The Effect of Aging Time at 600 °C on Tensile Properties of the 0.3Nb FeCrAl Alloy
Abstract
1. Introduction
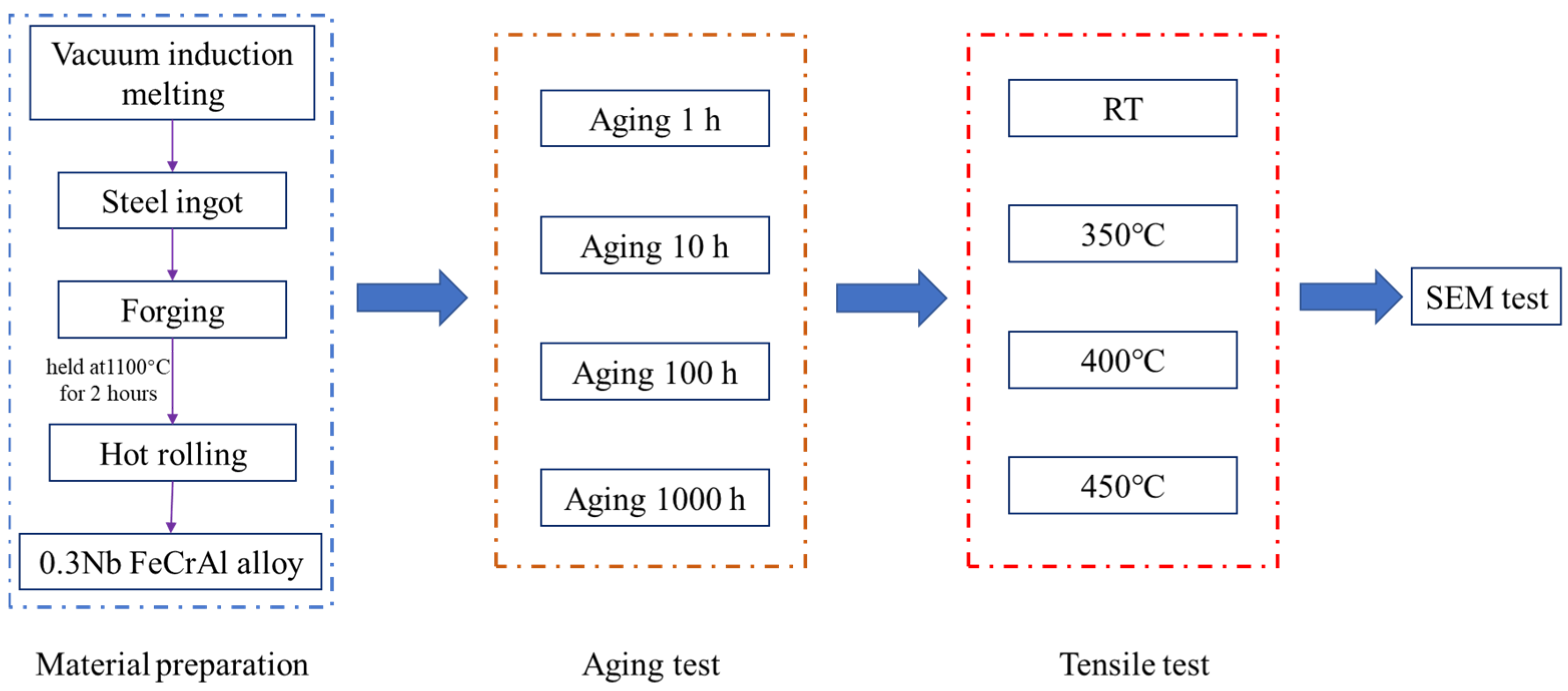
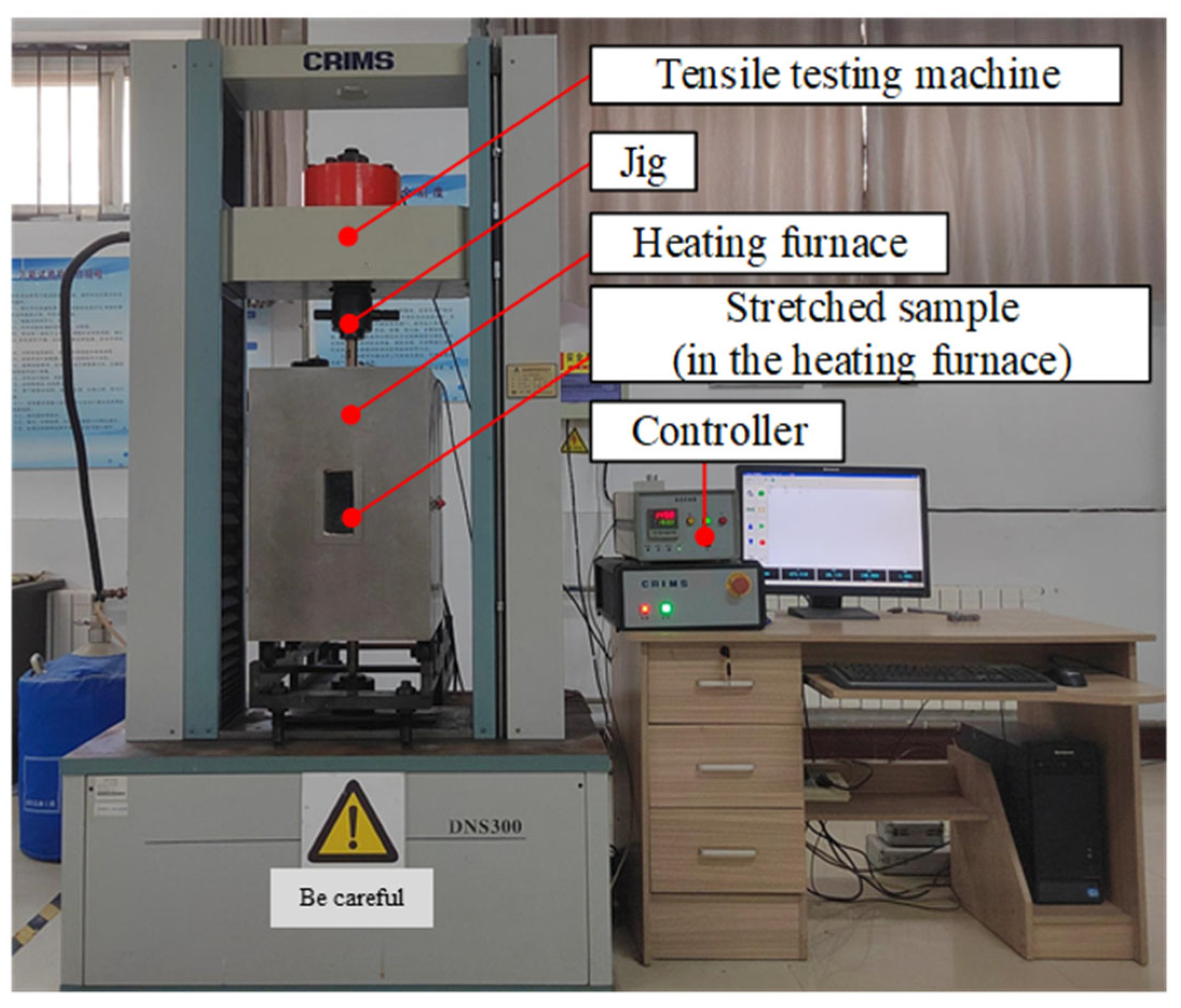
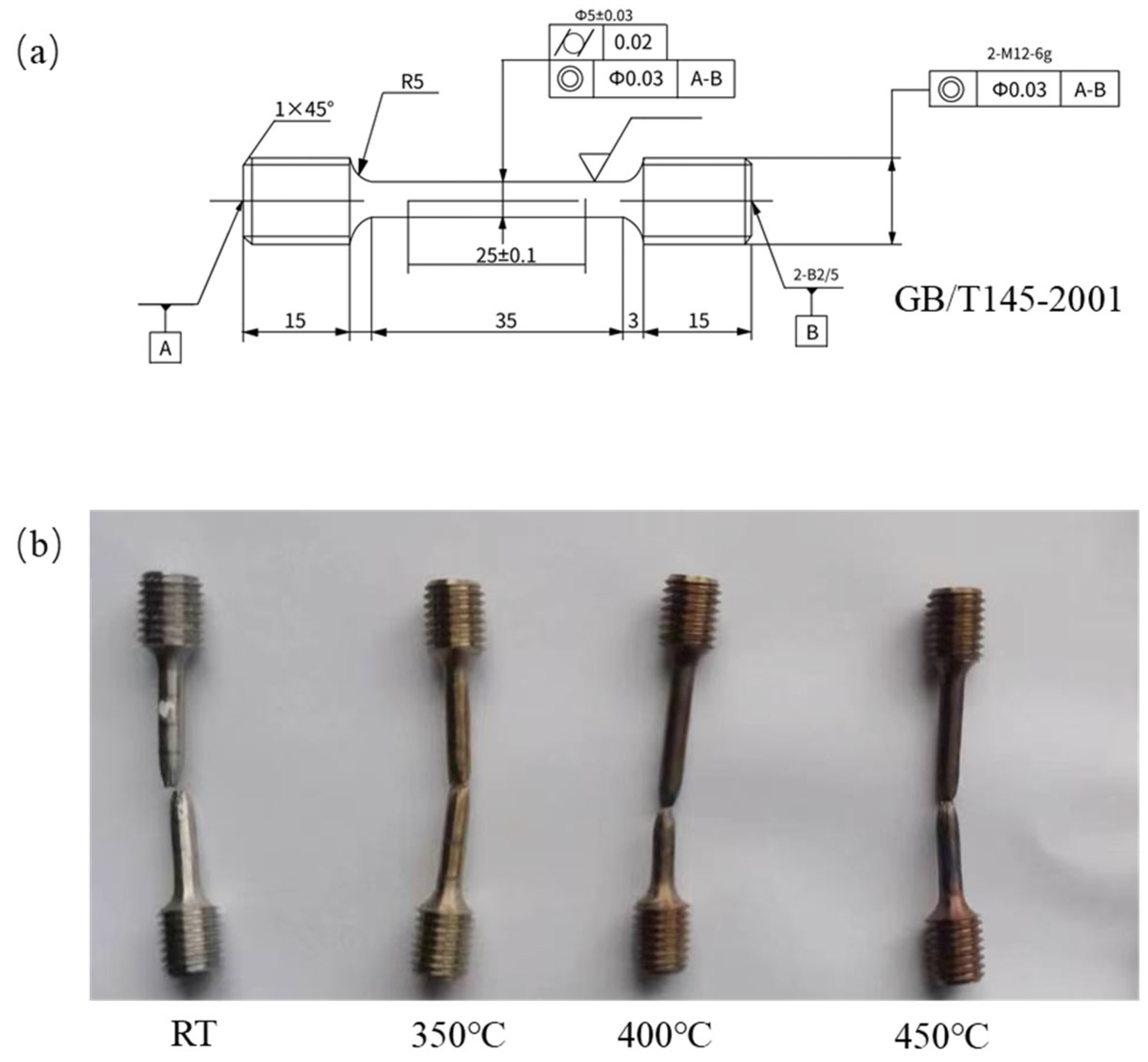
2. Materials and Methods
3. Results and Discussion
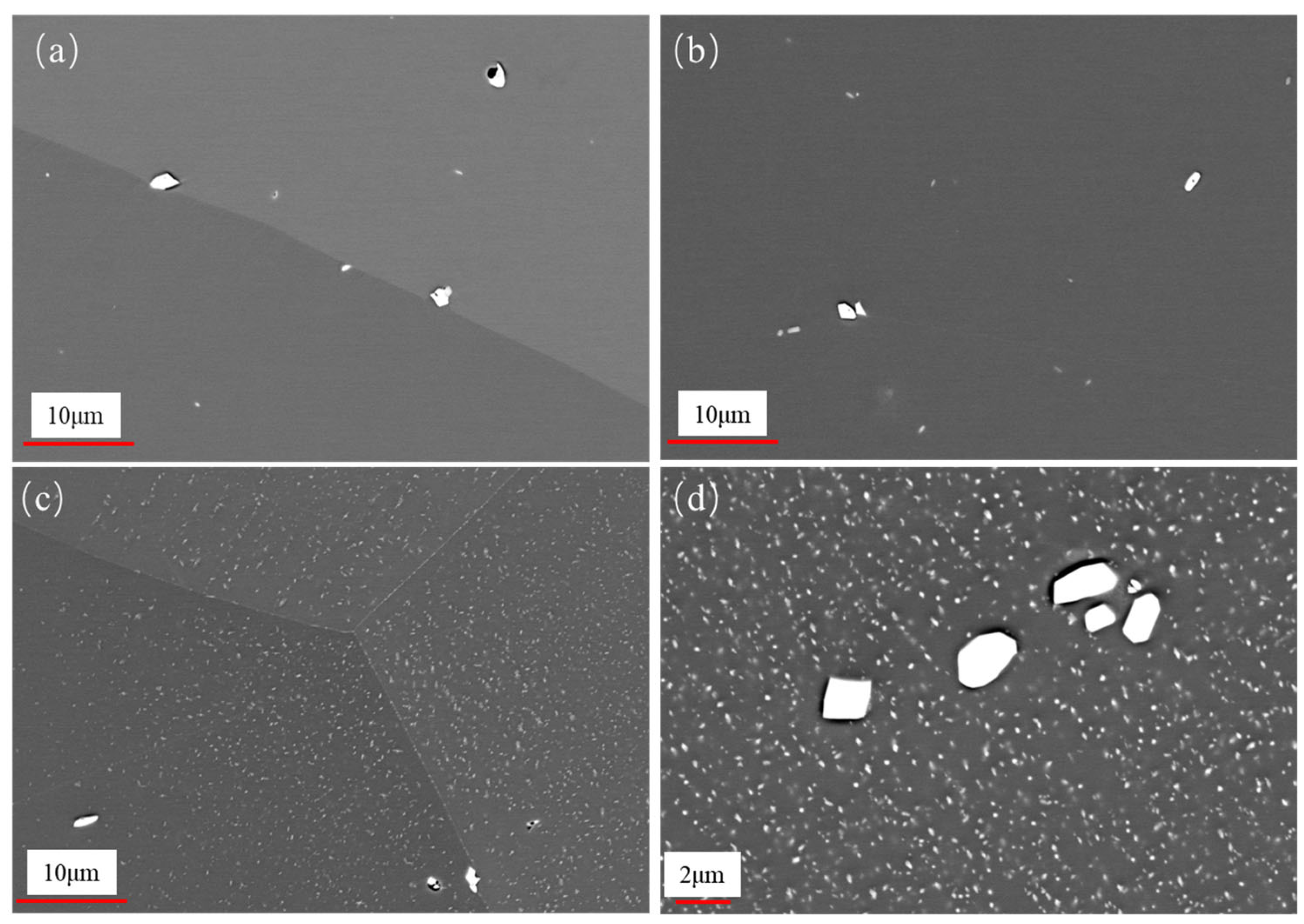
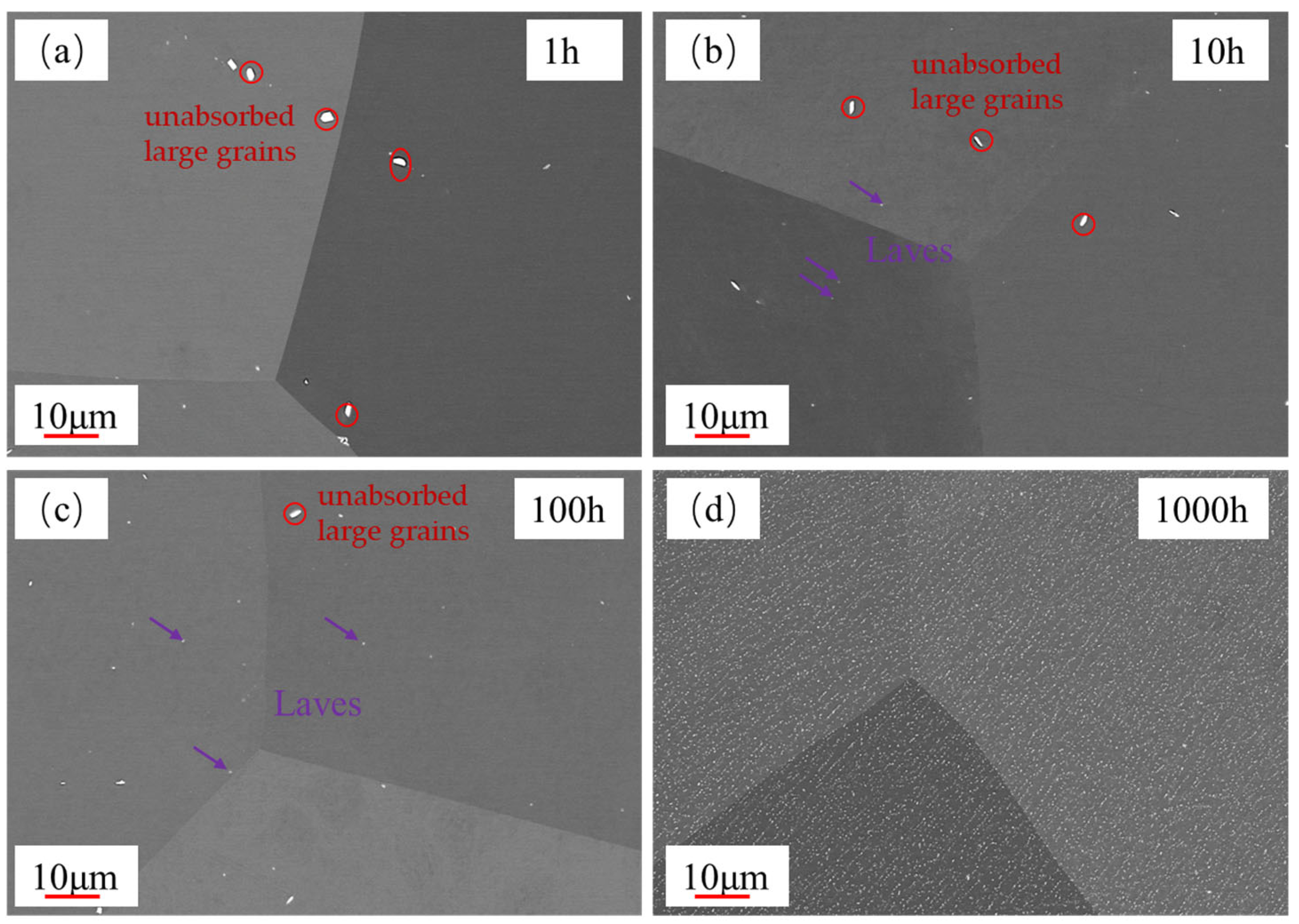
3.1. Microstructural Evolution During Aging
3.2. Evolution of Laves Phase During Aging
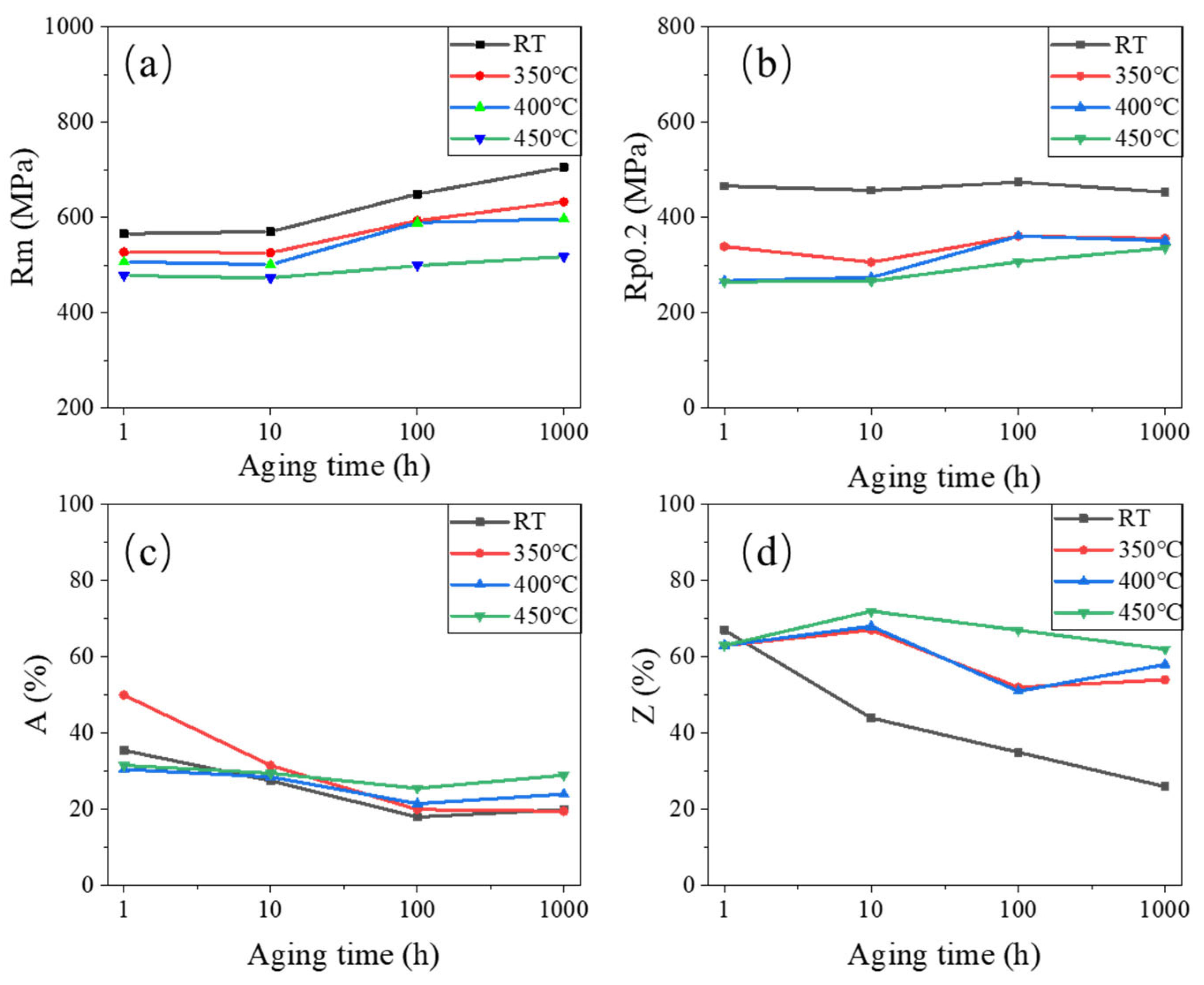
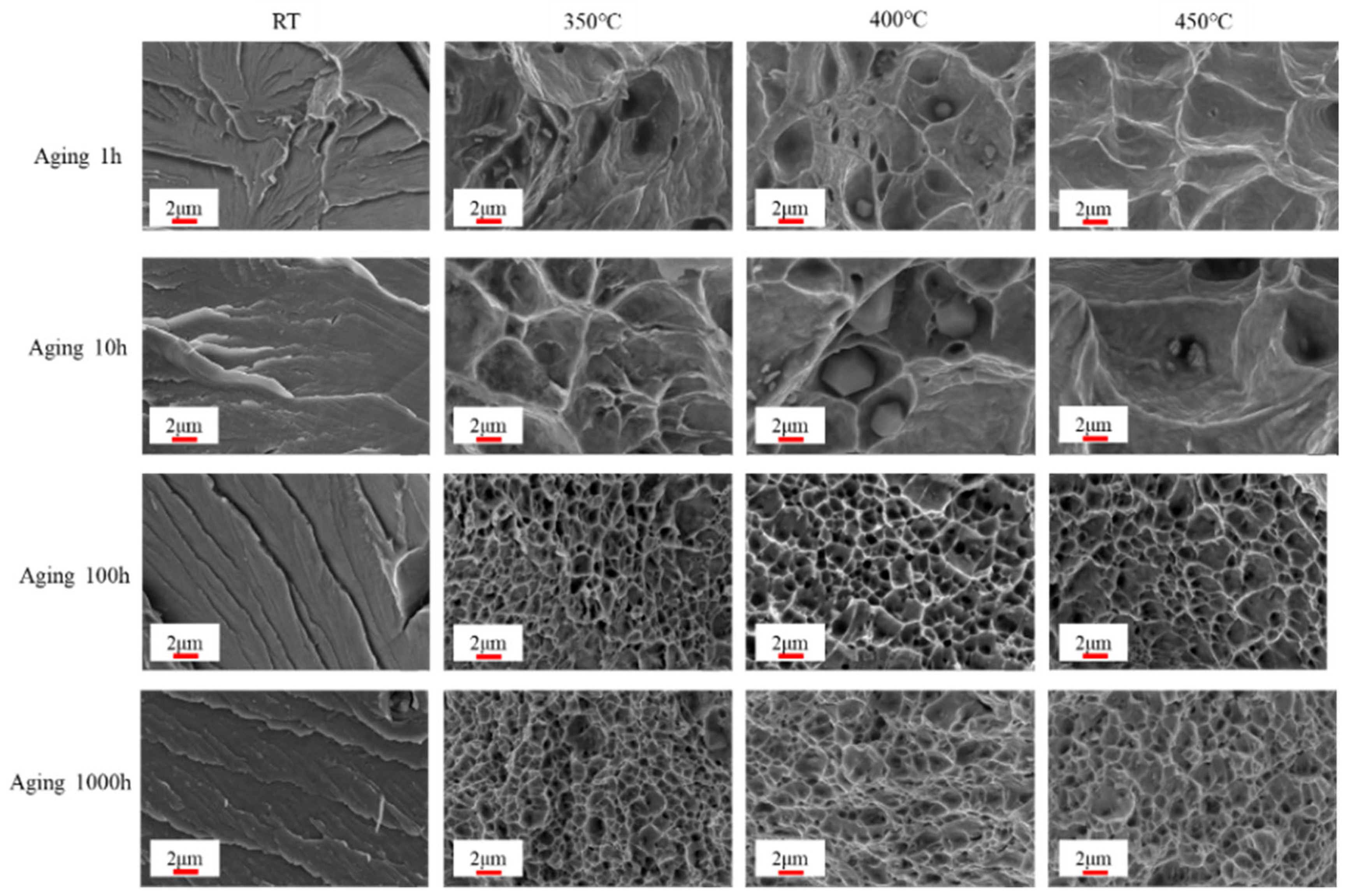
3.3. Tensile Properties After Aging
4. Conclusions
- (1)
- It was observed that the grain size of the 0.3Nb FeCrAl alloy remained relatively stable with increasing aging time. This suggests that the alloy’s grain size is not significantly affected by aging. However, the presence of Laves phase precipitates increased gradually with aging time, and these precipitates were evenly distributed within the crystals and along the crystal boundaries;
- (2)
- The tensile strength of the 0.3Nb FeCrAl alloy gradually increased with aging time at a constant tensile temperature. This indicates that aging can effectively improve the alloy’s tensile strength. Additionally, a higher amount of Laves phase precipitates resulted in finer grains and enhanced precipitation strengthening. Furthermore, as the tensile temperature increased, the tensile strength of the 0.3Nb FeCrAl alloy gradually decreased. Notably, the highest tensile strength was achieved at room temperature (RT);
- (3)
- The yield strength Rp0.2 of the 0.3Nb FeCrAl alloy tended to increase with aging time, indicating the presence of aging hardening. However, at the same aging time, the yield strength displayed a decreasing trend with increasing tensile temperature. This may be attributed to the softening of the ferrite phase. The rise in tensile temperature could lead to the softening of the main phase structure of FeCrAl alloy, resulting in a reduction in yield strength;
- (4)
- After aging for 100 h, both the A and Z gradually increased with higher tensile temperatures. This finding indicates that the plastic properties of the 0.3Nb FeCrAl alloy improved as the tensile temperature increased.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terrani, K.A. Accident tolerant fuel cladding development: Promise, status, and Challenges. J. Nucl. Mater. 2018, 501, 13–30. [Google Scholar]
- Pan, D.; Zhang, R.; Wang, H.; Lu, C.; Liu, Y. Formation and stability of oxide layer in FeCrAl fuel cladding material under high-temperature steam. J. Alloys Compd. 2016, 684, 549–555. [Google Scholar]
- Dryepondt, S.; Unocic, K.A.; Hoelzer, D.T.; Massey, C.P.; Pint, B.A. Development of low-Cr ODS FeCrAl alloys for accident-tolerant fuel cladding. J. Nucl. Mater. 2018, 501, 59–71. [Google Scholar]
- Huang, X.; Wang, H.; Qiu, S.; Zhang, Y.; Wu, B. Cold-rolling & annealing process for nuclear grade wrought FeCrAl cladding alloy to enhance the strength and ductility. J. Mater. Process. Technol. 2019, 277, 116434. [Google Scholar]
- Fang, X.Q.; Wang, J.B.; Liu, S.Y.; Wen, J.Z.; Song, H.Y.; Liu, H.T. Microstructure Evolution, Hot Deformation Behavior and Processing Maps of an FeCrAl Alloy. Materials 2024, 17, 1847. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Wang, H.; Jiang, Y.; Liu, R.; Jing, K.; Sun, M.; Zhang, R.; Qiu, S.; Xie, Z.; Deng, H.; et al. Effects of ZrC addition on the microstructure and mechanical properties of Fe-Cr-Al alloys fabricated by spark plasma sintering. J. Alloys Compd. 2019, 805, 1025–1033. [Google Scholar]
- Han, L.; Wang, Y.; Guan, H.; Yin, C.; Zhou, Z. Effect of oxide and carbide nanoparticle strengthening on the microstructure of FeCrAl alloy. Mater. Sci. Eng. 2025, 922, 147657. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Y.; An, X.; Zhang, J.; Kong, Q.; Wang, H.; Yao, W.; Wang, Q. Mechanical and corrosion properties of nano ZrC reinforced FeCrAl alloys. Anti-Corros. Methods Mater. 2024, 71, 55–64. [Google Scholar]
- Zhang, Y.; Sun, H.; Wang, H.; Wang, X.; An, X.; He, K. Effects of Cr element on the crystal structure, microstructure, and mechanical properties of FeCrAl alloys. Mater. Sci. Eng. 2021, 826, 142003. [Google Scholar]
- Qu, H.J.; Abouelella, H.; Chikhalikar, A.S.; Rajendran, R.; Roy, I.; Priedeman, J.L.; Umretiya, R.; Hoffman, A.; Wharry, J.; Rebak, R. Effect of Nickel on the Oxidation Behavior of Fecral Alloy in Simulated Pwr and Bwr Conditions. Corros. Sci. 2023, 216, 111093. [Google Scholar]
- Wang, H.; Guo, B.; An, X.; Zhang, Y. Influence of Cold-Rolling Reduction on Microstructure and Tensile Properties of Nuclear FeCrAl Alloy with Low Cr and Nb Contents. Acta Met. Sin. Engl. Ed. 2022, 35, 2101–2110. [Google Scholar]
- Sun, Z.; Bei, H.; Yamamoto, Y. Microstructural control of FeCrAl alloys using Mo and Nb additions. Mater. Charact. 2017, 13, 126–131. [Google Scholar]
- Wang, H.; Gao, Y.X.; Sun, M.; Yang, H.Y.; Li, G.; He, K.; Wang, X.P.; Jiang, W.B.; Fang, Q.F. Strengthening mechanism of Nb addition in Fe–13Cr–4.5Al–2Mo alloys assessed by internal friction measurement. J. Nucl. Mater. 2020, 542, 152461. [Google Scholar] [CrossRef]
- Sun, Z.; Edmondson, P.D.; Yamamoto, Y. Effects of Laves phase particles on recovery and recrystallization behaviors of Nb-containing FeCrAl alloys. Acta Mater. 2018, 144, 716–727. [Google Scholar] [CrossRef]
- Zheng, J.; Jia, Y.; Du, P.; Wang, H.; Qiu, S. Control of Laves Precipitation in a FeCrAl-based Alloy Through Severe Thermomechanical Processing. Materials 2019, 12, 2939. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; Chikhalikar, A.S.; Roy, I. Effect of aging and α′ segregation on oxidation and electrochemical behavior of FeCrAl alloys. J. Nucl. Mater. 2024, 588, 154751. [Google Scholar]
- Zhang, Y.; Wang, H.; An, X.; Chen, G.; Wang, Y. Dynamic strain aging behavior of accident tolerance fuel cladding FeCrAl-based alloy for advanced nuclear energy. J. Mater. Sci. 2021, 56, 8815–8834. [Google Scholar]
- Li, N.; Parker, S.S.; Wood, E.S.; Nelson, A.T. Oxide Morphology of a FeCrAl Alloy, Kanthal APMT, Following Extended Aging in Air at 300 °C to 600 °C. Metall. Mater. Trans. A 2018, 49, 2940–2950. [Google Scholar] [CrossRef]
- GB/T 145-2001; National Standard of the People’s Republic of China: Center Hole. China Standards Press: Beijing, China, 2001.
- Chen, G.; Wang, H.; Sun, H.; Zhang, Y.; Cao, P.; Wang, J. Effects of Nb-doping on the mechanical properties and high-temperature steam oxidation of annealing FeCrAl fuel cladding alloys. Mater. Sci. Eng. 2021, 803, 140500. [Google Scholar] [CrossRef]


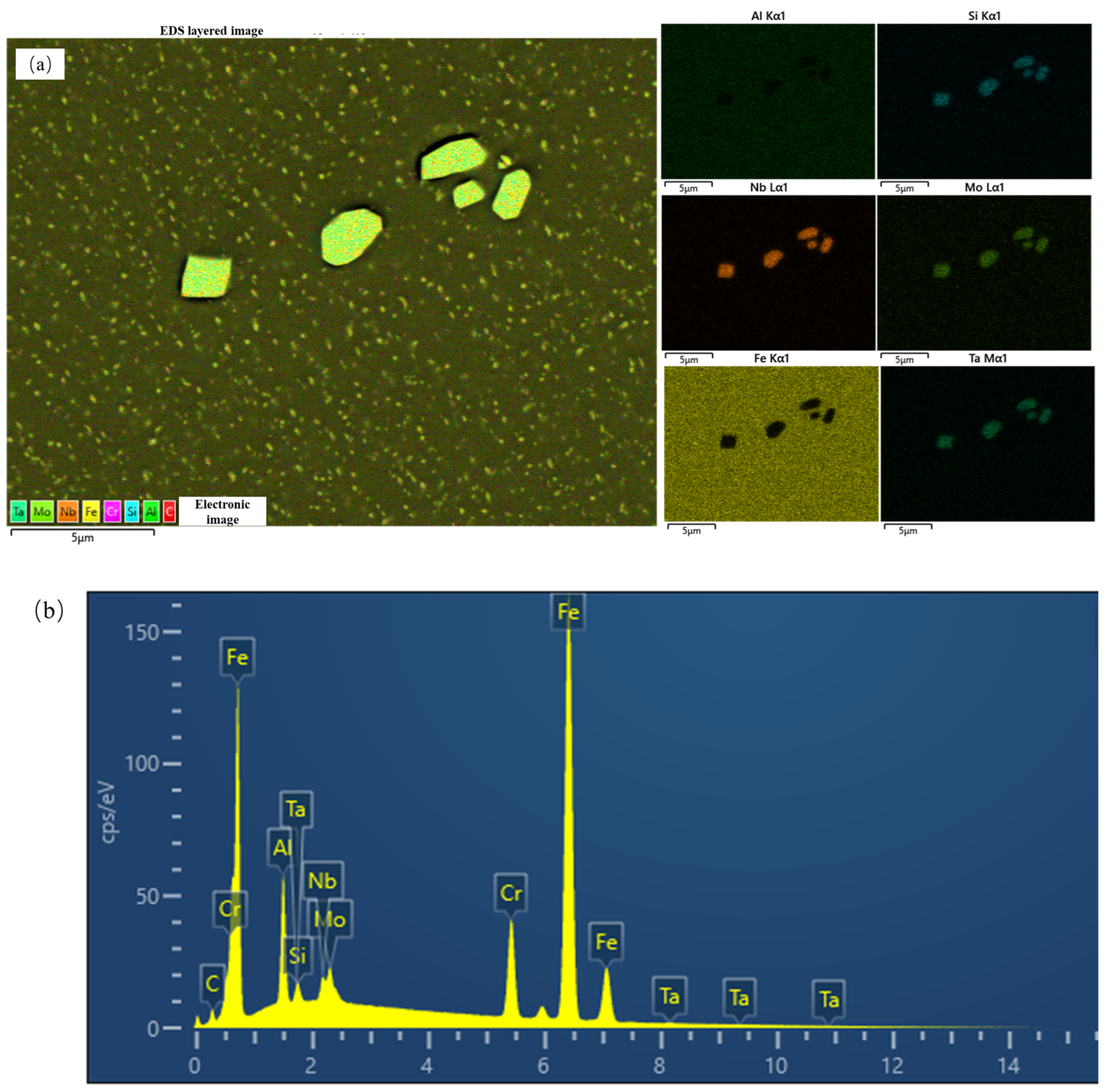






| Cu | Si | Mn | Nb | Mo | Al | Fe | Ti | Cr | Ni |
|---|---|---|---|---|---|---|---|---|---|
| 0.0146 | 0.0135 | 0.0091 | 0.3271 | 1.9598 | 5.3026 | 81.3614 | 0.1122 | 10.2866 | 0.1957 |
| Aging Time/h | Al | Si | Cr | Fe | Nb | Mo |
|---|---|---|---|---|---|---|
| 1 | 4.31 | 0.35 | 9.88 | 79.84 | 0.32 | 1.98 |
| 10 | 4.53 | 0.34 | 9.80 | 79.27 | 0.33 | 2.02 |
| 100 | 4.51 | 0.35 | 9.85 | 79.23 | 0.32 | 2.06 |
| 1000 | 4.43 | 0.36 | 9.91 | 79.36 | 0.34 | 2.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, L.; Sun, H.; Wu, G.; Lv, Z.; Xiong, Y. The Effect of Aging Time at 600 °C on Tensile Properties of the 0.3Nb FeCrAl Alloy. Materials 2025, 18, 1684. https://doi.org/10.3390/ma18071684
Tang L, Sun H, Wu G, Lv Z, Xiong Y. The Effect of Aging Time at 600 °C on Tensile Properties of the 0.3Nb FeCrAl Alloy. Materials. 2025; 18(7):1684. https://doi.org/10.3390/ma18071684
Chicago/Turabian StyleTang, Liping, Hongying Sun, Guijun Wu, Zhangquan Lv, and Yi Xiong. 2025. "The Effect of Aging Time at 600 °C on Tensile Properties of the 0.3Nb FeCrAl Alloy" Materials 18, no. 7: 1684. https://doi.org/10.3390/ma18071684
APA StyleTang, L., Sun, H., Wu, G., Lv, Z., & Xiong, Y. (2025). The Effect of Aging Time at 600 °C on Tensile Properties of the 0.3Nb FeCrAl Alloy. Materials, 18(7), 1684. https://doi.org/10.3390/ma18071684






