Porous La-Fe-O Perovskite as Catalyst for Combustion of Volatile Organic Compounds
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
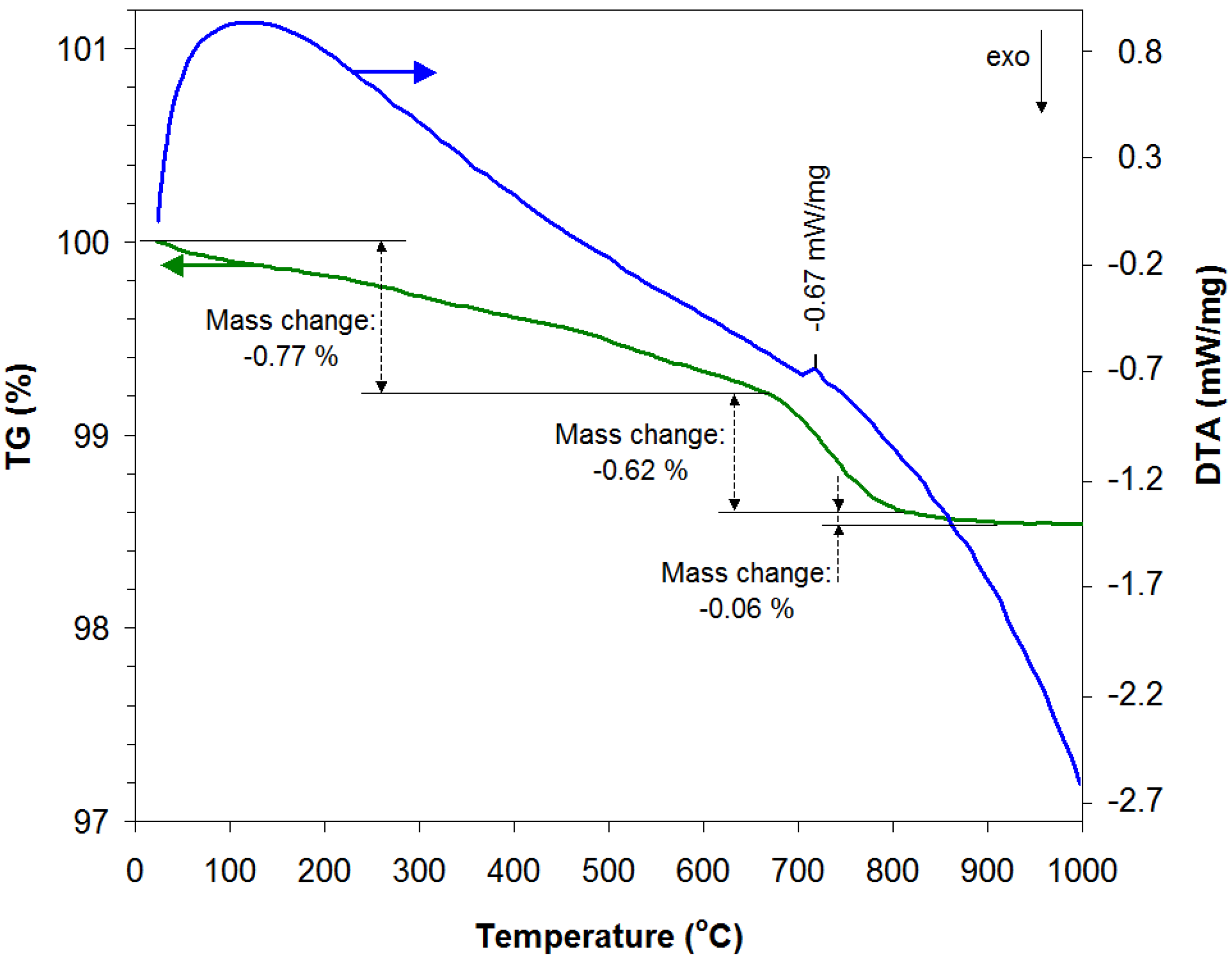
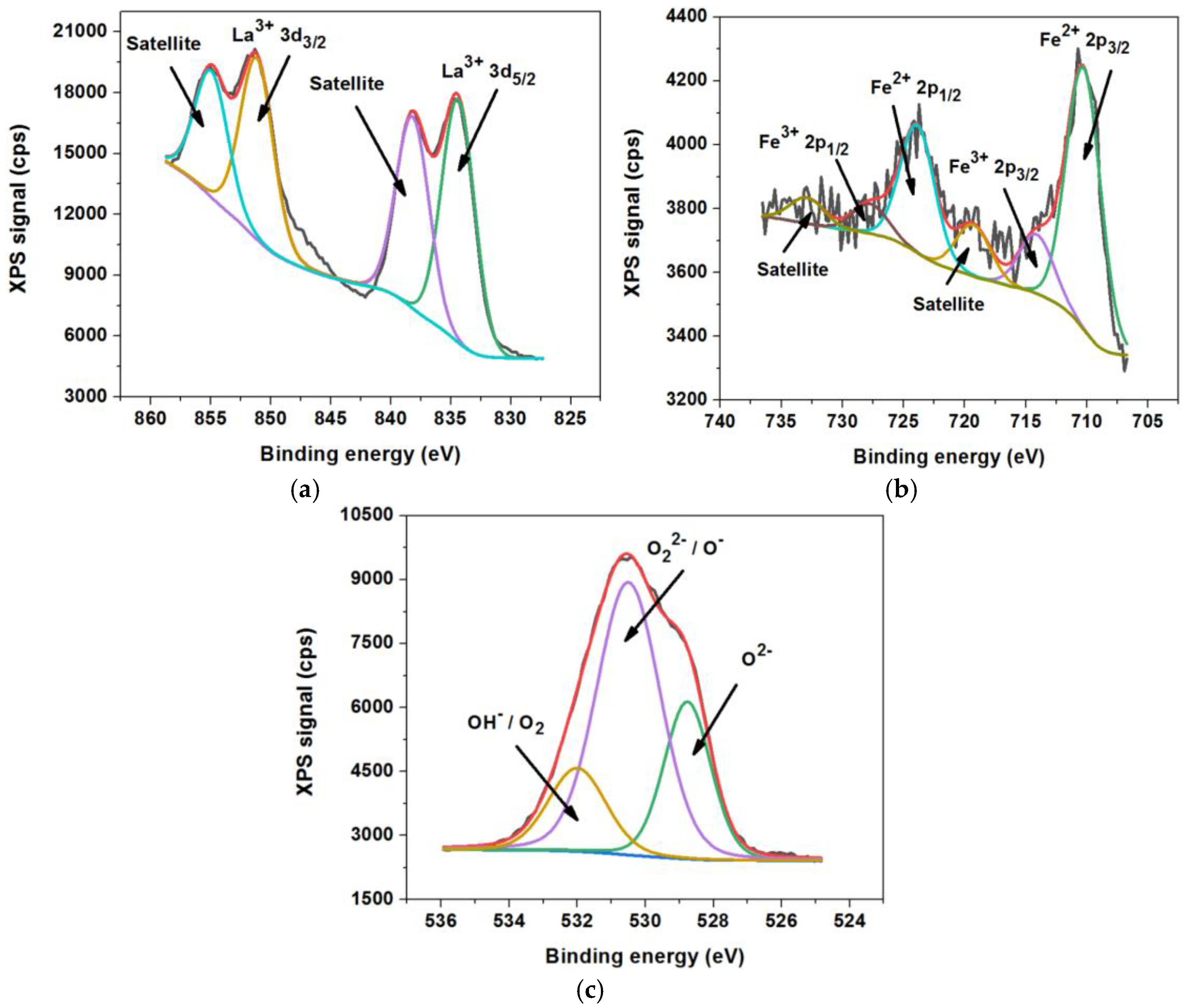
3.1. Structural and Morphological Characterization
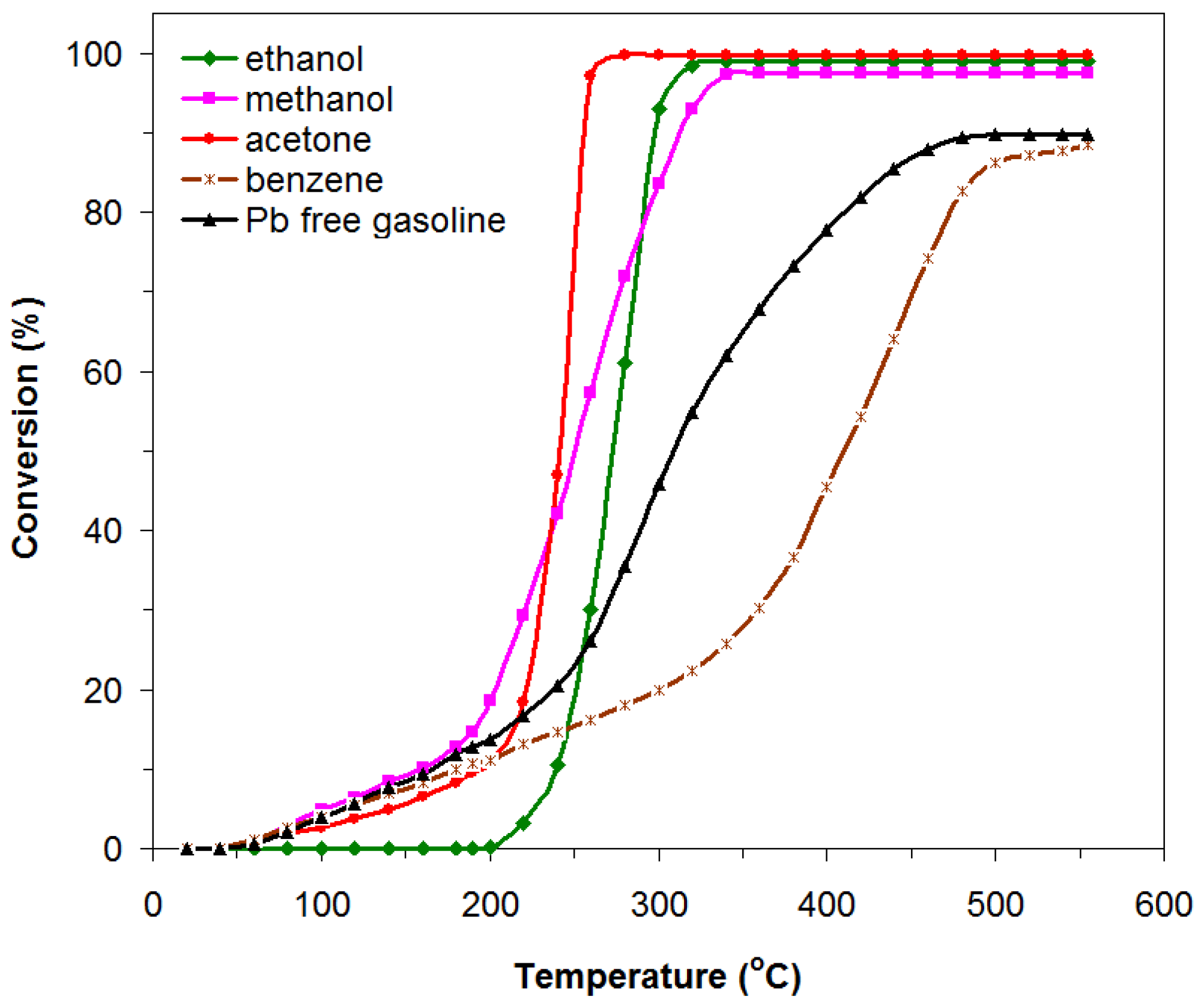
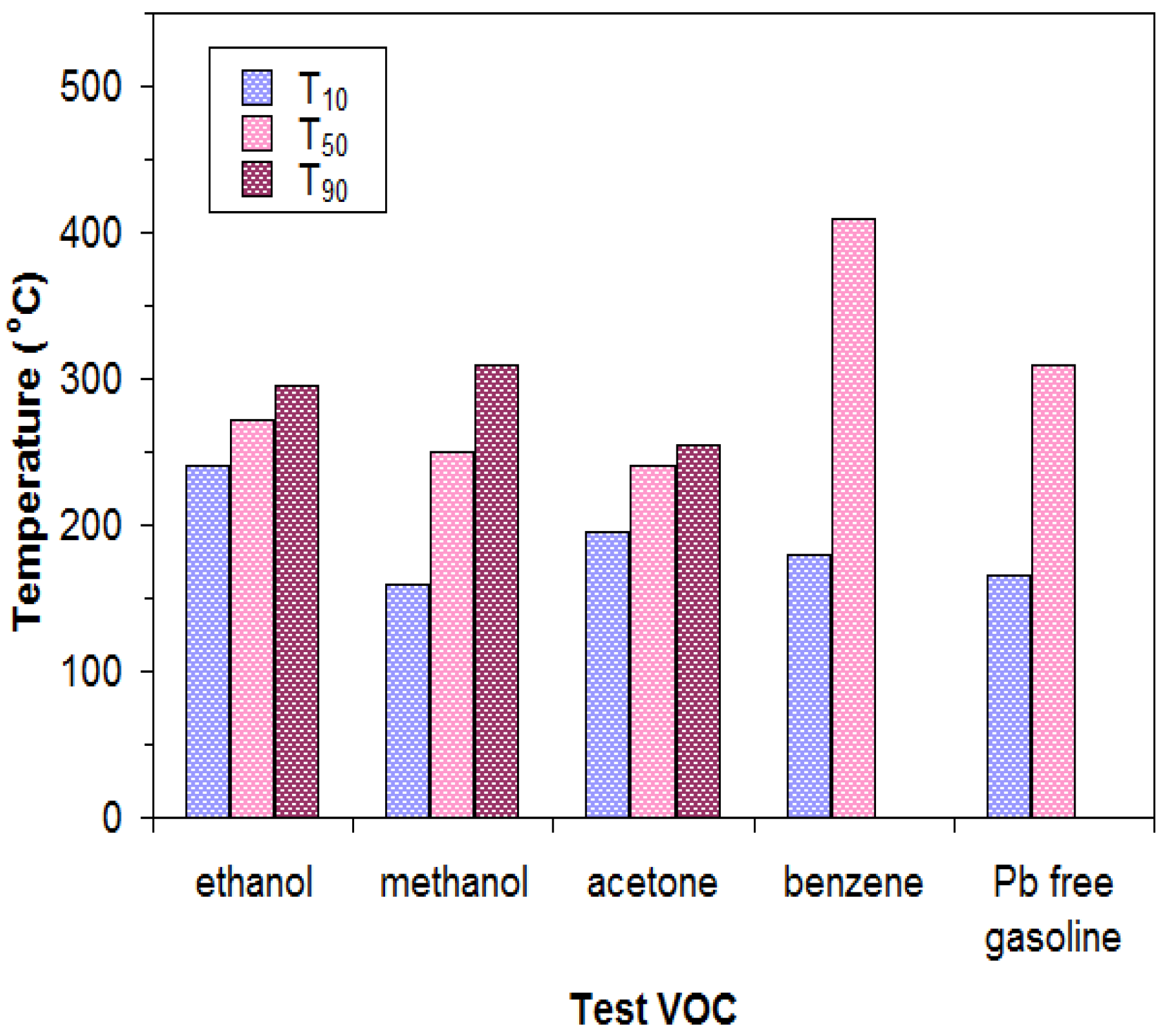
3.2. Catalytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tripathy, A.; Pramanik, S.; Manna, A.; Bhuyan, S.; Shah, N.F.A.; Radzi, Z.; Osman, N.A.A. Design and development for capacitive humidity sensor applications of Lead-free Ca, Mg, Fe, Ti-oxides-based electro-ceramics with improved sensing properties via physisorption. Sensors 2016, 16, 1135. [Google Scholar] [CrossRef]
- Kulwicki, B.M. Humidity sensors. J. Am. Ceram. Soc. 1991, 74, 697–708. [Google Scholar] [CrossRef]
- Traversa, E. Ceramic sensors for humidity detection: The state-of-the-art and future developments. Sens. Actuators B Chem. 1995, 23, 135–156. [Google Scholar] [CrossRef]
- Suhadi, D.R.; Awang, M.; Hassan, M.N.; Abdullah, R.; Azizi, H. Review of photochemical smog pollution in Jakarta Metropolitan, Indonesia. Am. J. Environ. Sci. 2005, 1, 110–118. [Google Scholar] [CrossRef]
- Doroftei, C. Nanostructured perovskites for catalytic combustion. In Nanostructures Book; IntechOpen: London, UK, 2019; Volume 5, pp. 75–93. ISBN 978-1-78985-739-9. [Google Scholar]
- Belotti, A.; Liu, J.; Curcio, A.; Wang, J.; Wang, Z.; Quattrocchi, E.; Effat, M.B.; Ciucci, F. Introducing Ag in Ba0.9La0.1FeO3-δ: Combining cationic substitution with metal particle decoration. Mater. Rep. Energy 2021, 1, 100018. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent advances in novel nanostructuring methods of perovskite electrocatalysts for energy-related applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Rezlescu, N.; Rezlescu, E.; Popa, P.D.; Doroftei, C.; Ignat, M. Characterization and catalytic properties of some perovskites. Compos. Part B-Eng. 2014, 60, 515–522. [Google Scholar] [CrossRef]
- Seiyama, T. Preparation and Application of Perovskite-Type Oxides; Tejuka, L.G., Fierro, G.L.G., Eds.; Marcel Decker: New York, NY, USA, 1993; p. 215. [Google Scholar]
- Tanaka, H.; Tan, I.; Uenishi, M.; Kimura, M.; Dohmae, K. Regeneration of palladium subsequent to solid solution and segregation in a perovskite catalyst: An Intelligent catalyst. Top Catal. 2001, 16–17, 63–70. [Google Scholar] [CrossRef]
- Seyfi, B.; Baghalha, M.; Kazeman, H. Modified LaCoO3 nano-perovskite catalysts for the environmental application of automotive CO oxidation. Chem. Eng. J. 2009, 143, 306–311. [Google Scholar] [CrossRef]
- Merino, N.A.; Barbero, B.P.; Grange, P.; Cadus, L.E. La1−xCaxCoO3 perovskite-type oxides: Preparation, characterization, stability, and catalytic potentiality for the total oxidation of propane. J. Catal. 2005, 231, 232–244. [Google Scholar] [CrossRef]
- Spinicci, R.; Tofanari, A.; Delmastrto, A.; Mazza, D.; Ronchetti, S. Catalytic properties of stoichiometric and non-stoichiometric LaFeO3 perovskite for total oxidation of methane. Mater. Chem. Phys. 2002, 76, 20–25. [Google Scholar] [CrossRef]
- Saracco, G.; Geobaldo, F.; Baldi, G. Methane combustion on Mg-doped LaMnO3 perovskite catalysts. Appl. Catal. B-Environ. 1999, 20, 277–288. [Google Scholar] [CrossRef]
- Kucharczyk, B.; Tylus, W. Partial substitution of lanthanum with silver in the LaMnO3 perovskite: Effect of the modification on the activity of monolithic catalysts in the reactions of methane and carbon oxide oxidation. Appl. Catal. A 2008, 335, 28–36. [Google Scholar] [CrossRef]
- Shengelaya, A.; Zhao, G.M.; Keller, H.; Muller, K.A. EPR evidence of Jahn–Teller polaron formation in La1-xCaxMnO3+y. Phys. Rev. Lett. 1996, 77, 5296–5299. [Google Scholar] [CrossRef] [PubMed]
- Oliva, C.; Forni, L.; Pasqualin, P.; D’Ambrosio, A.; Vishniakov, A.V. EPR analysis of La1-xMxMnO3+y (M = Ce, Eu, Sr) perovskitic catalysts for methane oxidation. Phys. Chem. Chem. Phys. 1999, 1, 355–360. [Google Scholar] [CrossRef]
- Oliva, C.; Forni, L.; D’Ambrosio, A.; Navarrini, F.; Stepanov, A.D.; Kagramanov, Z.D.; Mikhailichenko, A.I. Characterization by EPR and other techniques of La1−xCexCoO3+delta perovskitelike catalysts for methane flameless combustion. Appl. Catal. A 2001, 205, 245–252. [Google Scholar] [CrossRef]
- Nishihata, Y.; Mizuki, J.; Akao, T.; Tanaka, H.; Uenishi, M.; Kimura, M.; Okamoto, T.; Hamada, N. Self-regeneration of a Pd-perovskite catalyst for utomotive emissions control. Nature 2002, 418, 164–167. [Google Scholar] [CrossRef]
- Wu, M.; Chen, S.; Xiang, W. Oxygen vacancy induced performance enhancement of toluene catalytic oxidation using LaFeO3 perovskite oxides. Chem. Eng. J. 2020, 387, 124101. [Google Scholar] [CrossRef]
- Doroftei, C. Formaldehyde sensitive Zn-doped LPFO thin films obtained by rf sputtering. Sens. Actuators B 2016, 231, 793–799. [Google Scholar] [CrossRef]
- Pecchi, G.; Reyes, P.; Zamora, R.; Cadus, L.E.; Barbero, B.P. Catalytic combustion of methane over LaFeO3 perovskites: The influence of coprecipitation pH and ageing time. J. Chil. Chem. Soc. 2006, 51, 1001–1005. [Google Scholar] [CrossRef]
- Liu, F.; Li, Z.; Ma, H.; Gao, Z. Surface composition and catalytic activity of La-Fe mixed oxides for methane oxidation. Appl. Surf. Sci. 2015, 351, 709–714. [Google Scholar] [CrossRef]
- Saberi, M.H.; Mortazavi, Y.; Chodadadi, A.A. Dual selective Pt/SnO2 sensor to CO and propane in exhaust gases of gasoline engines using Pt/LaFeO3 filter. Sens. Actuators B 2015, 206, 617–623. [Google Scholar] [CrossRef]
- Costa, A.C.F.M.; Diniz, A.P.A.; Gama, L.; Morelli, M.R.; Kiminami, R.H.G.A. Comparison of Ni-Zn ferrite powder preparation by combustion reaction using different syntesization routes. J. Metastable Nanocrystalline Mater. 2004, 20–21, 582–587. [Google Scholar] [CrossRef]
- Tanaka, H.; Tan, I.; Uenishi, M.; Kajita, N.; Taniguchi, M.; Kaneko, K.; Mitachi, S.; Kimura, M.; Narita, K.; Sato, N. Perovskite-Type Composite Oxide, Catalist Composition and Method for Producing Perovskite-Type Composite Oxide. Patent US 7601325, 13 October 2009. [Google Scholar]
- Svensson, E.E.; Nassos, S.; Boutonnet, M.; Järås, S.G. Microemulsion synthesis of MgO-supported LaMnO3 for catalytic combustion of methane. Catal Today 2006, 117, 484–490. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Wei, Z.; Du, X.; Gong, M.; Chen, Y. Preparation of high performance methane combustion catalyst and its application to natural gas catalytic combustion fan-boiler. Chin. J. Catal. 2006, 27, 823–826. [Google Scholar] [CrossRef]
- Xanthopoulou, G. Catalitic properties of the SHS products. Adv. Sci. Technol. 2010, 63, 287–296. [Google Scholar]
- Battiston, A.A.; Bitter, J.H.; Heijboer, W.M.; de Groot, F.M.F.; Koningsberger, D.C. Reactivity of Fe-binuclear complexes in over-exchanged Fe/ZSM5, studied by in situ XAFS spectroscopy Part 1: Heat treatment in He and O2. J. Catal. 2003, 215, 279–293. [Google Scholar] [CrossRef]
- Doroftei, C.; Leontie, L. Porous nanostructured gadolinium aluminate for high-sensitivity humidity sensors. Materials 2021, 14, 7102. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 2004. [Google Scholar]
- Rezlescu, N.; Rezlescu, E.; Popa, P.D.; Doroftei, C.; Ignat, M. Nanostructured GdAlO3 perovskite, a new possible catalyst for combustion of volatile organic compounds. J. Mater. Sci. 2013, 48, 4297–4304. [Google Scholar] [CrossRef]
- Doroftei, C.; Popa, P.D.; Rezlescu, E.; Rezlescu, N. Nanocrystalline SrMnO3 powder as catalyst for hydrocarbon combustion. J. Alloys Compd. 2014, 584, 195–198. [Google Scholar] [CrossRef]
- Janbutrach, Y.; Hunpratub, S.; Swatsitang, E. Ferromagnetism and optical properties of La1−xAlxFeO3 nanopowders. Nanoscale Res. Lett. 2014, 9, 498. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Thakur, A.K. Effect of co-substitution on structural, optical, dielectric and magnetic behavior of LaFeO3. J. Alloys Compd. 2017, 695, 3579–3588. [Google Scholar] [CrossRef]
- Huang, S.; Qin, H.; Song, P.; Liu, X.; Li, L.; Zhang, R.; Hu, J.; Yan, H.; Jiang, M. The formaldehyde sensitivity of LaFe1−xZnxO3-based gas sensor. J. Mater. Sci. 2007, 42, 9973–9977. [Google Scholar] [CrossRef]
- Doroftei, C. Nanocrystalline FeMnO3 powder as catalyst for combustion of volatile organic compounds. Nanomaterials 2024, 14, 521. [Google Scholar] [CrossRef]
- Doroftei, C.; Leontie, L. Synthesis and characterization of some nanostructured composite oxides for low temperature catalytic combustion of dilute propane. RSC Adv. 2017, 7, 27863–27871. [Google Scholar] [CrossRef]
- Vasquez, R.P. X-ray photoemission measurements of La1−xCaxCoO3 (x = 0, 0.5). Phys. Rev. B 1996, 54, 14938–14941. [Google Scholar] [CrossRef]
- Zhang, X.D.; Zhang, W.L.; Cai, Z.X.; Li, Y.K.; Yamauchi, Y.; Guo, X. LaFeO3 porous hollow micro-spindles for NO2 sensing. Ceram. Int. 2019, 45, 5240–5248. [Google Scholar] [CrossRef]
- Huang, L.; Cheng, L.; Pan, S.; Yao, Q.; Long, Q.; Wang, M.; Chen, Y.; Zhou, H. Influence of A-site doping barium on structure, magnetic and microwave absorption properties of LaFeO3 ceramics powders. J. Rare Earths 2022, 40, 1106–1117. [Google Scholar] [CrossRef]
- Li, M.; Xu, W.; Wang, W.; Liu, Y.; Cui, B.; Guo, X. Facile synthesis of specific FeMnO3 hollow sphere/graphene composites and their superior electrochemical energy storage performances for supercapacitor. J. Power Sources 2014, 248, 465–473. [Google Scholar] [CrossRef]
- Lobo, L.S.; Rubankumar, A. Investigation on structural and electrical properties of FeMnO3 synthesized by sol-gel method. Ionics 2019, 25, 1341–1350. [Google Scholar] [CrossRef]
- Tang, Q.; Jiang, L.; Liu, J.; Wang, S.; Sun, G. Effect of surface manganese valence of manganese oxides on the activity of the oxygen reduction reaction in alkaline media. ACS Catal. 2014, 4, 457–463. [Google Scholar] [CrossRef]
- Yuan, W.; Cheng, L.; Xia, T.; Chen, Y.; Long, Q.; Yao, Q.; Pan, S.; Hu, C. Effect of Fe doping on the lattice structure, microscopic morphology and microwave absorption properties of LaCo1−xFexO3. J. Alloys Compd. 2022, 926, 166839. [Google Scholar] [CrossRef]
- Zhou, G.; Gui, B.; Xie, H.; Yang, F.; Chen, Y.; Chen, S.; Zheng, X. Influence of CeO2 morphology on the catalytic oxidation of ethanol in air. J. Ind. Eng. Chem. 2014, 20, 160–165. [Google Scholar] [CrossRef]
- Gomez-Cuaspud, J.A.; Schmal, M. Effect of metal oxides concentration over supported cordierite monoliths on the partial oxidation of ethanol. Appl. Catal. B Environ. 2014, 148–149, 1–10. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, X.; Jin, J.; Di, X.; Liang, C.; Liu, Z. Insight into catalytic properties of Co3O4-CeO2 binary oxides for propane total oxidation. Chin. Fournal Catal. 2020, 41, 679–690. [Google Scholar] [CrossRef]
- Rezlescu, N.; Rezlescu, E.; Popa, P.D.; Doroftei, C.; Ignat, M. Scandium substituted nickel-cobalt ferrite nanoparticles for catalyst applications. Appl. Catal. B Environ. 2014, 158, 70–75. [Google Scholar] [CrossRef]
- Burgos, N.; Paulis, M.; Antxustegi, M.M.; Montes, M. Deep oxidation of VOC mixtures with platinum supported on Al2O3/Al monoliths. Appl. Catal. B Environ. 2002, 38, 251–258. [Google Scholar] [CrossRef]
- Deng, Q.; Li, X.M.; Peng, Z.S.; Long, Y.F.; Xiang, L.M.; Cai, T.J. Catalytic performance and kinetics of Au/γ-Al2O3 catalysts for low-temperature combustion of light alcohols. Treans. Nonferrous Met. Soc. China 2010, 20, 437–442. [Google Scholar] [CrossRef]
- Ge, Y.; Fu, K.; Zhao, Q.; Ji, N.; Song, C.; Ma, D.; Liu, Q. Performance study of modified Pt catalysts for the complete oxidation of acetone. Chem. Eng. Sci. 2019, 206, 499–506. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Xie, S.; Liu, Y.; Dai, H.; Guo, G.; Deng, J. Supported ultralow loading Pt catalysts with high H2O-, CO2-, and SO2-resistance for acetone removal. Appl. Catal. A 2019, 579, 106–115. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Oikonomopoulos, E.; Kanistras, D.; Ioannides, T. Complete oxidation of ethanol over alkali-promoted Pt/Al2O3 catalysts. Appl. Catal. B 2006, 65, 62–69. [Google Scholar] [CrossRef]
- Kundakovic, L.; Stephanopoulos, M.F. Cu- and Ag-modified cerium oxide catalysts for methane oxidation. J. Catal. 1998, 179, 203–221. [Google Scholar] [CrossRef]
- Arandiyan, H.; Dai, H.; Ji, K.; Sun, H.; Li, J. Pt nanoparticles embedded in colloidal crystal template derived 3D ordered macroporous Ce0.6Zr0.3Y0.1O2: Highly efficient catalysts for methane combustion. ACS Catal. 2015, 5, 1781–1793. [Google Scholar] [CrossRef]
- Dong, F.; Suda, A.; Tanabe, T.; Nagai, Y.; Sobukawa, H.; Shinjoh, H.; Sugiura, M.; Descorme, C.; Duprez, D. Dynamic oxygen mobility and a new insight into the role of Zr atoms in three-way catalysts of Pt/CeO2–ZrO2. Catal. Today 2004, 93–95, 827–832. [Google Scholar] [CrossRef]
- Milt, V.G.; Ulla, M.A.; Lombardo, E.A. Cobalt-containing catalysts for the high temperature combustion of methane. Catal. Lett. 2000, 65, 67–73. [Google Scholar] [CrossRef]
- Feng, S.; Yang, W.; Wang, Z. Synthesis of porous NiFe2O4 microparticles and its catalytic properties for methane combustion. Mater. Sci. Eng. B 2011, 176, 1509–1512. [Google Scholar] [CrossRef]


| Catalyst | VOC | T10 (°C) | T50 (°C) | T90 (°C) | T99 (°C) | References |
|---|---|---|---|---|---|---|
| LaFeO3 | Acetone | 195 | 240 | 255 | 270 | This work |
| Pt/Al2O3 | Acetone | – | 222 | 246 | 259 | [53] |
| Pt-Zr/Al2O3 | Acetone | – | 245 | 261 | 281 | [53] |
| Pt-TiO2 | Acetone | – | 220 | 244 | 260 | [53] |
| Pt-Zr/TiO2 | Acetone | – | 235 | 256 | 275 | [53] |
| Pt/Al2O3/Al | Acetone | – | ~208 | ~270 | ~341 | [51] |
| 0.11 wt% Pt/TiO2 | Acetone | 162 | 220 | 260 | – | [54] |
| 0.17 wt% Pt/TiO2 | Acetone | 143 | 205 | 245 | – | [54] |
| 0.46 wt% Pt/TiO2 | Acetone | 140 | 192 | 231 | – | [54] |
| 1.40 wt% Pt/TiO2 | Acetone | 135 | 180 | 208 | – | [54] |
| LaFeO3 | Ethanol | 240 | 272 | 295 | 310 | This work |
| 1.5 wt% Au/γ-Al2O3 | Ethanol | – | – | – | ~290 | [52] |
| 2÷2.5 wt% Au/γ-Al2O3 | Ethanol | – | – | – | ~280 | [52] |
| Pt/Al2O3 | Ethanol | – | 158 | 220 | 240 | [55] |
| VOCs/ LaFeO3 | Conversion at 270 °C (%) | Conversion at 550 °C (%) | Reaction Rate * (μmol s−1 m−2) | Apparent Activation Energy ** (KJ/mol) |
|---|---|---|---|---|
| Ethanol | 45.54 | 99.03 | 1.066 × 10−2 | 133.449 |
| Methanol | 64.54 | 97.54 | 1.818 × 10−2 | 20.166 |
| Acetone | 99.05 | 99.70 | 8.166 × 10−2 | 21.631 |
| Benzene | 15.76 | 88.50 | 0.300 × 10−2 | 22.238 |
| Pb-free gasoline | 30.80 | 89.70 | 0.645 × 10−2 | 22.237 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doroftei, C.; Murariu, G.; Dobromir, M. Porous La-Fe-O Perovskite as Catalyst for Combustion of Volatile Organic Compounds. Materials 2025, 18, 2008. https://doi.org/10.3390/ma18092008
Doroftei C, Murariu G, Dobromir M. Porous La-Fe-O Perovskite as Catalyst for Combustion of Volatile Organic Compounds. Materials. 2025; 18(9):2008. https://doi.org/10.3390/ma18092008
Chicago/Turabian StyleDoroftei, Corneliu, Gabriel Murariu, and Marius Dobromir. 2025. "Porous La-Fe-O Perovskite as Catalyst for Combustion of Volatile Organic Compounds" Materials 18, no. 9: 2008. https://doi.org/10.3390/ma18092008
APA StyleDoroftei, C., Murariu, G., & Dobromir, M. (2025). Porous La-Fe-O Perovskite as Catalyst for Combustion of Volatile Organic Compounds. Materials, 18(9), 2008. https://doi.org/10.3390/ma18092008







