Fragmentation of Cu2O Oxides Caused by Various States of Stress Resulting from Extreme Plastic Deformation
Abstract
1. Introduction
2. Materials and Methods
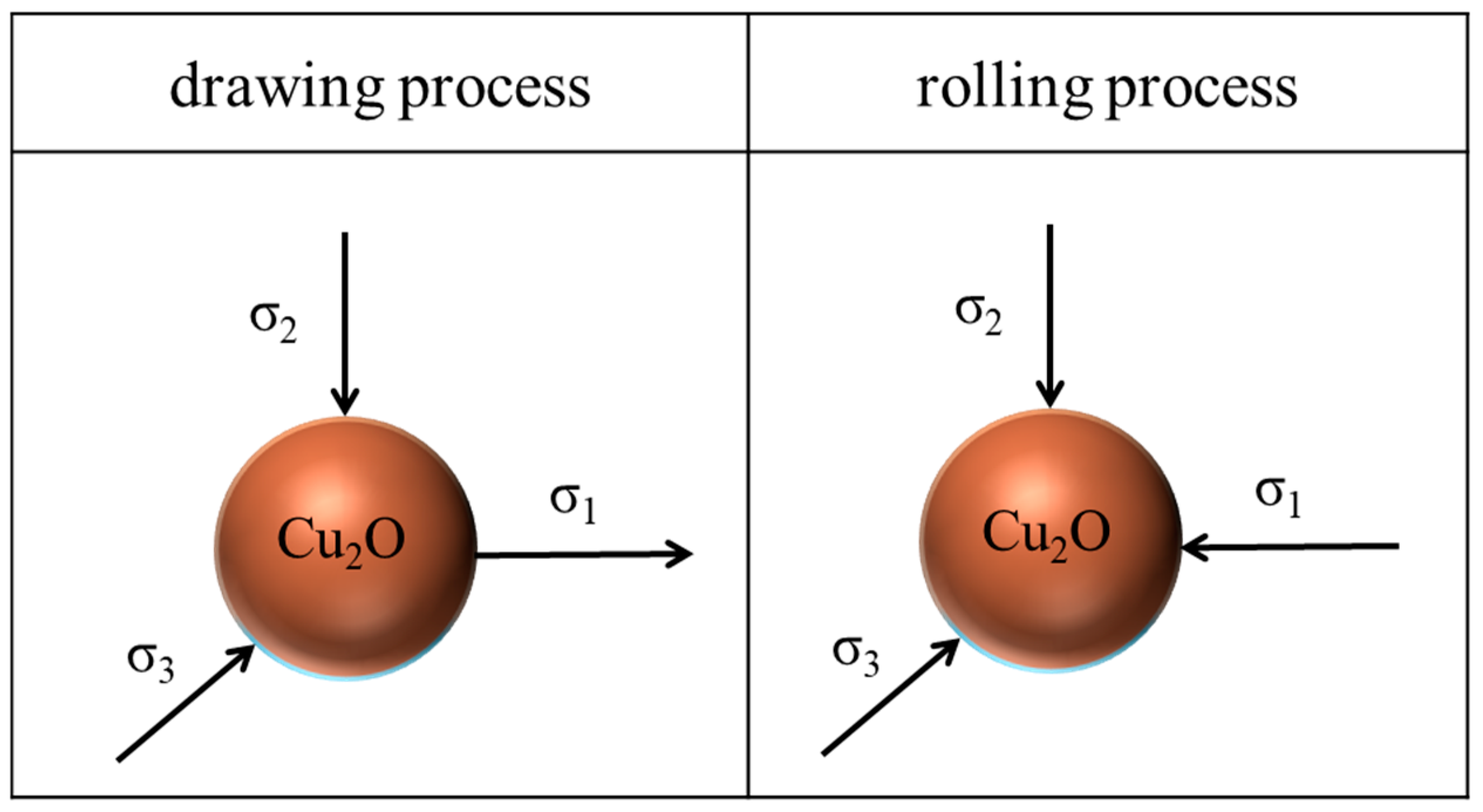
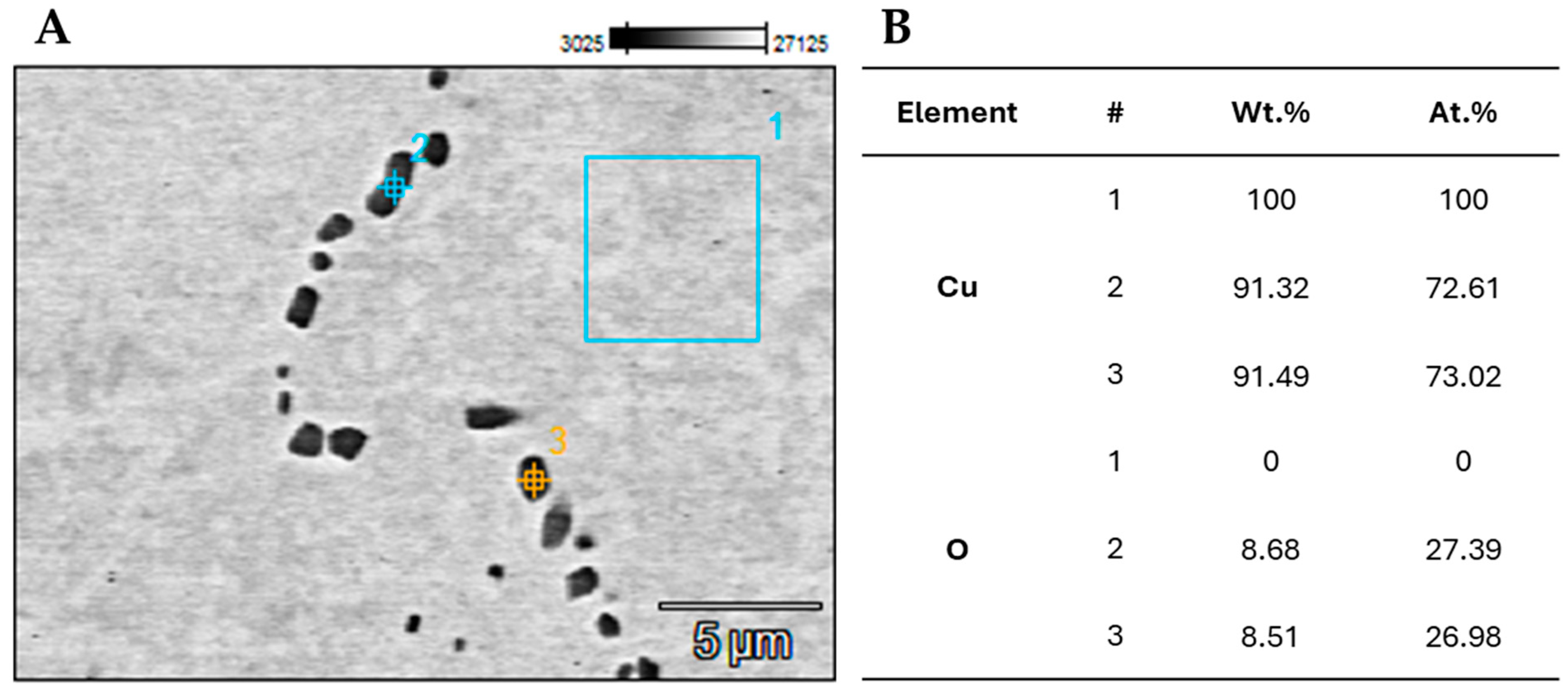
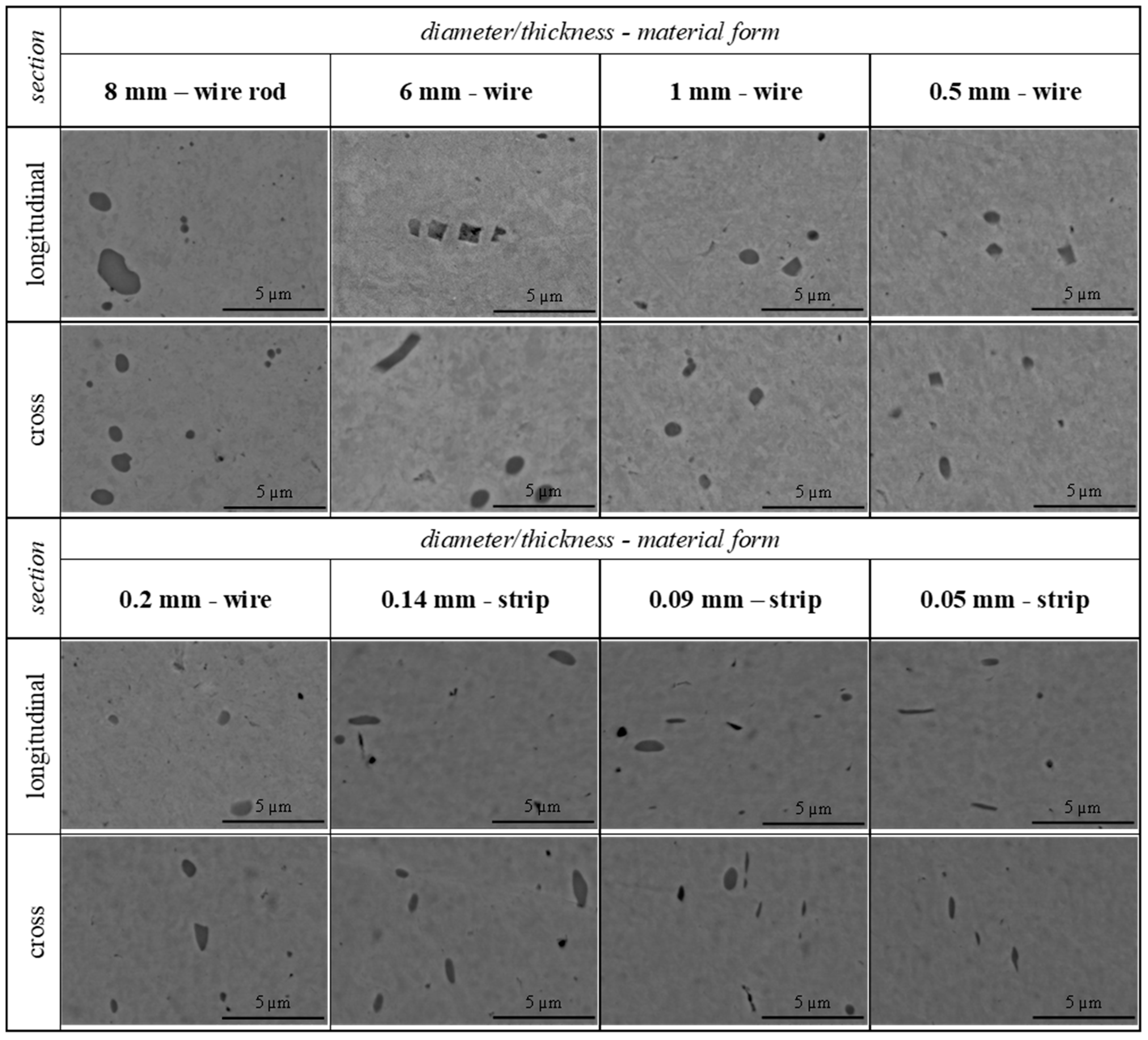
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mao, Q.; Liu, Y.; Zhao, Y. A review on copper alloys with high strength and high electrical conductivity. J. Alloys. Compd. 2024, 990, 174456. [Google Scholar] [CrossRef]
- Lu, L.; Shen, Y.; Chen, X.; Qian, L.; Lu, K. Ultrahigh strength and high electrical conductivity in copper. Science 2004, 304, 422–426. [Google Scholar] [CrossRef]
- Strzępek, P. The Assessment of Abrasion Resistance of Casted Non-Ferrous Metals and Alloys with the Use of 3D Scanning. Processes 2024, 12, 2200. [Google Scholar] [CrossRef]
- Chandra, K.; Mahanti, A.; Singh, A.P.; Joshi, N.S.; Kain, V. Metallurgical Investigation on Embrittlement of Copper Cable of an Electric Motor. J. Fail. Anal. Preven 2019, 19, 598–603. [Google Scholar] [CrossRef]
- Jiménez-Ruiz, E.; Lostado-Lorza, R.; Berlanga-Labari, C. A Comprehensive Review of Fatigue Strength in Pure Copper Metals (DHP, OF, ETP). Metals 2024, 14, 464. [Google Scholar] [CrossRef]
- Liu, P.; Tong, L.; Wang, J.; Shi, L.; Tang, H. Challenges and developments of copper wire bonding technology. Microelectron. Reliab. 2012, 52, 1092–1098. [Google Scholar] [CrossRef]
- Kinas, I.; Tan, E.; Can, H. The Effect of Oxygen Content on Mechanical and Conductivity Properties of Copper Rods Produced by Contirod and Up-Cast Continuous Casting Methods. Int. J. Sci. Technol. Res. 2018, 4, 384–391. [Google Scholar]
- Jakani, S.; Mathon, M.H.; Gerber, P.; Benyoucef, M.; Novion, C.H.; Baudin, T. Influence of Oxygen Content on the Static Recrystallization of ETP Copper. Mater. Sci. Forum. 2004, 467–470, 471–476. [Google Scholar] [CrossRef]
- Norasethasopon, S. Chevron Crack Initiation in Multi-Pass Drawing of Inclusion Copper Shaped-Wire. J. Met. Mater. Miner. 2011, 21, 1–8. [Google Scholar]
- Gavrish, P.A.; Perig, A.V.; Gribkov, E.P.; Berezshnaya, O.V. Reducing the risk of formation of the eutectic Cu-Cu2O during welding of copper with steel by improving treatment preparation technology. Adv. Mater. Process. Technol. 2021, 7, 400–416. [Google Scholar] [CrossRef]
- Goto, I.; Aso, S.; Ohguchi, K.; Kurosawa, K.; Suzuki, H.; Hayashi, H.; Shionoya, J. Effect of Solidification Conditions on the Deformation Behavior of Pure Copper Castings. Mater. Trans. 2019, 60, 2–9. [Google Scholar] [CrossRef]
- Helbert, A.L.; Moya, A.; Jil, T.; Andrieux, M.; Ignat, M.; Brisset, F.; Baudin, T. Copper Refinement from Anode to Cathode and Then to Wire Rod: Effects of Impurities on Recrystallization Kinetics and Wire Ductility. Microsc. Microanal. 2015, 21, 1153–1166. [Google Scholar] [CrossRef]
- Mysik, R.K.; Brusnitsyn, S.V.; Sulitsin, A.V.; Sokolov, I.A. Investigation of Microstructure of Oxygen-Containing Copper. KnE Eng. 2019, 1, 128–134. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, W.; Wu, M.; Li, R.; Dong, X. Influence of Low Oxygen Content on the Recrystallization Behavior of Rolled Copper Foil. Oxid. Met. 2018, 90, 203–215. [Google Scholar] [CrossRef]
- Pantazopoulos, G.; Vazdirvanidis, A.; Contopoulos, I. Cracking of Electrolytic Tough Pitch Copper Plates During Hot Rolling. J. Fail. Anal. Preven. 2019, 19, 858–865. [Google Scholar] [CrossRef]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. Handbook of Mineralogy: Halides, Hydroxides, Oxides; Mineral Data Pub.: Tucson, AZ, USA, 1997; Volume 3. [Google Scholar]
- Goto, I.; Aso, S.; Ohguchi, K.; Kurosawa, K.; Suzuki, H.; Hayashi, H.; Shionoya, J. Deformation Behavior of Pure Copper Castings with As-Cast Surfaces for Electrical Parts. J. Mater. Eng. Perform. 2019, 28, 3835–3843. [Google Scholar] [CrossRef]
- Komori, K. Ductile Fracture in Metal Forming: Modeling and Simulation. Acad. Press. 2020, 1, 49–94. [Google Scholar] [CrossRef]
- Elices, M.; Llorca, J. Models of fibre fracture. In Fiber Fracture; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2002; pp. 27–56. [Google Scholar] [CrossRef]
- Son, S.B.; Lee, Y.K.; Kang, S.H.; Chung, H.S.; Cho, J.S.; Moon, J.T.; Oh, K.H. A numerical approach on the inclusion effects in ultrafine gold wire drawing process. Eng. Fail. Anal. 2011, 18, 1272–1278. [Google Scholar] [CrossRef]
- Cho, H.; Jo, H.H.; Lee, S.G.; Kim, B.M.; Kim, Y.J. Effect of reduction ratio, inclusion size and distance between inclusions on wire breaks in Cu fine wiredrawing. J. Mater. Process. Technol. 2002, 130–131, 416–420. [Google Scholar] [CrossRef]
- Norasethasopon, S.; Yoshida, K. Influences of inclusion shape and size in drawing of copper shaped-wire. J. Mater. Process. Technol. 2006, 172, 400–406. [Google Scholar] [CrossRef]
- Yilmaz, M. Failures during the production and usage of steel wires. J. Mater. Process. Technol. 2006, 171, 232–239. [Google Scholar] [CrossRef]
- Sachana, S.; Morishita, K.; Miyahara, H. Oxide and Heat Treatment Microstructure Evolution of Melted Mark on Copper Wire under Various Heat Treatment Conditions. Mater. Trans. 2023, 64, 2302–2308. [Google Scholar] [CrossRef]
- Das, A.; Sivaprasada, S.; Tarafder, M.; Das, S.K.; Tarafder, S. Estimation of damage in high strength steels. Appl. Soft Comput. 2013, 13, 1033–1042. [Google Scholar] [CrossRef]
- Zasadzińska, M.; Knych, T. The morphology of eutectic copper oxides I (Cu2O) in the processing of wire rod and wires made from ETP grade copper. Arch. Metall. Mater. 2019, 64, 1611–1616. [Google Scholar] [CrossRef]
- Loginov, Y.N.; Demakov, S.L.; Ivanova, M.A.; Illarionov, A.G.; Karabanalov, M.S.; Stepatov, S.I. Effect of Annealing on Properties of Hot Rolled Electrical Copper. Phys. Met. Metallogr. 2015, 116, 393–400. [Google Scholar] [CrossRef]
- Loginov, Y.N.; Demakov, S.L.; Illarionov, A.G.; Ivanova, M.A. Interaction of a Copper Oxide Particle with Copper in Drawing. Russ. Metall. (Met.) 2012, 11, 947–953. [Google Scholar] [CrossRef]
- Strzępek, P.; Mamala, A.; Zasadzińska, M.; Kiesiewicz, G.; Knych, T.A. Shape Analysis of the Elastic Deformation Region throughout the Axi-Symmetric Wire Drawing Process of ETP Grade Copper. Materials 2021, 14, 4713. [Google Scholar] [CrossRef]
- Scardaci, V. Copper Nanowires for Transparent Electrodes: Properties, Challenges and Applications. Appl. Sci. 2021, 11, 8035. [Google Scholar] [CrossRef]
- Li, X.S.; Wang, Y.M.; Yin, C.R.; Yin, Z.X. Copper nanowires in recent electronic applications: Progress and perspectives. J. Mater. Chem. C. 2020, 8, 849–872. [Google Scholar] [CrossRef]
- Zhao, S.; Han, F.; Li, J.; Meng, X.; Huang, W.; Cao, D.; Zhang, G.; Sun, R.; Wong, C.-P. Advancements in Copper Nanowires: Synthesis, Purification, Assemblies, Surface Modification, and Applications. Small 2018, 14, 1800047. [Google Scholar] [CrossRef]
- Avitzur, B. Metal Forming. Processes and Analysis; McGraw-Hill Book Company: New York, NY, USA, 1968. [Google Scholar]
- Banganayi, C.; Nyembwe, K.; Mageza, K. Annealer curve characteristics of electrolytically refined tough pitch copper (Cu-ETP) and oxygen free up-cast copper (Cu-OF) for electrical cable wires. Results Mater. 2020, 8, 100146. [Google Scholar] [CrossRef]
- Mishra, A.; Kad, B.K.; Gregori, F.; Meyers, M.A. Microstructural evolution in copper subjected to severe plastic deformation: Experiments and analysis. Acta. Mater. 2007, 55, 13–28. [Google Scholar] [CrossRef]
- Cao, Y.; Ni, S.; Liao, X.; Song, M.; Zhu, Y. Structural evolutions of metallic materials processed by severe plastic deformation. Mater. Sci. Eng. R Rep. 2018, 133, 1–59. [Google Scholar] [CrossRef]
- Shih, M.H.; Yu, C.Y.; Kao, P.W.; Chang, C.P. Microstructure and flow stress of copper deformed to large plastic strains. Scr. Mater. 2001, 45, 793–799. [Google Scholar] [CrossRef]
- Zasadzińska, M.; Smyrak, B.; Knych, T.; Strzępek, P. Defects analysis of copper wires manufactured in industrial conditions. Metalurgija 2022, 61, 774–776. [Google Scholar]
- Hallstedt, B.; Risold, D.; Gauckle, L.J. Thermodynamic assessment of the copper-oxygen system. J. Phase Equilibria 1994, 15, 483–499. [Google Scholar] [CrossRef]
- Schramm, L.; Behr, G.; Löser, W.; Wetzig, K. Thermodynamic reassessment of the Cu-O phase diagram. J. Phase Equilibria Diffus. 2005, 26, 605–612. [Google Scholar] [CrossRef]
- Aurubis Official Website. Available online: https://www.aurubis.com/en/products/copper-products/rod-and-specialty-wire/wire-rod/Productionprocess (accessed on 14 March 2025).
- Okamoto, H.; Schlesinger, M.E.; Mueller, E.M. ASM Handbook, Vol. III: Alloy Phase Diagrams; ASM International: Geauga, OH, USA, 1992. [Google Scholar]
- Schmid, R. A thermodynamic Analysis of the Cu-O System with an Associated Solution Model. Metall. Trans. B 1983, 14, 473–481. [Google Scholar] [CrossRef]
- Zasadzińska, M.; Knych, T.; Smyrak, B.; Strzępek, P. Investigation of the Dendritic Structure Influence on the Electrical and Mechanical Properties Diversification of the Continuously Casted Copper Strand. Materials 2020, 13, 5513. [Google Scholar] [CrossRef]
- Blicharski, M. Odkształcanie I Pękanie; UWND AGH: Kraków, Poland, 2002. (In Polish) [Google Scholar]
- Norasethasopon, S.; Yoshida, K. Prediction of chevron crack initiation in inclusion copper shaped-wire drawing. Eng. Fail. Anal. 2008, 15, 378–393. [Google Scholar] [CrossRef]
- Martin, E.; Leguillon, D.; Sevecek, O.; Bermejo, R. Understanding the tensile strength of ceramics in the presence of small critical flaws. Eng. Fract. Mech. 2018, 201, 167–175. [Google Scholar] [CrossRef]
- Leguillon, D.; Martin, E.; Sevecek, O.; Bermejo, R. What is the tensile strength of a ceramic to be used in numerical models for predicting crack initiation? Int. J. Fract. 2018, 212, 89–103. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zasadzińska, M. Fragmentation of Cu2O Oxides Caused by Various States of Stress Resulting from Extreme Plastic Deformation. Materials 2025, 18, 1736. https://doi.org/10.3390/ma18081736
Zasadzińska M. Fragmentation of Cu2O Oxides Caused by Various States of Stress Resulting from Extreme Plastic Deformation. Materials. 2025; 18(8):1736. https://doi.org/10.3390/ma18081736
Chicago/Turabian StyleZasadzińska, Małgorzata. 2025. "Fragmentation of Cu2O Oxides Caused by Various States of Stress Resulting from Extreme Plastic Deformation" Materials 18, no. 8: 1736. https://doi.org/10.3390/ma18081736
APA StyleZasadzińska, M. (2025). Fragmentation of Cu2O Oxides Caused by Various States of Stress Resulting from Extreme Plastic Deformation. Materials, 18(8), 1736. https://doi.org/10.3390/ma18081736






