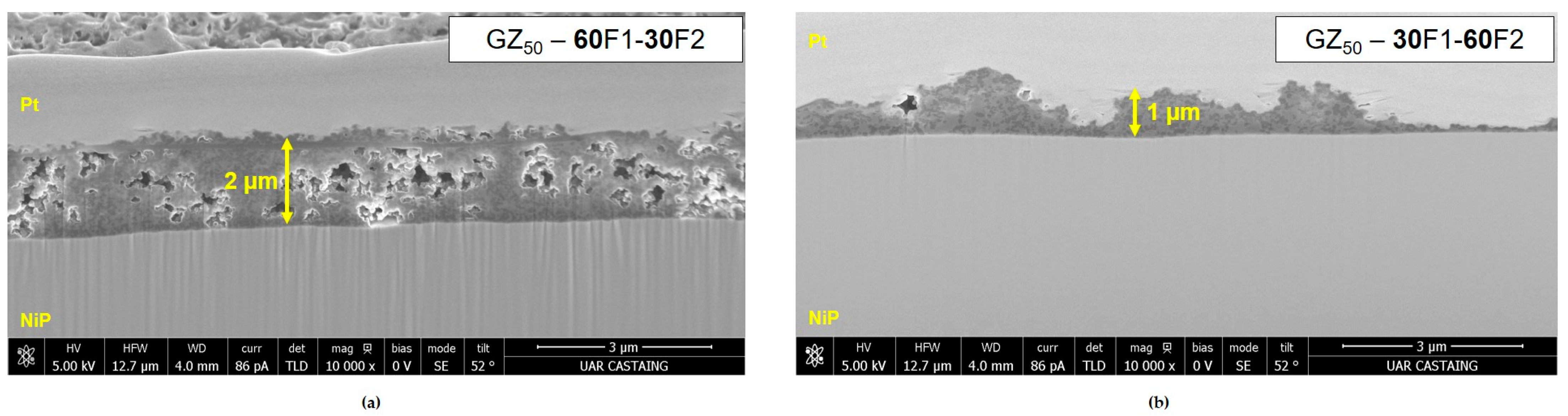
| GZ50-60F1-30F2 | F1 + F2 | 5.0 ± 0.5 | 52 | 2 ± 0.2 | 109 ± 8 | 500 h | ||
| GZ50-45F1-45F2 | F1 + F2 | 5.0 ± 0.5 | 53 | 0.7 ± 0.3 | 122 ± 13 | 500 h | ||
| GZ50-30F1-60F2 | F1 + F2 | 3.0 ± 0.5 | 51 | 1 ± 0.5 | 134 ± 12 | 336 h | ||
| GZ50-90F2 | F2 | 2.0 ± 0.5 | 52 | / | 1004 ± 42 | 336 h | ||
| Formulation | mcond/minsu | Viscosity (mPa·s) | FP (±1 °C) |
|---|---|---|---|
| GZ50-200F3 | 2 | 8 ± 1 | 55 |
| GZ50-150F3 | 1.5 | 4 ± 1 | 52 |
| GZ50-120F3 | 1.2 | 4 ± 0.5 | 52 |
| GZ50-100F3 | 1 | 3 ± 1 | 52 |
| GZ50-90F3 | 0.9 | 3 ± 0.5 | 50 |
| GZ50-75F3 | 0.75 | 3 ± 0.5 | 48 |
| GZ50-50F3 | 0.5 | 3 ± 0.5 | 50 |
| Formulation | Sol’s Characteristics | |||
|---|---|---|---|---|
| mcond/minsu | H | Viscosity (mPa·s) | Flash Point (±1 °C) | |
| GZ100-100F3 | 1 | 100 | 1.3 ± 0.5 | 65 |
| GZ75-100F3 | 75 | 2 ± 0.5 | 58 | |
| GZ60-100F3 | 60 | 3.0 ± 0.4 | 55 | |
| GZ50-100F3 | 50 | 3 ± 1 | 52 | |
| GZ35-100F3 | 35 | 6 ± 1 | 45 | |
| GZ20-100F3 | 20 | 13 ± 3 | 38 | |
| Formulation | Sol’s Characteristics | Coating’s Characteristics | |||||
|---|---|---|---|---|---|---|---|
| mcond/minsu | H | Viscosity (mPa·s) | Flash Point (±1 °C) | e (µm) | R□ (mΩ□) | NSS Duration | |
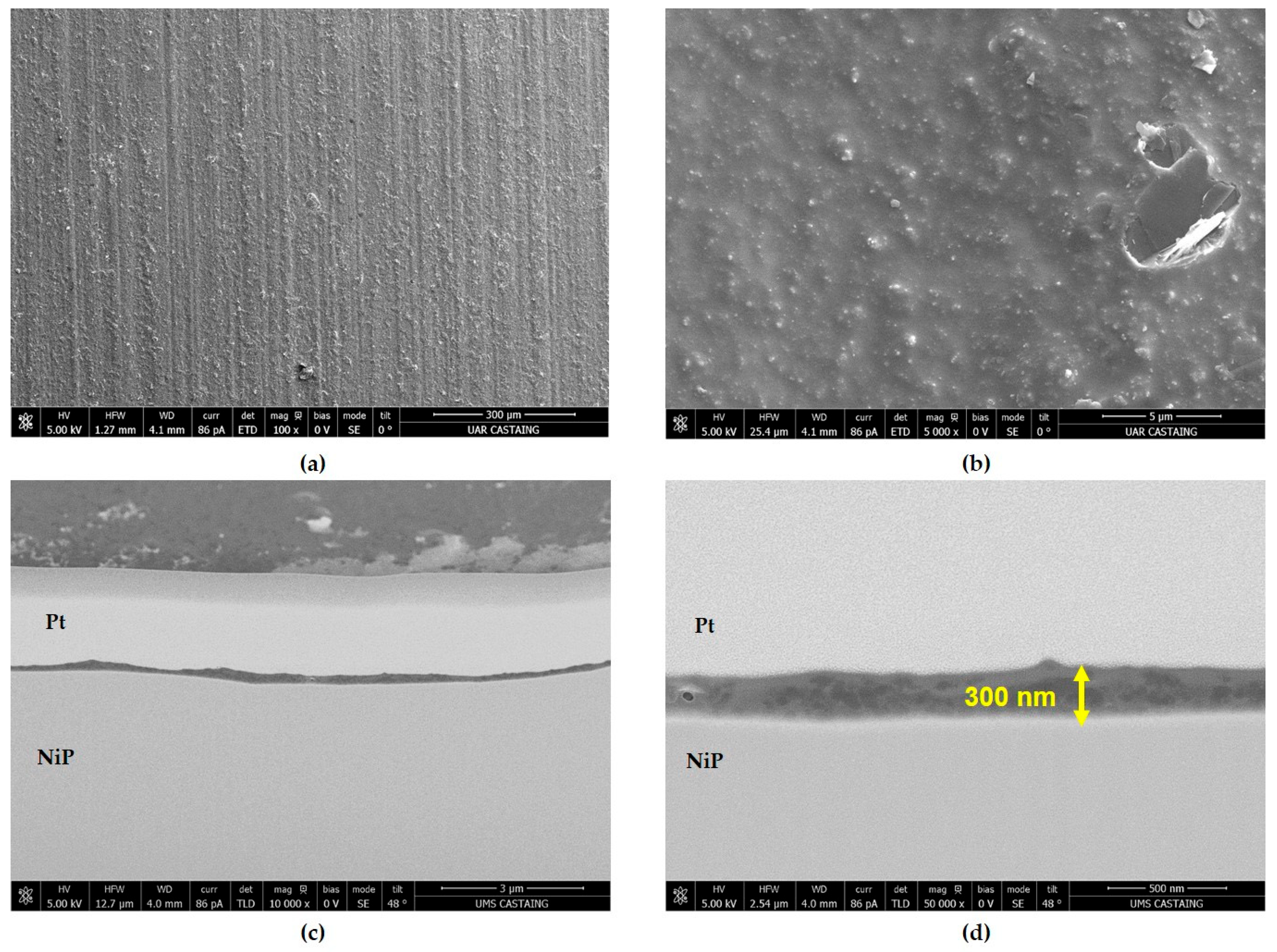
| GZ75-90F3 | 0.90 | 75 | 3.0 ± 0.5 | 59 | 0.3 | 200 | 500 h |
| GZ75-90F3-20EG | 75 | 3.3 ± 0.5 | 62 | 0.1–0.3 | 174 | 1000 h | |
| GZ75-90F3-30EG | 75 | 3.3 ± 0.5 | 66 | 0.1–0.5 | 148 | 1000 h | |
| GZ75-90F3-1PVP | 75 | 3.0 ± 0.5 | 57 | - | - | - | |
| GZ75-90F3-5PVP | 75 | 3.6 ± 0.5 | 59 | - | - | - | |
| GZ75-90F3-1PVA | 75 | 2.0 ± 0.5 | 56 | - | - | - | |
| GZ75-90F3-5PVA | 75 | 3.0 ± 0.5 | 58 | - | - | - | |
| GZ75-90F3-1PEG | 75 | 4.0 ± 0.5 | 57 | - | - | - | |
| GZ75-90F3-5PEG | 75 | 11 ± 1 | 57 | - | - | - | |
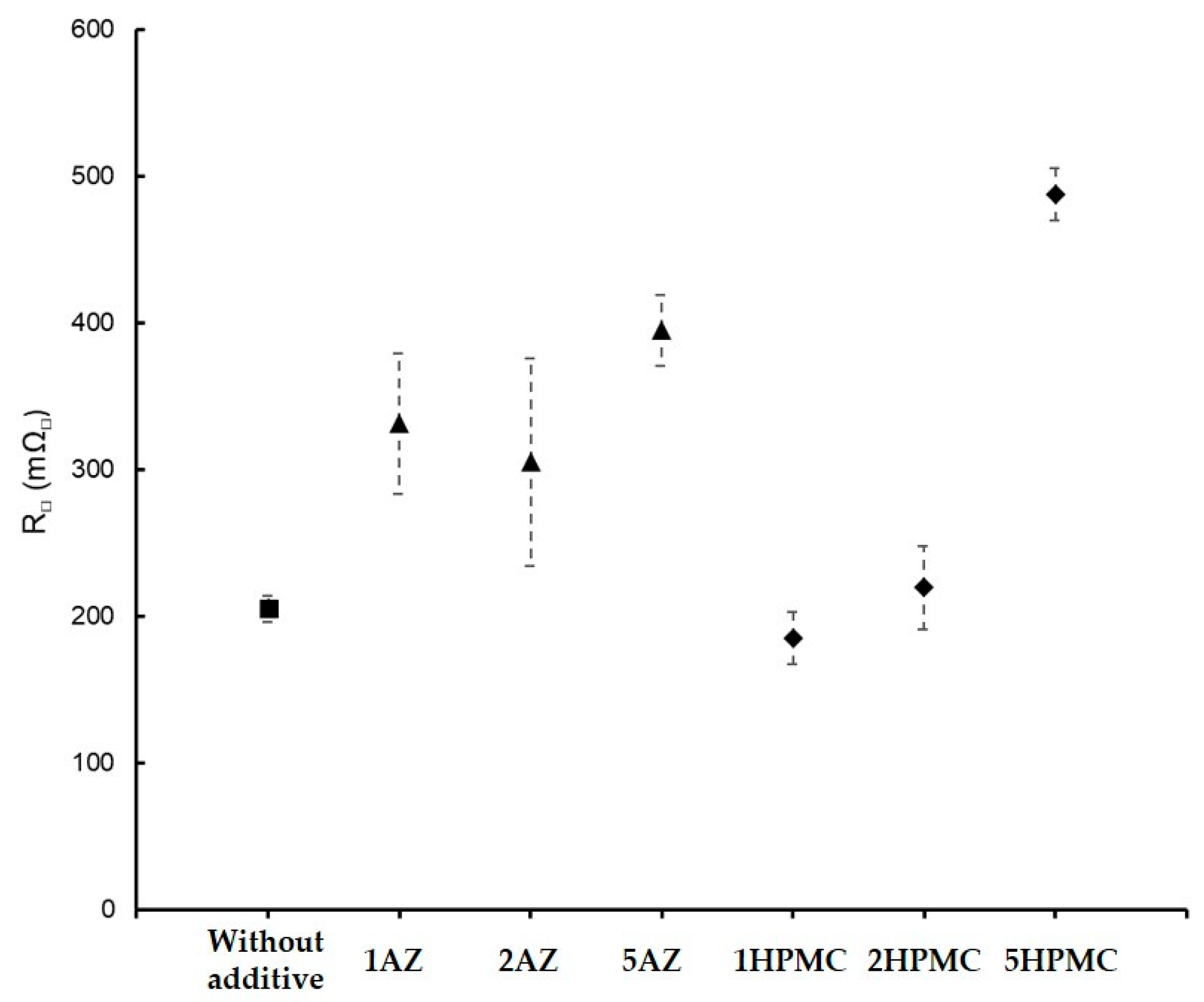
| GZ75-90F3-1AZ | 75 | 8 ± 1 | 58 | - | 331 | 500 h | |
| GZ75-90F3-2AZ | 75 | 14 ± 2 | 59 | 2 ± 0.5 | 305 | 500 h | |
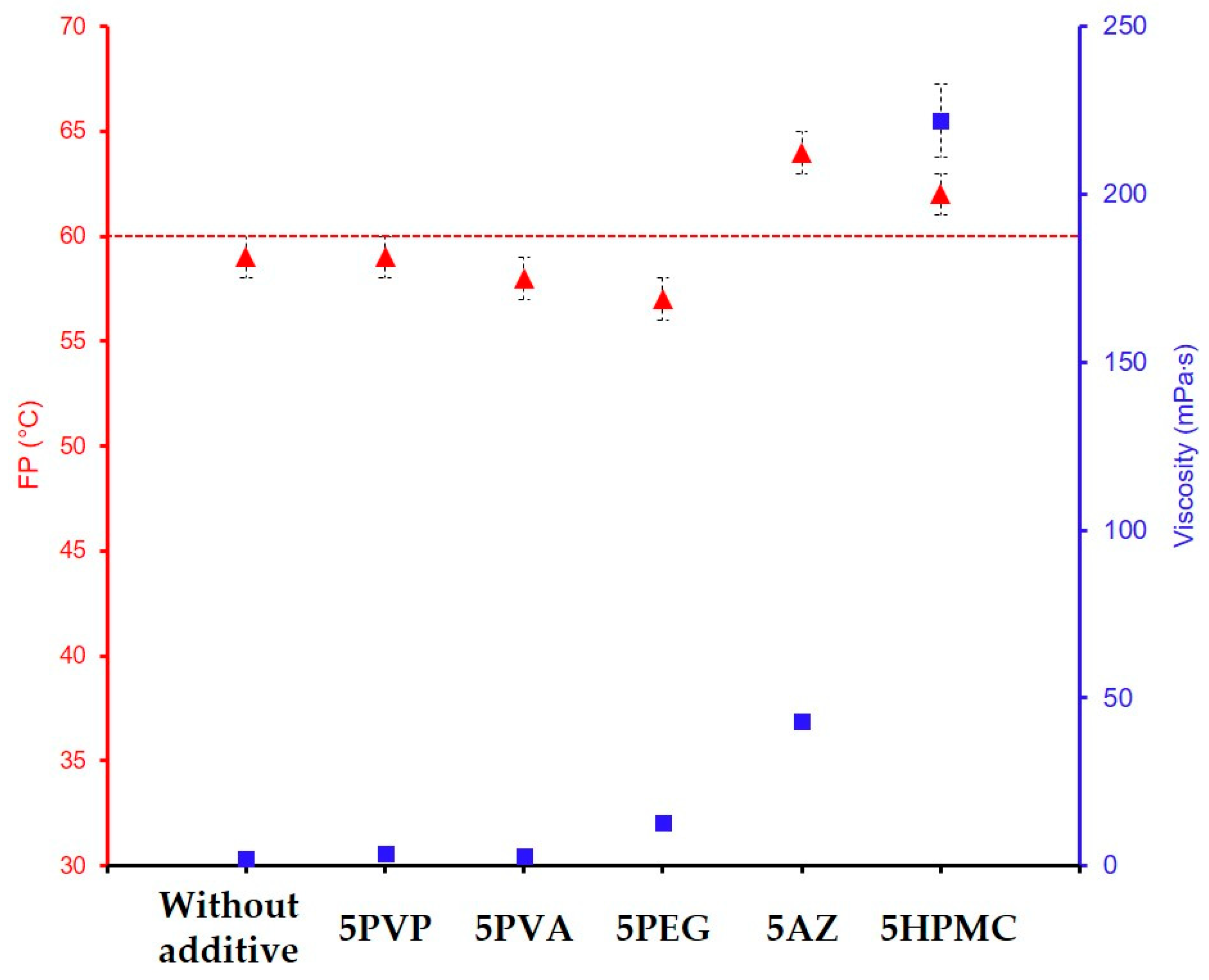
| GZ75-90F3-5AZ | 75 | 43 ± 2 | 64 | 3.3 ± 0.3 | 395 | 500 h | |
| GZ75-90F3-1HPMC | 75 | 10.3 ± 0.5 | 61 | - | 185 | 500 h | |
| GZ75-90F3-2HPMC | 75 | 23 ± 1 | 62 | 3.4 ± 0.3 | 231 | 500 h | |
| GZ75-90F3-5HPMC | 75 | 222 ± 11 | 63 | 6.6 ± 0.5 | 488 | 500 h | |
| Formulations | Viscosity (mPa·s) | FP (±1 °C) | e (µm) | R□ (mΩ□) | NSS Duration |
|---|---|---|---|---|---|
| GZ75-90F3 | 3 (±0.5) | 59 | 0.3 ± 0.1 | 200 ± 9 | 500 h |
| GZ75-90F3-2AZ | 14 (±2) | 59 | 2.8 ± 0.3 | 305 ± 71 | 500 h |
| GZ75-90F3-5AZ | 43 (±2) | 64 | 3.3 ± 0.3 | 395 ± 24 | |
| GZ75-90F3-2HPMC | 23 (±1) | 62 | 3.4 ± 0.2 | 219 ± 28 | |
| GZ75-90F3-5HPMC | 222 (±11) | 62 | 6.5 ± 0.3 | 488 ± 18 | 500 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genet, C.; Azougaghe, H.; Bréniaux, E.; Montpellaz, R.; Gressier, M.; Ansart, F.; Gavard, O.; Menu, M.-J. Multifunctional Sol–Gel Coatings for Both Anticorrosion and Electrical Conduction Properties. Materials 2025, 18, 2011. https://doi.org/10.3390/ma18092011
Genet C, Azougaghe H, Bréniaux E, Montpellaz R, Gressier M, Ansart F, Gavard O, Menu M-J. Multifunctional Sol–Gel Coatings for Both Anticorrosion and Electrical Conduction Properties. Materials. 2025; 18(9):2011. https://doi.org/10.3390/ma18092011
Chicago/Turabian StyleGenet, Clément, Hiba Azougaghe, Edouard Bréniaux, Robin Montpellaz, Marie Gressier, Florence Ansart, Olivier Gavard, and Marie-Joëlle Menu. 2025. "Multifunctional Sol–Gel Coatings for Both Anticorrosion and Electrical Conduction Properties" Materials 18, no. 9: 2011. https://doi.org/10.3390/ma18092011
APA StyleGenet, C., Azougaghe, H., Bréniaux, E., Montpellaz, R., Gressier, M., Ansart, F., Gavard, O., & Menu, M.-J. (2025). Multifunctional Sol–Gel Coatings for Both Anticorrosion and Electrical Conduction Properties. Materials, 18(9), 2011. https://doi.org/10.3390/ma18092011






