Non-Isothermal Crystallization Behavior of a Zr-Based Amorphous Alloy Composite Prepared by Selective Laser Melting
Highlights
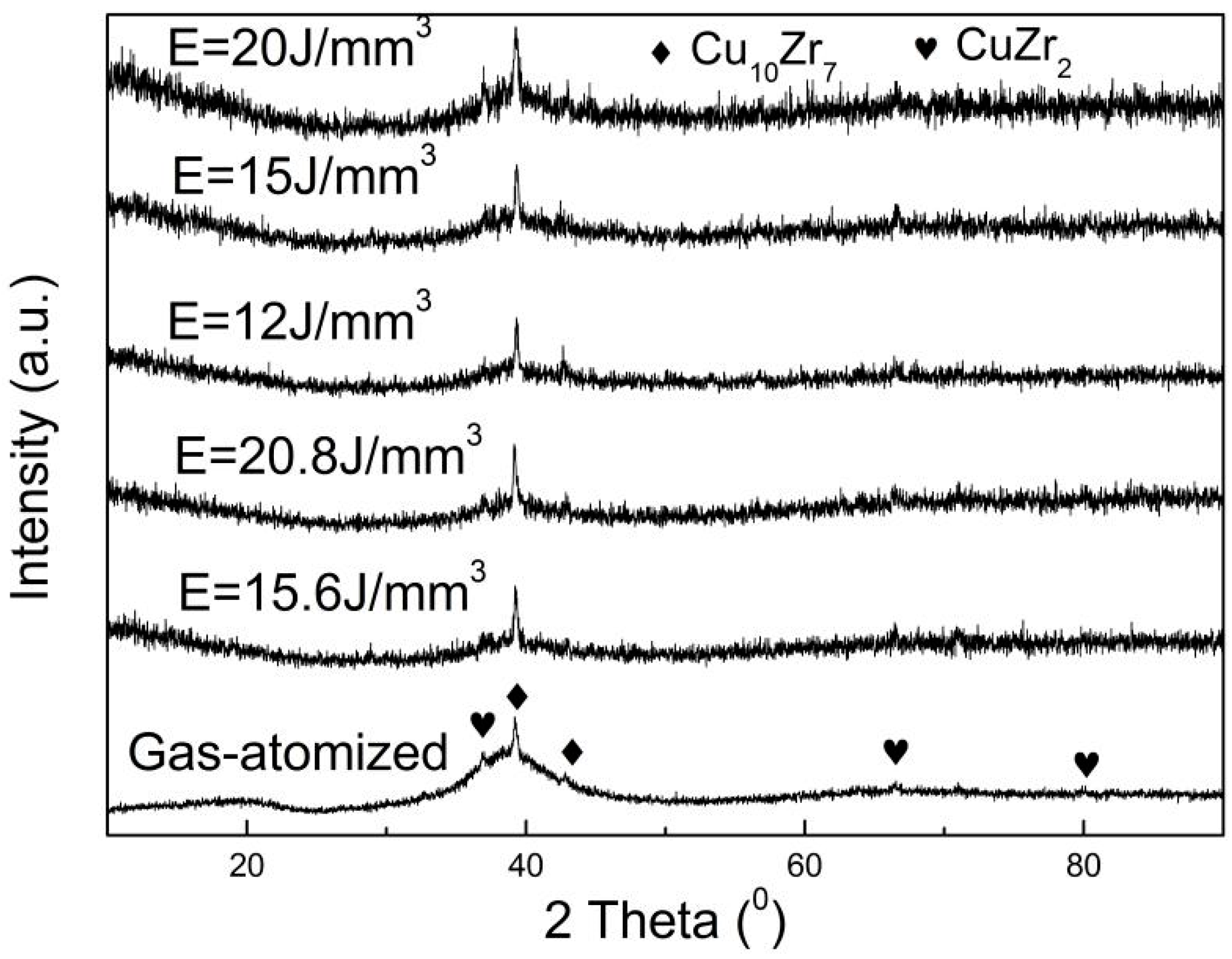
- The crystallization phases are Cu10Zr7 and CuZr2 for both gas-atomized powder and SLMed samples.
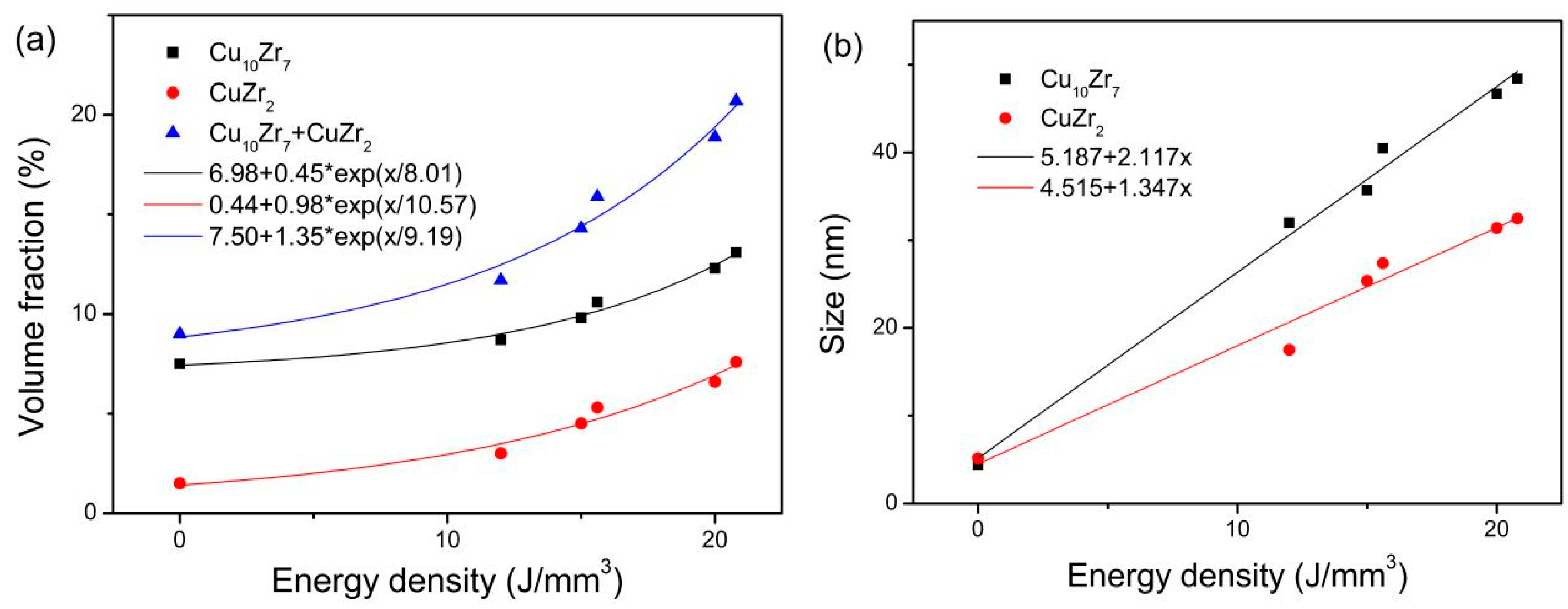
- The dependence of volume fraction of Cu10Zr7 and CuZr2 on energy density can be fitted by an exponential function.
- The crystalline size of Cu10Zr7 and CuZr2 linearly increases with increasing energy density.
- The crystallization enthalpy ΔHx exponential changed with the amorphous content.
- The thermal stability is larger for the gas-atomized powders than for the SLMed bulk ones
Abstract
1. Introduction
2. Experimental
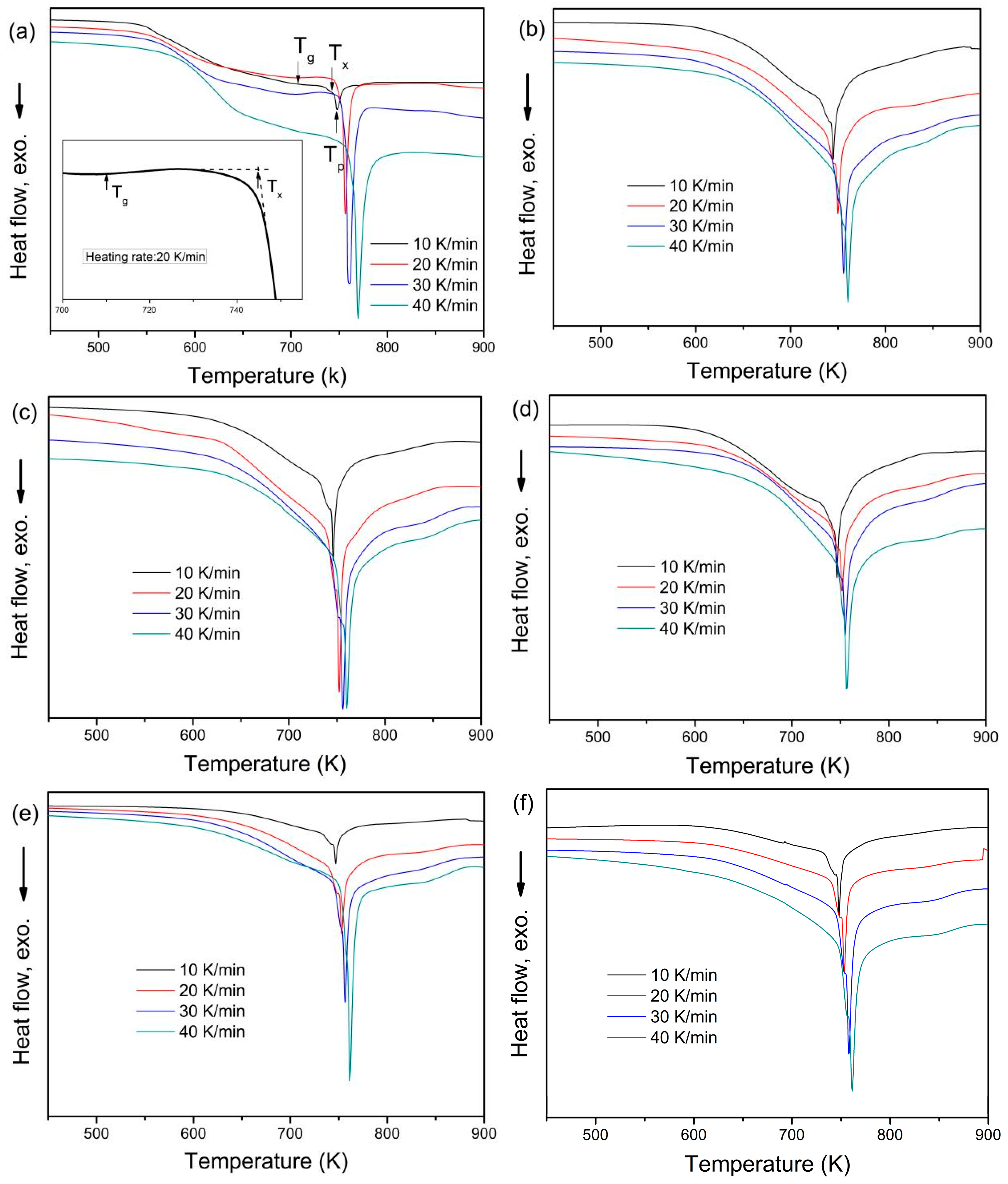
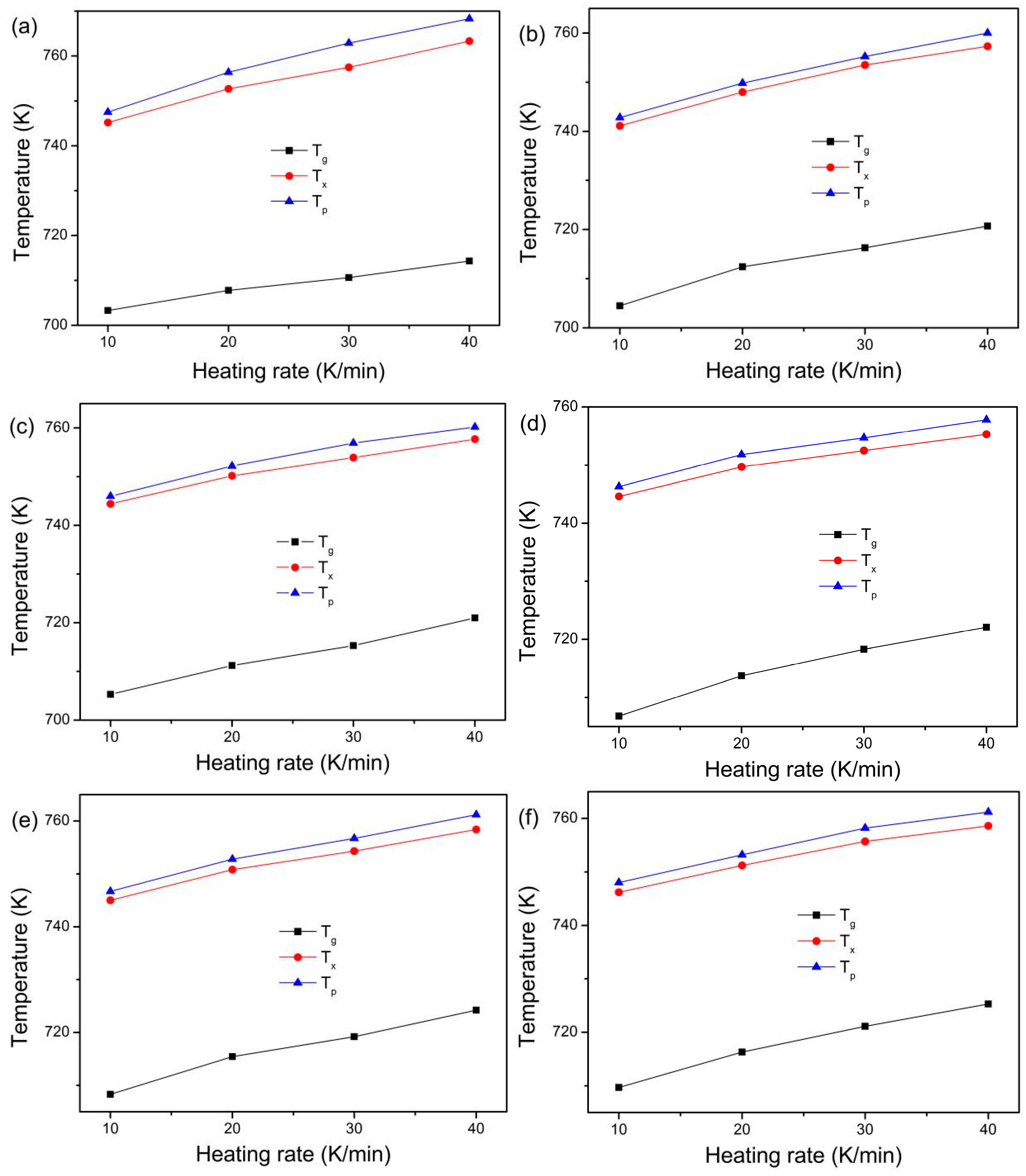
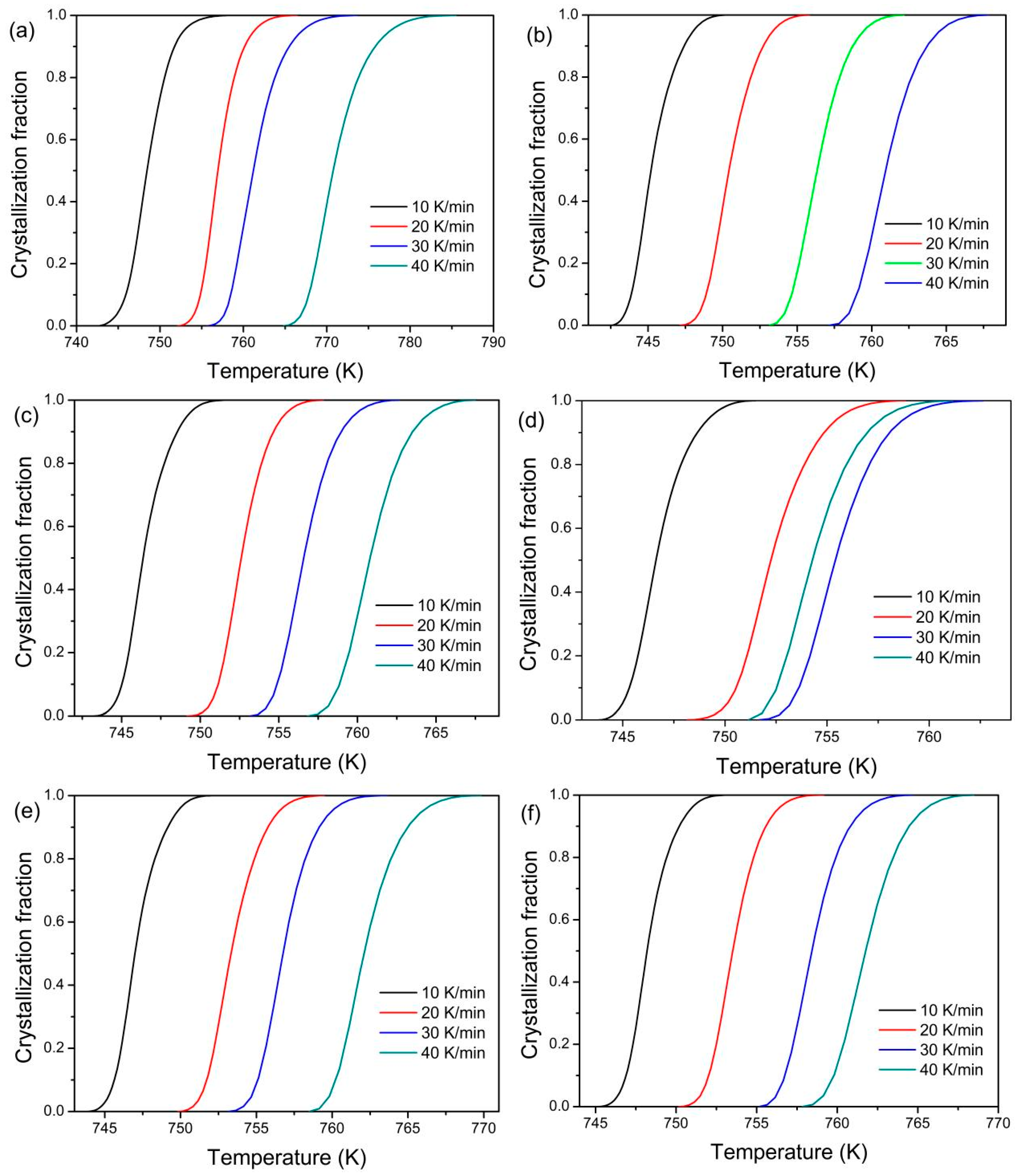
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prabhu, Y.; Vincent, S.; Bhatt, J. Thermodynamic modelling to optimize glass forming composition in multicomponent Zr-Cu-Co-Al system. Mater. Today Proc. 2020, 28, 1239–1244. [Google Scholar]
- Han, K.M.; Jiang, H.; Wang, Y.M.; Qiang, J.B.; Yu, C.Y. Antimicrobial Zr-based bulk metallic glasses for surgical devices ap-plications. J. Non-Cryst. Solids 2021, 564, 120827. [Google Scholar]
- Wada, T.; Zhang, T.; Inoue, A. Formation and high mechanical strength of bulk glassy alloys in Zr-Al-Co-Cu System. Mater. Trans. 2003, 44, 1839–1844. [Google Scholar] [CrossRef]
- Zhang, T.; Inoue, A. Formation, thermal and mechanical properties of bulk glassy alloys in Zr–Al–Co and Zr–Al–Co–Cu systems. Mater. Sci. Eng. A 2004, 375–377, 432–435. [Google Scholar]
- Wang, Z.; Ketov, S.V.; Sun, B.A.; Chen, C.L.; Churyumov, A.Y.; Louzguine-Luzgin, D.V. Eutectic crystallization during fracture of Zr–Cu–Co–Al metallic glass. Mater. Sci. Eng. A 2016, 657, 210–214. [Google Scholar]
- Han, K.; Qiang, J.; Wang, Y.; Häussler, P. Zr-Al-Co-Cu bulk metallic glasses for biomedical devices applications. J. Alloy. Compd. 2017, 729, 144–149. [Google Scholar]
- Kuo, C.; Huang, J.; Li, J.; Jang, J.; Lin, C.; Nieh, T. Effects of B2 precipitate size on transformation-induced plasticity of Cu–Zr–Al glassy alloys. J. Alloy. Compd. 2013, 590, 453–458. [Google Scholar]
- Wu, Y.; Xiao, Y.; Chen, G.; Liu, C.T.; Lu, Z. Bulk metallic glass composites with transformation-mediated work-hardening and ductility. Adv. Mater. 2010, 22, 2770–2773. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, C.; Xing, W.; Liu, L. 3D printing of Zr-based bulk metallic glasses with complex geometries and enhanced catalytic properties. Intermetallics 2018, 94, 22–28. [Google Scholar]
- Wu, M.-W.; Ni, K.; Lei, Y.; Xiong, X.-X.; Chuang, Y.-T.; Lin, Q.-E.; Wang, P.; Ramasamy, P.; Eckert, J. Mechanical behavior of CuZrAl metallic glass scaffolds fabricated by selective laser melting. Mater. Lett. 2023, 341, 124242. [Google Scholar]
- Yang, C.; Ouyang, D.; Zhang, L.; Zhang, Y.; Tong, X.; Ke, H.; Chan, K.; Wang, W. The enhancement of damage tolerance of 3D-printed high strength architected metallic glasses by unit cell shape design. Addit. Manuf. 2024, 85, 104125. [Google Scholar]
- Aliyu, A.A.A.; Panwisawas, C.; Shinjo, J.; Puncreobutr, C.; Reed, R.C.; Poungsiri, K.; Lohwongwatana, B. Laser-based additive manufacturing of bulk metallic glasses: Recent advances and future perspectives for biomedical applications. J. Mater. Res. Technol. 2023, 23, 2956–2990. [Google Scholar]
- Wu, W.; Li, X.; Liu, Q.; Fuh, J.Y.H.; Zheng, A.; Zhou, Y.; Ren, L.; Li, G. Additive manufacturing of bulk metallic glass: Principles, materials and prospects. Mater. Today Adv. 2022, 16, 100319. [Google Scholar]
- Luo, H.; Fan, A.; Liao, W.; Du, Y. Effect of laser power on the structure and wear performance of laser additively manufactured Cu45Zr45Al6Ti4 metallic glass coating. Surf. Coat. Technol. 2024, 482, 130706. [Google Scholar]
- Liu, H.; Jiang, Y.; Yang, D.; Jiang, Q.; Yang, W. Pores and cracks in the metallic glasses prepared by laser powder bed fusion. J. Mater. Res. Technol. 2023, 26, 3070–3089. [Google Scholar]
- Pauzon, C.; Daudin, R.; Robaut, F.; Berthomé, G.; Blandin, J.-J. Laser powder bed fusion spatters of Zr-Cu-Al-Nb metallic glass. J. Alloy. Compd. 2023, 976, 173073. [Google Scholar]
- Rodríguez-Sánchez, M.; Sadanand, S.; Ghavimi, A.; Busch, R.; Tiberto, P.; Ferrara, E.; Barrera, G.; Thorsson, L.; Wachter, H.; Gallino, I.; et al. Relating laser powder bed fusion process parameters to (micro)structure and to soft magnetic behaviour in a Fe-based bulk metallic glass. Materialia 2024, 35, 102111. [Google Scholar]
- Li, B.; Yakubov, V.; Nomoto, K.; Ringer, S.P.; Gludovatz, B.; Li, X.; Kruzic, J.J. Superior mechanical properties of a Zr-based bulk metallic glass via laser powder bed fusion process control. Acta Mater. 2024, 266, 1196. [Google Scholar]
- Frey, M.; Wegner, J.; Barreto, E.S.; Ruschel, L.; Neuber, N.; Adam, B.; Riegler, S.S.; Jiang, H.-R.; Witt, G.; Ellendt, N.; et al. Laser powder bed fusion of Cu-Ti-Zr-Ni bulk metallic glasses in the Vit101 alloy system. Addit. Manuf. 2023, 66, 103467. [Google Scholar]
- Ouyang, D.; Zhang, P.; Zhang, C.; Liu, L. Understanding of crystallization behaviors in laser 3D printing of bulk metallic glasses. Appl. Mater. Today 2021, 23, 100988. [Google Scholar]
- Pacheco, V.; Karlsson, D.; Marattukalam, J.J.; Stolpe, M.; Hjörvarsson, B.; Jansson, U.; Sahlberg, M. Thermal stability and crystallization of a Zr-based metallic glass produced by suction casting and selective laser melting. J. Alloy. Compd. 2020, 825, 153995. [Google Scholar] [CrossRef]
- Ouyang, D.; Li, N.; Liu, L. Structural heterogeneity in 3D printed Zr-based bulk metallic glass by selective laser melting. J. Alloy. Compd. 2018, 740, 603–609. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, C.; Pan, J.; Ouyang, D.; Liu, L. Toughening additive manufactured Zr-based bulk metallic glass composites by martensite phase transformation. J. Mater. Sci. Technol. 2023, 170, 95–102. [Google Scholar] [CrossRef]
- Gao, X.; Liu, Z.; Li, J.; Liu, E.; Yue, C.; Zhao, K.; Yang, G. Selective laser melting of CuZr-based metallic glass composites. Mater. Lett. 2020, 259, 126724. [Google Scholar] [CrossRef]
- Kozachkov, H.; Kolodziejska, J.; Johnson, W.L.; Hofmann, D.C. Effect of cooling rate on the volume fraction of B2 phases in a CuZrAlCo metallic glass matrix composite. Intermetallics 2013, 39, 89–93. [Google Scholar] [CrossRef]
- Cai, A.; Zhou, G.; Li, P.; Ding, D.; An, Q.; Li, Y.; Yang, Q.; Mao, H. Mechanical, wetting and corrosion properties of a Zr-based amorphous alloy composite consolidated by spark plasma sintering. J. Non-Cryst. Solids 2023, 621, 122758. [Google Scholar] [CrossRef]
- Han, X.; Kaban, I.; Orava, J.; Cheng, Q.; Sun, Y.H.; Soldatov, I.; Zimmermann, M.V.; Song, K.; Nielsch, K. Phase-formation maps of CuZrAlCo metallic glass explored by in situ ultrafast techniques. Acta Mater. 2022, 241, 118371. [Google Scholar] [CrossRef]
- Lan, S.; Wu, Z.; Wei, X.; Zhou, J.; Lu, Z.; Neuefeind, J.; Wang, X.-L. Structure origin of a transition of classic-to-avalanche nucleation in Zr-Cu-Al bulk metallic glasses. Acta Mater. 2018, 149, 108–118. [Google Scholar] [CrossRef]
- Wang, C.; Schmelzer, J.W.; Zhang, L.; Zhang, L.; Wang, L.; Schick, C.; Gao, Y.; Zhao, B. Effect of cooling rate on the crystallization behaviors of Mg65Zn30Ca5 metallic glass composites. Intermetallics 2024, 169, 108295. [Google Scholar] [CrossRef]
- Yang, Z.; Markl, M.; Körner, C. Comprehensive numerical investigation of laser powder bed fusion process conditions for bulk metallic glasses. Addit. Manuf. 2024, 81, 104026. [Google Scholar] [CrossRef]
- Sohrabi, N.; Ivas, T.; Jhabvala, J.; Schawe, J.E.; Löffler, J.F.; Ghasemi-Tabasi, H.; Logé, R.E. Quantitative prediction of crystallization in laser powder bed fusion of a Zr-based bulk metallic glass with high oxygen content. Mater. Des. 2024, 239, 112744. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar]
- Bing, L.; Li, Y.H.; Yang, K.; Li, J.S.; Fan, X.H. Effect of yttrium addition on the non-isothermal crystallization kinetics and fragility of Cu-Zr-Al bulk metallic glass. Thermochim. Acta 2016, 642, 105–110. [Google Scholar]
- Zhu, M.; Li, J.; Yao, L.; Jian, Z.; Chang, F.; Yang, G. Non-isothermal crystallization kinetics and fragility of (Cu46Zr47Al7)97Ti3 bulk metallic glass investigated by differential scanning calorimetry. Thermochim. Acta 2013, 565, 132–136. [Google Scholar] [CrossRef]
- Cai, A.; Li, P.; Ding, D.; An, Q.; Zhou, G.; Yang, Q.; Lin, Y.; Mao, H. Crystallization behavior of a series of Zr-based metallic glasses. Thermochim. Acta 2022, 717, 179346. [Google Scholar]
- An, Q.; Zhou, G.; Cai, A.; Li, P.; Ding, D.; Zhou, G.; Yang, Q.; Mao, H. Effect of Ti and Al ratio on glass forming ability and crystallization behavior of Zr-Cu-Al-Ti alloy powders. Thermochim. Acta 2022, 710, 179363. [Google Scholar] [CrossRef]
- Cai, A.; Li, P.; Ding, D.; An, Q.; Zhou, G.; Li, Y.; Mao, H. Preparation and crystallization behavior of Cu-Zr-Ti amorphous composite powders. J. Non-Cryst. Solids 2023, 625, 122758. [Google Scholar] [CrossRef]
- Cai, A.; Li, P.; Ding, D.; An, Q.; Zhou, G.; Yang, Q.; Lin, Y.; Mao, H. Crystallization kinetics of Cu50Zr40Ti10 amorphous powder. Thermochim. Acta 2022, 714, 179261. [Google Scholar]
- Lu, W.; Yan, B.; Huang, W.-H. Complex primary crystallization kinetics of amorphous Finemet alloy. J. Non-Cryst. Solids 2005, 351, 3320–3324. [Google Scholar] [CrossRef]
- Cai, A.; Zhou, G.; Ding, D.; Wu, H.; An, Q.; Zhou, G.; Yang, Q.; Li, P. Effect of Ti addition on crystallization behavior of a Zr-based bulk metallic glass. Thermochim. Acta 2022, 709, 179159. [Google Scholar]
- Yang, Z.; Al-Mukadam, R.; Stolpe, M.; Markl, M.; Deubener, J.; Körner, C. Isothermal crystallization kinetics of an industrial-grade Zr-based bulk metallic glass. J. Non-Cryst. Solids 2021, 573, 121145. [Google Scholar]
- Cui, J.; Li, J.; Wang, J.; Kou, H.; Qiao, J.; Gravier, S.; Blandin, J. Crystallization kinetics of Cu38Zr46Ag8Al8 bulk metallic glass in different heating conditions. J. Non-Cryst. Solids 2014, 404, 7–12. [Google Scholar]
- Gao, Q.; Jian, Z.Y.; Xu, J.F.; Zhu, M.; Chang, F.G.; Han, A.M. Crystallization kinetics of the Cu50Zr50 metallic glass under iso-thermal conditions. J. Solid State Chem. 2016, 244, 116–119. [Google Scholar]
- Qiao, J.; Pelletier, J. Isochronal and isothermal crystallization in Zr55Cu30Ni5 Al10 bulk metallic glass. Trans. Nonferrous Met. Soc. China 2012, 22, 577–584. [Google Scholar]
- Sohrabi, S.; Gholamipour, R. Effect of Nb minor addition on the crystallization kinetics of Zr-Cu-Al-Ni metallic glass. J. Non-Cryst. Solids 2021, 560, 120731. [Google Scholar]
- Pauly, S.; Das, J.; Mattern, N.; Kim, D.H.; Eckert, J. Phase formation and thermal stability in Cu–Zr–Ti(Al) metallic glasses. Intermetallics 2009, 17, 453–462. [Google Scholar]
- Qiao, J.; Pelletier, J. Crystallization kinetics in Cu46Zr45Al7Y2 bulk metallic glass by differential scanning calorimetry (DSC). J. Non-Cryst. Solids 2011, 357, 2590–2594. [Google Scholar]
- Blazquez, J.; Conde, C.; Conde, A. Non-isothermal approach to isokinetic crystallization processes: Application to the nanocrystallization of HITPERM alloys. Acta Mater. 2005, 53, 2305–2311. [Google Scholar]
- Sun, N.; Liu, X.; Lu, K. An explanation to the anomalous avrami exponent. Scr. Mater. 1996, 34, 1201–1207. [Google Scholar]
- Arroyave, A.; Eagar, T.W.; Kaufman, L. Thermodynamic assessment of the Cu-Ti-Zr system. J. Alloys Compd. 2003, 351, 158–170. [Google Scholar]
- Sohrabi, N.; Schawe, J.E.K.; Jhabvala, J.; Löffler, J.F.; Logé, R.E. Critical crystallization properties of an industrial-grade Zr-based metallic glass used in additive manufacturing. Scr. Mater. 2021, 199, 113861. [Google Scholar] [CrossRef]
- Kim, J.; Lee, D.; Shin, S.; Lee, C. Phase evolution in Cu54Ni6Zr22Ti18 bulk metallic glass Nd: YAG laser weld. Mater. Sci. Eng. A 2006, 434, 194–201. [Google Scholar] [CrossRef]
- Sun, H.; Flores, K. Laser deposition of a Cu-based metallic glass powder on a Zr-based glass substrate. J. Mater. Res. 2008, 23, 2692–2703. [Google Scholar] [CrossRef]
- Ouyang, D.; Xing, W.; Li, N.; Li, Y.; Liu, L. Structural evolutions in 3D-printed Fe-based metallic glass fabricated by selective laser melting. Addit. Manuf. 2018, 23, 246–252. [Google Scholar] [CrossRef]
- Lindwall, J.; Lundbäck, A.; Marattukalam, J.J.; Ericsson, A. Virtual Development of Process Parameters for Bulk Metallic Glass Formation in Laser-Based Powder Bed Fusion. Materials 2022, 15, 450. [Google Scholar] [CrossRef] [PubMed]
- Marattukalam, J.J.; Pacheco, V.; Karlsson, D.; Riekehr, L.; Lindwall, J.; Forsberg, F.; Jansson, U.; Sahlberg, M.; Hjörvarsson, B. Development of process parameters for selective laser melting of a Zr-based bulk metallic glass. Addit. Manuf. 2020, 33, 101124. [Google Scholar] [CrossRef]
- Lindwall, J.; Ericsson, A.; Marattukalam, J.J.; Hassila, C.-J.; Karlsson, D.; Sahlberg, M.; Fisk, M.; Lundbäck, A. Simulation of phase evolution in a Zr-based glass forming alloy during multiple laser remelting. J. Mater. Res. Technol. 2022, 16, 1165–1178. [Google Scholar] [CrossRef]
- Christian, J.W. (Ed.) The Theory of Transformation in Metals and Alloys; Pergamon Press: London, UK, 1965. [Google Scholar]
- Henderson, D.W. Experimental analysis of non-isothermal transformations involving nucleation and growth. J. Therm. Anal. Calorim. 1979, 15, 325–331. [Google Scholar] [CrossRef]
- Wu, Y.; Li, B.; Zhu, Y.; Yuan, X.; Yan, T.; Zhang, H.; Fu, H.; Zhang, H.; Zhang, L. Crystallization path and non-isothermal kinetics of the Zr59.5Cu14.4Ni11.6Al9.7Nb4.8 metallic glass under different heating rates. Scr. Mater. 2024, 254, 116339. [Google Scholar] [CrossRef]



| Laser Power (W) | Scanning Velocity (mm/s) | Layer Thickness (mm) | Hatch Spacing (mm) | Energy Density (J/mm3) |
|---|---|---|---|---|
| 75 | 2000 | 0.08 | 0.03 | 15.6 |
| 75 | 1500 | 0.08 | 0.03 | 20.8 |
| 90 | 2500 | 0.1 | 0.03 | 12 |
| 90 | 2000 | 0.1 | 0.03 | 15 |
| 90 | 1500 | 0.1 | 0.03 | 20 |
| Energy Density (J/mm3) | 0 | 12 | 15 | 15.6 | 20 | 20.8 |
|---|---|---|---|---|---|---|
| Cu10Zr7 (Vol.%) | 7.5 | 8.7 | 9.8 | 10.6 | 12.3 | 13.1 |
| CuZr2 (Vol.%) | 1.5 | 3.0 | 4.5 | 5.3 | 6.6 | 7.6 |
| Cu10Zr7 (nm) | 4.4 | 32.0 | 35.7 | 40.5 | 46.7 | 48.4 |
| CuZr2 (nm) | 5.2 | 17.5 | 25.4 | 27.4 | 31.4 | 32.5 |
| Power Density (J/mm3) | 0 | 12 | 15 | 15.6 | 20 | 20.8 |
|---|---|---|---|---|---|---|
| ΔHx (J/g) | 20.2 | 15.6 | 14.5 | 13.2 | 13.0 | 12.6 |
| ΔTx (K) | 45.8 | 35.3 | 35.4 | 36.5 | 34.8 | 38.4 |
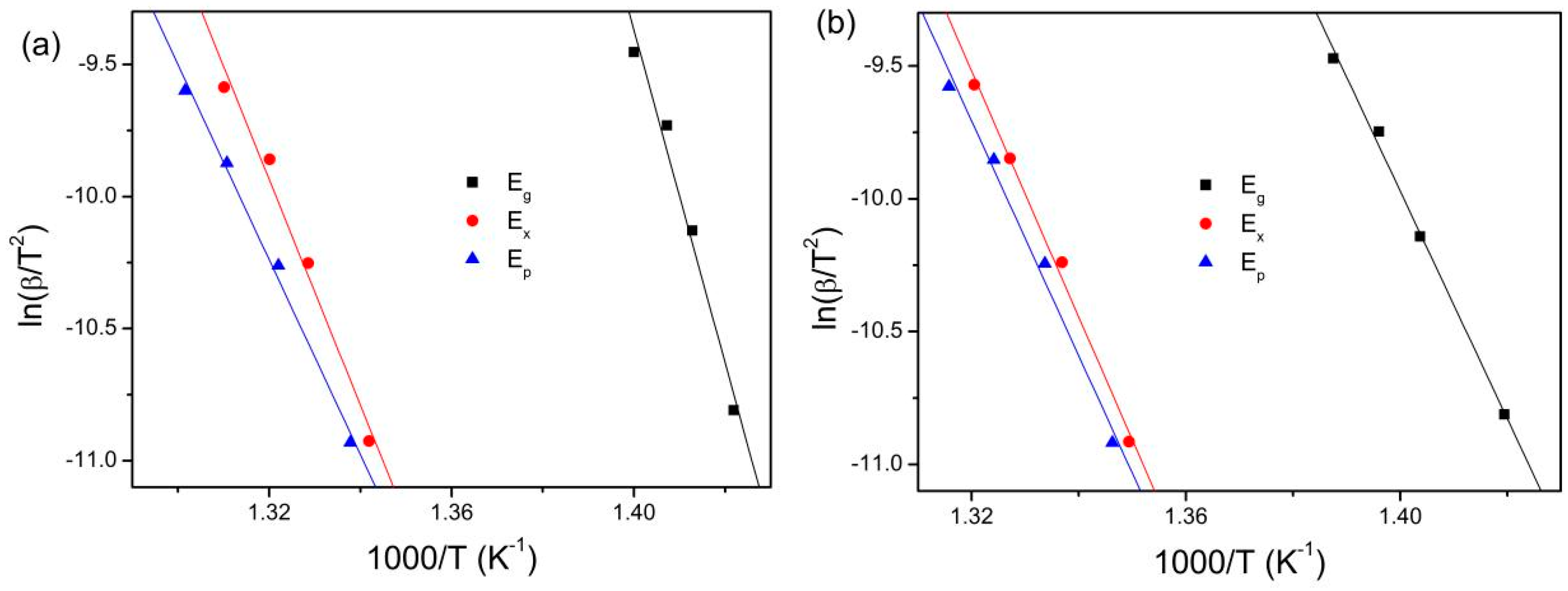
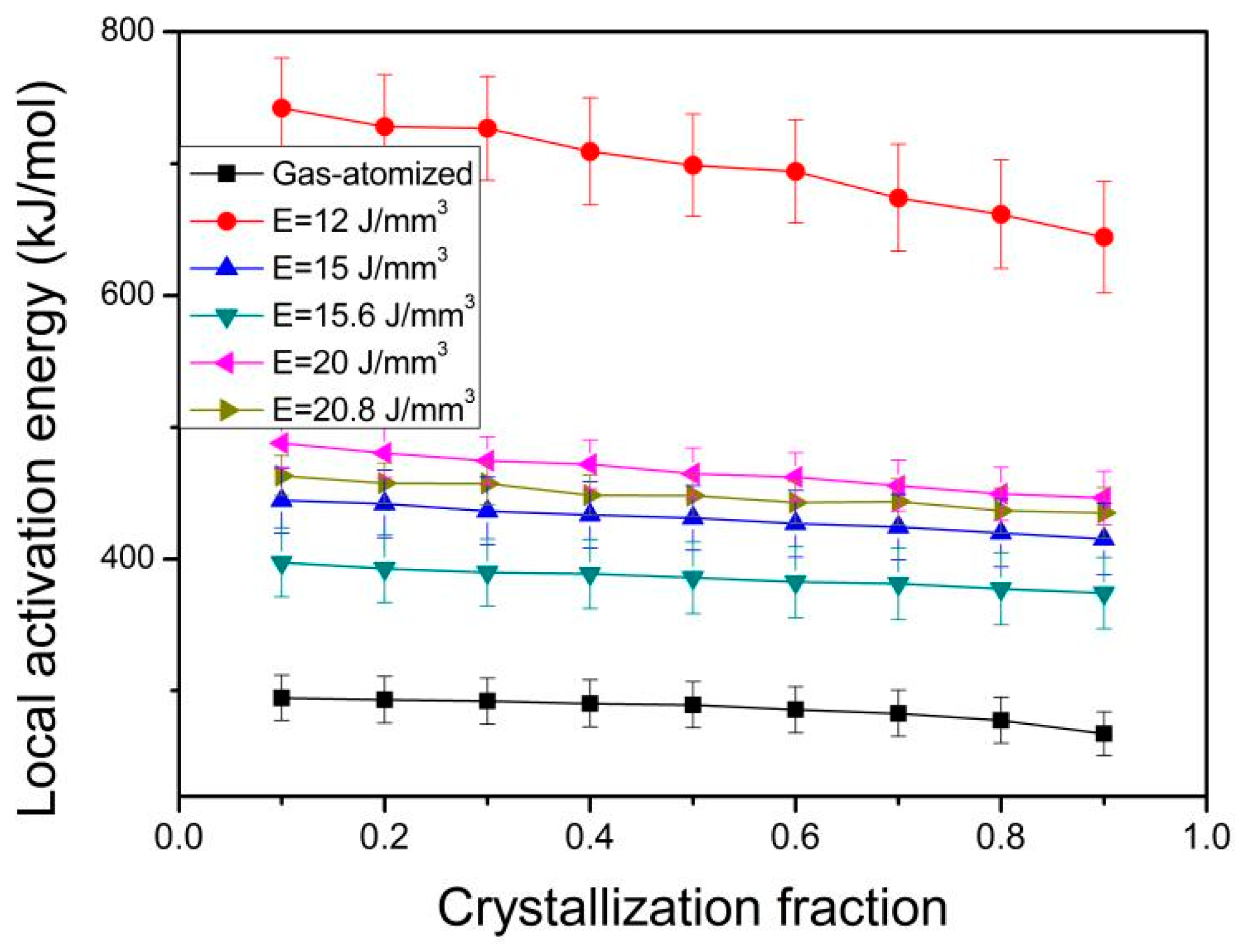
| Eg (kJ/mol) | 523.6 | 374.8 | 368.1 | 356.1 | 370.5 | 365.4 |
| Ex (kJ/mol) | 355.5 | 602.3 | 482.0 | 384.7 | 506.8 | 482.9 |
| Ep (kJ/mol) | 306.9 | 564.0 | 446.3 | 367.2 | 475.2 | 446.3 |
| Power Density (J/mm3) | 0 | 12 | 15 | 15.6 | 20 | 20.8 |
|---|---|---|---|---|---|---|
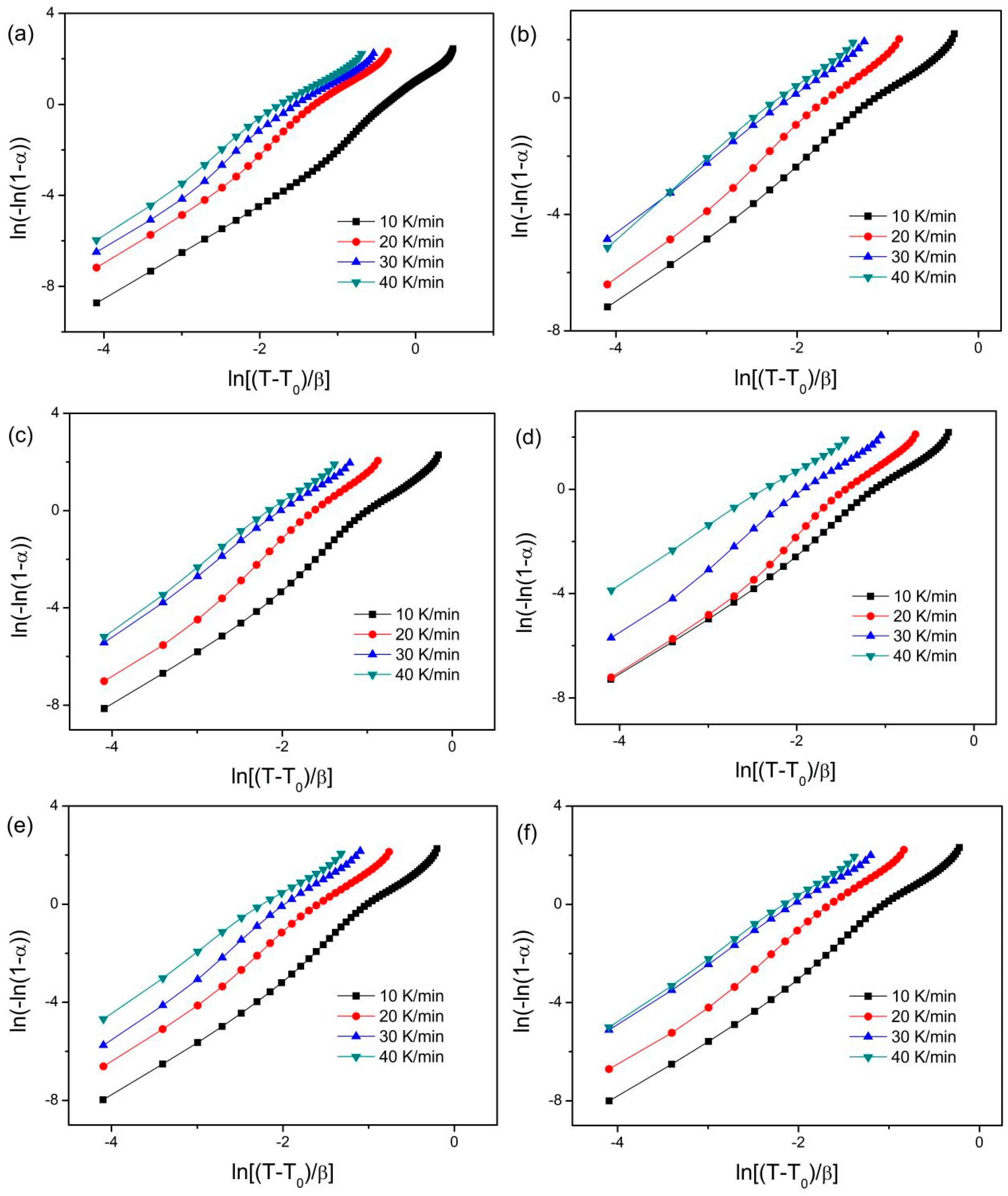
| 10 K/min | 2.5 | 2.4 | 2.7 | 2.7 | 2.5 | 2.6 |
| 20 K/min | 2.5 | 2.5 | 2.7 | 2.6 | 2.5 | 2.5 |
| 30 K/min | 2.5 | 2.5 | 2.6 | 2.5 | 2.4 | 2.5 |
| 40 K/min | 2.4 | 2.5 | 2.5 | 2.4 | 2.5 | 2.5 |
| Everaged n(α) | 2.5 | 2.5 | 2.6 | 2.6 | 2.5 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, Q.; Li, R.; Hu, Y.; Luo, Y.; Cai, A.; Li, Y.; Mao, H.; Li, S. Non-Isothermal Crystallization Behavior of a Zr-Based Amorphous Alloy Composite Prepared by Selective Laser Melting. Materials 2025, 18, 1631. https://doi.org/10.3390/ma18071631
An Q, Li R, Hu Y, Luo Y, Cai A, Li Y, Mao H, Li S. Non-Isothermal Crystallization Behavior of a Zr-Based Amorphous Alloy Composite Prepared by Selective Laser Melting. Materials. 2025; 18(7):1631. https://doi.org/10.3390/ma18071631
Chicago/Turabian StyleAn, Qi, Rui Li, Yalin Hu, Yun Luo, Anhui Cai, Yixian Li, Hong Mao, and Sheng Li. 2025. "Non-Isothermal Crystallization Behavior of a Zr-Based Amorphous Alloy Composite Prepared by Selective Laser Melting" Materials 18, no. 7: 1631. https://doi.org/10.3390/ma18071631
APA StyleAn, Q., Li, R., Hu, Y., Luo, Y., Cai, A., Li, Y., Mao, H., & Li, S. (2025). Non-Isothermal Crystallization Behavior of a Zr-Based Amorphous Alloy Composite Prepared by Selective Laser Melting. Materials, 18(7), 1631. https://doi.org/10.3390/ma18071631






