Corrosion-Resistant and Conductive Coatings on 316L Stainless Steel Bipolar Plates Fabricated by Hot Rolling
Abstract
1. Introduction
2. Materials and Methods
2.1. Pre-Treatment of 316LSS
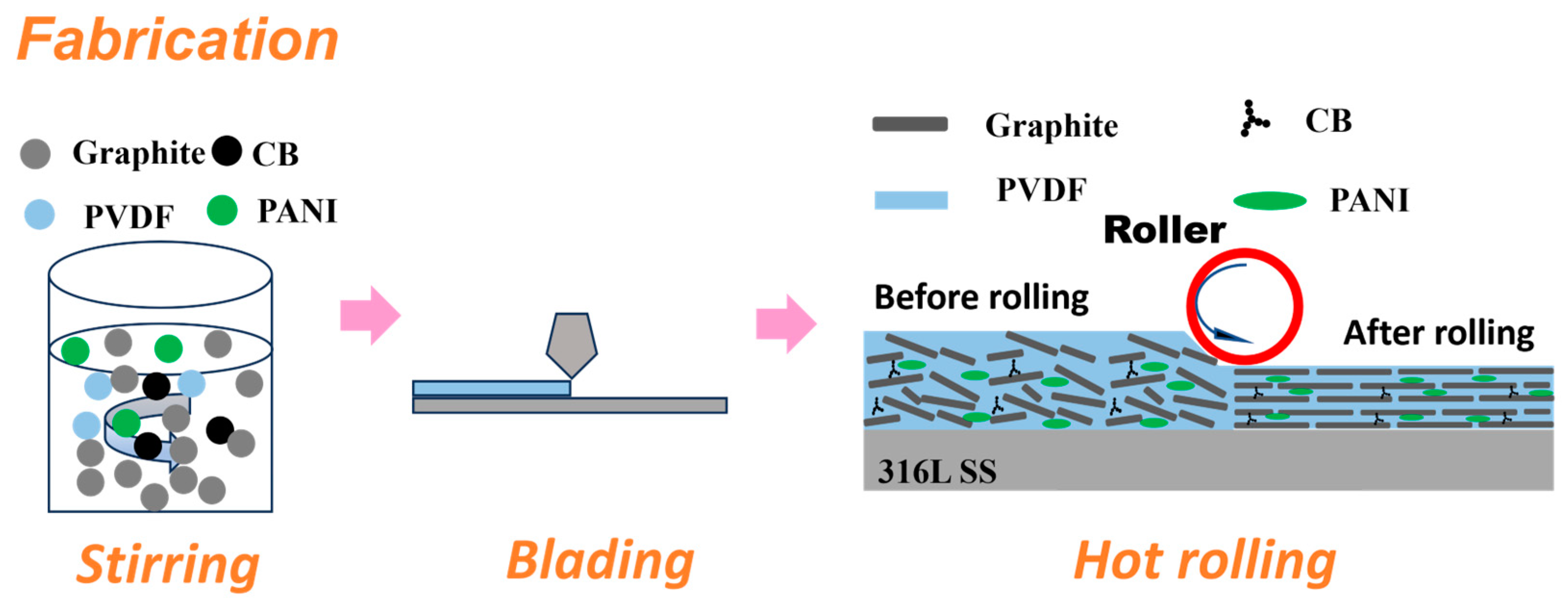
2.2. Coating Preparation
2.3. Analyses
2.3.1. Microstructure Characterization
2.3.2. Electrochemical Testing
2.3.3. Surface Hydrophobicity Assessment
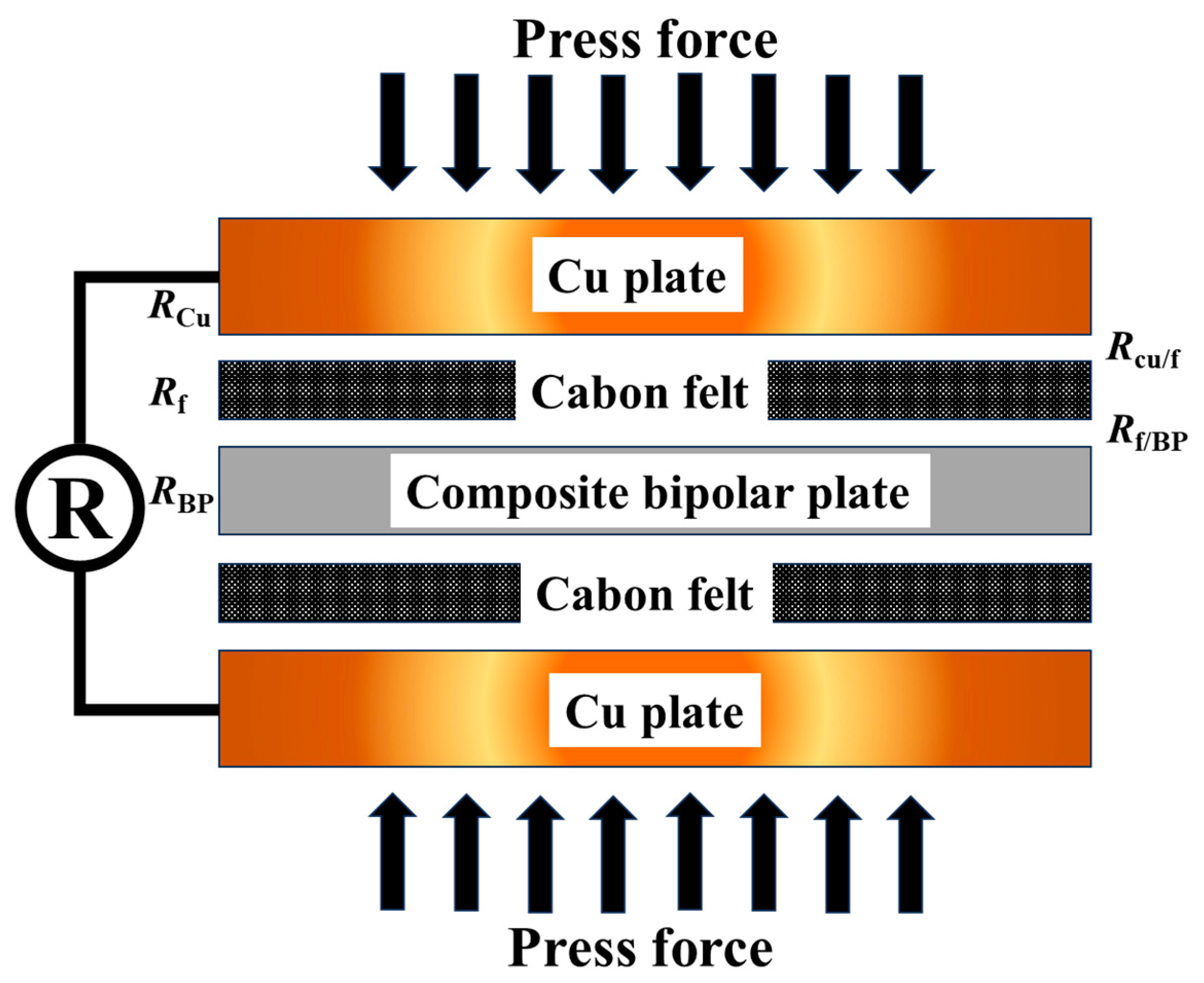
2.3.4. Interfacial Contact Resistance (ICR) Test
3. Results
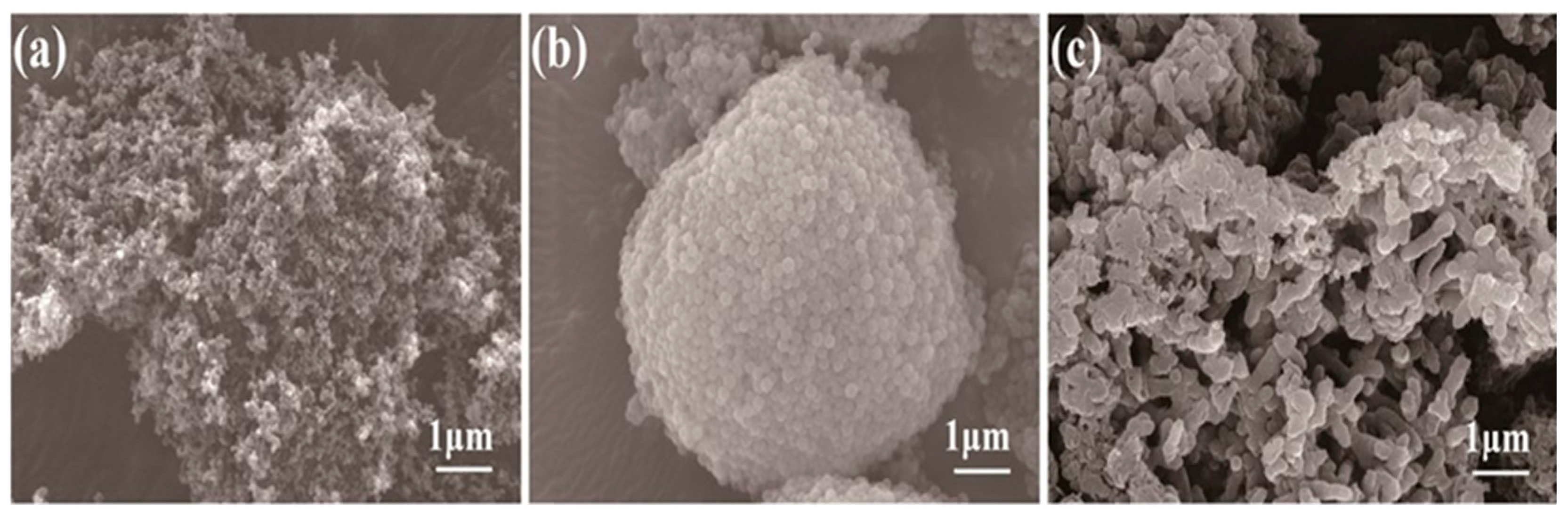
3.1. Coating Characterization
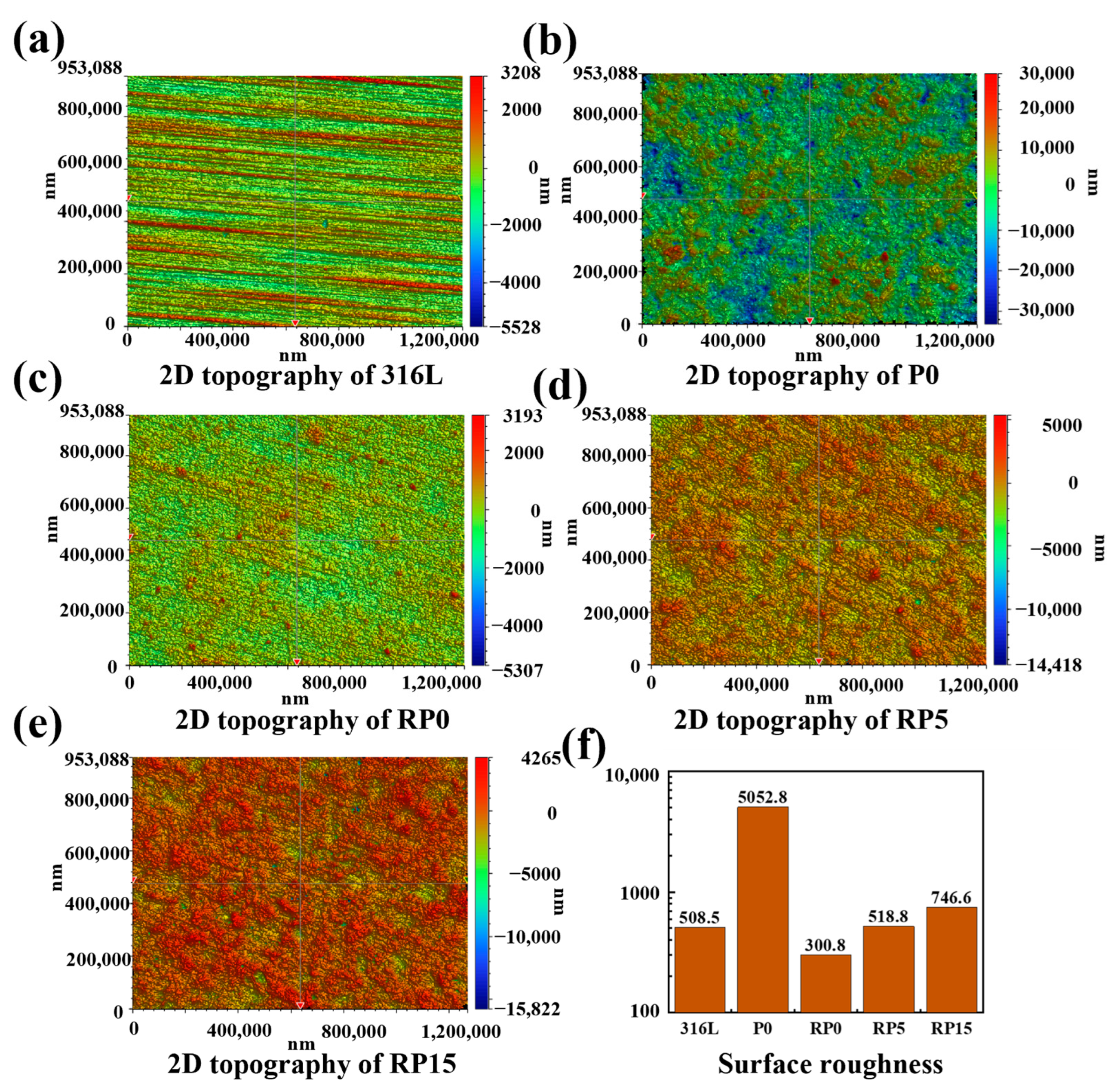
3.2. Roughness
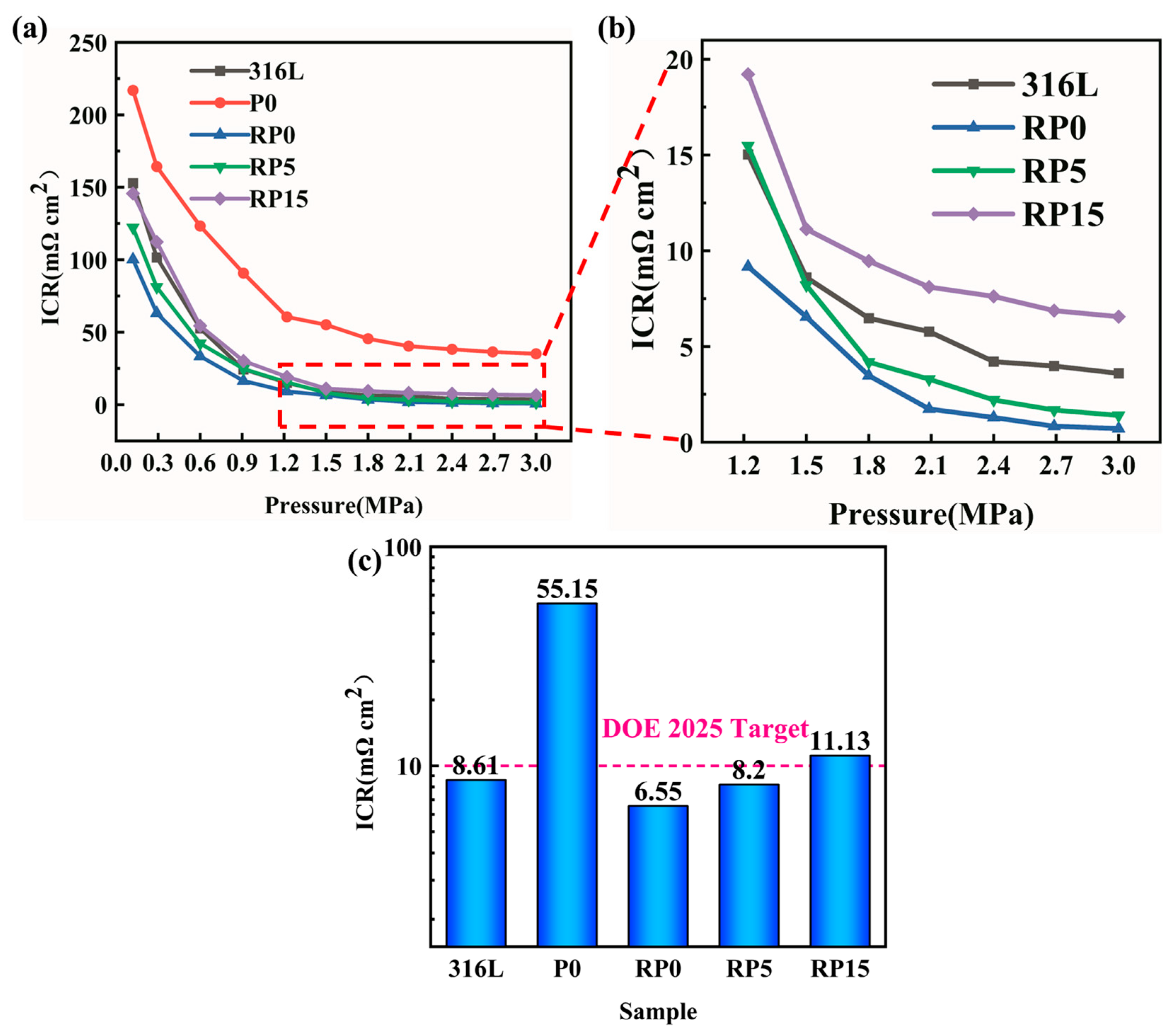
3.3. Interfacial Contact Resistance (ICR)
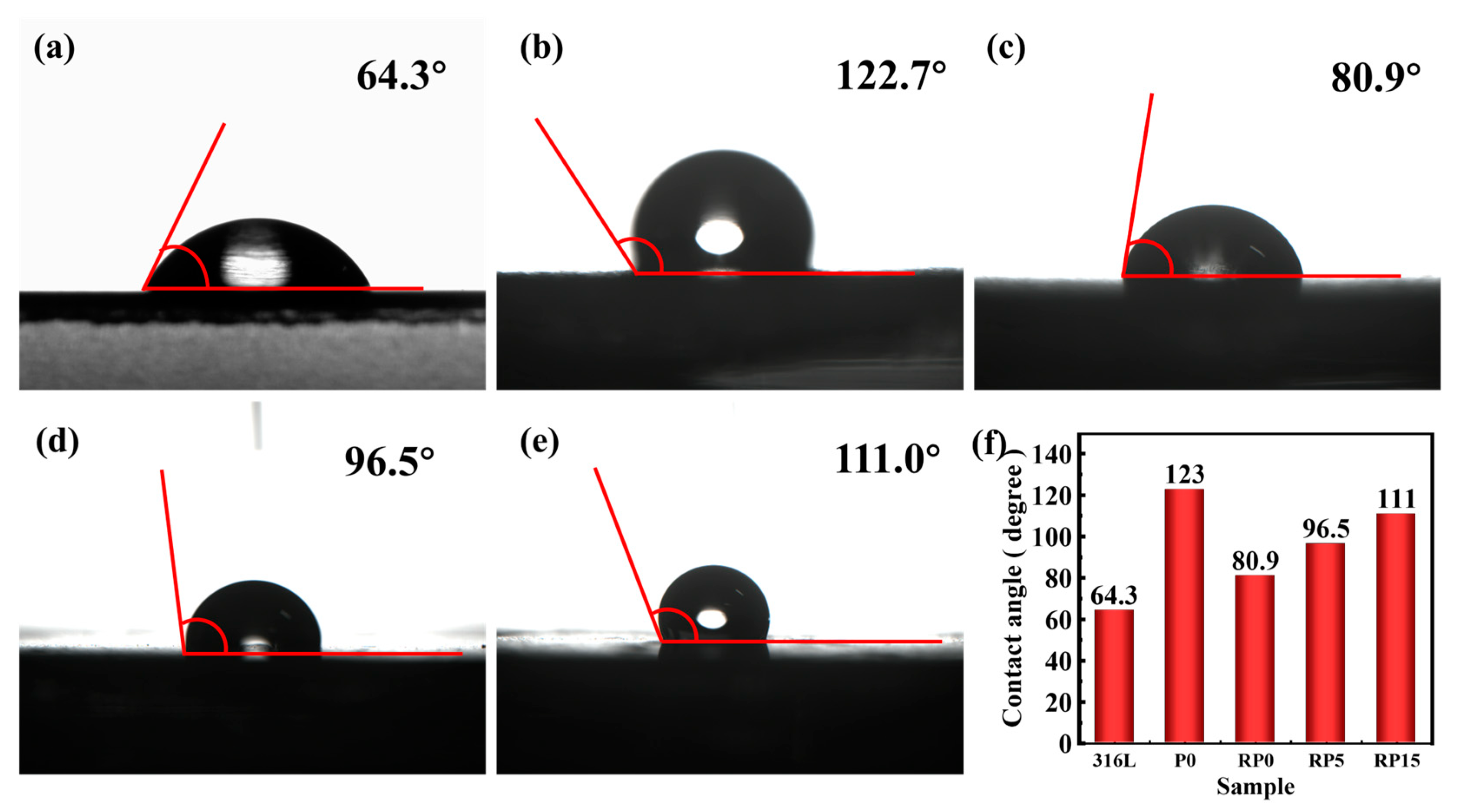
3.4. Contact Angle
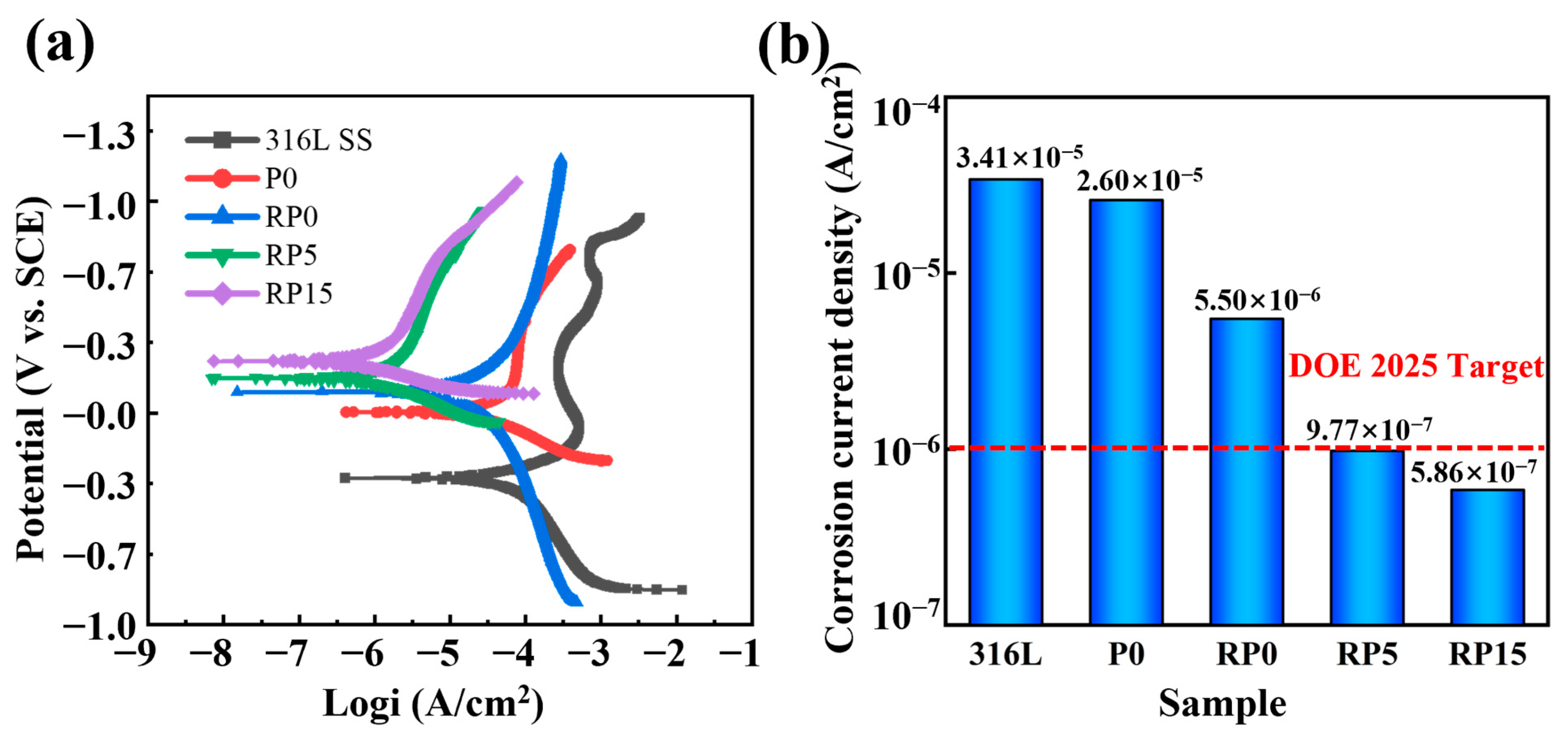
3.5. Electrochemical Measurement
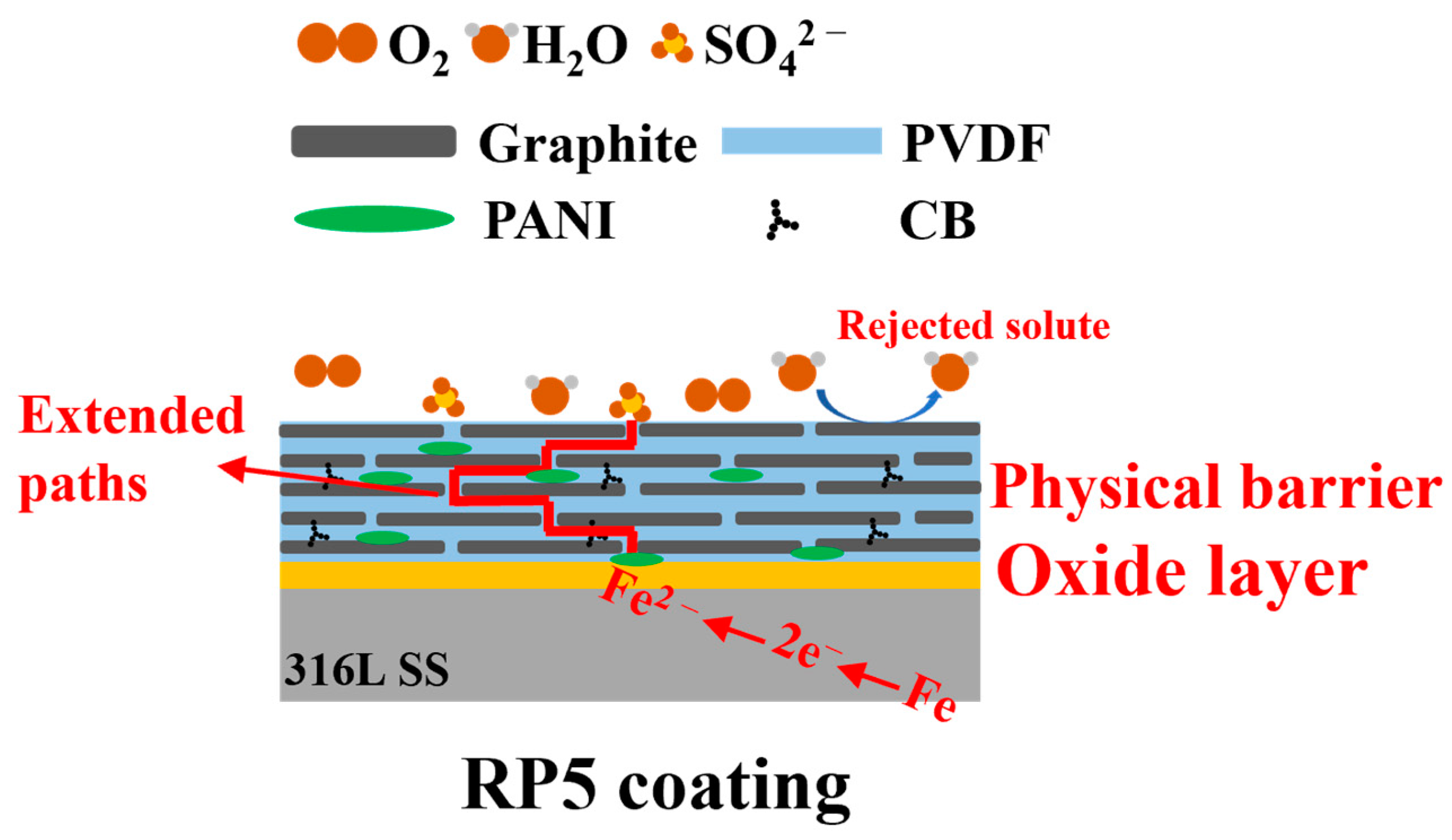
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antunes, R.A.; de Oliveira, M.C.L.; Ett, G.; Ett, V. Carbon materials in composite bipolar plates for polymer electrolyte membrane fuel cells: A review of the main challenges to improve electrical performance. J. Power Sources 2011, 196, 2945–2961. [Google Scholar] [CrossRef]
- Gao, X.; Chen, J.; Xu, R.; Zhen, Z.; Zeng, X.; Chen, X.; Cui, L. Research progress and prospect of the materials of bipolar plates for (PEMFCs). Int. J. Hydrogen Energy 2024, 50, 711–743. [Google Scholar] [CrossRef]
- Xiong, K.; Wu, W.; Wang, S.; Zhang, L. Modeling, design, materials and fabrication of bipolar plates for proton exchange membrane fuel cell: A review. Appl. Energy 2021, 301, 117443. [Google Scholar] [CrossRef]
- Tang, A.; Crisci, L.; Bonville, L.; Jankovic, J. An overview of bipolar plates in proton exchange membrane fuel cells. J. Renew. Sustain. Energy 2021, 13, 022701. [Google Scholar] [CrossRef]
- Bohackova, T.; Ludvik, J.; Kouril, M. Metallic Material Selection and Prospective Surface Treatments for Proton Exchange Membrane Fuel Cell Bipolar plates—A Review. Materials 2021, 14, 2682. [Google Scholar] [CrossRef]
- Li, W.; Xie, Z.; Zeng, H. Current status of research on composite bipolar plates for proton exchange membrane fuel cells (PEMFCs): Nanofillers and structure optimization. RSC Adv. 2024, 14, 7172–7194. [Google Scholar] [CrossRef]
- Chen, J.; Qin, N.; Jin, L.; Pan, X.; Zheng, J.; Ming, P.; Zhang, C.; Zheng, J.P. Tailoring flexible-segment-rich resin network structure by multi-step copolymerization for improved composite bipolar plates of proton exchange membrane fuel cell. Compos. Sci. Technol. 2024, 247, 110422. [Google Scholar] [CrossRef]
- Huang, Q.; Tong, Y.; Hu, B.; Huang, J.; Cao, X.; Yang, Z.; He, G. High-performance polybenzoxazine based composites PEMFC bipolar plates with a multi-layer structure for surface enrichment of conductive phase. Int. J. Hydrogen Energy 2023, 48, 32540–32552. [Google Scholar] [CrossRef]
- Kuan, Y.-D.; Ciou, C.-W.; Shen, M.-Y.; Wang, C.-K.; Fitriani, R.Z.; Lee, C.-Y. Bipolar plates design and fabrication using graphite reinforced composite laminate for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2021, 46, 16801–16814. [Google Scholar] [CrossRef]
- Guo, S.; Tian, C.; Pan, H.; Tang, X.; Han, L.; Wang, J. Research on the Springback Behavior of 316LN Stainless Steel in Micro-Scale Bending Processes. Materials 2022, 15, 6373. [Google Scholar] [CrossRef]
- Modanloo, V.; Talebi-Ghadikolaee, H.; Alimirzaloo, V.; Elyasi, M. Fracture prediction in the stamping of titanium bipolar plates for PEM fuel cells. Int. J. Hydrogen Energy 2021, 46, 5729–5739. [Google Scholar] [CrossRef]
- Alam, M.A.; Jahan, A.; Suzuki, E.; Yashiro, H. Corrosion behavior and surface structure analysis of pure aluminum immersed in fluoride-sulfate solutions simulating polymer electrolyte membrane fuel cell-produced water. Fuel Cells 2024, 24, 67–77. [Google Scholar] [CrossRef]
- Stein, T.; Ein-Eli, Y. Challenges and Perspectives of Metal-Based Proton Exchange Membrane’s Bipolar plates: Exploring Durability and Longevity. Energy Technol. 2020, 8, 2000007. [Google Scholar] [CrossRef]
- Novalin, T.; Eriksson, B.; Proch, S.; Bexell, U.; Moffatt, C.; Westlinder, J.; Lagergren, C.; Lindbergh, G.; Lindstrom, R.W. Demonstrating the absence of metal ion contamination in operando PEM fuel cells utilizing unmodified stainless steel bipolar plates. Appl. Energy 2023, 349, 121669. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Lee, I.-S.; Choi, J.-Y.; Jun, S.; Kim, D.; Cha, B.-C.; Kim, D.-W. Corrosion Behavior of Niobium-Coated 316L Stainless Steels as Metal Bipolar plates for Polymer Electrolyte Membrane Fuel Cells. Materials 2021, 14, 4972. [Google Scholar] [CrossRef]
- Feng, K.; Shen, Y.; Sun, H.; Liu, D.; An, Q.; Cai, X.; Chu, P.K. Conductive amorphous carbon-coated 316L stainless steel as bipolar plates in polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2009, 34, 6771–6777. [Google Scholar] [CrossRef]
- Kumar, A.; Ricketts, M.; Hirano, S. Ex situ evaluation of nanometer range gold coating on stainless steel substrate for automotive polymer electrolyte membrane fuel cell bipolar plates. J. Power Sources 2010, 195, 1401–1407. [Google Scholar] [CrossRef]
- Bi, F.; Hou, K.; Yi, P.; Peng, L.; Lai, X. Mechanisms of growth, properties and degradation of amorphous carbon films by closed field unbalanced magnetron sputtering on stainless steel bipolar plates for PEMFCs. Appl. Surf. Sci. 2017, 422, 921–931. [Google Scholar] [CrossRef]
- Haghighat Ghahfarokhi, H.; Saatchi, A.; Monirvaghefi, S. Corrosion investigation of chromium nitride and chromium carbide coatings for PEM fuel cell bipolar plates in simulated cathode condition. Fuel Cells 2016, 16, 356–364. [Google Scholar] [CrossRef]
- Wang, L.; Tao, Y.; Zhang, Z.; Wang, Y.; Feng, Q.; Wang, H.; Li, H. Molybdenum carbide coated 316L stainless steel for bipolar plates of proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2019, 44, 4940–4950. [Google Scholar] [CrossRef]
- Wang, Y.; Shao, W.; Wu, S.; Yang, W.; Chen, J. Research Progress on Surface Modification of Bipolar plates for Proton Exchange Membrane Fuel Cells. Rare Met. Mater. Eng. 2024, 53, 902–932. [Google Scholar] [CrossRef]
- Show, Y.; Nakashima, T.; Fukami, Y. Anticorrosion coating of carbon nanotube/polytetrafluoroethylene composite film on the stainless steel bipolar plates for proton exchange membrane fuel cells. J. Nanomater. 2013, 2013, 378752. [Google Scholar] [CrossRef]
- Sharma, S.; Zhang, K.; Gupta, G.; Santamaria, D.G. Exploring PANI-TiN nanoparticle coatings in a PEFC environment: Enhancing corrosion resistance and conductivity of stainless steel bipolar plates. Energies 2017, 10, 1152. [Google Scholar] [CrossRef]
- Husby, H.; Kongstein, O.E.; Oedegaard, A.; Seland, F. Carbon-polymer composite coatings for PEM fuel cell bipolar plates. Int. J. Hydrogen Energy 2014, 39, 951–957. [Google Scholar] [CrossRef]
- Liu, S.; Liu, L.; Meng, F.; Li, Y.; Wang, F. Protective Performance of Polyaniline-Sulfosalicylic Acid/Epoxy Coating for 5083 Aluminum. Materials 2018, 11, 292. [Google Scholar] [CrossRef]
- Avasarala, B.; Haldar, P. Effect of surface roughness of composite bipolar plates on the contact resistance of a proton exchange membrane fuel cell. J. Power Sources 2009, 188, 225–229. [Google Scholar] [CrossRef]
- Lin, C.-H. Surface roughness effect on the metallic bipolar plates of a proton exchange membrane fuel cell. Appl. Energy 2013, 104, 898–904. [Google Scholar] [CrossRef]
- Xu, R.; Jin, X.; Bi, H.; Zhang, Z.; Li, M. Effect of surface roughness on contact resistance and electrochemical corrosion behavior of 446 stainless steel in simulated anode environments for proton exchange membrane fuel cell. J. Solid State Electrochem. 2024, 28, 3087–3098. [Google Scholar] [CrossRef]
- Hamzah, M.; Qadariyah, L.; Purniawan, A.; Hidayat, A.S.; Chitraningrum, N.; Taufany, F. Use of Ni/CNT Particles as Additive Fillers in Ebonite Bipolar plates for Proton-Exchange Membrane Fuel Cells. ACS Omega 2024, 9, 43513–43522. [Google Scholar] [CrossRef]
- Lv, B.; Shao, Z.; He, L.; Gou, Y.; Sun, S. A novel graphite/phenolic resin bipolar plates modified by doping carbon fibers for the application of proton exchange membrane fuel cells. Prog. Nat. Sci.-Mater. Int. 2020, 30, 876–881. [Google Scholar] [CrossRef]
- Mao, X.; Li, Y.; Li, Y.; Zhu, D.; Yu, W.; Ji, Y.; Wang, D.; Hu, X. Simultaneous electrical and mechanical properties improvement for composite expanded graphite bipolar plates by incorporating surface-activated carbon black paving on the carbonized melamine foam skeleton. Compos. Sci. Technol. 2024, 257, 110822. [Google Scholar] [CrossRef]
- Kahveci, E.E.; Taymaz, I. Experimental study on performance evaluation of PEM fuel cell by coating bipolar plates with materials having different contact angle. Fuel 2019, 253, 1274–1281. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, H.J.; Kim, S.G.; Ahn, S.H. Evaluation of graphite composite bipolar plates for PEM (proton exchange membrane) fuel cell: Electrical, mechanical, and molding properties. J. Mater. Process. Technol. 2007, 187, 425–428. [Google Scholar] [CrossRef]
- Sadeghian, Z.; Hadidi, M.R.; Salehzadeh, D.; Nemati, A. Hydrophobic octadecylamine-functionalized graphene/TiO2 hybrid coating for corrosion protection of copper bipolar plates in simulated proton exchange membrane fuel cell environment. Int. J. Hydrogen Energy 2020, 45, 15380–15389. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Yu, S.; Liu, Y.; Shi, B.; Hu, E.; Hei, H. Investigation of hydrophobic and anti-corrosive behavior of Cr-DLC film on stainless steel bipolar plates. Diam. Relat. Mater. 2022, 129, 109352. [Google Scholar] [CrossRef]
- Yang, G.; Liu, F.; Hou, N.; Peng, S.; He, C.; Fang, P. Preparation of One-Dimensional Polyaniline Nanotubes as Anticorrosion Coatings. Materials 2022, 15, 3192. [Google Scholar] [CrossRef]



| Sample | Graphite (wt%) | PVDF (wt%) | CB (wt%) | PANI (wt%) | Method |
|---|---|---|---|---|---|
| P0 | 65 | 30 | 5 | 0 | blade |
| RP0 | 65 | 30 | 5 | 0 | blade + hot rolling |
| RP5 | 65 | 30 | 5 | 5 | blade + hot rolling |
| RP15 | 65 | 30 | 5 | 15 | blade + hot rolling |
| Specimen | Ecorr (mV) | jcorr (μA cm−2) |
|---|---|---|
| 316L | −308 | 34.127 |
| P0 | 4 | 26.074 |
| RP0 | 99 | 5.498 |
| RP5 | 165 | 0.977 |
| RP15 | 245 | 0.586 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Wang, Z.; Xiao, L.; Zha, Y.; Deng, G.; Li, S.; Cai, Z.; Liu, S. Corrosion-Resistant and Conductive Coatings on 316L Stainless Steel Bipolar Plates Fabricated by Hot Rolling. Materials 2025, 18, 1831. https://doi.org/10.3390/ma18081831
Zhao X, Wang Z, Xiao L, Zha Y, Deng G, Li S, Cai Z, Liu S. Corrosion-Resistant and Conductive Coatings on 316L Stainless Steel Bipolar Plates Fabricated by Hot Rolling. Materials. 2025; 18(8):1831. https://doi.org/10.3390/ma18081831
Chicago/Turabian StyleZhao, Xiaojun, Zihao Wang, Lairong Xiao, Yitao Zha, Guanzhi Deng, Shaohao Li, Zhenyang Cai, and Sainan Liu. 2025. "Corrosion-Resistant and Conductive Coatings on 316L Stainless Steel Bipolar Plates Fabricated by Hot Rolling" Materials 18, no. 8: 1831. https://doi.org/10.3390/ma18081831
APA StyleZhao, X., Wang, Z., Xiao, L., Zha, Y., Deng, G., Li, S., Cai, Z., & Liu, S. (2025). Corrosion-Resistant and Conductive Coatings on 316L Stainless Steel Bipolar Plates Fabricated by Hot Rolling. Materials, 18(8), 1831. https://doi.org/10.3390/ma18081831









