The Structural Effect of a Composite Solid Electrolyte on Electrochemical Performance and Fire Safety
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
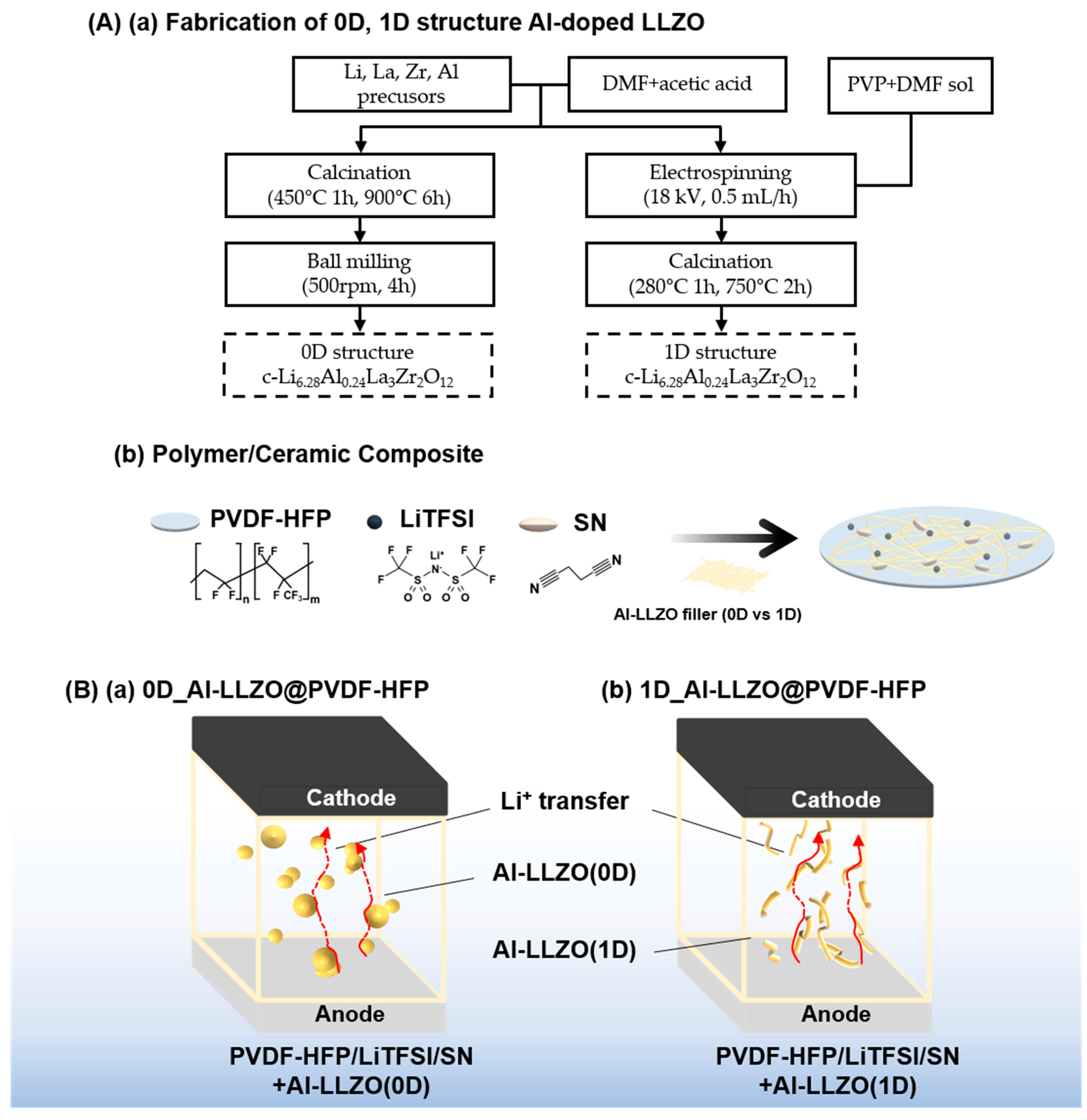
2.2. Synthesis of Al-LLZO(0D) and Al-LLZO(1D)
2.3. Preparation of Composite Solid Electrolyte
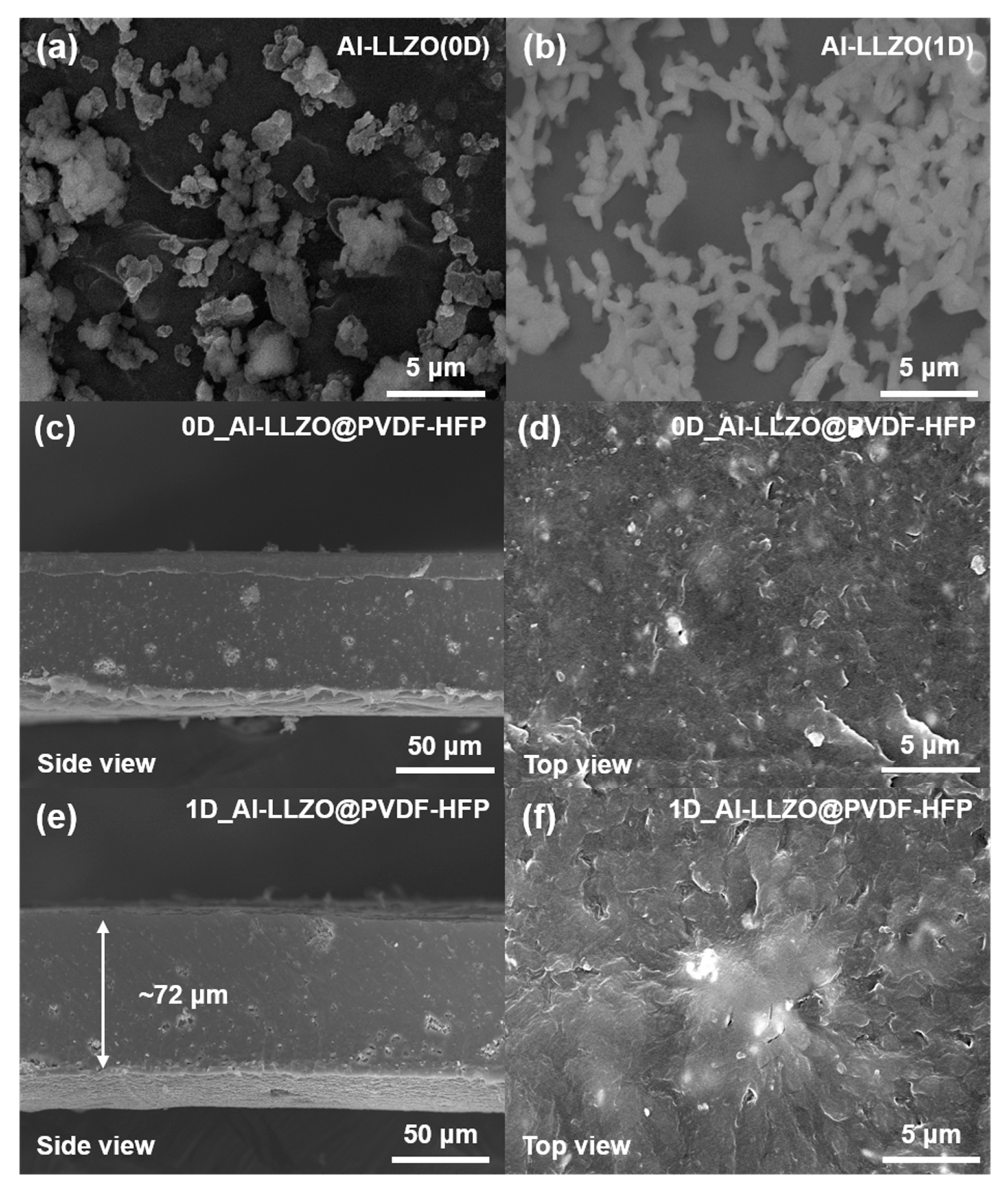
2.4. Material Characterizations
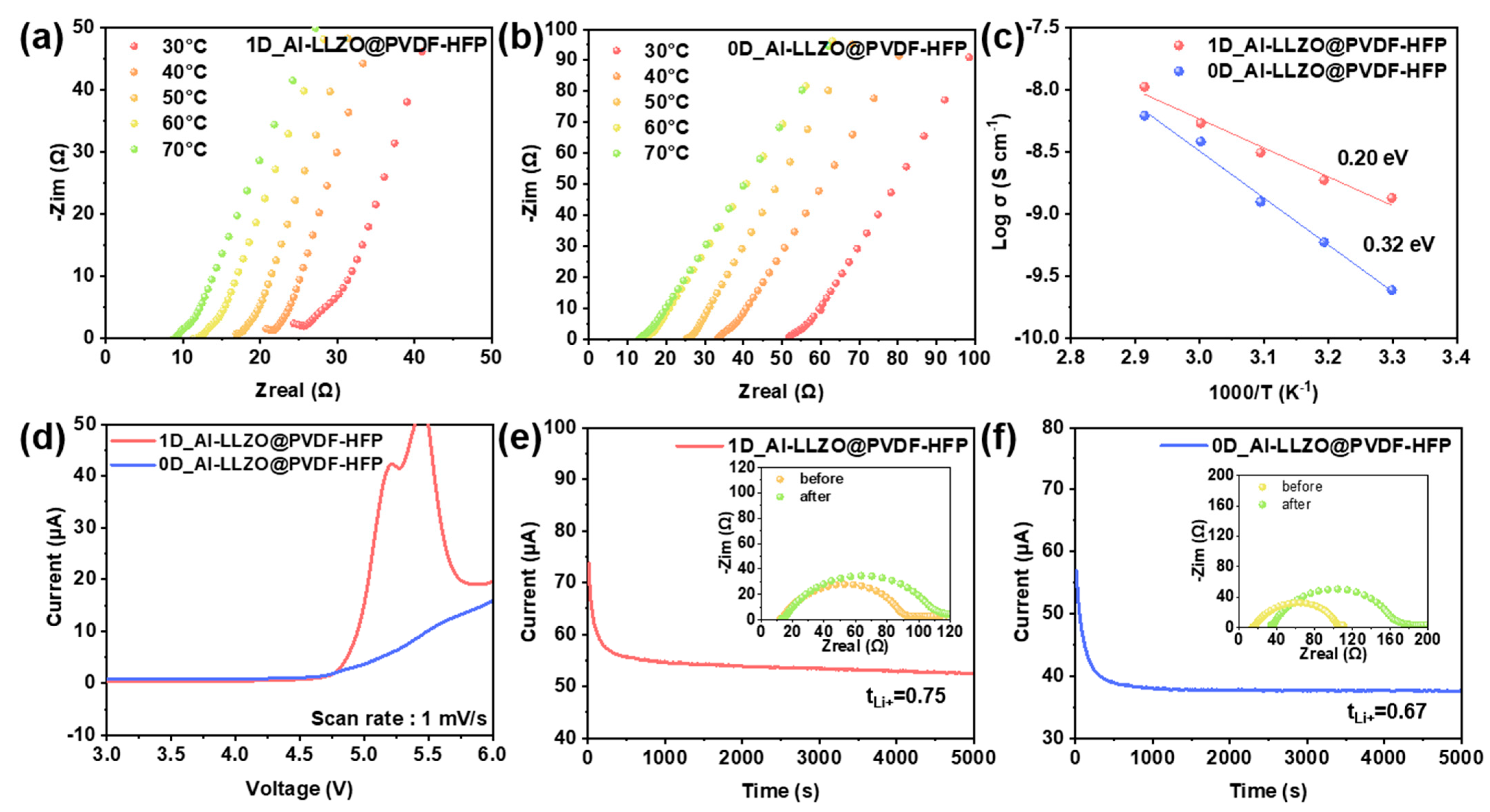
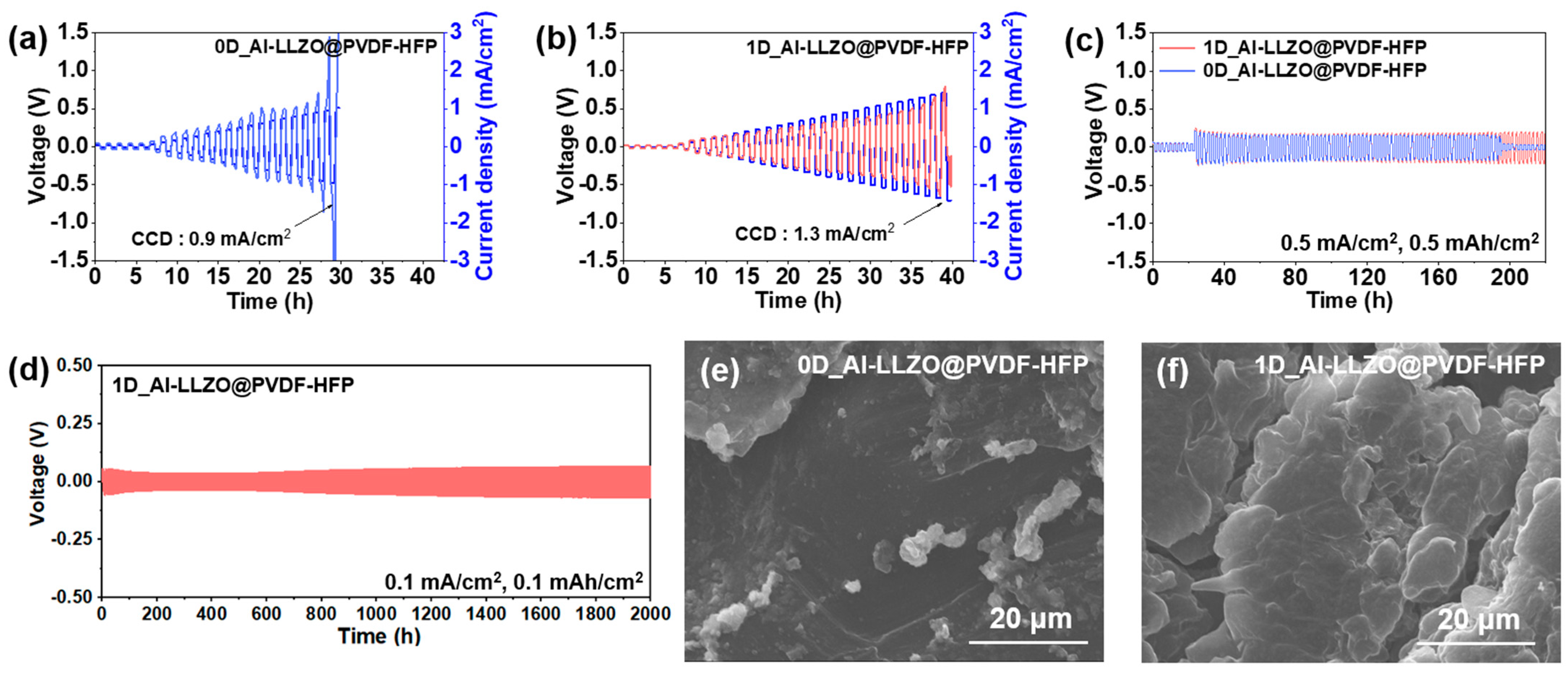
2.5. Electrochemical Characterizations
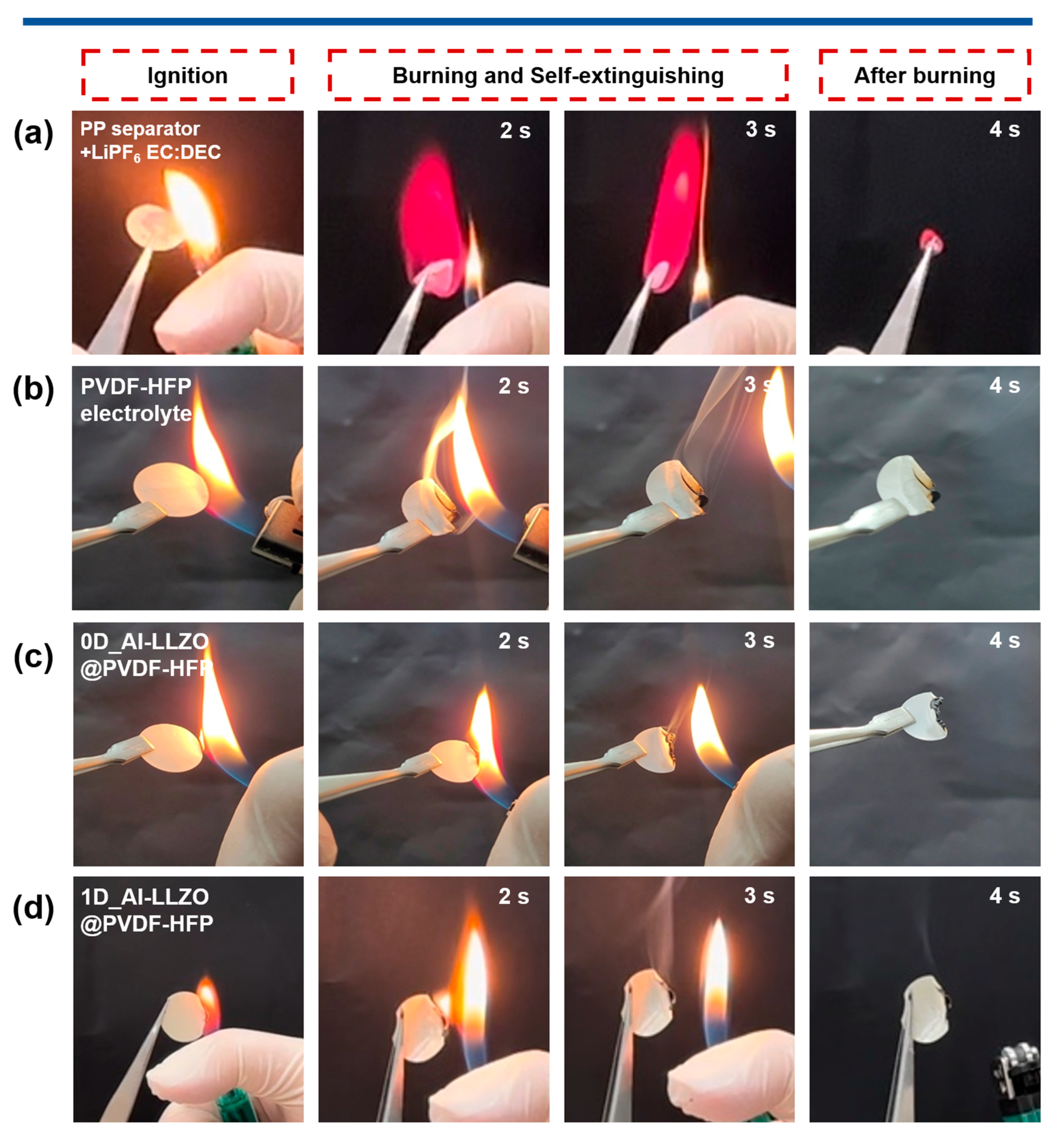
2.6. Fire Safety Test
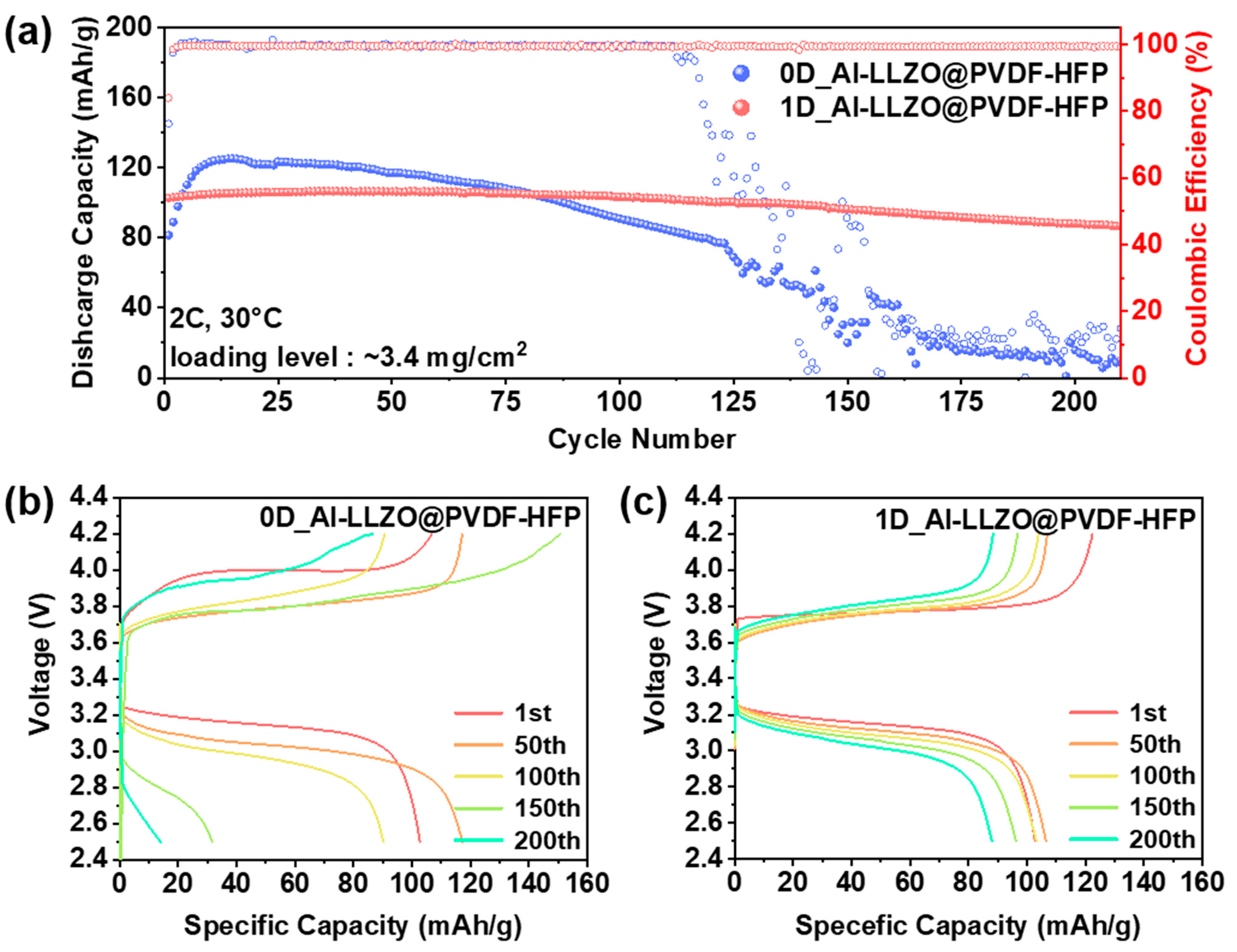
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaguemont, J.; Bardé, F. A Critical Review of Lithium-Ion Battery Safety Testing and Standards. Appl. Therm. Eng. 2023, 231, 121014. [Google Scholar] [CrossRef]
- Su, L.; Jarvis, K.; Charalambous, H.; Dolocan, A.; Manthiram, A. Stabilizing High-Nickel Cathodes with High-Voltage Electrolytes. Adv. Funct. Mater. 2023, 33, 2213675. [Google Scholar] [CrossRef]
- Khan, F.M.N.U.; Rasul, M.G.; Sayem, A.S.M.; Mandal, N. Maximizing Energy Density of Lithium-Ion Batteries for Electric Vehicles: A Critical Review. Energy Rep. 2023, 9, 11–21. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, D.; Wang, J.; Yang, K.; Wang, Z.; Chen, Z.; Zhang, S.; Zhang, C.; Yang, X. Inorganic Crosslinked Supramolecular Binder with Fast Self-Healing for High Performance Silicon Based Anodes in Lithium-Ion Batteries. J. Colloid Interface Sci. 2022, 625, 373–382. [Google Scholar] [CrossRef]
- Lee, H.G.; Kim, S.Y.; Lee, J.S. Dynamic Observation of Dendrite Growth on Lithium Metal Anode during Battery Charging/Discharging Cycles. NPJ Comput. Mater. 2022, 8, 103. [Google Scholar] [CrossRef]
- Menkin, S.; Fritzke, J.B.; Larner, R.; de Leeuw, C.; Choi, Y.; Gunnarsdóttir, A.B.; Grey, C.P. Insights into Soft Short Circuit-Based Degradation of Lithium Metal Batteries. Faraday Discuss. 2023, 248, 277–297. [Google Scholar] [CrossRef]
- Gebert, F.; Longhini, M.; Conti, F.; Naylor, A.J. An Electrochemical Evaluation of State-of-the-Art Non-Flammable Liquid Electrolytes for High-Voltage Lithium-Ion Batteries. J. Power Sources 2023, 556, 232412. [Google Scholar] [CrossRef]
- Khan, F.M.N.U.; Rasul, M.G.; Sayem, A.S.M.; Mandal, N.K. Design and Optimization of Lithium-Ion Battery as an Efficient Energy Storage Device for Electric Vehicles: A Comprehensive Review. J. Energy Storage 2023, 71, 108033. [Google Scholar] [CrossRef]
- Lim, J.H.; Won, J.H.; Kim, M.K.; Jung, D.S.; Kim, M.; Park, C.; Koo, S.M.; Oh, J.M.; Jeong, H.M.; Sohn, H.; et al. Synthesis of Flower-like Manganese Oxide for Accelerated Surface Redox Reactions on Nitrogen-Rich Graphene of Fast Charge Transport for Sustainable Aqueous Energy Storage. J. Mater. Chem. A 2022, 10, 7668–7676. [Google Scholar] [CrossRef]
- Jeong, Y.; Park, J.; Lee, S.; Oh, S.H.; Kim, W.J.; Ji, Y.J.; Park, G.Y.; Seok, D.; Shin, W.H.; Oh, J.M.; et al. Iron Oxide-Carbon Nanocomposites Modified by Organic Ligands: Novel Pore Structure Design of Anode Materials for Lithium-Ion Batteries. J. Electroanal. Chem. 2022, 904, 115905. [Google Scholar] [CrossRef]
- Hwang, K.; Kim, N.; Jeong, Y.; Sohn, H.; Yoon, S. Controlled Nanostructure of a Graphene Nanosheet-TiO2 Composite Fabricated via Mediation of Organic Ligands for High-Performance Li Storage Applications. Int. J. Energy Res. 2021, 45, 16189–16203. [Google Scholar] [CrossRef]
- Seok, D.; Shin, W.H.; Kang, S.W.; Sohn, H. Piezoelectric Composite of BaTiO3-Coated SnO2 Microsphere: Li-Ion Battery Anode with Enhanced Electrochemical Performance Based on Accelerated Li+ Mobility. J. Alloys Compd. 2021, 870, 159267. [Google Scholar] [CrossRef]
- Seok, D.; Jeong, Y.; Han, K.; Yoon, D.Y.; Sohn, H. Recent Progress of Electrochemical Energy Devices: Metal Oxide-Carbon Nanocomposites as Materials for next-Generation Chemical Storage for Renewable Energy. Sustainability 2019, 11, 3694. [Google Scholar] [CrossRef]
- Hwang, K.; Sohn, H.; Yoon, S. Mesostructured Niobium-Doped Titanium Oxide-Carbon (Nb-TiO2-C) Composite as an Anode for High-Performance Lithium-Ion Batteries. J. Power Sources 2018, 378, 225–234. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Yun, X.; Gao, P.; Zheng, C.; Xiao, P. Critical Review of Fluorinated Electrolytes for High-Performance Lithium Metal Batteries. Adv. Funct. Mater. 2023, 33, 2300502. [Google Scholar] [CrossRef]
- Wang, R.; Cui, W.; Chu, F.; Wu, F. Lithium Metal Anodes: Present and Future. J. Energy Chem. 2020, 48, 145–159. [Google Scholar] [CrossRef]
- Park, D.; Shin, W.; Sohn, H. The Enhanced Electrochemical Performance of Lithium Metal Batteries through the Piezoelectric Protective Layer. Membr. J. 2023, 33, 13–22. [Google Scholar] [CrossRef]
- Lee, S.; Choi, S.; Hyun, J.; Kim, D.; Park, Y.; Yu, J.; Jeon, S.; Park, J.; Shin, W.; Sohn, H. Nanostructured PVdF-HFP/TiO2 Composite as Protective Layer on Lithium Metal Battery Anode with Enhanced Electrochemical Performance. Membr. J. 2021, 31, 417–425. [Google Scholar] [CrossRef]
- Lee, S.; Seok, D.; Jeong, Y.; Sohn, H. Surface Modification of Li Metal Electrode with PDMS/GO Composite Thin Film: Controlled Growth of Li Layer and Improved Performance of Lithium Metal Battery (LMB). Membr. J. 2020, 30, 38–45. [Google Scholar] [CrossRef]
- Jeong, Y.; Seok, D.; Lee, S.; Shin, W.; Sohn, H. Polymer/Inorganic Nanohybrid Membrane on Lithium Metal Electrode: Effective Control of Surficial Growth of Lithium Layer and Its Improved Electrochemical Performance. Membr. J. 2020, 30, 30–37. [Google Scholar] [CrossRef]
- Kim, J.S.; Yoon, G.; Kim, S.; Sugata, S.; Yashiro, N.; Suzuki, S.; Lee, M.J.; Kim, R.; Badding, M.; Song, Z.; et al. Surface Engineering of Inorganic Solid-State Electrolytes via Interlayers Strategy for Developing Long-Cycling Quasi-All-Solid-State Lithium Batteries. Nat. Commun. 2023, 14, 782. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, Y.; Wang, J.; Liang, J.; Wang, C.; Sun, Q.; Lin, X.; Adair, K.R.; Luo, J.; Wang, D.; et al. A Novel Organic “Polyurea” Thin Film for Ultralong-Life Lithium-Metal Anodes via Molecular-Layer Deposition. Adv. Mater. 2019, 31, 1806541. [Google Scholar] [CrossRef]
- Liu, S.; Liu, W.; Ba, D.; Zhao, Y.; Ye, Y.; Li, Y.; Liu, J. Filler-Integrated Composite Polymer Electrolyte for Solid-State Lithium Batteries. Adv. Mater. 2023, 35, 2110423. [Google Scholar] [CrossRef]
- Xue, W.; Yang, Y.; Yang, Q.; Liu, Y.; Wang, L.; Chen, C.; Cheng, R. The Effect of Sintering Process on Lithium Ionic Conductivity of Li6.4Al0.2La3Zr2O12 Garnet Produced by Solid-State Synthesis. RSC Adv. 2018, 8, 13083–13088. [Google Scholar] [CrossRef]
- Ren, Y.; Danner, T.; Moy, A.; Finsterbusch, M.; Hamann, T.; Dippell, J.; Fuchs, T.; Müller, M.; Hoft, R.; Weber, A.; et al. Oxide-Based Solid-State Batteries: A Perspective on Composite Cathode Architecture. Adv. Energy Mater. 2023, 13, 2201939. [Google Scholar] [CrossRef]
- Randrema, X.; Leteyi Mfiban, I.; Soler, M.; Profatilova, I.; Berthault, M.; Ramos, R.; Lavie, J.; De Vito, E.; Blanc, L.; Diry, S.; et al. Towards a Practical Use of Sulfide Solid Electrolytes in Solid-State Batteries: Impact of Dry Room Exposure on H2S Release and Material Properties. Batter. Supercaps 2024, 7, e202300380. [Google Scholar] [CrossRef]
- Morino, Y.; Otoyama, M.; Okumura, T.; Kuratani, K.; Shibata, N.; Ito, D.; Sano, H. Concerted Influence of H2O and CO2: Moisture Exposure of Sulfide Solid Electrolyte Li4SnS4. ACS Omega 2024, 9, 38523–38531. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.S.; Miara, L.; Wang, Y.; Jung, S.K.; Park, S.Y.; Song, Z.; Kim, H.; Badding, M.; Chang, J.M.; et al. High-Energy and Durable Lithium Metal Batteries Using Garnet-Type Solid Electrolytes with Tailored Lithium-Metal Compatibility. Nat. Commun. 2022, 13, 1883. [Google Scholar] [CrossRef]
- Siller, V.; Morata, A.; Eroles, M.N.; Arenal, R.; Gonzalez-Rosillo, J.C.; López Del Amo, J.M.; Tarancón, A. High Performance LATP Thin Film Electrolytes for All-Solid-State Microbattery Applications. J. Mater. Chem. A 2021, 9, 17760–17769. [Google Scholar] [CrossRef]
- Tan, F.; An, H.; Li, N.; Du, J.; Peng, Z. A Study on Li0.33La0.55TiO3 solid Electrolyte with High Ionic Conductivity and Its Application in Flexible All-Solid-State Batteries. Nanoscale 2021, 13, 11518–11524. [Google Scholar] [CrossRef]
- Sun, H.; Kang, S.; Cui, L. Prospects of LLZO Type Solid Electrolyte: From Material Design to Battery Application. Chem. Eng. J. 2023, 454, 140375. [Google Scholar] [CrossRef]
- Halder, B.; Mohamed, M.G.; Kuo, S.W.; Elumalai, P. Review on Composite Polymer Electrolyte Using PVDF-HFP for Solid-State Lithium-Ion Battery. Mater. Today Chem. 2024, 36, 101926. [Google Scholar] [CrossRef]
- Xiong, Z.; Wang, Z.; Zhou, W.; Liu, Q.; Wu, J.F.; Liu, T.H.; Xu, C.; Liu, J. 4.2V Polymer All-Solid-State Lithium Batteries Enabled by High-Concentration PEO Solid Electrolytes. Energy Storage Mater. 2023, 57, 171–179. [Google Scholar] [CrossRef]
- Liu, X.; Xin, X.; Shen, L.; Gu, Z.; Wu, J.; Yao, X. Poly(Methyl Methacrylate)-Based Gel Polymer Electrolyte for High-Performance Solid State Li-O2Battery with Enhanced Cycling Stability. ACS Appl. Energy Mater. 2021, 4, 3975–3982. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, C.Z.; Yuan, H.; Cheng, X.B.; Huang, J.Q.; Zhang, Q. Critical Current Density in Solid-State Lithium Metal Batteries: Mechanism, Influences, and Strategies. Adv. Funct. Mater. 2021, 31, 2009925. [Google Scholar] [CrossRef]
- Wu, N.; Chien, P.H.; Qian, Y.; Li, Y.; Xu, H.; Grundish, N.S.; Xu, B.; Jin, H.; Hu, Y.Y.; Yu, G.; et al. Enhanced Surface Interactions Enable Fast Li + Conduction in Oxide/Polymer Composite Electrolyte. Angew. Chem. 2020, 132, 4160–4166. [Google Scholar] [CrossRef]
- Yao, Z.; Zhu, K.; Li, X.; Zhang, J.; Li, J.; Wang, J.; Yan, K.; Liu, J. Double-Layered Multifunctional Composite Electrolytes for High-Voltage Solid-State Lithium-Metal Batteries. ACS Appl. Mater. Interfaces 2021, 13, 11958–11967. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, J.; Chang, W.; Tan, Y.; Lai, C.; Peng, Y.; Liu, H. Interface Strategies for Enhancing the Lithium-Ion Transport of Composite Polymer Electrolytes toward High-Performance Solid-State Batteries. ACS Appl. Energy Mater. 2024, 7, 4303–4313. [Google Scholar] [CrossRef]
- Overhoff, G.M.; Ali, M.Y.; Brinkmann, J.P.; Lennartz, P.; Orthner, H.; Hammad, M.; Wiggers, H.; Winter, M.; Brunklaus, G. Ceramic-in-Polymer Hybrid Electrolytes with Enhanced Electrochemical Performance. ACS Appl. Mater. Interfaces 2022, 14, 53636–53647. [Google Scholar] [CrossRef]
- Wu, J.; Wu, X.; Wang, W.; Wang, Q.; Zhou, X.; Liu, Y.; Liu, Y.; Guo, B. Dense PVDF-Type Polymer-in-Ceramic Electrolytes for Solid State Lithium Batteries. RSC Adv. 2020, 10, 22417–22421. [Google Scholar] [CrossRef]
- He, M.; Mo, C.; Lu, Z.; Huang, Y.; Qiu, Z.; Li, W.; Liao, Y. Ceramic-in-Polymer Solid Electrolyte Reinforced by in-Situ Polymerization of PEGDA Interlayer for Lithium Metal Battery. Solid State Ion. 2023, 395, 116217. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, M.; Qian, S.; Ling, H.Y.; Wu, Z.; Liu, X.; Yan, C.; Zhang, S. Functional Additives for Solid Polymer Electrolytes in Flexible and High-Energy-Density Solid-State Lithium-Ion Batteries. Carbon Energy 2021, 3, 929–956. [Google Scholar] [CrossRef]
- Yan, C.; Zhu, P.; Jia, H.; Zhu, J.; Selvan, R.K.; Li, Y.; Dong, X.; Du, Z.; Angunawela, I.; Wu, N.; et al. High-Performance 3-D Fiber Network Composite Electrolyte Enabled with Li-Ion Conducting Nanofibers and Amorphous PEO-Based Cross-Linked Polymer for Ambient All-Solid-State Lithium-Metal Batteries. Adv. Fiber Mater. 2019, 1, 101–111. [Google Scholar] [CrossRef]
- Chen, F.; Yang, D.; Zha, W.; Zhu, B.; Zhang, Y.; Li, J.; Gu, Y.; Shen, Q.; Zhang, L.; Sadoway, D.R. Solid Polymer Electrolytes Incorporating Cubic Li7La3Zr2O12 for All-Solid-State Lithium Rechargeable Batteries. Electrochim. Acta 2017, 258, 1106–1114. [Google Scholar] [CrossRef]
- Yang, T.; Zheng, J.; Cheng, Q.; Hu, Y.-Y.; Chan, C.K. Composite Polymer Electrolytes with Li7La3Zr2O12 Garnet-Type Nanowires as Ceramic Fillers: Mechanism of Conductivity Enhancement and Role of Doping and Morphology. ACS Appl. Mater. Interfaces 2017, 9, 21773–21780. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, T.; Huang, S.; Mi, J.; Zhang, Y.; Travas-Sejdic, J.; Turner, A.P.; Gao, W.; Cao, P. Constructing a PVDF-Based Composite Solid-State Electrolyte with High Ionic Conductivity Li6.5La3Zr1.5Ta0.1Nb0.4O12 for Lithium Metal Battery. J. Power Sources 2023, 564, 232849. [Google Scholar] [CrossRef]
- Han, J.-Y.; Kim, S.; Park, D.U.; Kang, D.; Oh, J.-H.; Hyun, D.-E.; Lee, D.-W.; Im, H.; Oh, J.-M.; Koo, S.-M.; et al. Electrospun 1D Al-LLZO Incorporated PVDF-HFP Composite Electrolyte with Fast Li+ Pathway Derived from Highway-Traction Effect for High Performance Lithium Metal Batteries. Chem. Eng. J. 2025, 505, 159627. [Google Scholar] [CrossRef]
- Keller, M.; Appetecchi, G.B.; Kim, G.-T.; Sharova, V.; Schneider, M.; Schuhmacher, J.; Roters, A.; Passerini, S. Electrochemical Performance of a Solvent-Free Hybrid Ceramic-Polymer Electrolyte Based on Li7La3Zr2O12 in P(EO)15LiTFSI. J. Power Sources 2017, 353, 287–297. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, K.; Liu, K.; Li, J.; Ma, C.; Zhou, L.; Du, Y. Study of a Composite Solid Electrolyte Made from a New Pyrrolidone-Containing Polymer and LLZTO. J. Colloid Interface Sci. 2020, 580, 389–398. [Google Scholar] [CrossRef]
- Ding, Y.; He, B.; Wang, D.; Avdeev, M.; Li, Y.; Shi, S. Software for Evaluating Ionic Conductivity of Inorganic–Polymer Composite Solid Electrolytes. Energy Mater. Adv. 2023, 4, 0041. [Google Scholar] [CrossRef]
- Zha, W.; Li, W.; Ruan, Y.; Wang, J.; Wen, Z. In Situ Fabricated Ceramic/Polymer Hybrid Electrolyte with Vertically Aligned Structure for Solid-State Lithium Batteries. Energy Storage Mater. 2021, 36, 171–178. [Google Scholar] [CrossRef]
- Shi, C.; Song, J.; Zhang, Y.; Wang, X.; Jiang, Z.; Sun, T.; Zhao, J. Revealing the Mechanisms of Lithium-Ion Transport and Conduction in Composite Solid Polymer Electrolytes. Cell Rep. Phys. Sci. 2023, 4, 101321. [Google Scholar] [CrossRef]
- Cao, S.; Chen, F.; Shen, Q.; Zhang, L. Dual-Coordination-Induced Poly(vinylidene fluoride)/Li6.4 Ga0.2La3Zr2O12/Succinonitrile Composite Solid Electrolytes Toward Enhanced Rate Performance in All-Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2023, 15, 37422–37432. [Google Scholar] [CrossRef] [PubMed]
- Din, M.M.U.; Häusler, M.; Fischer, S.M.; Ratzenböck, K.; Chamasemani, F.F.; Hanghofer, I.; Henninge, V.; Brunner, R.; Slugovc, C.; Rettenwander, D. Role of Filler Content and Morphology in LLZO/PEO Membranes. Front. Energy Res. 2021, 9, 711610. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, P.; Cheng, Z.; Mao, J.; Jiang, L.; Ni, C.; Park, S.; Deng, K.; Hu, Y.; Fu, K.K. Flexible, Mechanically Robust, Solid-State Electrolyte Membrane with Conducting Oxide-Enhanced 3D Nanofiber Networks for Lithium Batteries. Nano Lett. 2021, 21, 7070–7078. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Ma, W.; Dong, H.; Zhang, K.; Wang, R. Low-Temperature Synthesis of Cubic Phase Li7La3Zr2O12 via Sol-Gel and Ball Milling Induced Phase Transition. J. Power Sources 2019, 412, 189–196. [Google Scholar] [CrossRef]
- Qian, X.; Gu, N.; Cheng, Z.; Yang, X.; Wang, E.; Dong, S. Methods to Study the Ionic Conductivity of Polymeric Electrolytes using A.C. Impedance Spectroscopy. J. Solid State Electrochem. 2001, 6, 8–15. [Google Scholar] [CrossRef]
- Aziz, S.B.; Woo, T.J.; Kadir, M.F.Z.; Ahmed, H.M. A Conceptual Review on Polymer Electrolytes and Ion Transport Models. J. Sci. Adv. Mater. Devices 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Wang, A.; Pei, D.; Liu, Z.; Huang, S.; Cao, G.; Jin, H.; Hou, S. Exploring High Li+ Transference Number Solid-State Electrolytes Based on a Poly(ε-caprolactone) Polymer Matrix with Efficient Lithium Salt Dissociation for Applications in Lithium-Metal Batteries. ACS Appl. Energy Mater. 2023, 6, 8221–8228. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, Z.H.; Wang, Z.M.; Zhang, Q.; Ye, Y.S.; Lu, J.H.; Rahman, Z.U.; Zhang, Z.W. Ultrathin Solid Composite Electrolyte Based on Li6.4La3Zr1.4Ta0.6O12/PVDF-HFP/LiTFSI/Succinonitrile for High-Performance Solid-State Lithium Metal Batteries. ACS Appl. Energy Mater. 2020, 3, 9428–9435. [Google Scholar] [CrossRef]
- Alarco, P.J.; Abu-Lebdeh, Y.; Abouimrane, A.; Armand, M. The Plastic-Crystalline Phase of Succinonitrile as a Universal Matrix for Solid-State Ionic Conductors. Nat. Mater. 2004, 3, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Zha, W.; Li, J.; Li, W.; Sun, C.; Wen, Z. Anchoring Succinonitrile by Solvent-Li+ Associations for High-Performance Solid-State Lithium Battery. J. Chem. Eng. 2021, 406, 126754. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Y.; Guo, X.; Ding, P.; Lin, Z.; Yu, H. Interface Science in Polymer-based Composite Solid Electrolytes in Lithium Metal Batteries. SusMat 2022, 2, 264–292. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Jiang, K.; Shen, Z.; Passerini, S.; Chen, M. Accelerating the Development of LLZO in Solid-State Batteries Toward Commercialization: A Comprehensive Review. Small 2024, 20, 2402035. [Google Scholar] [CrossRef]
- Guo, Y.; Cheng, J.; Zeng, Z.; Li, Y.; Zhang, H.; Li, D.; Ci, L. Li2CO3: Insights into Its Blocking Effect on Li-Ion Transfer in Garnet Composite Electrolytes. ACS Appl. Energy Mater. 2022, 5, 2853–2861. [Google Scholar] [CrossRef]
- Lin, Y.; Wu, M.; Sun, J.; Zhang, L.; Jian, Q.; Zhao, T. A High-Capacity, Long-Cycling All-Solid-State Lithium Battery Enabled by Integrated Cathode/Ultrathin Solid Electrolyte. Adv. Energy Mater. 2021, 11, 2101612. [Google Scholar] [CrossRef]
- Balasubramaniam, R.; Nam, C.W.; Aravindan, V.; Seol, J.c.; Ajeya, K.V.; Jung, H.Y.; Lee, Y.S. Composite Solid Electrolyte for High Voltage Solid-State Li-Metal Battery. ChemElectroChem 2022, 9, e202200317. [Google Scholar] [CrossRef]
- Mishra, R.; Singh, S.K.; Gupta, H.; Tiwari, R.K.; Meghnani, D.; Patel, A.; Tiwari, A.; Tiwari, V.K.; Singh, R.K. Polar β-Phase PVdF-HFP-Based Freestanding and Flexible Gel Polymer Electrolyte for Better Cycling Stability in a Na Battery. Energy Fuels 2021, 35, 15153–15165. [Google Scholar] [CrossRef]
- He, W.; Ding, H.; Chen, X.; Yang, W. Three-Dimensional LLZO/PVDF-HFP Fiber Network-Enhanced Ultrathin Composite Solid Electrolyte Membrane for Dendrite-Free Solid-State Lithium Metal Batteries. J. Membr. Sci. 2023, 665, 121095. [Google Scholar] [CrossRef]
- Yao, Z.; Qi, F.; Ye, L.; Sun, Q.; Gu, X.; Yang, X.; Zhu, K. Composite Polymer Electrolyte Based on Poly(Vinylidene Fluoride-Hexafluoropropylene) (PVDF-HFP) for Solid-State Batteries. Heliyon 2024, 10, e28097. [Google Scholar] [CrossRef]
- Celik, M.; Kızılaslan, A.; Can, M.; Cetinkaya, T.; Akbulut, H. Electrochemical Investigation of PVDF: HFP Gel Polymer Electrolytes for Quasi-Solid-State Li-O2 Batteries: Effect of Lithium Salt Type and Concentration. Electrochim. Acta 2021, 371, 137824. [Google Scholar] [CrossRef]
- Du, S.-Y.; Ren, G.-X.; Zhang, N.; Liu, X.-S. High-Performance Poly(Vinylidene Fluoride-Hexafluoropropylene)-Based Composite Electrolytes with Excellent Interfacial Compatibility for Room-Temperature All-Solid-State Lithium Metal Batteries. ACS Omega 2022, 7, 19631–19639. [Google Scholar] [CrossRef]
- Li, J.; Zhang, T.; Hui, X.; Zhu, R.; Sun, Q.; Li, X.; Yin, L. Competitive Li+ Coordination in Ionogel Electrolytes for Enhanced Li-Ion Transport Kinetics. Adv. Sci. 2023, 10, 2300226. [Google Scholar] [CrossRef]
- Elbouazzaoui, K.; Nkosi, F.; Brandell, D.; Mindemark, J.; Edström, K. Ionic Transport in Solid-State Composite Poly(Trimethylene Carbonate)–Li6.7Al0.3La3Zr2O12 Electrolytes: The Interplay between Surface Chemistry and Ceramic Particle Loading. Electrochim. Acta 2023, 462, 142785. [Google Scholar] [CrossRef]
- Suo, L.; Oh, D.; Lin, Y.; Zhuo, Z.; Borodin, O.; Gao, T.; Wang, F.; Kushima, A.; Wang, Z.; Kim, H.-C.; et al. How Solid-Electrolyte Interphase Forms in Aqueous Electrolytes. J. Am. Chem. Soc. 2017, 139, 18670–18680. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Tang, J.; Huang, X.; Ao, X.; Tian, B. Building Bulk and Interface Dual Fast Li+ Conducting Pathway in Composite Solid Polymer Electrolyte Membrane for All–Solid–State Lithium–Metal Batteries. Batter. Supercaps 2024, 7, e202400075. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, L.; Li, Y.; Jin, Y.; Politis, D.J.; Liu, H.; Wang, H.; Liu, M.; He, Y.-B.; Wang, L. Weakening Ionic Coordination for High Ionic Conductivity Composite Solid Electrolytes. ACS Energy Lett. 2024, 9, 2109–2115. [Google Scholar] [CrossRef]
- Liu, M.; Guan, X.; Liu, H.; Ma, X.; Wu, Q.; Ge, S.; Zhang, H.; Xu, J. Composite Solid Electrolytes Containing Single-Ion Lithium Polymer Grafted Garnet for Dendrite-Free, Long-Life All-Solid-State Lithium Metal Batteries. Chem. Eng. J. 2022, 445, 136436. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, Y.; Li, X.; Yu, J.; Yan, J.; Ding, B. Solid-State Lithium Metal Batteries with Extended Cycling Enabled by Dynamic Adaptive Solid-State Interfaces. Adv. Mater. 2021, 33, 2008084. [Google Scholar] [CrossRef]
- Zhang, H.; An, X.; Lu, Z.; Liu, L.; Cao, H.; Xu, Q.; Liu, H.; Ni, Y. A Three-Dimensional Interconnected Li7La3Zr2O12 Framework Composite Solid Electrolyte Utilizing Lignosulfonate/Cellulose Nanofiber Bio-Template for High-Performance Lithium Ion Batteries. J. Power Sources 2020, 477, 228752. [Google Scholar] [CrossRef]
- Counihan, M.J.; Powers, D.J.; Barai, P.; Hu, S.; Zagorac, T.; Zhou, Y.; Lee, J.; Connell, J.G.; Chavan, K.S.; Gilmore, I.S.; et al. Understanding the Influence of Li7La3Zr2O12 Nanofibers on Critical Current Density and Coulombic Efficiency in Composite Polymer Electrolytes. ACS Appl. Mater. Interfaces 2023, 15, 26047–26059. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhao, L.; Zhang, J.; Li, Q.; Sun, D.; Ren, Y.; Tang, Y.; Jin, G.; Wang, H. La2O3 Filler’s Stabilization of Residual Solvent in Polymer Electrolyte for Advanced Solid-State Lithium-Metal Batteries. Small Sci. 2023, 3, 2300017. [Google Scholar] [CrossRef]
- Peng, J.; Lu, D.; Wu, S.; Yang, N.; Cui, Y.; Ma, Z.; Liu, M.; Shi, Y.; Sun, Y.; Niu, J.; et al. Lithium Superionic Conductive Nanofiber-Reinforcing High-Performance Polymer Electrolytes for Solid-State Batteries. J. Am. Chem. Soc. 2024, 146, 11897–11905. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Chen, L.; Ma, J.; Lai, C.; Huang, Y.; Mi, J.; Biao, J.; Zhang, D.; Shi, P.; Xia, H.; et al. Stable Interface Chemistry and Multiple Ion Transport of Composite Electrolyte Contribute to Ultra-Long Cycling Solid-State LiNi0.8Co0.1Mn0.1O2/Lithium Metal Batteries. Angew. Chem. Int. Ed. Engl. 2021, 60, 24668–24675. [Google Scholar] [CrossRef]
- Zeng, T.; Yan, Y.; He, M.; Zheng, R.; Du, D.; Ren, L.; Zhou, B.; Shu, C. Boosted Li+ transference Number Enabled: Via Interfacial Engineering for Dendrite-Free Lithium Metal Anodes. Chem. Commun. 2021, 57, 12687–12690. [Google Scholar] [CrossRef]
- Li, J.; Jing, M.-X.; Li, R.; Li, L.-X.; Huang, Z.-H.; Yang, H.; Liu, M.-Q.; Hussain, S.; Xiang, J.; Shen, X.-Q. Al2O3 Fiber-Reinforced Polymer Solid Electrolyte Films with Excellent Lithium-Ion Transport Properties for High-Voltage Solid-State Lithium Batteries. ACS Appl. Polym. Mater. 2022, 4, 7144–7151. [Google Scholar] [CrossRef]
- Beshahwured, S.L.; Wu, Y.S.; Wu, S.H.; Chien, W.C.; Jose, R.; Lue, S.J.; Yang, C.C. Flexible Hybrid Solid Electrolyte Incorporating Ligament-Shaped Li6.25Al0.25La3Zr2O12 Filler for All-Solid-State Lithium-Metal Batteries. Electrochim. Acta 2021, 366, 137348. [Google Scholar] [CrossRef]
- Xu, H.; Huang, S.; Qian, J.; Liu, S.; Li, L.; Zhao, X.; Zhang, W. Safe Solid-State PEO/TPU/LLZO Nano Network Polymer Composite Gel Electrolyte for Solid-State Lithium Batteries. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 130040. [Google Scholar] [CrossRef]
- Chen, H.; Adekoya, D.; Hencz, L.; Ma, J.; Chen, S.; Yan, C.; Zhao, H.; Cui, G.; Zhang, S. Stable Seamless Interfaces and Rapid Ionic Conductivity of Ca–CeO2/LiTFSI/PEO Composite Electrolyte for High-Rate and High-Voltage All-Solid-State Battery. Adv. Energy Mater. 2020, 10, 2000049. [Google Scholar] [CrossRef]
- Cheng, B.; Du, P.; Xiao, J.; Zhan, X.; Zhu, L. Improving the Ionic Conductivity and Anode Interface Compatibility of LLZO/PVDF Composite Polymer Electrolytes by Compositional Tuning. ACS Appl. Mater. Interfaces 2024, 16, 31648–31656. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Li, S.-P.; Fan, L.-Z.; Nan, C.-W.; Goodenough, J.B. PEO/Garnet Composite Electrolytes for Solid-State Lithium Batteries: From “Ceramic-in-Polymer” to “Polymer-in-Ceramic”. Nano Energy 2018, 46, 176–184. [Google Scholar] [CrossRef]


| Sample Name | Full Name |
|---|---|
| PVDF-HFP | PVDF-HFP/LiTFSI/SN |
| 0D_Al-LLZO@PVDF-HFP | Al-LLZO(0D)+PVDF-HFP/LiTFSI/SN |
| 1D_Al-LLZO@PVDF-HFP | Al-LLZO(1D)+PVDF-HFP/LiTFSI/SN |
| Sample | σ (S/cm) | Ea (eV) | tLi+ | Electrochemical Window (V) |
|---|---|---|---|---|
| 0D_Al-LLZO @PVDF-HFP | 0.67 × 10−4 (30 °C) | 0.32 | 0.67 | 4.65 |
| 1D_Al-LLZO @PVDF-HFP | 1.40 × 10−4 (30 °C) | 0.20 | 0.78 | 4.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, H.; Park, D.U.; Lee, Y.J.; Moon, J.; Lee, S.; Choi, T.-M.; Lee, T.; Lee, G.; Oh, J.-M.; Shin, W.H.; et al. The Structural Effect of a Composite Solid Electrolyte on Electrochemical Performance and Fire Safety. Materials 2025, 18, 1536. https://doi.org/10.3390/ma18071536
Im H, Park DU, Lee YJ, Moon J, Lee S, Choi T-M, Lee T, Lee G, Oh J-M, Shin WH, et al. The Structural Effect of a Composite Solid Electrolyte on Electrochemical Performance and Fire Safety. Materials. 2025; 18(7):1536. https://doi.org/10.3390/ma18071536
Chicago/Turabian StyleIm, Hwiyun, Dae Ung Park, Yong Jae Lee, Junseok Moon, Sanglim Lee, Tae-Min Choi, Taek Lee, Giwon Lee, Jong-Min Oh, Weon Ho Shin, and et al. 2025. "The Structural Effect of a Composite Solid Electrolyte on Electrochemical Performance and Fire Safety" Materials 18, no. 7: 1536. https://doi.org/10.3390/ma18071536
APA StyleIm, H., Park, D. U., Lee, Y. J., Moon, J., Lee, S., Choi, T.-M., Lee, T., Lee, G., Oh, J.-M., Shin, W. H., Pyo, S. G., Seubsai, A., & Sohn, H. (2025). The Structural Effect of a Composite Solid Electrolyte on Electrochemical Performance and Fire Safety. Materials, 18(7), 1536. https://doi.org/10.3390/ma18071536








