Co-Doped ErFeO3 for Dual-Band Laser Absorption with High-Temperature Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Synthesis of ErFe(1−x)CoxO3
2.3. Characterization
3. Results and Discussion
3.1. Formation and Deformation of EFCO
3.2. Optical Properties Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lavrov, A.; Utkin, A.B.; Vilar, R.; Fernandes, A. Application of lidar in ultraviolet, visible and infrared ranges for early forest fire detection. Appl. Phys. B Lasers Opt. 2003, 76, 87–95. [Google Scholar] [CrossRef]
- Spiessberger, S.; Schiemangk, M.; Wicht, A.; Wenzel, H.; Brox, O.; Erbert, G. Narrow Linewidth DFB Lasers Emitting Near a Wavelength of 1064 nm. J. Light. Technol. 2010, 28, 2611–2616. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H. Tunable fiber laser based photoacoustic gas sensor for early fire detection. Infrared Phys. Technol. 2014, 65, 1–4. [Google Scholar] [CrossRef]
- Dupont, A.; Caminat, P.; Bournot, P.; Gauchon, J.P. Enhancement of material ablation using 248, 308, 532, 1064 nm laser pulse with a water film on the treated surface. J. Appl. Phys. 1995, 78, 2022–2028. [Google Scholar] [CrossRef]
- Xu, H.; Hao, L.; Liu, H.; Dong, S.; Wu, Y.; Liu, Y.; Cao, B.; Wang, Z.; Ling, C.; Li, S.; et al. Flexible SnSe Photodetectors with Ultrabroad Spectral Response up to 10.6 μm Enabled by Photobolometric Effect. ACS Appl. Mater. Interfaces 2020, 12, 35250–35258. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Jiang, X.; Zeng, J.; Liao, X.; Chen, Y.; Xiao, S.; Zhao, F.; Chen, H.; Yang, J. Multispectral camouflage nanostructure design based on a particle swarm optimization algorithm for color camouflage, infrared camouflage, laser stealth, and heat dissipation. Opt. Express 2023, 31, 44811–44822. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, X.; Kang, Q.; Liu, H.; Yu, K.; Zhang, K. Visible color camouflage and infrared, laser band stealth, compatible with dual-band radiative heat dissipation. Int. Commun. Heat Mass Transf. 2024, 159, 108265. [Google Scholar] [CrossRef]
- Chai, X.; Zhu, D.; Liu, Y.; Qing, Y.; Luo, F.; Huang, Z.; Li, P.; Chen, Q. In-situ construction of Cr2O3@ATO hybrid pigment towards synergetic enhancement of visible light-infrared-radar compatible stealth. J. Colloid Interface Sci. 2023, 645, 570–579. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, Q.; Zhao, B.; Zhang, B.; Murugadoss, V.; Wu, S.; Ding, T.; Guo, Z. Facile bioactive yeast cell templated synthesis of laser stealth antimony doped tin oxide hollow microspheres. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123965. [Google Scholar] [CrossRef]
- Shi, T.; Zheng, Z.; Liu, H.; Wu, D.; Wang, X. Flexible and foldable composite films based on polyimide/phosphorene hybrid aerogel and phase change material for infrared stealth and thermal camouflage. Compos. Sci. Technol. 2022, 217, 109127. [Google Scholar] [CrossRef]
- Hu, J.; Hu, Y.; Ye, Y.; Shen, R. Unique applications of carbon materials in infrared stealth: A review. Chem. Eng. J. 2023, 452, 139147. [Google Scholar] [CrossRef]
- Wu, Y.; Tan, S.; Zhao, Y.; Liang, L.; Zhou, M.; Ji, G. Broadband multispectral compatible absorbers for radar, infrared and visible stealth application. Prog. Mater. Sci. 2023, 135, 101088. [Google Scholar] [CrossRef]
- Guo, S.-S.; Zhu, J.-P.; Song, Z.; Ren, Q.-G.; Feng, T.; Zhang, Q.-T.; Wang, L.-X. Multispectral ErBO3@ATO porous composite microspheres with laser and electromagnetic wave compatible absorption. Rare Met. 2023, 42, 2406–2418. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Q.; Tao, C.; Hong, Y.; Xu, Z.; Shen, W.; Kaur, S.; Ghosh, P.; Qiu, M. Multispectral camouflage for infrared, visible, lasers and microwave with radiative cooling. Nat. Commun. 2021, 12, 1805. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Xiao, C.; Fu, L.; Xu, J.; Zhang, D.; Zhou, H.; Fan, T. Compatible Stealth Metasurface for Laser and Infrared with Radiative Thermal Engineering Enabled by Machine Learning. Adv. Funct. Mater. 2023, 33, 2212068. [Google Scholar] [CrossRef]
- Feng, X.; Pu, M.; Zhang, F.; Pan, R.; Wang, S.; Gong, J.; Zhang, R.; Guo, Y.; Li, X.; Ma, X.; et al. Large-Area Low-Cost Multiscale-Hierarchical Metasurfaces for Multispectral Compatible Camouflage of Dual-Band Lasers, Infrared and Microwave. Adv. Funct. Mater. 2022, 32, 2205547. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, W.; Qian, M.; Shen, P.; Liu, Y. Multiband metamaterial emitters for infrared and laser compatible stealth with thermal management based on dissipative dielectrics. Photonics Res. 2023, 11, 290–298. [Google Scholar] [CrossRef]
- Han, P.; Huang, X.; Zhang, Q. Laser stealth absorbent of samarium oxysulfide prepared by flux method. Rare Met. 2011, 30, 616–620. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, Z.; Gao, L.; Zhen, Y.; Liu, L.; Liu, Y.; Xu, F. Laser stealth and laser protection properties of oxide-carbide coatings prepared by atmospheric plasma spraying. Ceram. Int. 2022, 48, 31389–31396. [Google Scholar] [CrossRef]
- Feng, X.; Xie, X.; Pu, M.; Ma, X.; Guo, Y.; Li, X.; Luo, X. Hierarchical metamaterials for laser-infrared-microwave compatible camouflage. Opt. Express 2020, 28, 9445–9453. [Google Scholar] [CrossRef]
- Ur Rahman, A.; Shah, A.H.; Rehman, F.; Ahmad, F.; Waqas, M.; Rahman, K.U. Dual-band absorption due to simultaneous excitation of TE and TM modes in ITO-ES metamaterial for IR stealth technology. Opt. Commun. 2023, 549, 129875. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, M.; Lang, Y.; Tian, C.; Wei, H.; Wang, C.-A. Preparation and characterization of monodispersed spherical Fe2O3@SiO2 reddish pigments with core–shell structure. J. Adv. Ceram. 2019, 8, 39–46. [Google Scholar] [CrossRef]
- Medeiros, P.N.; Gomes, Y.F.; Bomio, M.R.D.; Santos, I.M.G.; Silva, M.R.S.; Paskocimas, C.A.; Li, M.S.; Motta, F.V. Influence of variables on the synthesis of CoFe2O4 pigment by the complex polymerization method. J. Adv. Ceram. 2015, 4, 135–141. [Google Scholar] [CrossRef]
- Wendusu; Yoshida, T.; Masui, T.; Imanaka, N. Novel environmentally friendly inorganic red pigments based on calcium bismuth oxides. J. Adv. Ceram. 2015, 4, 39–45. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, M.; Guan, Y.; Huang, X. Laser absorption and infrared stealth properties of Al/ATO composites. Ceram. Int. 2019, 45, 14312–14315. [Google Scholar] [CrossRef]
- Hao, F.; Li, D.; Shan, Y.; Yu, X.; Chen, K. The hydrothermal synthesis and properties of Sm2O(CO3)2·xH2O using g-C3N4 as raw material. Mater. Chem. Phys. 2020, 241, 122356. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, S.; Song, Z.; Feng, C.; Feng, T.; Zhang, Q.; Wang, L. Reinforced near-infrared compatible absorption performance of Er4Al2O9 based on Sm3+/Dy3+ co-doping. J. Mater. Sci. Mater. Electron. 2023, 34, 736. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, F.; Li, Z.; Cheng, Z.; Hu, J. Research on Infrared Emissivity and Laser Reflectivity of Sn1−xErxO2 Micro/Nanofibers Based on First-Principles. J. Renew. Mater. 2023, 11, 921–936. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, C.; Li, P.; Wang, W.; Xiao, W.; Sun, T.; Zhang, J. Optical and Photocatalytic Properties of Cobalt-Doped LuFeO3 Powders Prepared by Oxalic Acid Assistance. Molecules 2023, 28, 5730. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Lan, Y.-S.; Zeng, Z.-Y.; Chen, X.-R.; Chen, Q.-F. Magnetic structures and optical properties of rare-earth orthoferrites RFeO3 (R = Ho, Er, Tm and Lu). Solid State Commun. 2019, 288, 10–17. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, Z.; Xi, Z.; Yang, Y.; Wu, M.; Li, Y.; Li, X.; Wang, Q.; Li, C.; Wu, D.; et al. Flexoelectric-induced photovoltaic effects and tunable photocurrents in flexible LaFeO3 epitaxial heterostructures. J. Mater. 2022, 8, 281–287. [Google Scholar] [CrossRef]
- Sati, P.C.; Kumar, M.; Chhoker, S.; Jewariya, M. Influence of Eu substitution on structural, magnetic, optical and dielectric properties of BiFeO3 multiferroic ceramics. Ceram. Int. 2015, 41, 2389–2398. [Google Scholar] [CrossRef]
- Dhiman, M.; Singhal, S. Effect of doping of different rare earth (europium, gadolinium, dysprosium and neodymium) metal ions on structural, optical and photocatalytic properties of LaFeO3 perovskites. J. Rare Earths 2019, 37, 1279–1287. [Google Scholar] [CrossRef]
- Raji, R.K.; Kurapati, V.; Ramachandran, T.; Muralidharan, M.; Suriakarthick, R.; Dhilip, M.; Hamed, F. Tweaking the red emission, magneto, and dielectrical properties of perovskite type-LaFeO3 in the presence of Co substitution. J. Mater. Sci. Mater. Electron. 2020, 31, 7998–8014. [Google Scholar] [CrossRef]
- Shen, H.; Feng, P.; Jiang, G.; Xian, Q. Synthesis of Mn-doped ErFeO3 with enhanced photo and vibration catalytic activities. J. Sol-Gel Sci. Technol. 2020, 95, 230–238. [Google Scholar] [CrossRef]
- Fang, L.; Yang, Q.; Xu, M.; Cao, J.; Tang, P.; Chen, H. Preparation of Nanosized Ni2+-Doped ErFeO3 by Microwave Assisted Process and its Visible-Light Photocatalytic Activity. Key Eng. Mater. 2017, 748, 418–422. [Google Scholar]
- Li, L.; Li, Y.; Qiao, J.; Du, M. Developing high-Q × f value MgNb2–xTaxO6(0 ≤ x ≤ 0.8) columbite ceramics and clarifying the impact mechanism of dielectric loss: Crystal structure, Raman vibrations, microstructure, lattice defects, chemical bond characteristics, structural parameters, and microwave dielectric properties in-depth studies. J. Mater. Sci. Technol. 2023, 146, 186–199. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Glazer, A. Simple ways of determining perovskite structures. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1975, 31, 756–762. [Google Scholar] [CrossRef]
- Huo, G.; Song, D.; Yang, Q.; Dong, F. Structure, magnetic and electrical transport properties of the perovskites Sm1−xCaxFe1−xMnxO3. Ceram. Int. 2008, 34, 497–503. [Google Scholar] [CrossRef]
- Park, J.; Xu, B.; Pan, J.; Zhang, D.; Lany, S.; Liu, X.; Luo, J.; Qi, Y. Accurate prediction of oxygen vacancy concentration with disordered A-site cations in high-entropy perovskite oxides. npj Comput. Materials 2023, 9, 29. [Google Scholar] [CrossRef]
- Pazhanivelu, V.; Suriakarthi, K.; Murugaraj, R.; Venkateswaran, C. Ionic radii correlative study on LaFe0.5Cr0.5O3 and YbFe0.5Cr0.5O3 magnetic double perovskites. Phys. B Condens. Matter 2021, 607, 412717. [Google Scholar]
- Rai, A.; Thakur, A.K. Effect of co-substitution on structural, optical, dielectric and magnetic behavior of LaFeO3. J. Alloys Compd. 2017, 695, 3579–3588. [Google Scholar] [CrossRef]
- Mino, L.; Mandrile, L.; Iannarelli, L.; Portesi, C.; Martra, G.; Rossi, A.M. Vibrational spectroscopy. In Characterization of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 457–480. [Google Scholar]
- Bruce, I.J.; Taylor, J.; Todd, M.; Davies, M.J.; Borioni, E.; Sangregorio, C.; Sen, T. Synthesis, characterisation and application of silica-magnetite nanocomposites. J. Magn. Magn. Mater. 2004, 284, 145–160. [Google Scholar] [CrossRef]
- Azad, F.; Maqsood, A. Fabrication, structural characterization, dielectric and electrical parameters of the synthesized nano-crystalline erbium oxide. Electron. Mater. Lett. 2014, 10, 557–563. [Google Scholar] [CrossRef]
- Chen, H.; Fan, H.; Su, N.; Hong, R.; Lu, X. Highly hydrophobic polyaniline nanoparticles for anti-corrosion epoxy coatings. Chem. Eng. J. 2021, 420, 130540. [Google Scholar] [CrossRef]
- Mulushoa, S.Y.; Wegayehu, M.T.; Aregai, G.T.; Murali, N.; Reddi, M.S.; Babu, B.V.; Arunamani, T.; Samatha, K. Synthesis of Spinel MgFe2O4 Ferrite Material and Studying its Structural and Morphological Properties Using Solid State Method. Chem. Sci. Trans. 2017, 6, 653–661. [Google Scholar]
- Koshizuka, N.; Ushioda, S. Inelastic-light-scattering study of magnon softening in ErFeO3. Phys. Rev. B 1980, 22, 5394–5399. [Google Scholar] [CrossRef]
- Gupta, H.C.; Kumar Singh, M.; Tiwari, L.M. Lattice dynamic investigation of Raman and infrared wavenumbers at the zone center of orthorhombic RFeO3 (R = Tb, Dy, Ho, Er, Tm) perovskites. J. Raman Spectrosc. 2002, 33, 67–70. [Google Scholar] [CrossRef]
- Weber, M.C.; Guennou, M.; Zhao, H.J.; Íñiguez, J.; Vilarinho, R.; Almeida, A.; Moreira, J.A.; Kreisel, J. Raman spectroscopy of rare-earth orthoferrites RFeO3 (R=La, Sm, Eu, Gd, Tb, Dy). Phys. Rev. B 2016, 94, 214103. [Google Scholar] [CrossRef]
- Warshi, M.K.; Gautam, K.; Kumar, A.; Sagdeo, A.; Kim, J.-H.; Sagdeo, P.R. Unusual Lattice Dilation and Strong Electron–Phonon Coupling in Erbium Ferrite Perovskite. J. Phys. Chem. C 2024, 128, 9652–9661. [Google Scholar] [CrossRef]
- Iliev, M.N.; Abrashev, M.V. Raman phonons and Raman Jahn–Teller bands in perovskite-like manganites. J. Raman Spectrosc. 2001, 32, 805–811. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, B.; Li, Y.; Xu, X.; Sun, G.; Cao, J.; Wang, Y. Synthesis of spindle-like Co-doped LaFeO3 porous microstructure for high performance n-butanol sensor. Sens. Actuators B Chem. 2021, 343, 130125. [Google Scholar] [CrossRef]
- Bazin, D.; Kovács, I.; Guczi, L.; Parent, P.; Laffon, C.; De Groot, F.; Ducreux, O.; Lynch, J. Genesis of Co/SiO2 Catalysts: XAS Study at the Cobalt LIII, II Absorption Edges. J. Catal. 2000, 189, 456–462. [Google Scholar] [CrossRef]
- Papaefthimiou, V.; Dintzer, T.; Dupuis, V.; Tamion, A.; Tournus, F.; Hillion, A.; Teschner, D.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R.; et al. Nontrivial redox behavior of nanosized cobalt: New insights from ambient pressure X-ray photoelectron and absorption spectroscopies. ACS Nano 2011, 5, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wu, Y.; Liu, T.; Wang, X.; Yan, J.; Yin, A. Interface structure and deuterium permeation properties of Er2O3/SiC multilayer film prepared by RF magnetron sputtering. Int. J. Hydrog. Energy 2015, 40, 5701–5706. [Google Scholar] [CrossRef]
- Chen, P.; Jiang, L.; Yang, S.; Chen, H.; He, J.; Wang, Y.; An, J. Effects of combustion agents on the synthesis of perovskite erbium ferrite (ErFeO3) nanocrystalline powders fabricated by auto-propagating combustion process. Inorg. Chem. Commun. 2019, 101, 164–171. [Google Scholar] [CrossRef]
- Polat, O.; Coskun, M.; Yildirim, Y.; Roupcova, P.; Sobola, D.; Sen, C.; Durmus, Z.; Caglar, M.; Turut, A. Variation in the dielectric and magnetic characteristics of multiferroic LuFeO3 as a result of cobalt substitution at Fe sites. J. Alloys Compd. 2023, 963, 170939. [Google Scholar] [CrossRef]
- He, L.; Chu, Y.-B.; Dai, N.-L.; Li, J.-Y. Silicate-based erbium-doped fiber extended to L-band and its amplification performance. Acta Phys. Sin. 2022, 71, 154204. [Google Scholar] [CrossRef]
- Landi, S.; Segundo, I.R.; Freitas, E.; Vasilevskiy, M.; Carneiro, J.; Tavares, C.J. Use and misuse of the Kubelka-Munk function to obtain the band gap energy from diffuse reflectance measurements. Solid State Commun. 2022, 341, 114573. [Google Scholar] [CrossRef]
- Wang, L.; Ran, X.; Xiao, B.; Lei, L.; Zhu, J.; Xi, X.; Feng, G.; Li, R.; Feng, J. Visible light assisted Fenton degradation of oxytetracycline over perovskite ErFeO3/porous g-C3N4 nanosheets p-n heterojunction. J. Environ. Chem. Eng. 2022, 10, 108330. [Google Scholar] [CrossRef]
- Pearson, R.G. Absolute electronegativity and hardness: Application to inorganic chemistry. Inorg. Chem. 1988, 27, 734–740. [Google Scholar] [CrossRef]
- Rhaman, M.M.; Matin, M.A.; Hossain, M.N.; Mozahid, F.A.; Hakim, M.A.; Islam, M.F. Bandgap engineering of cobalt-doped bismuth ferrite nanoparticles for photovoltaic applications. Bull. Mater. Sci. 2019, 42, 190. [Google Scholar] [CrossRef]
- Husain, S.; Keelani, A.O.A.; Khan, W. Influence of Mn substitution on morphological, thermal and optical properties of nanocrystalline GdFeO3 orthoferrite. Nano-Struct. Nano-Objects 2018, 15, 17–27. [Google Scholar] [CrossRef]
- Zhang, L.; Jaroniec, M. Toward designing semiconductor-semiconductor heterojunctions for photocatalytic applications. Appl. Surf. Sci. 2018, 430, 2–17. [Google Scholar] [CrossRef]
- Shakur, H.R. A detailed study of physical properties of ZnS quantum dots synthesized by reverse micelle method. Phys. E Low-Dimens. Syst. Nanostructures 2011, 44, 641–646. [Google Scholar] [CrossRef]
- Wang, C.X.; Hu, K.; Li, L. Study on Preparation and Infrared Absorbency of CeO2 Nanocrystals. Laser Infrared 2006, 36, 400–402. [Google Scholar]
- Li, L.-W.; Lü, Y.; Niu, C.-H. Fabrication and optical property study of SmCrO3 powder. Laser Infrared 2018, 48, 83–87. [Google Scholar]
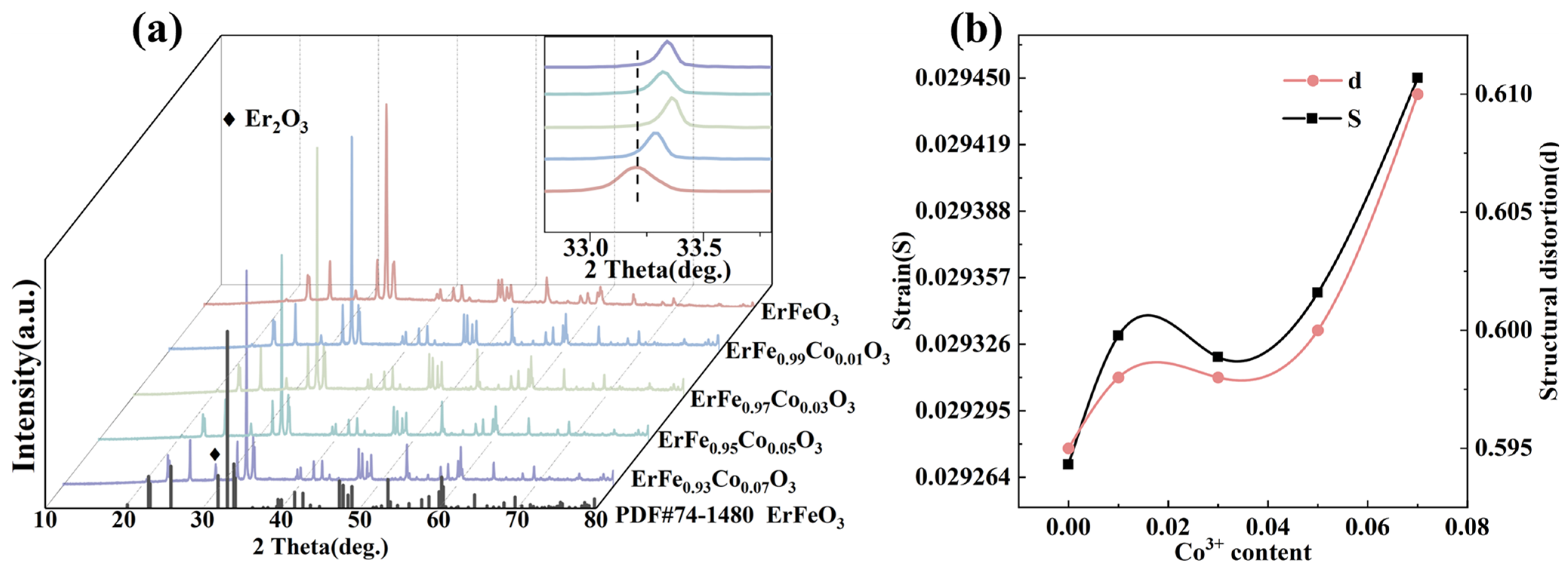

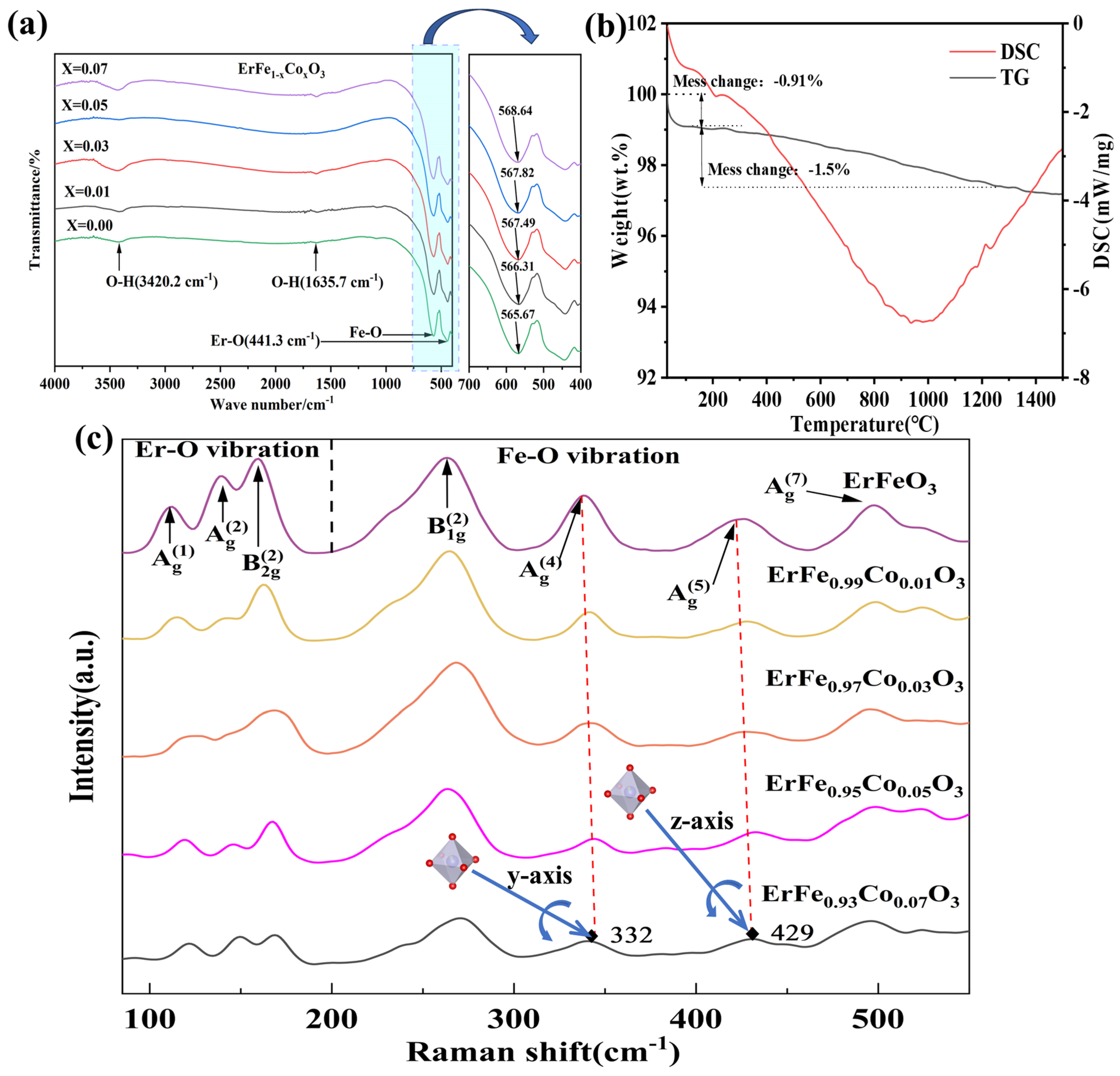
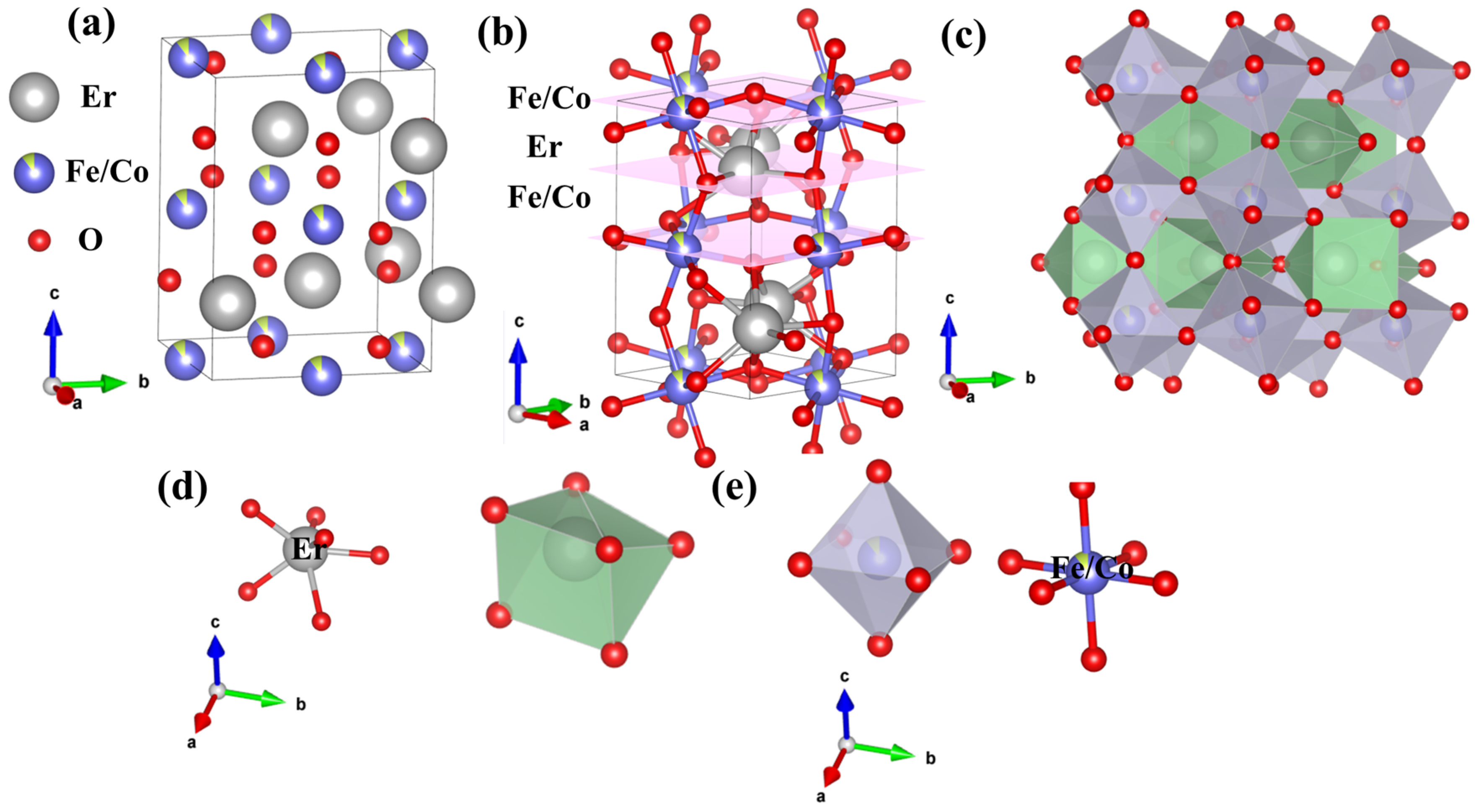
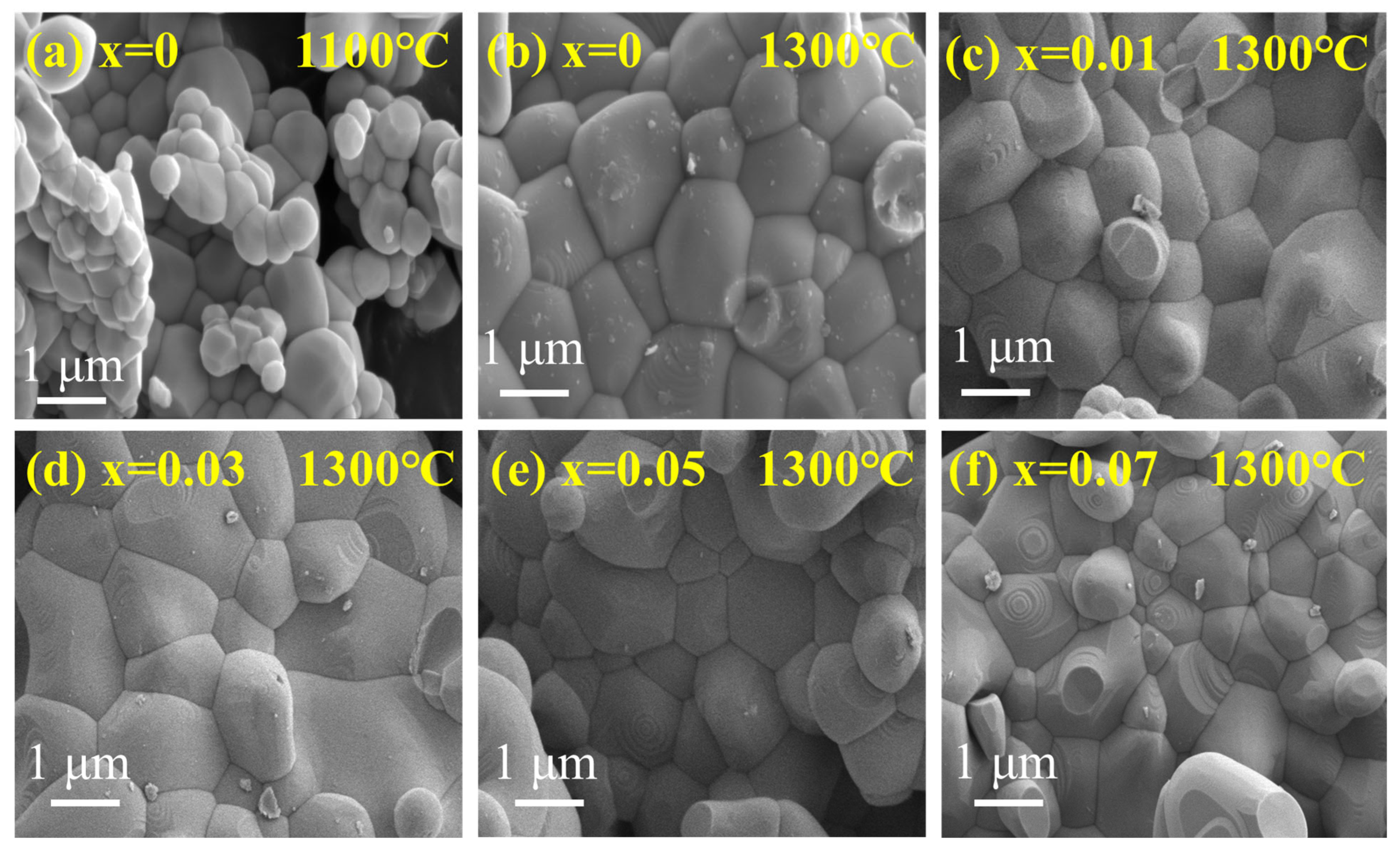
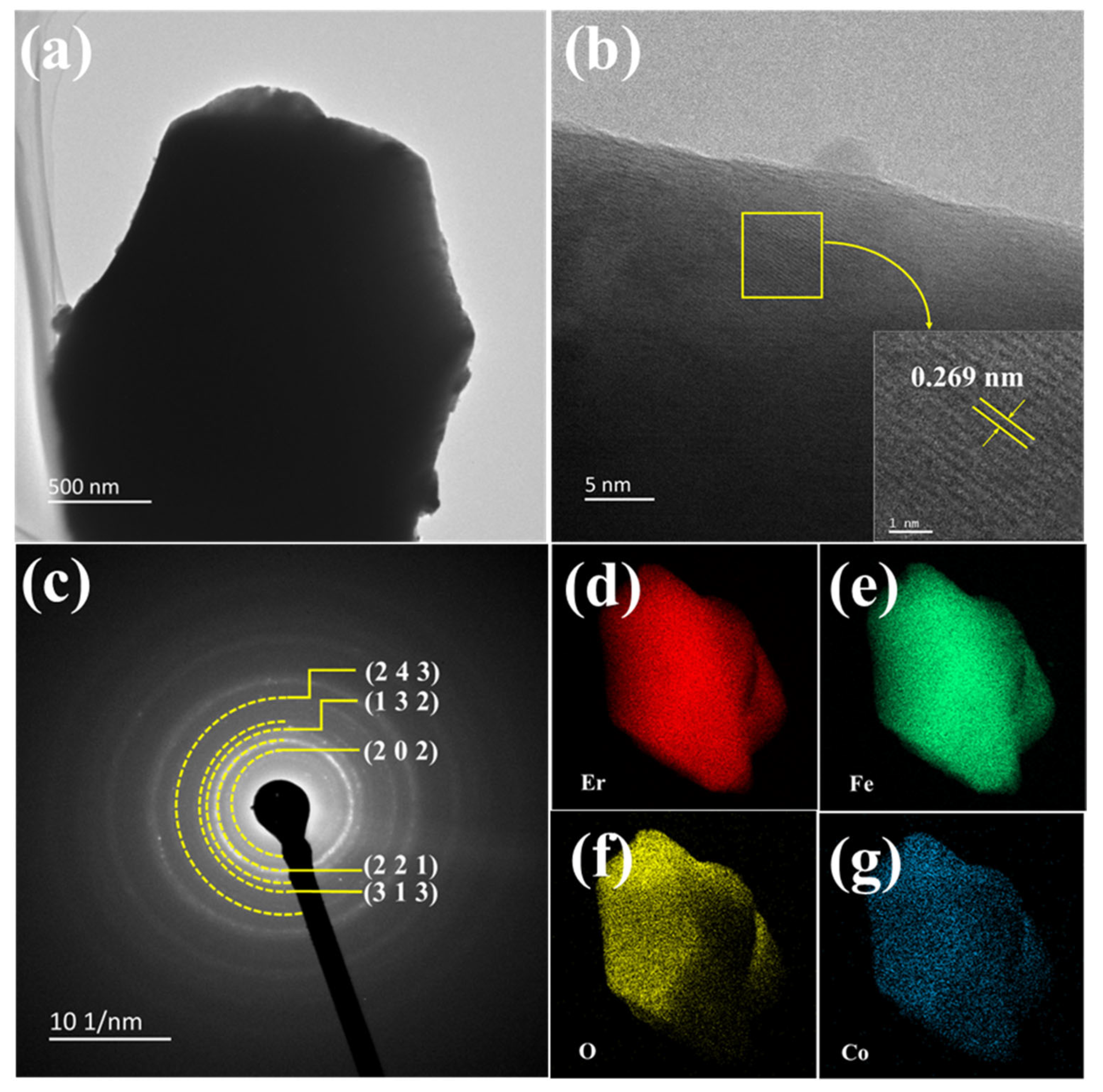
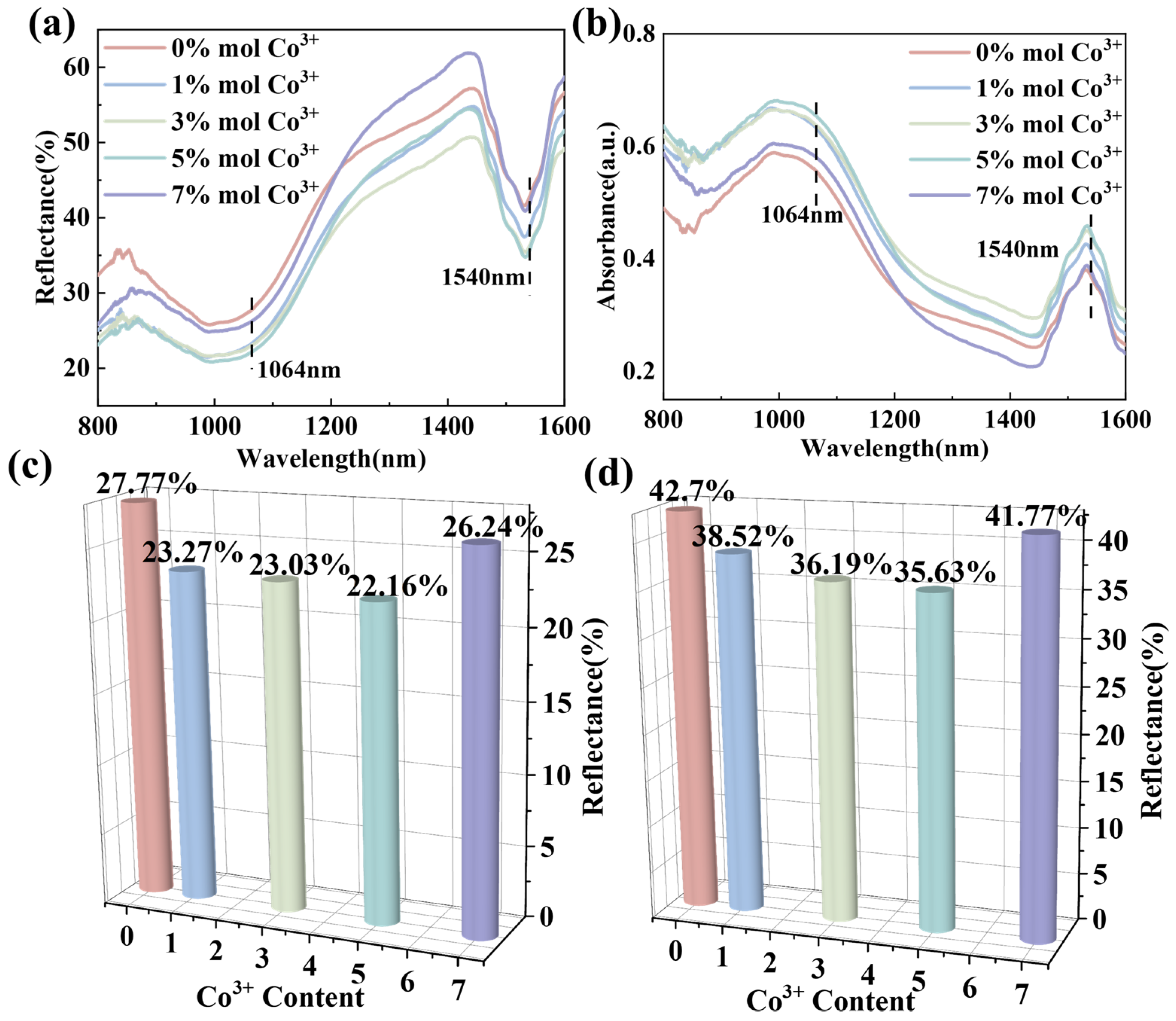
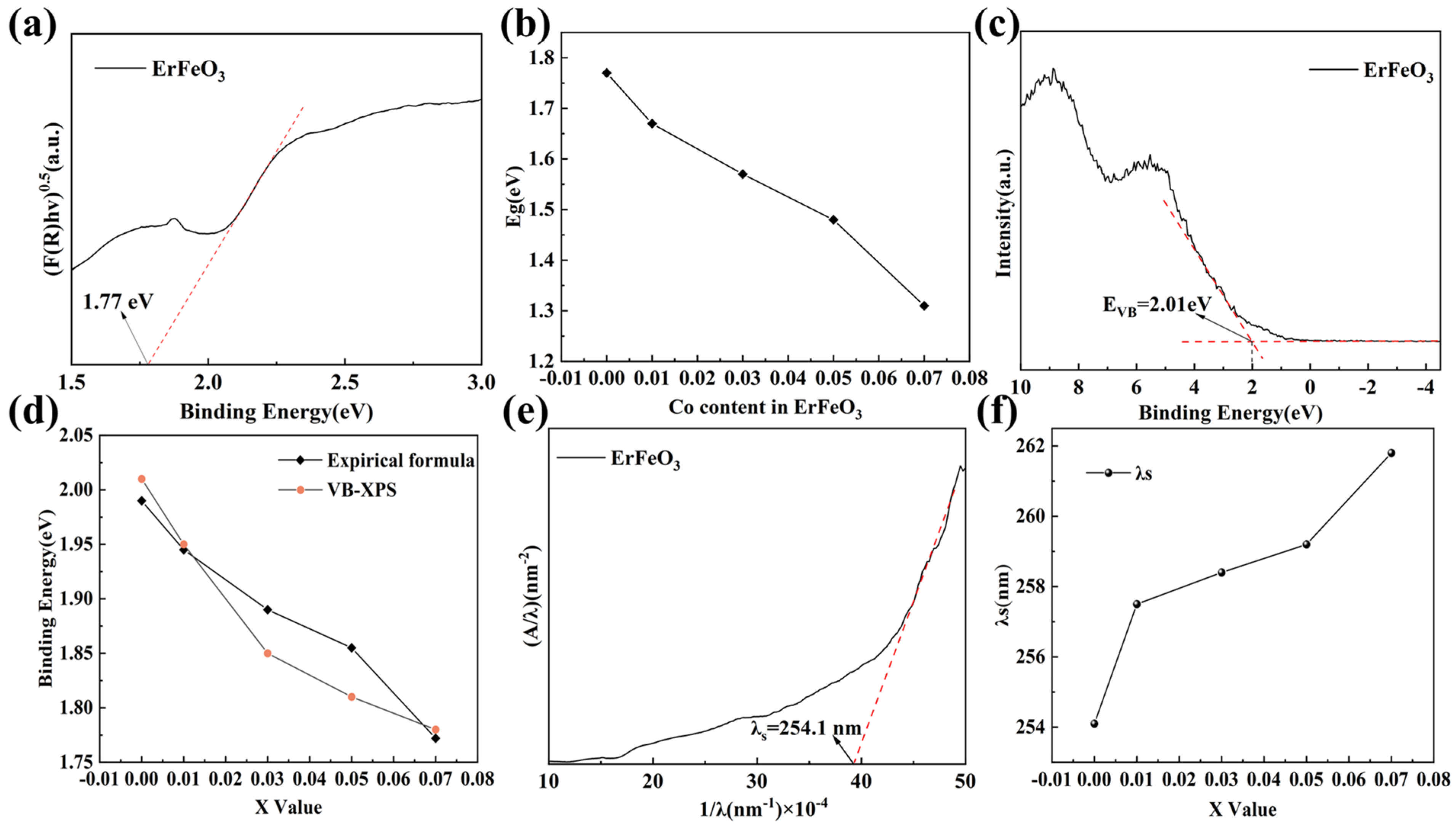
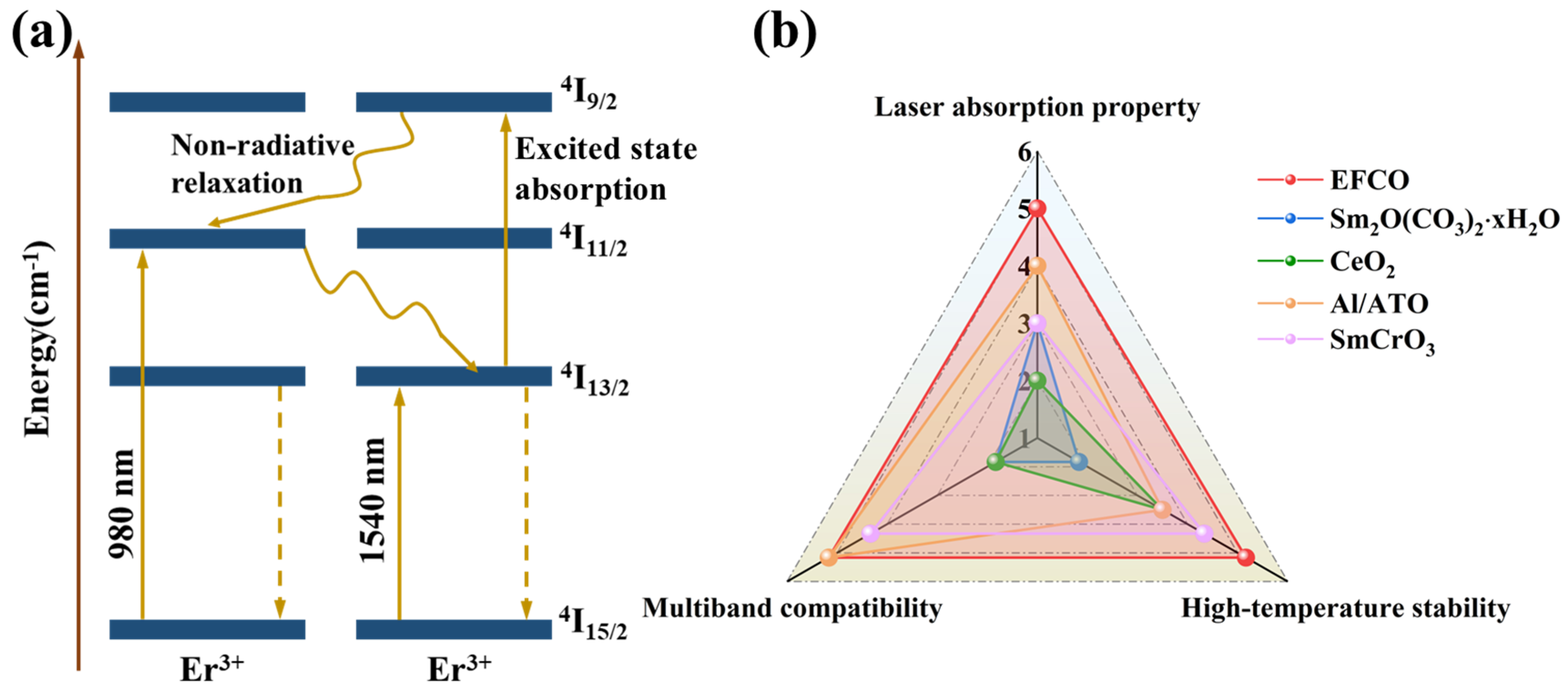
| ErFe1−xCoxO3 | X = 0.00 | X = 0.01 | X = 0.03 | X = 0.05 | X = 0.07 |
|---|---|---|---|---|---|
| a (Å) | 5.2648 | 5.2629 | 5.2629 | 5.2609 | 5.2589 |
| b (Å) | 5.5823 | 5.5810 | 5.5808 | 5.5791 | 5.5781 |
| c (Å) | 7.5966 | 7.5934 | 7.5923 | 7.5885 | 7.5847 |
| V (Å3) | 223.263 | 223.03 | 222.99 | 222.734 | 222.492 |
| Fe-O(1) (Å) | 2.0043 (10) | 2.0033 (5) | 2.0030 (5) | 2.0021 (4) | 2.0011 (3) |
| Fe-O(2) (Å) | 2.0292 (8) | 2.0284 (4) | 2.0284 (4) | 2.0277 (4) | 2.0273 (4) |
| Fe-O(1)-Fe (°) | 142.752 (3) | 142.747 (12) | 142.741 (11) | 142.737 (10) | 142.732 (8) |
| Fe-O(2)-Fe (°) | 144.231 (11) | 144.234 (5) | 144.237 (5) | 144.239 (5) | 144.243 (4) |
| d | 0.595 | 0.598 | 0.598 | 0.600 | 0.610 |
| S | 0.0585 | 0.05866 | 0.05863 | 0.0587 | 0.0589 |
| Rwp (%) | 7.218 | 5.379 | 5.968 | 4.466 | 4.157 |
| Raman Shift (cm−1) | Atomic Motions in EFCO | Mode |
|---|---|---|
| 116 | Er3+-O2− vibrations | Ag(1) |
| 141 | Er3+-O2− vibrations | Ag(2) |
| 163 | Er3+-O2− vibrations | B2g(2) |
| 263 | Co-rotations of FeO6 octahedra | B1g(2) |
| 332 | Rotation of FeO6 octahedra around the y-axis | Ag(4) |
| 429 | Rotation of FeO6 octahedra around the z-axis | Ag(5) |
| 501 | Scissor-like bending of O(1)-Fe-O(2) [51] | Ag(7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Pan, L.; Meng, F.; Feng, X.; Zhang, Q.; Hou, Y.; Wang, L. Co-Doped ErFeO3 for Dual-Band Laser Absorption with High-Temperature Stability. Materials 2025, 18, 1861. https://doi.org/10.3390/ma18081861
Liu R, Pan L, Meng F, Feng X, Zhang Q, Hou Y, Wang L. Co-Doped ErFeO3 for Dual-Band Laser Absorption with High-Temperature Stability. Materials. 2025; 18(8):1861. https://doi.org/10.3390/ma18081861
Chicago/Turabian StyleLiu, Rui, Linghao Pan, Fanqi Meng, Xia Feng, Qitu Zhang, Yi Hou, and Lixi Wang. 2025. "Co-Doped ErFeO3 for Dual-Band Laser Absorption with High-Temperature Stability" Materials 18, no. 8: 1861. https://doi.org/10.3390/ma18081861
APA StyleLiu, R., Pan, L., Meng, F., Feng, X., Zhang, Q., Hou, Y., & Wang, L. (2025). Co-Doped ErFeO3 for Dual-Band Laser Absorption with High-Temperature Stability. Materials, 18(8), 1861. https://doi.org/10.3390/ma18081861







