Effect of CdO on the Structural and Spectroscopic Properties of Germanium–Tellurite Glass
Abstract
1. Introduction
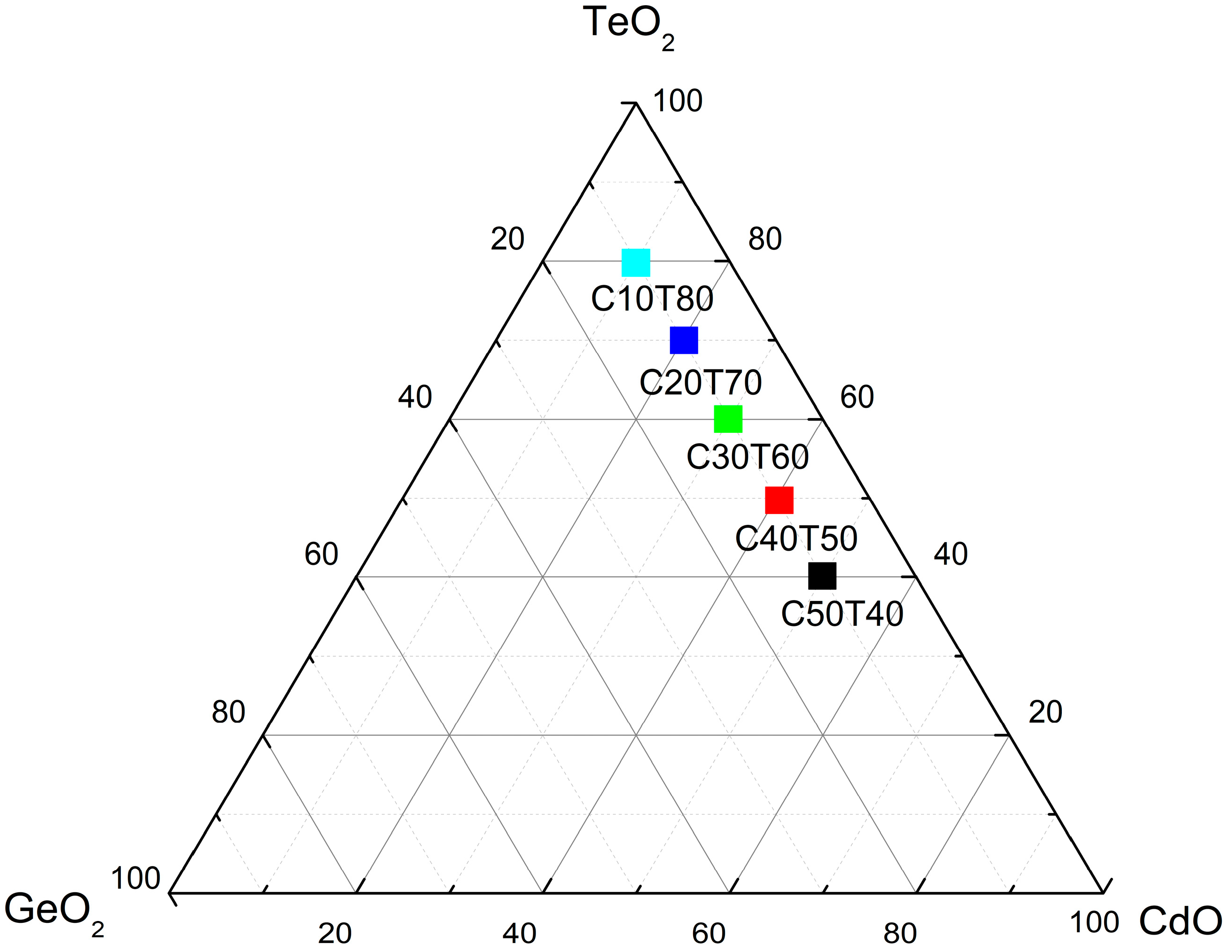
2. Materials and Methods
3. Results and Discussion
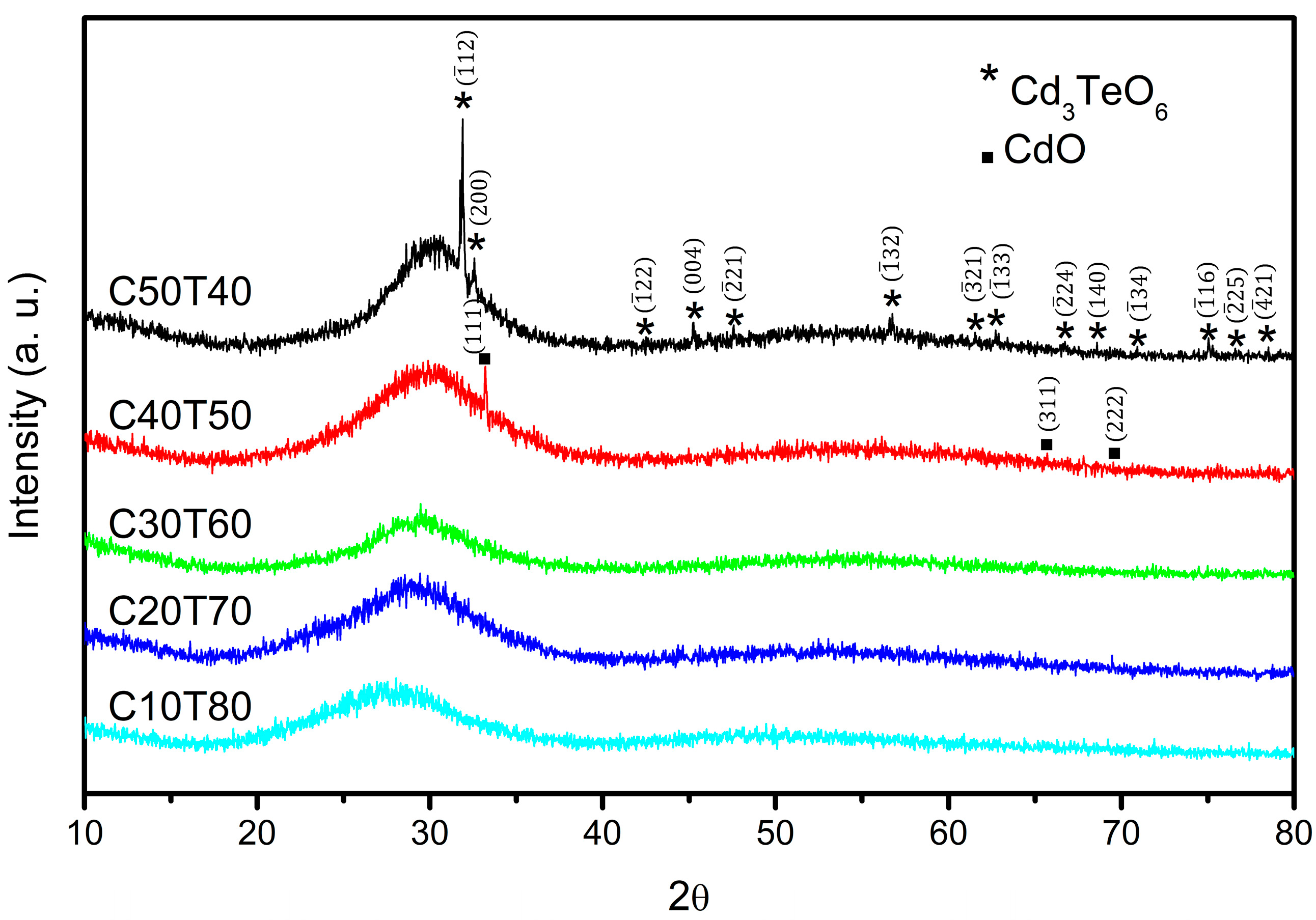
3.1. X-Ray Diffraction Analysis
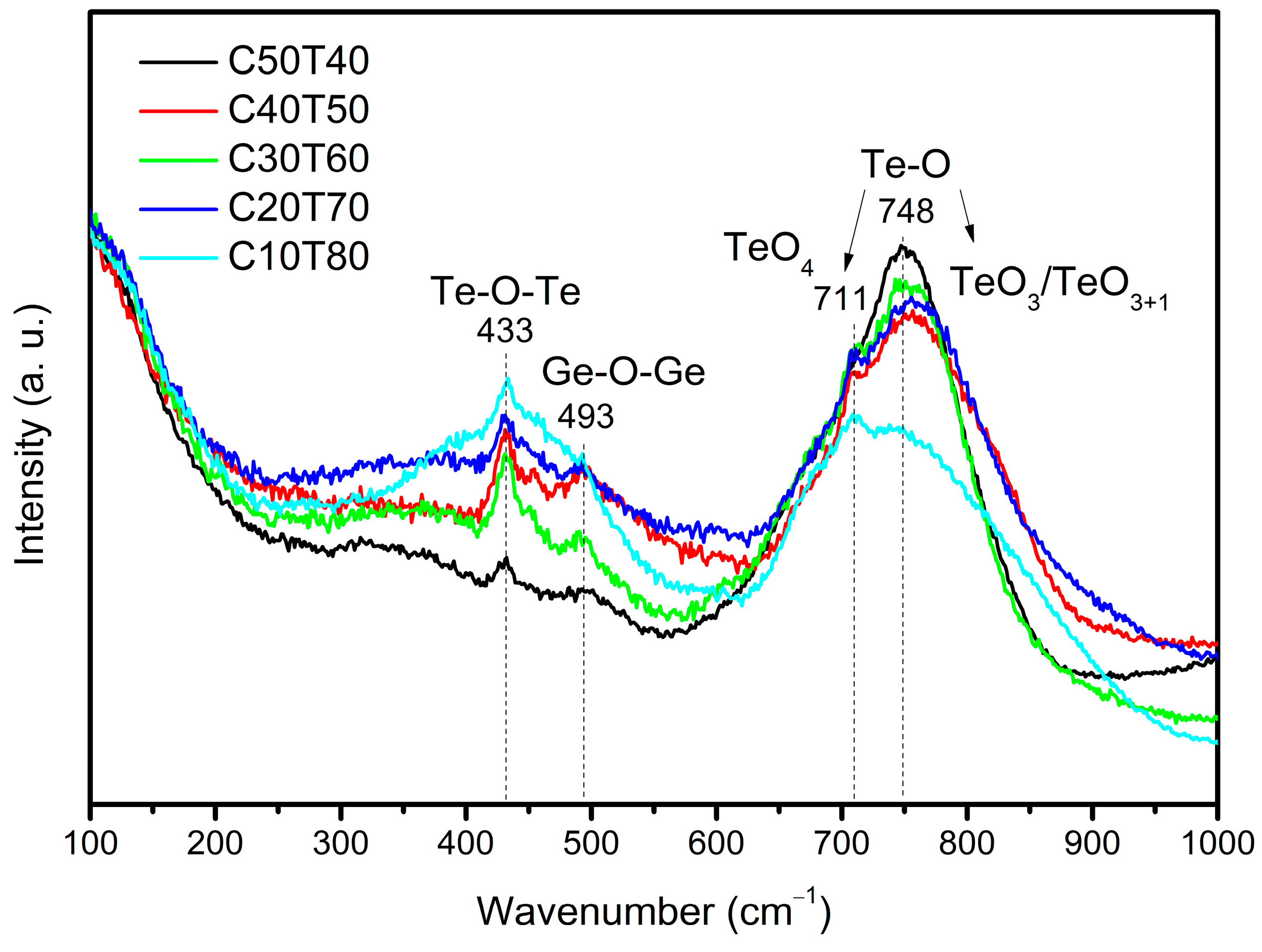
3.2. Raman Spectroscopy
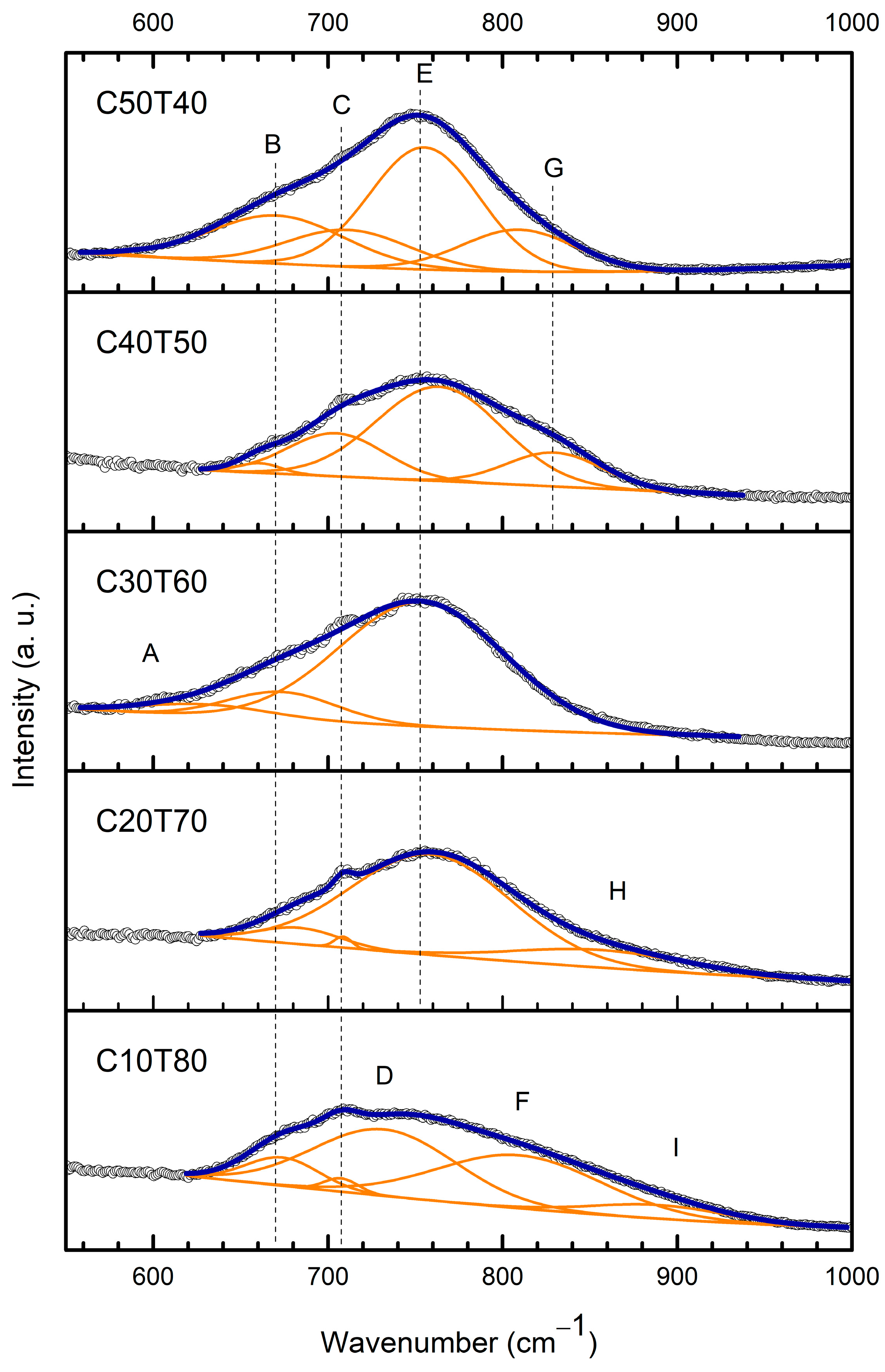
3.3. Raman Deconvolution
| Band Label | Location (cm−1) | Band Assignment | References |
|---|---|---|---|
| A | 621–630 | Stretching modes of O-Te-O in TeO4 tbp units, and/or ring strain from Ge-O-Ge bending vibrations. | [35,36] |
| B | 661–683 | Stretching modes of O-Te-O in TeO4 tbp units, antisymmetric vibrations of Te-O bonds in TeO4, TeO3+1 and TeO3 units. | [32,37,38,39,40] |
| C | 705–709 | Assigned to the deformation modes of Ge atoms in the glassy network. | [37,41] |
| D | 732 | Stretching vibration between tellurium and non-bridging oxygen (NBO) atoms. | [37,41] |
| E | 754–764 | Asymmetric vibrations of Te-O-Te symmetric bridges in γ-TeO2, stretching vibrations from groups TeO3+1 and [TeO3]2− with three terminal oxygens. | [5,35,42,43,46,54] |
| The peaks around 713 and 747 are assigned to stretching vibrations of TeO3/TeO3+1 unit. | [42] | ||
| F | 810 | Characteristic band of γ-TeO2, short Te-O- bonds with NBOs form TeO4 units; LO split of the antisymmetric stretching vibrations into Q2 units. | [32,35,36,50] |
| G | 830 | Could arise from that in Ge Q3-species. | [41] |
| H | 856 | Assigned to the stretching vibrations of NBO in Q3 species (GeO4 with three bridging oxygen atoms). | [41,51] |
| I | 889 | Stretching vibrations from Te=O in TeO3 isolated units, TO split of the antisymmetric stretching vibrations of Ge-O-Ge in Q3 units. | [35] |
3.4. XPS Analysis
3.5. Optical Absorption
3.6. Refractive Index
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| XPS | X-ray photoelectron spectroscopy |
| IR | Infrared |
| NIR | Near Infrared |
| UV | Ultraviolet |
| LEDs | Light-Emitting Diodes |
| XRD | X-ray diffraction |
| OA | Optical absorption |
| NBOs | Non-bridging oxygen atoms |
| FWHM | Full Width at Half Maximum |
| BOs | Bridging oxygen atoms |
References
- Wang, Z.; Zhang, B.; Liu, J.; Song, Y.; Zhang, H. Recent Developments in Mid-Infrared Fiber Lasers: Status and Challenges. Opt. Laser Technol. 2020, 132, 106497. [Google Scholar] [CrossRef]
- Jlassi, I.; Elhouichet, H.; Hraiech, S.; Ferid, M. Effect of Heat Treatment on the Structural and Optical Properties of Tellurite Glasses Doped Erbium. J. Lumin. 2012, 132, 832–840. [Google Scholar] [CrossRef]
- Kamalaker, V.; Upender, G.; Ramesh, C.; Chandra Mouli, V. Raman Spectroscopy, Thermal and Optical Properties of TeO2–ZnO–Nb2O5–Nd2O3 Glasses. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 89, 149–154. [Google Scholar] [CrossRef]
- Himei, Y.; Miura, Y.; Nanba, T.; Osaka, A. X-Ray Photoelectron Spectroscopy of Alkali Tellurite Glasses. J. Non-Cryst. Solids 1997, 211, 64–71. [Google Scholar] [CrossRef]
- Upender, G.; Vardhani, C.P.; Suresh, S.; Awasthi, A.M.; Mouli, V.C. Structure, Physical and Thermal Properties of WO3–GeO2–TeO2 Glasses. Mater. Chem. Phys. 2010, 121, 335–341. [Google Scholar] [CrossRef]
- Rachkovskaya, G.E.; Zakharevich, G.B. Germanate Lead-Tellurite Glasses for Optical Light Filters. Glass Ceram. 2012, 68, 385–388. [Google Scholar] [CrossRef]
- Fares, H.; Jlassi, I.; Elhouichet, H.; Férid, M. Investigations of Thermal, Structural and Optical Properties of Tellurite Glass with WO3 Adding. J. Non-Cryst. Solids 2014, 396–397, 1–7. [Google Scholar] [CrossRef]
- Kaur, A.; Khanna, A.; Pesquera, C.; González, F.; Sathe, V. Preparation and Characterization of Lead and Zinc Tellurite Glasses. J. Non-Cryst. Solids 2010, 356, 864–872. [Google Scholar] [CrossRef]
- Ghribi, N.; Dutreilh-Colas, M.; Duclére, J.R.; Gouraud, F.; Chotard, T.; Karray, R.; Kabadou, A.; Thomas, P. Structural, Mechanical and Optical Investigations in the TeO2–Rich Part of the TeO2–GeO2–ZnO Ternary Glass System. Solid. State Sci. 2015, 40, 20–30. [Google Scholar] [CrossRef]
- Munemura, H.; Mitome, K.; Misawa, M.; Maruyama, K. Network Structure of M2O–TeO2 (M=Li, Na, Li0.62Na0.38) Glasses. J. Non-Cryst. Solids 2001, 293–295, 700–704. [Google Scholar] [CrossRef]
- Akagi, R.; Handa, K.; Ohtori, N.; Hannon, A.C.; Tatsumisago, M. High-Temperature Structure of K2O-TeO2 Glasses. J. Non-Cryst. Solids 1999, 256–257, 111–118. [Google Scholar] [CrossRef]
- Pulluru, C.R.; Kalluru, R.R.; Reddy, B.R.; Konovalova, T.A.; Kispert, L.D. Persistent Spectral Hole Burning in Europium-Doped Sodium Tellurite Glass. Appl. Phys. Lett. 2005, 87, 091107. [Google Scholar] [CrossRef]
- Sreenivasulu, V.; Upender, G.; Swapna; Vamsi Priya, V.; Chandra Mouli, V.; Prasad, M. Raman, DSC, ESR and Optical Properties of Lithium Cadmium Zinc Tellurite Glasses. Phys. B Condens. Matter 2014, 454, 60–66. [Google Scholar] [CrossRef]
- Cai, M.; Zhou, B.; Tian, Y.; Zhou, J.; Xu, S.; Zhang, J. Broadband Mid-Infrared 2.8 Μm Emission in Ho3+/Yb3+-Codoped Germanate Glasses. J. Lumin. 2016, 171, 143–148. [Google Scholar] [CrossRef]
- Promphet, N.; Rattanarat, P.; Rangkupan, R.; Chailapakul, O.; Rodthongkum, N. An Electrochemical Sensor Based on Graphene/Polyaniline/Polystyrene Nanoporous Fibers Modified Electrode for Simultaneous Determination of Lead and Cadmium. Sens. Actuators B Chem. 2015, 207, 526–534. [Google Scholar] [CrossRef]
- Wang, W.S.; Zhen, L.; Shao, W.Z.; Chen, Z.L. Sodium Chloride Induced Formation of Square-Shaped Cadmium Molybdate Nanoplates. Mater. Lett. 2014, 131, 292–294. [Google Scholar] [CrossRef]
- Nisha, K.D.; Navaneethan, M.; Hayakawa, Y.; Ponnusamy, S.; Muthamizhchelvan, C. Influence of Lanthanide Ion on the Morphology and Luminescence Properties of Cadmium Sulphide Nanocrystals. J. Alloys Compd. 2011, 509, 5816–5821. [Google Scholar] [CrossRef]
- Gupta, N.; Pal, B. The Synthesis, Structure, Optical and Photocatalytic Properties of Silica-Coated Cadmium Sulfide Nanocomposites of Different Shapes. J. Colloid. Interface Sci. 2012, 368, 250–256. [Google Scholar] [CrossRef]
- Vanalakar, S.A.; Mali, S.S.; Pawar, R.C.; Tarwal, N.L.; Moholkar, A.V.; Kim, J.A.; Kwon, Y.B.; Kim, J.H.; Patil, P.S. Synthesis of Cadmium Sulfide Spongy Balls with Nanoconduits for Effective Light Harvesting. Electrochim. Acta 2011, 56, 2762–2768. [Google Scholar] [CrossRef]
- Zhiyue, H.; Jingchang, Z.; Xiuying, Y.; Weiliang, C. Synthesis and Application in Solar Cell of Poly(3-Octylthiophene)/Cadmium Sulfide Nanocomposite. Sol. Energy Mater. Sol. Cells 2011, 95, 483–490. [Google Scholar] [CrossRef]
- Alvarado Rivera, J.; Pérez Hernández, C.G.; Zayas, M.E.; Álvarez, E. Nanocrystallization of the Cd3Al2Ge3O12 Garnet in Glasses of the CdO-TeO2-GeO2 System. In Advances in Glass Science and Technology; IntechOpen: London, UK, 2018; pp. 39–59. [Google Scholar]
- Medrano-Pesqueira, C.L.; Rodríguez-Carvajal, D.A. Structural Properties of Poly-Crystals Embedded in Glassy Matrix of the Ternary System CdO-TeO2-GeO2. J. Non-Cryst. Solids 2017, 475, 15–24. [Google Scholar] [CrossRef]
- Pérez Hernández, C.G. Síntesis de Nanoestructuras de Cd3Al2Ge3O12 Por La Técnica de Fusión de Polvos En Materiales Vítreos de La Matriz CdO-TeO2-GeO2 Con Propiedades Luminiscentes. Master’s Thesis, Universidad de Sonora, Hermosillo, Mexico, 2016. [Google Scholar]
- Rodríguez-Carvajal, D.A.; Meza-Rocha, A.N.; Caldiño, U.; Lozada-Morales, R.; Álvarez, E.; Zayas, M.E. Reddish-Orange, Neutral and Warm White Emissions in Eu3+, Dy3+ and Dy3+/Eu3+ Doped CdO-GeO2-TeO2 Glasses. Solid State Sci. 2016, 61, 70–76. [Google Scholar] [CrossRef]
- Mohammad, E.; Dariush, S. Study of Optical Absorption and Optical Band Gap Determination of Thin Amorphous TeO2–V2O5–MoO3 Blown Films. Indian J. Pure Appl. Phys. 2006, 44, 468–472. [Google Scholar]
- Hussin, R.; Leong, N.S.; Alias, N.S. Structural Investigation of Crystalline Host Phosphor Cadmium Tellurite Systems. J. Fundam. Sci. 2009, 5, 17–27. [Google Scholar] [CrossRef][Green Version]
- JCPDS 76-1007; Cadmium Tellurate Cd3TeO6. Inorganic Crystal Structure Database, International Union of Crystallography: Chester, England, 2019.
- JCPDS 75-0591; Monteponite, syn CdO. Inorganic Crystal Structure Database, International Union of Crystallography: Chester, England, 2019.
- Lesniak, M.; Zeid, J.; Starzyk, B.; Kochanowicz, M.; Kuwik, M.; Zmojda, J.; Miluski, P.; Baranowska, A.; Dorosz, J.; Pisarski, W.; et al. Investigation of the TeO2GeO2 Ratio on the Spectroscopic Properties of Eu3+-Doped Oxide Glasses for Optical Fiber Application. Materials 2022, 15, 117. [Google Scholar] [CrossRef]
- Koroleva, O.N.; Korobatova, N.M.; Shtenberg, M.V.; Ivanova, T.N. Structure of Glasses of the Li2O–K2O–GeO2 System: Raman Spectroscopic Data. Geochem. Int. 2019, 57, 331–340. [Google Scholar] [CrossRef]
- Jha, A.; Shen, S.; Naftaly, M. Structural Origin of Spectral Broadening of 1.5-Μm Emission in Er3+-Doped Tellurite Glasses. Phys. Rev. B 2000, 62, 6215–6227. [Google Scholar] [CrossRef]
- Hegazy, H.H.; Almojadah, S.; Reben, M. Absorption Spectra and Raman Gain Coefficient in Near-IR Region of Er3+ Ions Doped TeO2– Nb2O5–Bi2O3–ZnO Glasses. Opt. Laser Technol. 2015, 74, 138–144. [Google Scholar] [CrossRef]
- Senthil Murugan, G.; Ohishi, Y. Raman Spectroscopic Studies of TeO2-BaO-SrO-Nb2O5 Glasses: Structure-Property Correlations. J. Appl. Phys. 2004, 96, 2437–2442. [Google Scholar] [CrossRef]
- Murugan, G.S.; Ohishi, Y. Structural and Physical Properties of a Novel TeO2-BaO-SrO-Ta2O5 Glass System for Photonic Device Applications. J. Non-Cryst. Solids 2005, 351, 364–371. [Google Scholar] [CrossRef]
- Carrillo-Torres, R.C.; Saavedra-Rodríguez, G.; Alvarado-Rivera, J.; Caldiño, U.; Sánchez-Zeferino, R.; Alvarez-Ramos, M.E. Tunable Emission and Energy Transfer in TeO2-GeO2-ZnO and TeO2-GeO2-MgCl2 Glasses Activated with Eu3+/Dy3+ for Solid State Lighting Applications. J. Lumin. 2019, 212, 116–125. [Google Scholar] [CrossRef]
- Alvarado-Rivera, J.; Rodríguez-Carvajal, D.A.; Acosta-Enríquez, M.d.C.; Manzanares-Martínez, M.B.; Álvarez, E.; Lozada-Morales, R.; Díaz, G.C.; de Leon, A.; Zayas, M.E. Effect of CeO2 on the Glass Structure of Sodium Germanate Glasses. J. Am. Ceram. Soc. 2014, 97, 3494–3500. [Google Scholar] [CrossRef]
- Yadav, A.K.; Singh, P. A Review of the Structures of Oxide Glasses by Raman Spectroscopy. RSC Adv. 2015, 5, 67583–67609. [Google Scholar] [CrossRef]
- Kumar Ramamoorthy, R.; Bhatnagar, A.K. Effect of ZnO and PbO/ZnO on Structural and Thermal Properties of Tellurite Glasses. J. Alloys Compd. 2015, 623, 49–54. [Google Scholar] [CrossRef]
- Dewan, N.; Sreenivas, K.; Gupta, V. Properties of Crystalline γ-TeO2 Thin Film. J. Cryst. Growth 2007, 305, 237–241. [Google Scholar] [CrossRef]
- Sekiya, T.; Mochida, N.; Ohtsuka, A. Raman Spectra of MO–TeO2 (M = Mg, Sr, Ba and Zn) Glasses. J. Non-Cryst. Solids 1994, 168, 106–114. [Google Scholar] [CrossRef]
- Franco, D.F.; Fernandes, R.G.; Felix, J.F.; Mastelaro, V.R.; Eckert, H.; Afonso, C.R.M.; Messaddeq, Y.; Messaddeq, S.H.; Morency, S.; Nalin, M. Fundamental Studies of Magneto-Optical Borogermanate Glasses and Derived Optical Fibers Containing Tb3+. J. Mater. Res. Technol. 2021, 11, 312–327. [Google Scholar] [CrossRef]
- Dousti, M.R.; Sahar, M.R.; Amjad, R.J.; Ghoshal, S.K.; Awang, A. Surface Enhanced Raman Scattering and Up-Conversion Emission by Silver Nanoparticles in Erbium-Zinc-Tellurite Glass. J. Lumin. 2013, 143, 368–373. [Google Scholar] [CrossRef]
- García-Amaya, I.V.; Zayas, M.E.; Alvarado-Rivera, J.; Cortez-Valadez, M.; Pérez-Tello, M.; Cayetano-Castro, N.; Martínez-Suárez, F.; Mendoza-Córdova, A. Influence of Eu2O3 on Phase Crystallization and Nanocrystals Formation in Tellurite Glasses. J. Non-Cryst. Solids 2018, 499, 49–57. [Google Scholar] [CrossRef]
- Reza Dousti, M.; Sahar, M.R.; Ghoshal, S.K.; Amjad, R.J.; Samavati, A.R. Effect of AgCl on Spectroscopic Properties of Erbium Doped Zinc Tellurite Glass. J. Mol. Struct. 2013, 1035, 6–12. [Google Scholar] [CrossRef]
- Ghribi, N.; Dutreilh-Colas, M.; Duclère, J.; Hayakawa, T.; Carreaud, J.; Karray, R.; Kabadou, A. Thermal, Optical and Structural Properties of Glasses within the TeO2–TiO2–ZnO System. J. Alloys Compd. 2015, 622, 333–340. [Google Scholar] [CrossRef]
- Sahar, M.R.; Sulhadi, K.; Rohani, M.S. The Preparation and Structural Studies in the (80–x)TeO2–20ZnO–(x) Er2O3 Glass System. J. Non-Cryst. Solids 2008, 354, 1179–1181. [Google Scholar] [CrossRef]
- Noguera, O.; Smirnov, M.; Mirgorodsky, A.P.; Merle-Méjean, T.; Thomas, P.; Champarnaud-Mesjard, J.C. Ab Initio Study of the Polymer Molecules (TeO2)n as Model Systems for the Local Structure in TeO2 Glass. Phys. Rev. B 2003, 68, 094203. [Google Scholar] [CrossRef]
- Noguera, O.; Merle-Méjean, T.; Mirgorodsky, A.P.; Smirnov, M.B.; Thomas, P.; Champarnaud-Mesjard, J.C. Vibrational and Structural Properties of Glass and Crystalline Phases of TeO2. J. Non-Cryst. Solids 2003, 330, 50–60. [Google Scholar] [CrossRef]
- Champarnaud-Mesjard, J.C.; Blanchandin, S.; Thomas, P.; Mirgorodsky, A.; Merle-Mejean, T.; Frit, B. Crystal Structure, Raman Spectrum and Lattice Dynamics of a New Metastable Form of Tellurium Dioxide: γ–TeO2. J. Phys. Chem. Solids 2000, 61, 1499–1507. [Google Scholar] [CrossRef]
- Plotnichenko, V.G.; Koltashev, V.V.; Sokolov, V.O.; Popova, N.V.; Grishin, I.A.; Churbanov, M.F. Spectroscopic Properties of New BaCl2-BaO-TeO2 Tellurite Glasses for Fibre and Integrated Optics Applications. J. Phys. D Appl. Phys. 2008, 41, 015404. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Dong, G.; Qiu, J. Embedded Nanogratings in Germanium Dioxide Glass Induced by Femtosecond Laser Direct Writing. J. Opt. Soc. Am. B 2014, 31, 860–864. [Google Scholar] [CrossRef]
- Kang, S.; Xiao, X.; Pan, Q.; Chen, D.; Qiu, J.; Dong, G. Spectroscopic Properties in Er3+-Doped Germanotellurite Glasses and Glass Ceramics for Mid-Infrared Laser Materials. Sci. Rep. 2017, 7, 43186. [Google Scholar] [CrossRef]
- Parveen, N.; Jali, V.M.; Patil, S.D. Structure and Optical Properties of TeO2-GeO2 Glasses. Int. J. Adv. Sci. Eng. Technol. 2016, 4, 80–85. [Google Scholar]
- Tagiara, N.S.; Palles, D.; Simandiras, E.D.; Psycharis, V.; Kyritsis, A.; Kamitsos, E.I. Synthesis, Thermal and Structural Properties of Pure TeO2 Glass and Zinc-Tellurite Glasses. J. Non-Cryst. Solids 2017, 457, 116–125. [Google Scholar] [CrossRef]
- Barz, A.; Haase, T.; Meyer, K.; Stachel, D. Corrosion of Crucible Materials and Their Influence on Structure of Phosphate Glasses. Phosphorus Res. Bull. 1996, 6, 331–335. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dos Santos, I.M.G.; Moreira, R.C.M.; de Souza, A.G.; Paskocimas, C.A.; Leite, E.R.; Varela, J.A.; Longo, E. Corrosión En Crisol Cerámico Por Vidrios de Óxidos de Metales Pesados, Parte I: Estudio Fenomenológico Fecha. InterCeram Int. Ceram. Rev. 2003, 52, 198–205. [Google Scholar]
- Langer, D.W.; Vesely, C.J. Electronic Core Levels of Zinc Chalcogenides. Phys. Rev. B 1970, 2, 4885–4892. [Google Scholar] [CrossRef]
- Scrocco, M. X-Ray and Electron-Energy-Loss Spectra of Bi, Sb, Te and Bi2Te3, Sb2Te3 Chalcogenides. J. Electron. Spectros Relat. Phenom. 1990, 50, 171–184. [Google Scholar] [CrossRef]
- Molle, A.; Spiga, S.; Fanciulli, M. Stability and Interface Quality of GeO2 Films Grown on Ge by Atomic Oxygen Assisted Deposition. J. Chem. Phys. 2008, 129, 011104. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, D.; Thangaraj, R. XPS Study of Thermally Evaporated Ge-Sb-Te Amorphous Thin Films. AIP Conf. Proc. 2011, 1393, 141–142. [Google Scholar] [CrossRef]
- Wang, P.W.; Qi, Y.; Henderson, D.O. Oxygen Bonding in GeO2 Glass. J. Non-Cryst. Solids 1998, 224, 31–35. [Google Scholar] [CrossRef]
- Moholkar, A.V.; Agawane, G.L.; Sim, K.U.; Kwon, Y.B.; Choi, D.S.; Rajpure, K.Y.; Kim, J.H. Temperature Dependent Structural, Luminescent and XPS Studies of CdO:Ga Thin Films Deposited by Spray Pyrolysis. J. Alloys Compd. 2010, 506, 794–799. [Google Scholar] [CrossRef]
- Dahivade, P.B.; Pawar, S.N.; Pathan, H.M.; Lokhande, B.J. Temperature-Dependent Hydrothermal Synthesis of CdO Nanoparticles and Its Analysis for Supercapacitor Application. ES Energy Environ. 2024, 24, 1098. [Google Scholar] [CrossRef]
- Alam, A.E.; Ojo, A.A.; Jasinski, J.B.; Dharmadasa, I.M. Magnesium Incorporation in N-CdTe to Produce Wide Bandgap p-Type CdTe:Mg Window Layers. ChemEngineering 2018, 2, 59. [Google Scholar] [CrossRef]
- Larink, D.; Eckert, H. Mixed Network Former Effects in Tellurite Glass Systems: Structure/Property Correlations in the System (Na2O)1/3[(2TeO2)x(B2O3)1 − x]2/3. J. Non-Cryst. Solids 2015, 426, 150–158. [Google Scholar] [CrossRef]
- Zeng, C.; Ramos-Ruiz, A.; Field, J.A.; Sierra-Alvarez, R. Cadmium Telluride (CdTe) and Cadmium Selenide (CdSe) Leaching Behavior and Surface Chemistry in Response to PH and O2. J. Environ. Manag. 2015, 154, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Lozano, G.; Marega, E., Jr.; Rivera, V.A. XPS Analysis of Bridging and Non-Bridging Oxygen in Yb3+-Er3+-Tm3+-Doped Zinc-Tellurite Glasses. J. Non-Cryst. Solids 2021, 553, 120520. [Google Scholar] [CrossRef]
- Chowdari, B.V.R.; Kumari, R. Structure and Ionic Conduction in the Ag2O·WO3·TeO2 Glass System. J. Mater. Sci. 1998, 33, 3591–3599. [Google Scholar] [CrossRef]
- Bartolo-Pérez, P.; Farías, M.H.; Castro-Rodríguez, R.; Peña, J.L. XPS Analysis of Oxidation States of Te in CdTe Oxide Films Grown by Rf Sputtering with an Ar-NH3 Plasma. Superf. Vacío 2001, 12, 8–11. [Google Scholar]
- Mekki, A.; Khattak, G.D.; Wenger, L.E. XPS and Magnetic Studies of Vanadium Tellurite Glassses. J. Electron. Spectros Relat. Phenom. 2009, 175, 21–26. [Google Scholar] [CrossRef]
- Qing-Hui, L.; Dong-Hong, G.; Fu-Xi, G. Short-Wavelength Recording Properties of TeOx Thin Films. Chin. Phys. Lett. 2004, 21, 320–323. [Google Scholar] [CrossRef]
- Clabel, H.J.L.; Valverde, J.V.P.; Lozano, C.G.; Marega, E.; Mastelaro, V.R.; Mendonça, C.R. Nonlinear Optical Properties of La3+-Doped Tellurite-Zinc Glasses: Impact of Chemical Bonding and Physical Properties via Raman and XPS. Ceram. Int. 2024, 51, 844–855. [Google Scholar] [CrossRef]
- Sk, R.K.; Ansari, G.F.; Kumar, S.; Sathish, N.; Mudgal, M.; Ashiq, M. Structural, Thermal and X-Ray Photoelectron Spectroscopic Properties of Boro-Tellurite Based Quaternary Glass System. Opt. Mater. 2024, 156, 115989. [Google Scholar] [CrossRef]
- Xu, M.; Li, J.; Yang, Q.; Jiang, L.; He, J.; He, D.; Wang, Y.; Yang, Y. Morphology-Controlled Green Synthesis of Tellurium Nanostructures and Applications of Te/MXene Hybrid Structures. Mater. Adv. 2023, 4, 5668–5673. [Google Scholar] [CrossRef]
- Kamalaker, V.; Chanakya, N.; Madhuri, J.H.; Jahangeer, N.; Maheshwar Reddy, M.; Ramesh, C.; Muralikrishna, P.; Upender, G. Influence of Nb5+ and La3+ Ions on Physical Properties of the Quaternary TeO2-ZnO-Nb2O5-La2O3 Glass System. Ceram. Int. 2024, 50, 41472–41482. [Google Scholar] [CrossRef]
- Wang, P.; Cui, Y.; Zhao, S.; Ye, R.; Cai, M.; Calve, L.; Rocherulle, J.; Huang, F.; Ma, H.; Bai, G.; et al. Effects of Melting Temperature on Color-Changing in Germano-Tellurite Niobate Glass. Opt Laser Technol 2022, 156, 108627. [Google Scholar] [CrossRef]
- Dzhagan, V.; Mazur, N.; Kapush, O.; Selyshchev, O.; Karnaukhov, A.; Yeshchenko, O.A.; Danylenko, M.I.; Yukhymchuk, V.; Zahn, D.R.T. Core and Shell Contributions to the Phonon Spectra of CdTe/CdS Quantum Dots. Nanomaterials 2023, 13, 921. [Google Scholar] [CrossRef] [PubMed]
- Ukarande, A.; Sonawane, S.M.; Chaure, S.; Chaure, N.B. Wet-Electrochemical Growth of CdTe Layers for Photovoltaic Applications. J. Mater. Sci. Mater. Electron. 2022, 33, 22456–22468. [Google Scholar] [CrossRef]
- Tarasov, A.S.; Mikhailov, N.N.; Dvoretsky, S.A.; Menshchikov, R.V.; Uzhakov, I.N.; Kozhukhov, A.S.; Fedosenko, E.V.; Tereshchenko, O.E. Preparation of Atomically Clean and Structurally Ordered Surfaces of Epitaxial CdTe Films for Subsequent Epitaxy. Semiconductors 2021, 55, S62–S66. [Google Scholar] [CrossRef]
- Hart, R.T.; Zwanziger, J.W.; Werner-Zwanziger, U.; Yates, J.R. On the Spectral Similarity of Bridging and Nonbridging Oxygen in Tellurites. J. Phys. Chem. A 2005, 109, 7636–7641. [Google Scholar] [CrossRef]
- Tauc, J. Optical Properties of Amorphous Semiconductors. In Amorphous and Liquid Semiconductors; Plenum Publishing Company Ltd.: Rhode, Island, 1974; pp. 159–220. ISBN 9781467344791. [Google Scholar]
- El-Samanoudy, M.M.; Sabry, A.I.; Shaisha, E.E.; Bahgat, A.A. Optical and Infrared Spectra of Glasses in the TeO2-GeO2-Fe2O3 System. Phys. Chem. Glas. 1991, 32, 115–120. [Google Scholar]
- Japari, S.J.; Sayyed, M.I.; Yahya, A.K.; Anis, A.L.; Iskandar, S.M.; Zaid, M.H.M.; Azlan, M.N.; Hisam, R. Effects of Na2O on Optical and Radiation Shielding Properties of XNa2O-(20-x)K2O-30V2O550TeO2Mixed Alkali Glasses. Results Phys. 2021, 22, 103946. [Google Scholar] [CrossRef]
- Sadeq, M.S.; Morshidy, H.Y. Effect of Mixed Rare-Earth Ions on the Structural and Optical Properties of Some Borate Glasses. Ceram. Int. 2019, 45, 18327–18332. [Google Scholar] [CrossRef]
- Ramachari, D.; Yang, C.S.; Wada, O.; Uchino, T.; Pan, C.L. High-Refractive Index, Low-Loss Oxyfluorosilicate Glasses for Sub-THz and Millimeter Wave Applications. J. Appl. Phys. 2019, 125, 151609. [Google Scholar] [CrossRef]
- Lozada-Morales, R.; Hernández-Rodríguez, M.F.; Rubio-Rosas, E.; Espinosa-Cerón, Y.; Meza-Rocha, A.N.; Zayas, M.E.; Licona-Ibarra, R.; Carmona-Téllez, S. Glass Formation Area of the CdO-CuCl2-V2O5 Ternary System: Optical Properties as a Function of CuCl2 Content. J. Non-Cryst. Solids 2021, 566, 120896. [Google Scholar] [CrossRef]

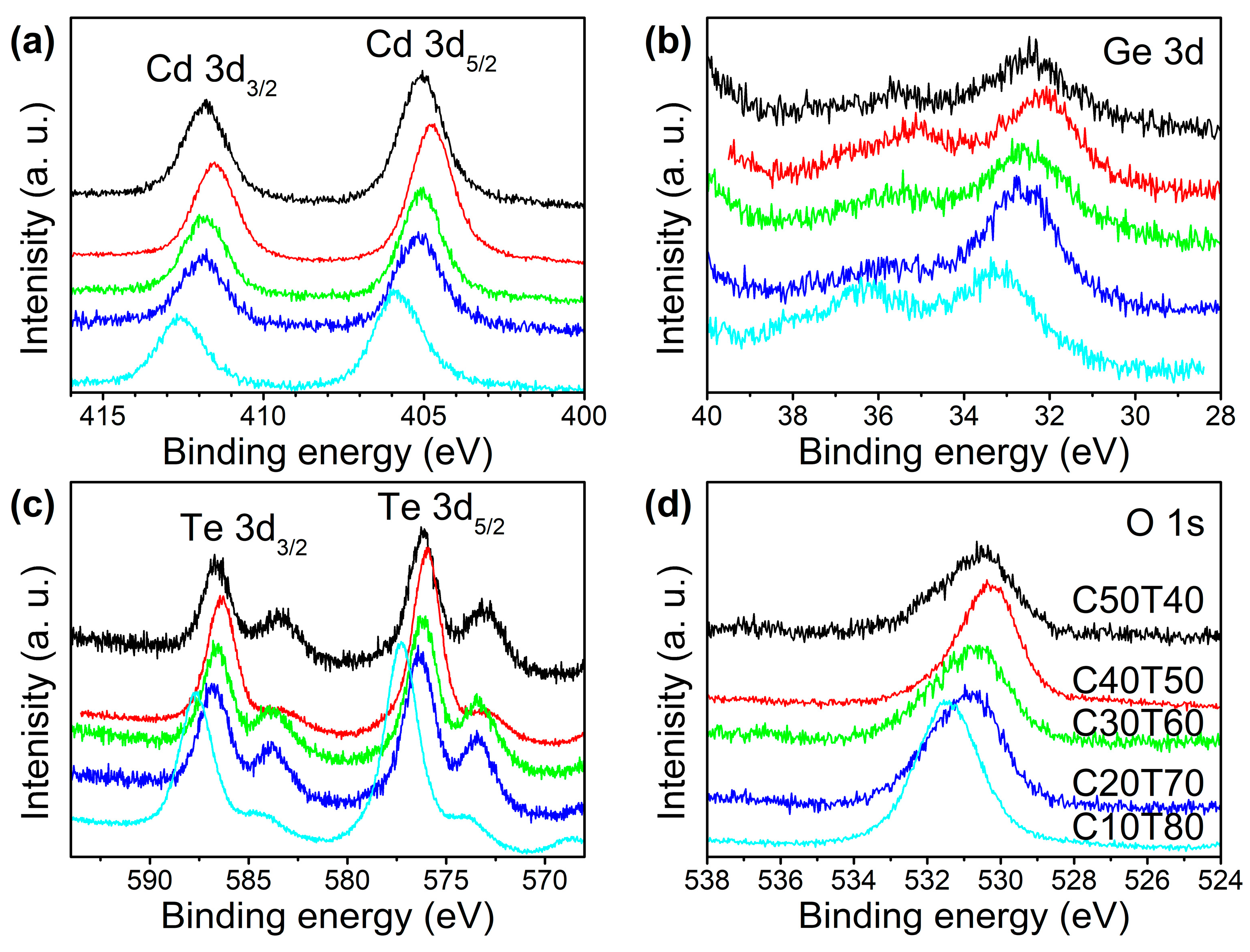
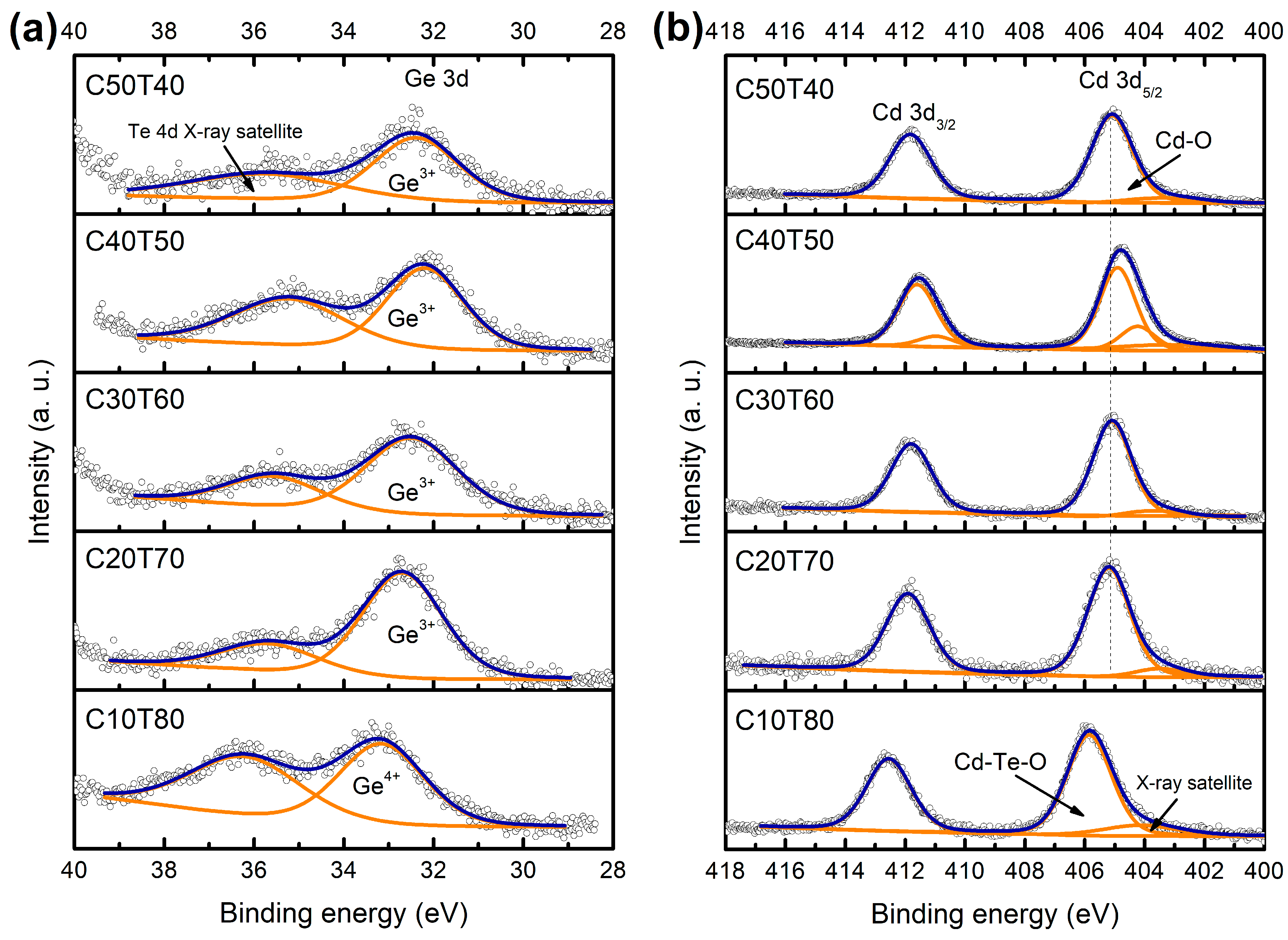
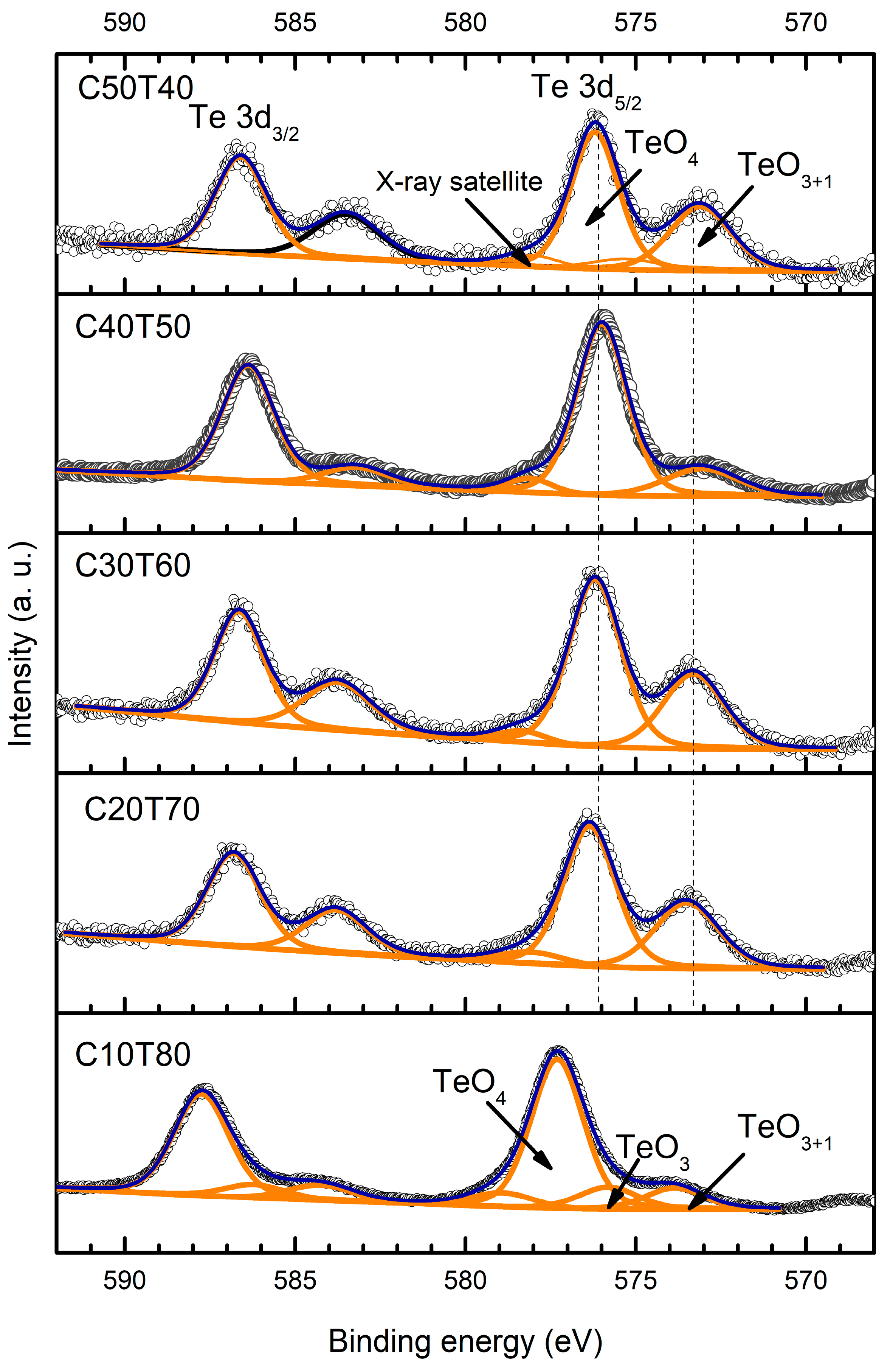
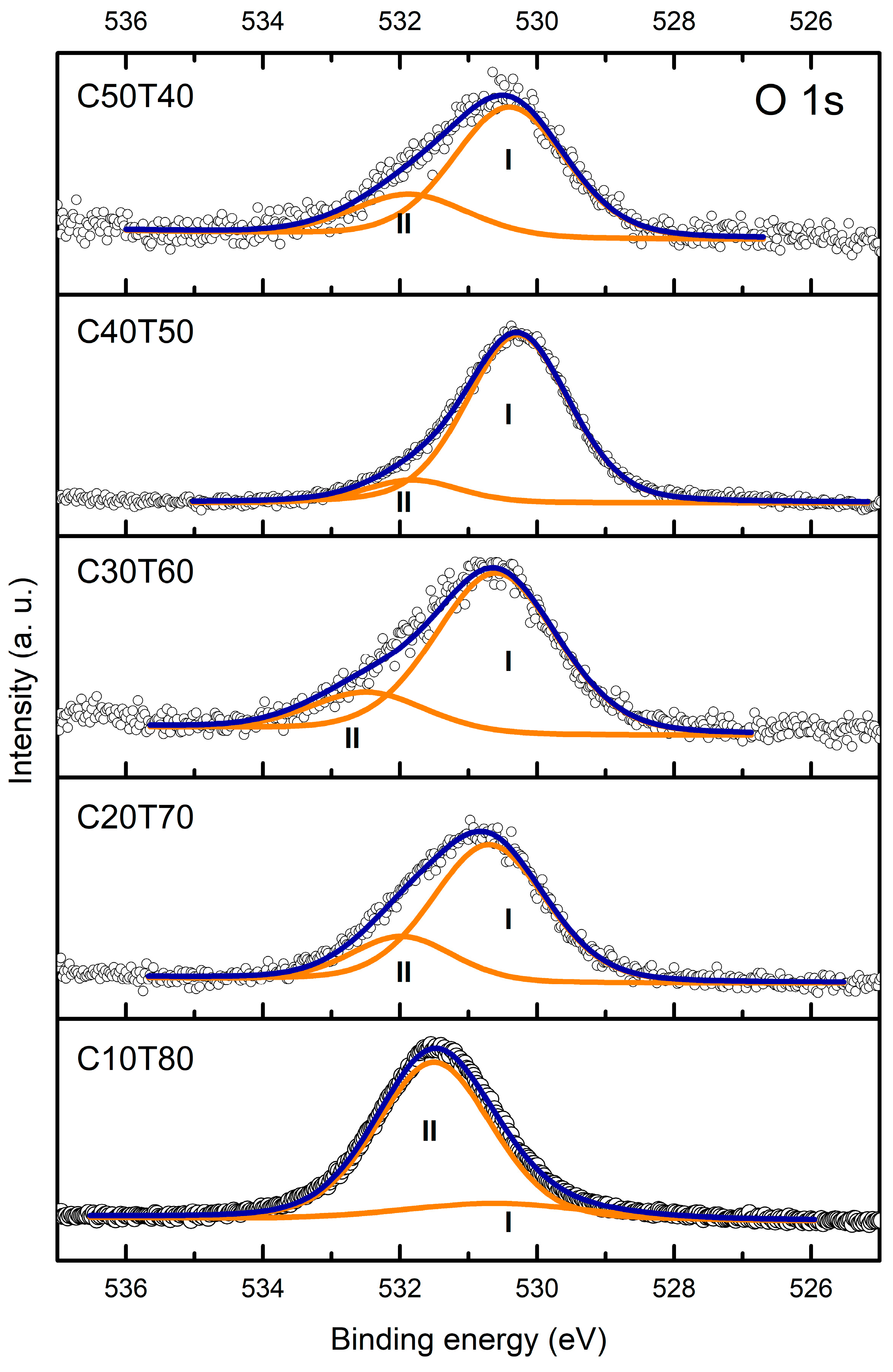

| Glass | Ge 3d | Cd | Te | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3d5/2 | 3d3/2 | 3d5/2 | 3d3/2 | |||||||
| eV | FWHM | eV | FWHM | eV | FWHM | eV | FWHM | eV | FWHM | |
| C50T40 | 32.4 | 2.24 | 405.2 | 1.65 | 411.9 | 1.70 | 573.1 | 2.13 | 583.5 | 2.28 |
| 576.2 | 1.65 | 586.6 | 1.79 | |||||||
| C40T50 | 32.2 | 1.97 | 404.2 | 1.37 | 410.9 | 1.44 | 573.0 | 253 | 583.2 | 2.26 |
| 404.9 | 1.42 | 411.6 | 1.49 | 576 | 1.75 | 586.4 | 1.80 | |||
| C30T60 | 32.5 | 2.44 | 405.1 | 1.51 | 411.8 | 1.6 | 573.3 | 2.13 | 583.75 | 2.31 |
| 576.2 | 1.81 | 586.6 | 1.72 | |||||||
| C20T70 | 32.7 | 2.08 | 405.2 | 1.66 | 411.9 | 1.71 | 573.5 | 2.13 | 583.8 | 2.12 |
| 576.3 | 1.78 | 586.8 | 1.85 | |||||||
| C10T80 | 33.2 | 5 | 405.85 | 1.75 | 412.6 | 1.71 | 573.85 | 2.02 | 584.25 | 2.21 |
| 575.8 | 2.02 | 586.2 | 2.10 | |||||||
| 577.3 | 1.80 | 587.75 | 1.92 | |||||||
| Glass | Component | Binding Energy (eV) | FWHM (eV) | Area (%) |
|---|---|---|---|---|
| C50T40 | OI OII | 530.4 531.9 | 1.96 1.97 | 75.9 24.1 |
| C40T50 | OI OII | 530.3 531.8 | 1.78 1.63 | 89.0 11.0 |
| C30T60 | OI OII | 530.6 532.5 | 2.13 2.03 | 80.7 19.3 |
| C20T70 | OI OII | 530.7 532.0 | 1.96 1.78 | 76.9 23.1 |
| C10T80 | OI OII | 530.6 531.5 | 1.99 3.23 | 15.0 85.0 |
| Sample | (eV) | |
|---|---|---|
| C50T40 | 2.68 | 2.489 |
| C40T50 | 2.87 | 2.432 |
| C30T60 | 2.77 | 2.461 |
| C20T70 | 2.33 | 2.605 |
| C10T80 | 2.32 | 2.609 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaya, I.V.G.; Rodríguez Carvajal, D.A.; Alvarado-Rivera, J.; Lozada-Morales, R.; Santos-Munguía, P.C.; Reyes, J.J.P.; Hernández-Abril, P.; Limón Reynosa, G.A.; Zayas, M.E. Effect of CdO on the Structural and Spectroscopic Properties of Germanium–Tellurite Glass. Materials 2025, 18, 1739. https://doi.org/10.3390/ma18081739
Amaya IVG, Rodríguez Carvajal DA, Alvarado-Rivera J, Lozada-Morales R, Santos-Munguía PC, Reyes JJP, Hernández-Abril P, Limón Reynosa GA, Zayas ME. Effect of CdO on the Structural and Spectroscopic Properties of Germanium–Tellurite Glass. Materials. 2025; 18(8):1739. https://doi.org/10.3390/ma18081739
Chicago/Turabian StyleAmaya, Iveth Viridiana García, David Alejandro Rodríguez Carvajal, Josefina Alvarado-Rivera, R. Lozada-Morales, Paula Cristina Santos-Munguía, Juan José Palafox Reyes, Pedro Hernández-Abril, Gloria Alicia Limón Reynosa, and Ma. Elena Zayas. 2025. "Effect of CdO on the Structural and Spectroscopic Properties of Germanium–Tellurite Glass" Materials 18, no. 8: 1739. https://doi.org/10.3390/ma18081739
APA StyleAmaya, I. V. G., Rodríguez Carvajal, D. A., Alvarado-Rivera, J., Lozada-Morales, R., Santos-Munguía, P. C., Reyes, J. J. P., Hernández-Abril, P., Limón Reynosa, G. A., & Zayas, M. E. (2025). Effect of CdO on the Structural and Spectroscopic Properties of Germanium–Tellurite Glass. Materials, 18(8), 1739. https://doi.org/10.3390/ma18081739







