A Novel Pre-Customized Saddle-Shape Soft Tissue Substitute for Volume Augmentation: An Ex Vivo Study in Pig Mandibles
Abstract
1. Introduction
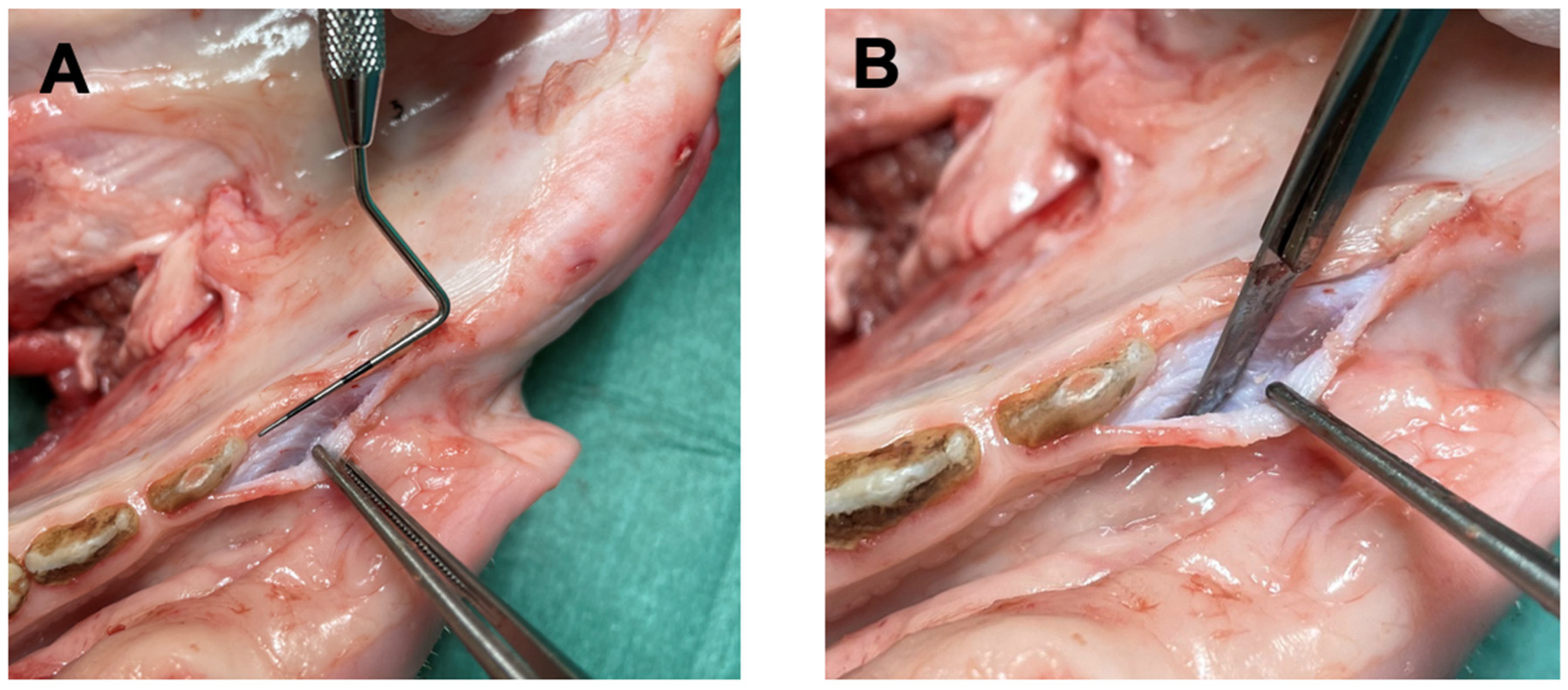
2. Materials and Methods
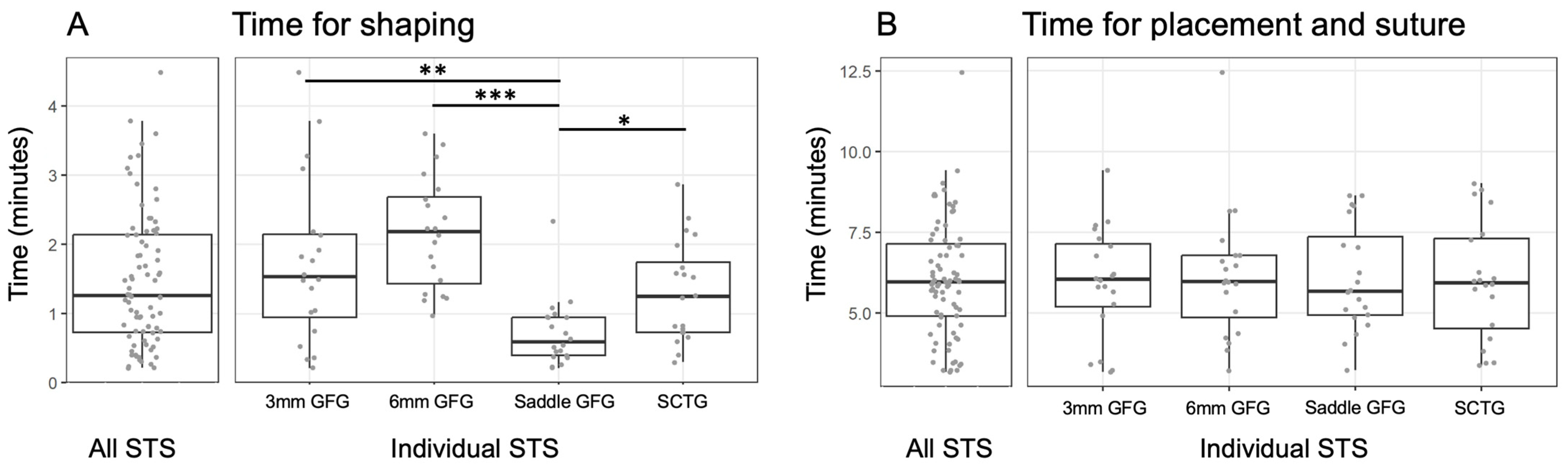
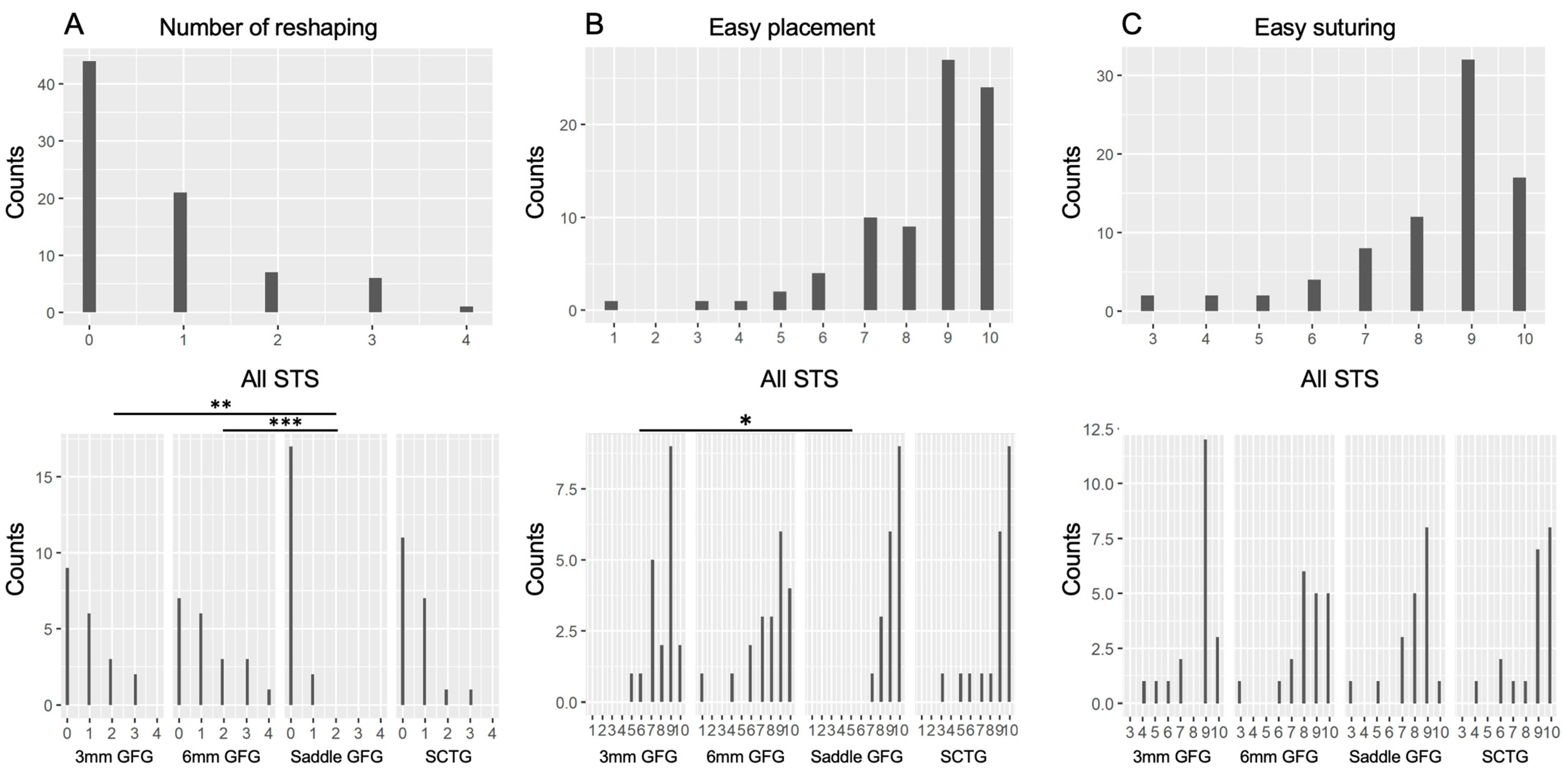
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCTG | Subepithelial connective tissue graft |
| STS | Soft tissue substitute |
| GFG/VCMX | Geistlich Fibro-Gide |
| CRO | Clinician Reported Outcomes |
References
- Van der Weijden, F.; Dell’Acqua, F.; Slot, D.E. Alveolar bone dimensional changes of post-extraction sockets in humans: A systematic review. J. Clin. Periodontol. 2009, 36, 1048–1058. [Google Scholar] [CrossRef]
- Ackali, A.; Trullenque- Eriksson, A.; Sun, C.; Petrie, A.; Nibali, L.; Donos, N. What is the effect of soft tissue thickness on crestal bone loss around dental implants? A systematic review. Clin. Oral Implant. Res. 2017, 28, 1046–1053. [Google Scholar] [CrossRef]
- Gobbato, L.; Avila Ortiz, G.; Sohrabi, K.; Wang, C.; Karimbux, N. The effect of keratinized mucosa width on peri-implant health: A systematic review. Int. J. Oral Maxillofac. Implant. 2013, 28, 1536–1545. [Google Scholar] [CrossRef]
- Naenni, N.; Lim, H.C.; Papageorgiou, S.N.; Hämmerle, C.H.F. Efficacy of lateral bone augmentation prior to implant placement: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 287–306. [Google Scholar] [CrossRef]
- Troeltzsch, M.; Troeltzsch, M.; Kauffmann, P.; Gruber, R.; Brockmeyer, P.; Moser, N.; Rau, A.; Schliephake, H. Clinical efficacy of grafting materials in alveolar ridge augmentation: A systematic review. J. Cranio. Maxill. Surg. 2016, 44, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ortiz, G.; Couso-Queiruga, E.; Pirc, M.; Chambrone, L.; Thoma, D.S. Outcome measures and methods of assessment of soft-tissue augmentation interventions in the context of dental implant therapy: A systematic review of clinical studies published in the last 10 years. Clin. Oral Implant. Res. 2023, 34 (Suppl. S25), 84–96. [Google Scholar] [CrossRef]
- Del Amo, F.S.L.; Yu, S.H.; Sammartino, G.; Sculean, A.; Zucchelli, G.; Rasperini, G.; Felice, P.; Pagni, G.; Iorio-Siciliano, V.; Grusovin, M.G.; et al. Peri-Implant Soft Tissue Management: Cairo Opinion Consensus Conference. Int. J. Environ. Res. Public Health 2020, 17, 2281. [Google Scholar] [CrossRef]
- Ashurko, I.; Tarasenko, S.; Magdalyanova, M.; Bokareva, S.; Balyasin, M.; Galyas, A.; Khadimova, M.; Zhornik, M.; Unkoviskiy, A. Comparative analysis of xenogeneic collagen matrix and autogenous subepithelial connective tissue graft to increase soft tissue volume around dental implants: A systematic review and meta-analysis. BMC Oral Health 2023, 23, 741. [Google Scholar] [CrossRef] [PubMed]
- Lissek, M.; Boeker, M.; Happe, A. How Thick Is the Oral Mucosa around Implants after Augmentation with Different Materials: A Systematic Review of the Effectiveness of Substitute Matrices in Comparison to Connective Tissue Grafts. Int. J. Mol. Sci. 2020, 21, 5043. [Google Scholar] [CrossRef]
- Thoma, D.S.; Buranawat, B.; Hammerle, C.H.; Held, U.; Jung, R.E. Efficacy of soft tissue augmentation around dental implants and in partially edentulous areas: A systematic review. J. Clin. Periodontol. 2014, 41 (Suppl. S15), S77–S91. [Google Scholar] [CrossRef]
- Cairo, F.; Barbato, L.; Tonelli, P.; Batalocco, G.; Pagavino, G.; Nieri, M. Xenogeneic collagen matrix versus connective tissue graft for buccal soft tissue augmentation at implant site. A randomized, controlled clinical trial. J. Clin. Periodontol. 2017, 44, 769–776. [Google Scholar] [CrossRef]
- Lorenzo, R.; Garcia, V.; Orsini, M.; Martin, C.; Sanz, M. Clinical efficacy of a xenogeneic collagen matrix in augmenting keratinized mucosa around implants: A randomized controlled prospective clinical trial. Clin. Oral Implants Res. 2012, 23, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Hilbe, M.; Bienz, S.P.; Sancho-Puchades, M.; Hammerle, C.H.; Jung, R.E. Palatal wound healing using a xenogeneic collagen matrix—Histological outcomes of a randomized controlled clinical trial. J. Clin. Periodontol. 2016, 43, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Nikolidakis, D.; Nikou, G.; Ivanovic, A.; Chapple, I.L.; Stavropoulos, A. Biomaterials for promoting periodontal regeneration in human intrabony defects: A systematic review. Periodontol. 2000 2015, 68, 182–216. [Google Scholar] [CrossRef]
- Wallace, D.G.; Rosenblatt, J. Collagen gel systems for sustained delivery and tissue engineering. Adv. Drug Deliv. Rev. 2003, 55, 1631–1649. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Hang, H.L.; Giannobile, W.V. Extracellular matrix-based scaffolding technologies for periodontal and peri-implant soft tissue regeneration. J. Periodontol. 2020, 91, 17–25. [Google Scholar] [CrossRef]
- Toledano, M.; Toledano-Osorio, M.; Carrasco-Carmona, A.; Vallecillo, C.; Toledano, R.; Medina-Castillo, A.L.; Osorio, R. State of the Art on Biomaterials for Soft Tissue Augmentation in the Oral Cavity. Part II: Synthetic Polymers-Based Biomaterials. Polymers 2020, 12, 1845. [Google Scholar] [CrossRef]
- Toledano, M.; Toledano-Osorio, M.; Carrasco-Carmona, A.; Vallecillo, C.; Toledano, R.; Medina-Castillo, A.L.; Osorio, R. State of the Art on Biomaterials for Soft Tissue Augmentation in the Oral Cavity. Part I: Natural Polymers-Based Biomaterials. Polymers 2020, 12, 1850. [Google Scholar] [CrossRef]
- Wolff, J.; Farre-Guasch, E.; Sandor, G.K.; Gibbs, S.; Jager, D.J.; Forouzanfar, T. Soft Tissue Augmentation Techniques and Materials Used in the Oral Cavity: An Overview. Implant Dent. 2016, 25, 427–434. [Google Scholar] [CrossRef]
- Eeckhout, C.; Bouckaert, E.; Verleyen, D.; De Bruyckere, T.; Cosyn, J. A 3-Year Prospective Study on a Porcine-Derived Acellular Collagen Matrix to Re-Establish Convexity at the Buccal Aspect of Single Implants in the Molar Area: A Volumetric Analysis. J. Clin. Med. 2020, 9, 1568. [Google Scholar] [CrossRef]
- Fickl, S.; Therese Kroger, A.; Dietrich, T.; Kebschull, M. Influence of soft tissue augmentation procedures around dental implants on marginal bone level changes-A systematic review. Clin. Oral Implants Res. 2021, 32 (Suppl. S21), 108–137. [Google Scholar] [CrossRef] [PubMed]
- Papi, P.; Pompa, G. The Use of a Novel Porcine Derived Acellular Dermal Matrix (Mucoderm) in Peri-Implant Soft Tissue Augmentation: Preliminary Results of a Prospective Pilot Cohort Study. Biomed. Res. Int. 2018, 2018, 6406051. [Google Scholar] [CrossRef]
- Puisys, A.; Deikuviene, J.; Vindasiute-Narbute, E.; Razukevicus, D.; Zvirblis, T.; Linkevicius, T. Connective tissue graft vs porcine collagen matrix after immediate implant placement in esthetic area: A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2022, 24, 141–150. [Google Scholar] [CrossRef]
- Artzi, Z.; Renert, U.; Pirc, M.; Netanely, E.; Thoma, D.S. Contour Changes Following Implant Placement and Concomitant Soft Tissue Augmentation Applying a Volume-Stable Collagen Matrix. Int. J. Periodontics Restor. Dent. 2022, 42, 515–522. [Google Scholar] [CrossRef]
- Cosyn, J.; Eeckhout, C.; De Bruyckere, T.; Eghbali, A.; Vervaeke, S.; Younes, F.; Christiaens, V. A multi-centre randomized controlled trial comparing connective tissue graft with collagen matrix to increase soft tissue thickness at the buccal aspect of single implants: 1-year results. J. Clin. Periodontol. 2022, 49, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Hammerle, C.H.F.; Jepsen, K.; Sailer, I.; Strasding, M.; Zeltner, M.; Cordaro, L.; di Torresanto, V.M.; Schwarz, F.; Zugh, O.; Akakpo, D.; et al. Efficacy of a collagen matrix for soft tissue augmentation after implant placement compared to connective tissue grafts: A multicenter, noninferiority, randomized controlled trial. Clin. Oral Implant. Res. 2023, 34, 999–1013. [Google Scholar] [CrossRef]
- Naenni, N.; Bienz, S.P.; Benic, G.I.; Jung, R.E.; Hämmerle, C.H.; Thoma, D.S. Volumetric and linear changes at dental implants following grafting with volume-stable three-dimensional collagen matrices or autogenous connective tissue grafts: 6-month data. Clin. Oral Investig. 2018, 22, 1185–1195. [Google Scholar] [CrossRef]
- Thoma, D.S.; Gasser, T.J.W.; Hammerle, C.H.F.; Strauss, F.J.; Jung, R.E. Soft tissue augmentation with a volume-stable collagen matrix or an autogenous connective tissue graft at implant sites: Five-year results of a randomized controlled trial post implant loading. J. Periodontol. 2023, 94, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Gasser, T.J.W.; Jung, R.E.; Hammerle, C.H.F. Randomized controlled clinical trial comparing implant sites augmented with a volume-stable collagen matrix or an autogenous connective tissue graft: 3-year data after insertion of reconstructions. J. Clin. Periodontol. 2020, 47, 630–639. [Google Scholar] [CrossRef]
- Zeltner, M.; Jung, R.E.; Hammerle, C.H.; Husler, J.; Thoma, D.S. Randomized controlled clinical study comparing a volume-stable collagen matrix to autogenous connective tissue grafts for soft tissue augmentation at implant sites: Linear volumetric soft tissue changes up to 3 months. J. Clin. Periodontol. 2017, 44, 446–453. [Google Scholar] [CrossRef]
- Thoma, D.S.; Zeltner, M.; Hilbe, M.; Hammerle, C.H.; Husler, J.; Jung, R.E. Randomized controlled clinical study evaluating effectiveness and safety of a volume-stable collagen matrix compared to autogenous connective tissue grafts for soft tissue augmentation at implant sites. J. Clin. Periodontol. 2016, 43, 874–885. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, T.; Strasding, M.; Liu, X.; Burkhardt, F.; Schafer, B.; Sailer, I.; Nesic, D. Design of customized soft tissue substitutes for posterior single-tooth defects: A proof-of-concept in vitro study. Clin. Oral Implants Res. 2021, 32, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Strasding, M.; Liu, X.; Schafer, B.; Liu, F.; Sailer, I.; Nesic, D. Design of customized soft tissue substitutes for anterior single-tooth and posterior double-tooth defects: An in vitro study. J. Esthet. Restor. Dent. 2023, 35, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Krithikadatta, J.; Gopikrishna, V.; Datta, M. CRIS Guidelines (Checklist for Reporting In-Vitro Studies): A concept note on the need for standardized guidelines for improving quality and transparency in reporting in-vitro studies in experimental dental research. J. Conserv. Dent. 2014, 17, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Hodges, J.L.; Lehmann, E.L. Estimates of Location Based on Rank-Tests. Ann. Math. Stat. 1963, 34, 598. [Google Scholar] [CrossRef]
- Gonzalez-Febles, J.; Romandini, M.; Laciar-Oudshoorn, F.; Noguerol, F.; Marruganti, C.; Bujaldon-Daza, A.; Zabalegui, I.; Sanz, M. Tunnel vs. coronally advanced flap in combination with a connective tissue graft for the treatment of multiple gingival recessions: A multi-center randomized clinical trial. Clin. Oral Investig. 2023, 27, 3627–3638. [Google Scholar] [CrossRef]
- Kofina, V.; Monfaredzadeh, M.; Rawal, S.Y.; Dentino, A.R.; Singh, M.; Tatakis, D.N. Patient-reported outcomes following guided bone regeneration: Correlation with clinical parameters. J. Dent. 2023, 136, 104605. [Google Scholar] [CrossRef]
- Lau, S.L.; Chow, L.K.; Leung, Y.Y. A Non-Invasive and Accurate Measurement of Gingival Thickness Using Cone-Beam Computerized Imaging for the Assessment of Planning Immediate Implant in the Esthetic Zone—A Pig Jaw Model. Implant. Dent. 2016, 25, 619–623. [Google Scholar] [CrossRef]
- Mardas, N.; Dereka, X.; Donos, N.; Dard, M. Experimental model for bone regeneration in oral and cranio-maxillo-facial surgery. J. Investig. Surg. 2014, 27, 32–49. [Google Scholar] [CrossRef]
- Rocchietta, I.; Schupbach, P.; Ghezzi, C.; Maschera, E.; Simion, M. Soft tissue integration of a porcine collagen membrane: An experimental study in pigs. Int. J. Periodontics Restor. Dent. 2012, 32, e34–e40. [Google Scholar]
- Eswaran, S.; Dowlatshahi, S.; Weltman, R.; Zhu, L.; Elangovan, S.; Lee, C.T. Preclinical teaching of periodontal surgical concepts using common instructional models: A comparative assessment. J. Dent. Educ. 2023, 87, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, A.; Ibrahim, S.S.A.; Ghalwash, D.; Adel-Khattab, D. Volumetric assessment of volume stable collagen matrix in maxillary single implant site development: A randomized controlled clinical trial. Clin. Implant Dent. Relat. Res. 2024, 26, 930–941. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, P.; De Angelis, S.; Passarelli, P.C.; Liguori, M.G.; Pompa, G.; Papi, P.; Manicone, P.F.; D’Addona, A. Clinical comparison of a xenogeneic collagen matrix versus subepithelial autogenous connective tissue graft for augmentation of soft tissue around implants. Int. J. Oral Maxillofac. Surg. 2021, 50, 956–963. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, P.; Rella, E.; Manicone, P.F.; Liguori, M.G.; De Rosa, G.; Cavalcanti, C.; Galeazzi, N.; D’Adonna, A. Xenogeneic collagen matrix versus connective tissue graft for soft tissue augmentation at immediately placed implants: A prospective clinical trial. Int. J. Oral Maxillofac. Surg. 2023, 52, 1097–1105. [Google Scholar] [CrossRef]
- Schmitt, C.M.; Bruckbauer, P.; Schlegel, K.A.; Buchbender, M.; Adler, W.; Matta, R.E. Volumetric soft tissue alterations in the early healing phase after peri- implant soft tissue contour augmentation with a porcine collagen matrix versus the autologous connective tissue graft: A controlled clinical trial. J. Clin. Periodontol. 2021, 48, 145–162. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strasding, M.; Sailer, I.; Merino-Higuera, E.; Zarauz, C.; Pitta, J.; Latyshev, A.; Wittmann, U.; Nesic, D. A Novel Pre-Customized Saddle-Shape Soft Tissue Substitute for Volume Augmentation: An Ex Vivo Study in Pig Mandibles. Materials 2025, 18, 1951. https://doi.org/10.3390/ma18091951
Strasding M, Sailer I, Merino-Higuera E, Zarauz C, Pitta J, Latyshev A, Wittmann U, Nesic D. A Novel Pre-Customized Saddle-Shape Soft Tissue Substitute for Volume Augmentation: An Ex Vivo Study in Pig Mandibles. Materials. 2025; 18(9):1951. https://doi.org/10.3390/ma18091951
Chicago/Turabian StyleStrasding, Malin, Irena Sailer, Elizabeth Merino-Higuera, Cristina Zarauz, Joao Pitta, Andrei Latyshev, Udo Wittmann, and Dobrila Nesic. 2025. "A Novel Pre-Customized Saddle-Shape Soft Tissue Substitute for Volume Augmentation: An Ex Vivo Study in Pig Mandibles" Materials 18, no. 9: 1951. https://doi.org/10.3390/ma18091951
APA StyleStrasding, M., Sailer, I., Merino-Higuera, E., Zarauz, C., Pitta, J., Latyshev, A., Wittmann, U., & Nesic, D. (2025). A Novel Pre-Customized Saddle-Shape Soft Tissue Substitute for Volume Augmentation: An Ex Vivo Study in Pig Mandibles. Materials, 18(9), 1951. https://doi.org/10.3390/ma18091951






