Effect of Citric Acid Concentration on the Transformation of Aragonite CaCO3 to Calcium Citrate Using Cockle Shells as a Green Calcium Source
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material Preparation
2.2. Calcium Citrate (CCT) Preparation
2.3. Material Characterizations
3. Results and Discussion
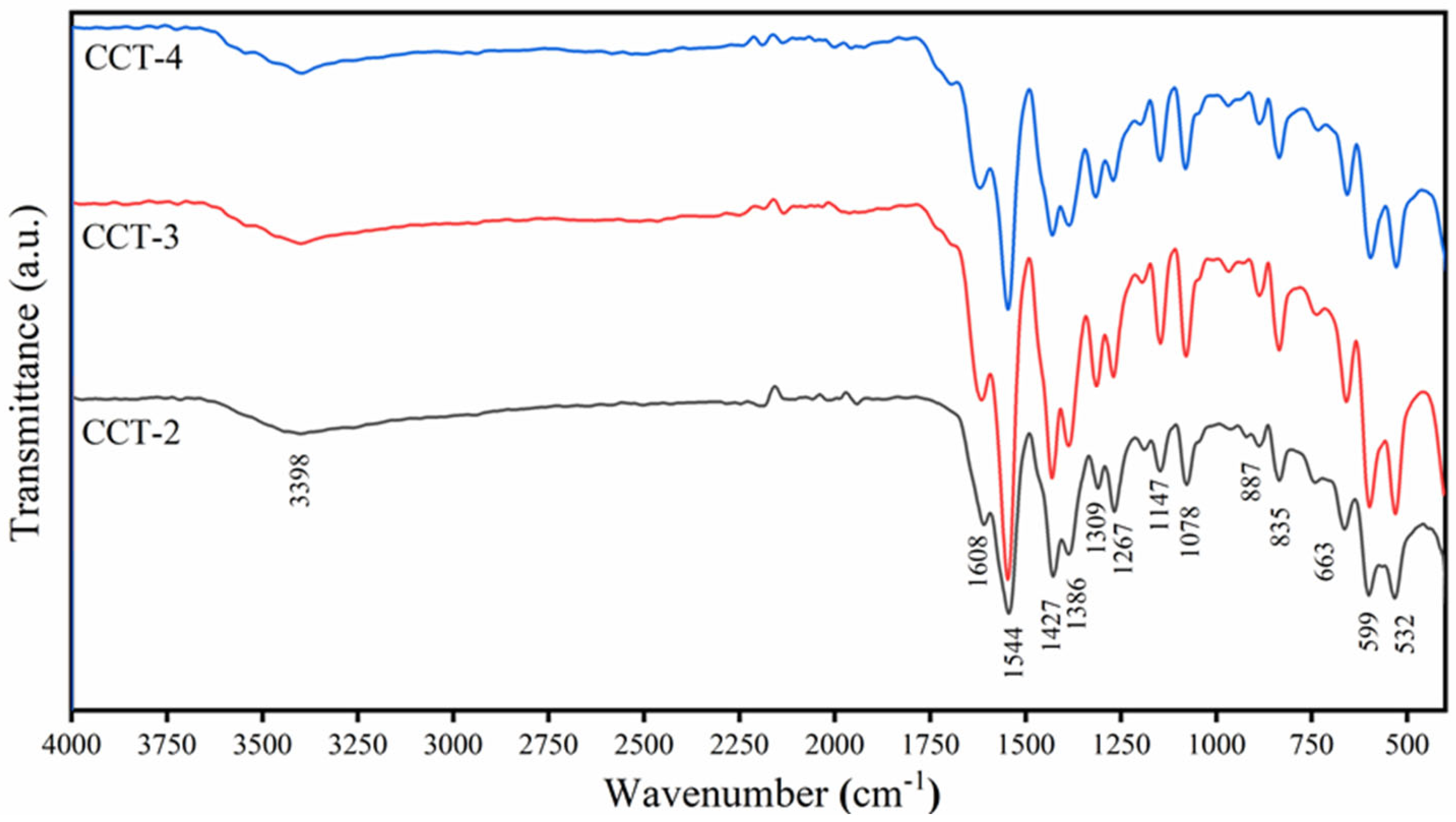
3.1. Functional Groups of CCT
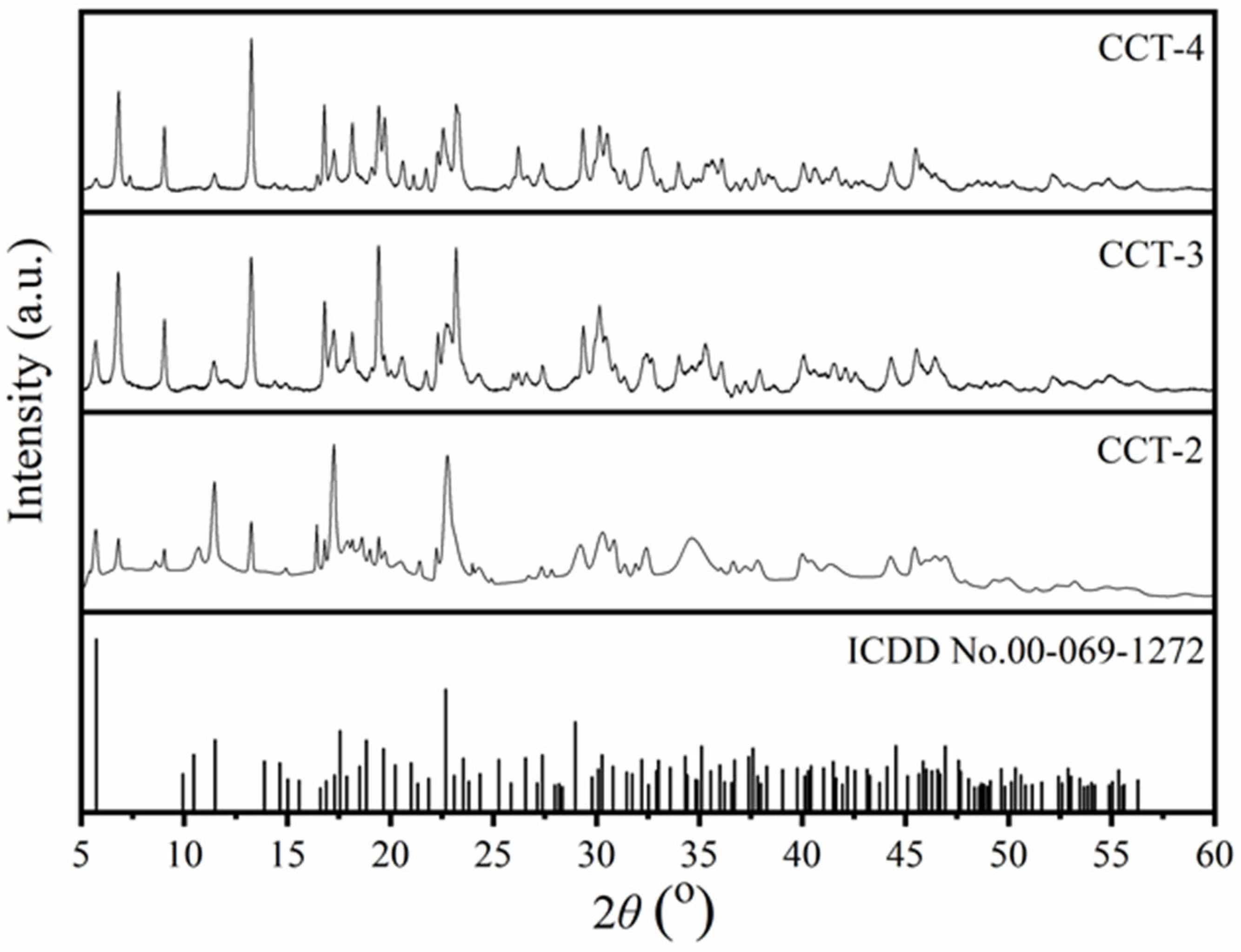
3.2. Crystal Structure of CCT
3.3. Thermal Decomposition of CCT
3.4. Purities of CCT Determined by XRF
3.5. Morphologies of CCT
3.6. Preparation Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hany, M.; Wuyts, S.; Abouelnasr, A.A.; Zidan, A.; Demerdash, H.M.; Hussein, H.A.S.M.; Arida, R.E.; Elsharkawi, S.M.; Kramers, C.; Torensma, B. Comparison of calcium citrate and calcium carbonate absorption in patients with a Roux-en-Y gastric bypass, sleeve gastrectomy, and one-anastomosis gastric bypass: A double-blind, randomized cross-over trial. Surg. Obes. Relat. Dis. 2025, 21, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Liu, Y.; Peng, H.; Li, K.; Li, C.; Jiang, S.; Chen, M.; Han, D.; Gong, J. Improving calcium citrate food functions through spherulitic growth in reactive crystallization and a mechanism study. Food Chem. 2023, 404, 134550. [Google Scholar] [CrossRef]
- Tondapu, P.; Provost, D.; Adams-Huet, B.; Sims, T.; Chang, C.; Sakhaee, K. Comparison of the absorption of calcium carbonate and calcium citrate after Roux-en-Y gastric bypass. Obes. Surg. 2009, 19, 1256–1261. [Google Scholar] [CrossRef]
- Wang, L.-m.; Wang, W.; Li, X.-C.; Peng, L.; Lin, Z.-Q. Calcium citrate: A new biomaterial that can enhance bone formation in situ. Chin. J. Traumatol. 2012, 15, 291–296. [Google Scholar]
- Kaduk, J.A. Crystal structure of aqua (citric acid)(hydrogen citrato) calcium monohydrate, [Ca(HC6H5O7)(H3C6H5O7)(H2O)]·H2O, from synchrotron X-ray powder data, and DFT-optimized crystal structure of existing calcium hydrogen citrate trihydrate, [Ca(HC6H5O7)(H2O)3]. Struct. Rep. 2020, 76, 1689–1693. [Google Scholar] [CrossRef]
- Kaduk, J.A. Crystal structures of tricalcium citrates. Powder Diffr. 2018, 33, 98–107. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Gao, Y.; Zhong, L.; Zou, Q.; Lai, X. Preparation and properties of calcium citrate nanosheets for bone graft substitute. Bioengineered 2016, 7, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Herdtweck, E.; Kornprobst, T.; Sieber, R.; Straver, L.; Plank, J. Crystal structure, synthesis, and properties of tri-calcium di-citrate tetra-hydrate [Ca3(C6H5O7)2(H2O)2]·2H2O. Z. Anorg. Allg. Chem. 2011, 637, 655–659. [Google Scholar] [CrossRef]
- Wei, S.; Sun, M. Transformation of calcite (CaCO3) into earlandite [Ca3(C6H5O7)2·4H2O] by the fungus Trichoderma asperellum BDH65. Int. Biodeterior. Biodegrad. 2021, 163, 105278. [Google Scholar] [CrossRef]
- Seesanong, S.; Seangarun, C.; Boonchom, B.; Phutphat, S.; Rungrojchaipon, P.; Montri, N.; Thompho, S.; Boonmee, W.; Laohavisuti, N. Efficient, green, and low-cost conversion of bivalve-shell wastes to value-added calcium lactate. ACS Omega 2023, 8, 27044–27055. [Google Scholar] [CrossRef]
- Sand, K.; Rodriguez-Blanco, J.; Makovicky, E.; Benning, L.G.; Stipp, S. Crystallization of CaCO3 in water–alcohol mixtures: Spherulitic growth, polymorph stabilization, and morphology change. Cryst. Growth Des. 2012, 12, 842–853. [Google Scholar] [CrossRef]
- Cubillas, P.; Köhler, S.; Prieto, M.; Chaïrat, C.; Oelkers, E.H. Experimental determination of the dissolution rates of calcite, aragonite, and bivalves. Chem. Geol. 2005, 216, 59–77. [Google Scholar] [CrossRef]
- Pan, L.; Li, Q.; Zhou, Y.; Song, N.; Yu, L.; Wang, X.; Xiong, K.; Yap, L.; Huo, J. Effects of different calcium sources on the mineralization and sand curing of CaCO3 by carbonic anhydrase-producing bacteria. RSC Adv. 2019, 9, 40827–40834. [Google Scholar] [CrossRef]
- Sasaki, K.; Yamashita, T.; Tsunekawa, M. Synthesis of Aragonite from Calcined Scallop Shells at Ambient Temperatures and Their Morphological Characterization by FE-SEM. Shigen-to-Sozai 2002, 118, 553–558. [Google Scholar] [CrossRef]
- Suwannasingha, N.; Kantavong, A.; Tunkijjanukij, S.; Aenglong, C.; Liu, H.-B.; Klaypradit, W. Effect of calcination temperature on structure and characteristics of calcium oxide powder derived from marine shell waste. J. Saudi Chem. Soc. 2022, 26, 101441. [Google Scholar] [CrossRef]
- Cölfen, H. Precipitation of carbonates: Recent progress in controlled production of complex shapes. Curr. Opin. Colloid Interface Sci. 2003, 8, 23–31. [Google Scholar] [CrossRef]
- Ramakrishna, C.; Thenepalli, T.; Han, C.; Ahn, J.-W. Synthesis of aragonite-precipitated calcium carbonate from oyster shell waste via a carbonation process and its applications. Korean J. Chem. Eng. 2017, 34, 225–230. [Google Scholar] [CrossRef]
- Gopi, S.; Subramanian, V.; Palanisamy, K. Aragonite–calcite–vaterite: A temperature influenced sequential polymorphic transformation of CaCO3 in the presence of DTPA. Mater. Res. Bull. 2013, 48, 1906–1912. [Google Scholar] [CrossRef]
- Jimoh, O.A.; Ariffin, K.S.; Hussin, H.B.; Temitope, A.E. Synthesis of precipitated calcium carbonate: A review. Carbonates Evaporites 2018, 33, 331–346. [Google Scholar] [CrossRef]
- Kirboga, S.; Oner, M. Effect of the experimental parameters on calcium carbonate precipitation. Chem. Eng. J. 2013, 32, 2119–2124. [Google Scholar]
- Zaitsev, A.V.; Astapov, I.A. Study of the Composition and Properties of Bivalve Mollusk Shells as Promising Bio-Indifferent Materials for Photocatalytic Applications (Example of Practical Use). Catalysts 2023, 14, 16. [Google Scholar] [CrossRef]
- Basumatary, S.F.; Brahma, S.; Hoque, M.; Das, B.K.; Selvaraj, M.; Brahma, S.; Basumatary, S. Advances in CaO-based catalysts for sustainable biodiesel synthesis. Green Energy Resour. 2023, 1, 100032. [Google Scholar] [CrossRef]
- Boey, P.-l.; Maniam, G.P.; Abd Hamid, S.; Ali, D.M.H. Utilization of waste cockle shell (Anadara granosa) in biodiesel production from palm olein: Optimization using response surface methodology. Fuel 2011, 90, 2353–2358. [Google Scholar] [CrossRef]
- Ruslan, H.N.; Muthusamy, K.; Jaafar, M.; Zamri, N.A.; Jaya, R.P. Mechanical properties of mortar with Anadara Granosa waste as partial sand replacement. Open Civ. Eng. J. 2023, 17, e187414952303280. [Google Scholar] [CrossRef]
- Mahmood, S.K.; Zakaria, M.Z.A.B.; Razak, I.S.B.A.; Yusof, L.M.; Jaji, A.Z.; Tijani, I.; Hammadi, N.I. Preparation and characterization of cockle shell aragonite nanocomposite porous 3D scaffolds for bone repair. Biochem. Biophys. Rep. 2017, 10, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Seesanong, S.; Wongchompoo, Y.; Boonchom, B.; Sronsri, C.; Laohavisuti, N.; Chaiseeda, K.; Boonmee, W. Economical and environmentally friendly track of biowaste recycling of scallop shells to calcium lactate. ACS Omega 2022, 7, 14756–14764. [Google Scholar] [CrossRef] [PubMed]
- Wong-Ng, W.; McMurdie, H.; Hubbard, C.; Mighell, A.D. JCPDS-ICDD research associateship (cooperative program with NBS/NIST). J. Res. Natl. Inst. Stand. Technol. 2001, 106, 1013. [Google Scholar] [CrossRef]
- Sronsri, C.; Boonchom, B. Thermal kinetic analysis of a complex process from a solid-state reaction by deconvolution procedure from a new calculation method and related thermodynamic functions of Mn0.90Co0.05Mg0.05HPO4·3H2O. Trans. Nonferrous Met. Soc. China 2018, 28, 1887–1902. [Google Scholar] [CrossRef]
- Khan, M.M. X-ray fluorescence spectroscopy. In Photocatalysts: Synthesis and Characterization Methods; Elsevier: Amsterdam, The Netherlands, 2025; pp. 225–237. [Google Scholar]
- Yuan, J.; Yang, L.; Yu, P.; Tang, N.; Liu, L.; Wang, W.; Wang, P.; Yang, Q.; Guo, S.; Li, J. Comparison and development of scanning electron microscope techniques for delicate plant tissues. Plant Sci. 2024, 340, 111963. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, X.; Wu, W. Application of fourier transform infrared (FTIR) spectroscopy in sample preparation: Material characterization and mechanism investigation. Adv. Sample Prep. 2024, 11, 100122. [Google Scholar] [CrossRef]
- Ding, X.-M.; Peng, L.; Wen, F.; Tan, Z.-W.; Mu, Z.-L. Simulated body fluid immersion method for assessing biological characteristics of calcium citrate. Chin. J. Tissue Eng. Res. 2013, 17, 6811. [Google Scholar]
- Ali, A.; Chiang, Y.W.; Santos, R.M. X-ray diffraction techniques for mineral characterization: A review for engineers of the fundamentals, applications, and research directions. Minerals 2022, 12, 205. [Google Scholar] [CrossRef]
- Liu, X.-C.; Kirkensgaard, J.J.; Skibsted, L.H. Hydrates of calcium citrate and their interconversion in relation to calcium bioaccessibility. Food Res. Int. 2021, 140, 109867. [Google Scholar] [CrossRef]
- El-Sayed, S.A.; Khass, T.M.; Mostafa, M.E. Thermal degradation behaviour and chemical kinetic characteristics of biomass pyrolysis using TG/DTG/DTA techniques. Biomass Convers. Biorefinery 2024, 14, 17779–17803. [Google Scholar] [CrossRef]
- Mirza, A.C.; Panchal, S.S.; Allam, A.A.; Othman, S.I.; Satia, M.; Mandhane, S.N. Syringic acid ameliorates cardiac, hepatic, renal and neuronal damage induced by chronic hyperglycaemia in Wistar rats: A behavioural, biochemical and histological analysis. Molecules 2022, 27, 6722. [Google Scholar] [CrossRef]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.; Kumar, C.S. Syringic acid (SA)—A review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef]
- Cahill, J.; Davis, V.; King, C.; Miller, L.; Norrgard, T.; Castano, C.E.; Mohammadi, R.; Rojas, J.; Goddard, B. Modeling and benchmarking XRF analysis using MCNP for applications in accident tolerant fuel and cladding. Prog. Nucl. Energy 2025, 178, 105487. [Google Scholar] [CrossRef]
- Janssens, K. X-Ray Fluorescence Analysis. In Handbook of Spectroscopy: Second, Enlarged Edition; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 449–506. [Google Scholar]
- Richter, R.F.; Vater, C.; Korn, M.; Ahlfeld, T.; Rauner, M.; Pradel, W.; Stadlinger, B.; Gelinsky, M.; Lode, A.; Korn, P. Treatment of critical bone defects using calcium phosphate cement and mesoporous bioactive glass providing spatiotemporal drug delivery. Bioact. Mater. 2023, 28, 402–419. [Google Scholar] [CrossRef]
- Ielo, I.; Calabrese, G.; De Luca, G.; Conoci, S. Recent advances in hydroxyapatite-based biocomposites for bone tissue regeneration in orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef]
- Khan, M.M. Scanning electron microscopy. In Photocatalysts: Synthesis and Characterization Methods; Elsevier: Amsterdam, The Netherlands, 2025; pp. 281–294. [Google Scholar]
- Moreno, J.; Peinado, R. Enological Chemistry; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Olatide, M.; Oladele, E.; Lajide, L.; Oluwasina, O. The effect of citric acid concentration, reaction temperature and time on the esterification of some varieties of yam flour in Nigeria. Discov. Chem. 2024, 1, 69. [Google Scholar] [CrossRef]


| Elemental Compositions | Elemental Contents/wt% | |||
|---|---|---|---|---|
| CCT-2 | CCT-3 | CCT-4 | ||
| Major composition | ||||
| Calcium oxide | CaO | 98.1778 | 98.1805 | 98.1857 |
| Minor compositions | ||||
| Sodium oxide | Na2O | 0.8520 | 0.8200 | 0.8410 |
| Magnesium oxide | MgO | 0.0462 | 0.0414 | 0.0470 |
| Aluminum oxide | Al2O3 | 0.0323 | 0.0331 | 0.0431 |
| Silicon dioxide | SiO2 | 0.0854 | 0.0903 | 0.0925 |
| Phosphorus pentoxide | P2O5 | 0.0193 | 0.0187 | 0.0209 |
| Sulfur trioxide | SO3 | 0.1370 | 0.1330 | 0.1430 |
| Chlorine | Cl | 0.0212 | 0.0192 | 0.0211 |
| Potassium oxide | K2O | 0.0123 | 0.0097 | 0.0126 |
| Cobalt oxide | Co2O3 | - | 0.0211 | - |
| Zinc oxide | ZnO | - | 0.0146 | - |
| Strontium oxide | SrO | 0.4350 | 0.4450 | 0.455 |
| Ferrous oxide | Fe2O3 | 0.1470 | 0.1450 | 0.1460 |
| Summations | ~100 | ~100 | ~100 | |
| Samples | Reaction Time (min) | Production Yields (%) | Soluble Fractions (%) |
|---|---|---|---|
| CCT-2 | 54 | 98.57 ± 1.46 | 92.51 ± 1.24 |
| CCT-3 | 67 | 93.14 ± 1.37 | 88.72 ± 1.33 |
| CCT-4 | 85 | 88.63 ± 1.42 | 84.17 ± 1.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chanwetprasat, P.; Seangarun, C.; Seesanong, S.; Boonchom, B.; Laohavisuti, N.; Boonmee, W.; Rungrojchaipon, P. Effect of Citric Acid Concentration on the Transformation of Aragonite CaCO3 to Calcium Citrate Using Cockle Shells as a Green Calcium Source. Materials 2025, 18, 2003. https://doi.org/10.3390/ma18092003
Chanwetprasat P, Seangarun C, Seesanong S, Boonchom B, Laohavisuti N, Boonmee W, Rungrojchaipon P. Effect of Citric Acid Concentration on the Transformation of Aragonite CaCO3 to Calcium Citrate Using Cockle Shells as a Green Calcium Source. Materials. 2025; 18(9):2003. https://doi.org/10.3390/ma18092003
Chicago/Turabian StyleChanwetprasat, Pantita, Chaowared Seangarun, Somkiat Seesanong, Banjong Boonchom, Nongnuch Laohavisuti, Wimonmat Boonmee, and Pesak Rungrojchaipon. 2025. "Effect of Citric Acid Concentration on the Transformation of Aragonite CaCO3 to Calcium Citrate Using Cockle Shells as a Green Calcium Source" Materials 18, no. 9: 2003. https://doi.org/10.3390/ma18092003
APA StyleChanwetprasat, P., Seangarun, C., Seesanong, S., Boonchom, B., Laohavisuti, N., Boonmee, W., & Rungrojchaipon, P. (2025). Effect of Citric Acid Concentration on the Transformation of Aragonite CaCO3 to Calcium Citrate Using Cockle Shells as a Green Calcium Source. Materials, 18(9), 2003. https://doi.org/10.3390/ma18092003







