Microstructural Optimization and Erosion–Corrosion Resistance of Cu-10Ni-3Al-1.8Fe-0.8Mn Alloy via Tailored Heat Treatment
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.2.1. Mechanical Properties Testing
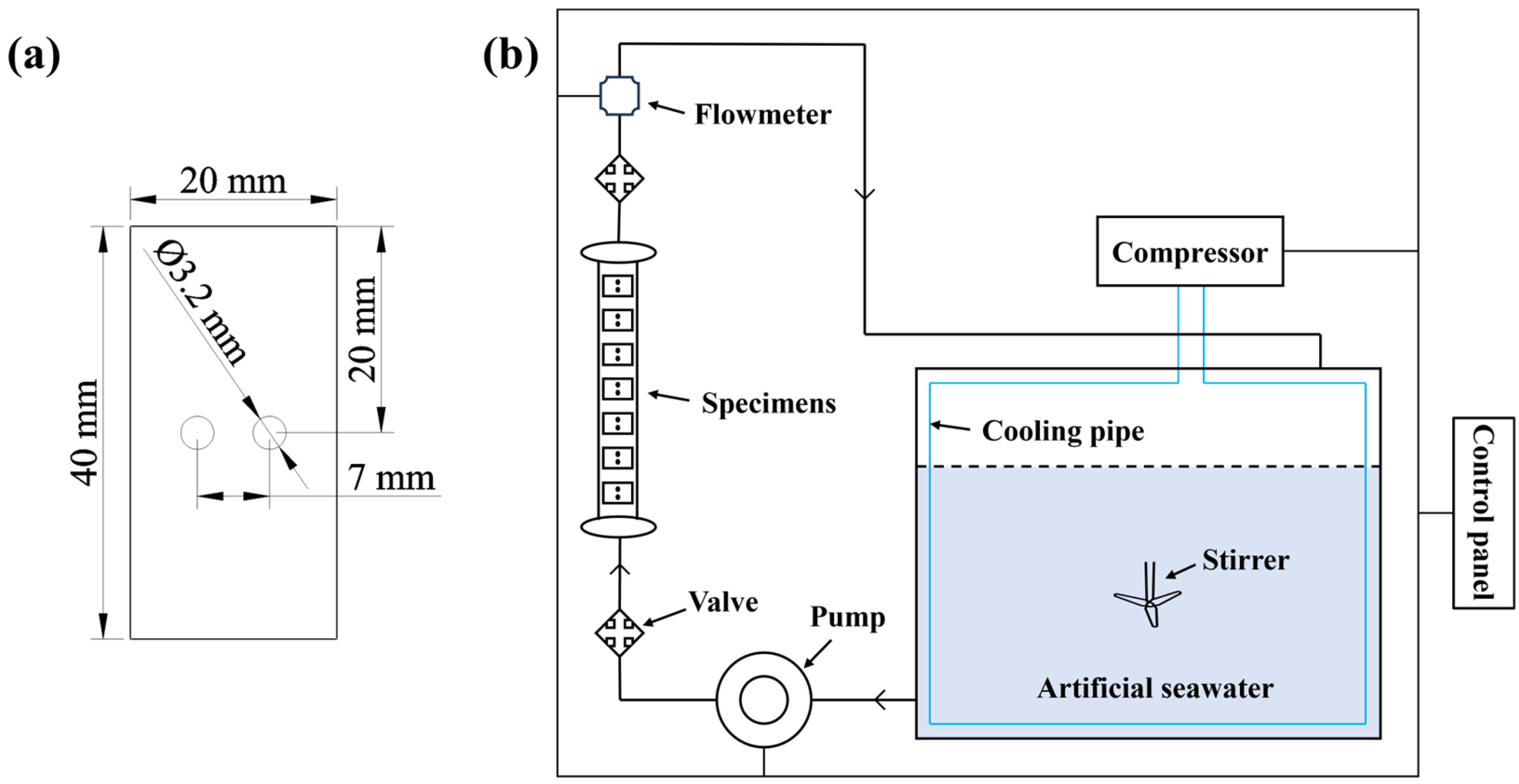
2.2.2. Erosion–Corrosion Testing
2.2.3. Microstructural Characterization
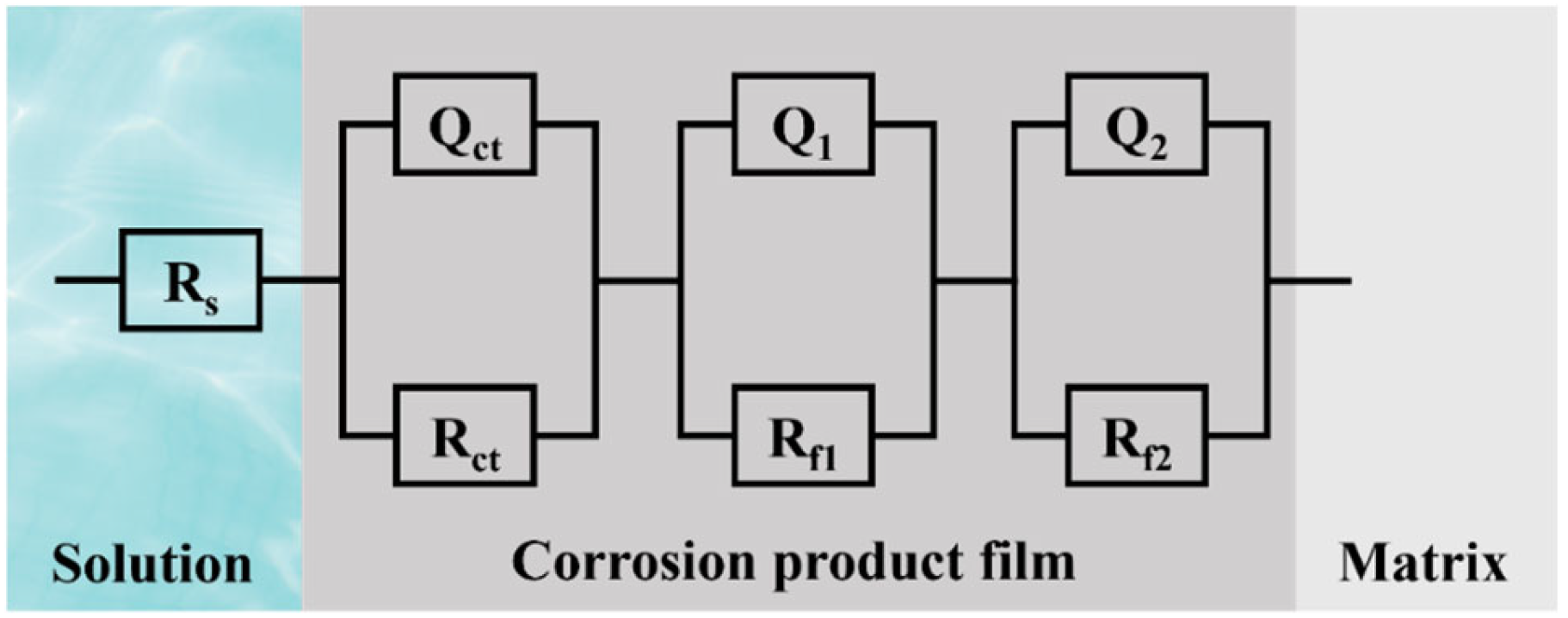
2.2.4. EIS Testing
3. Results and Discussion
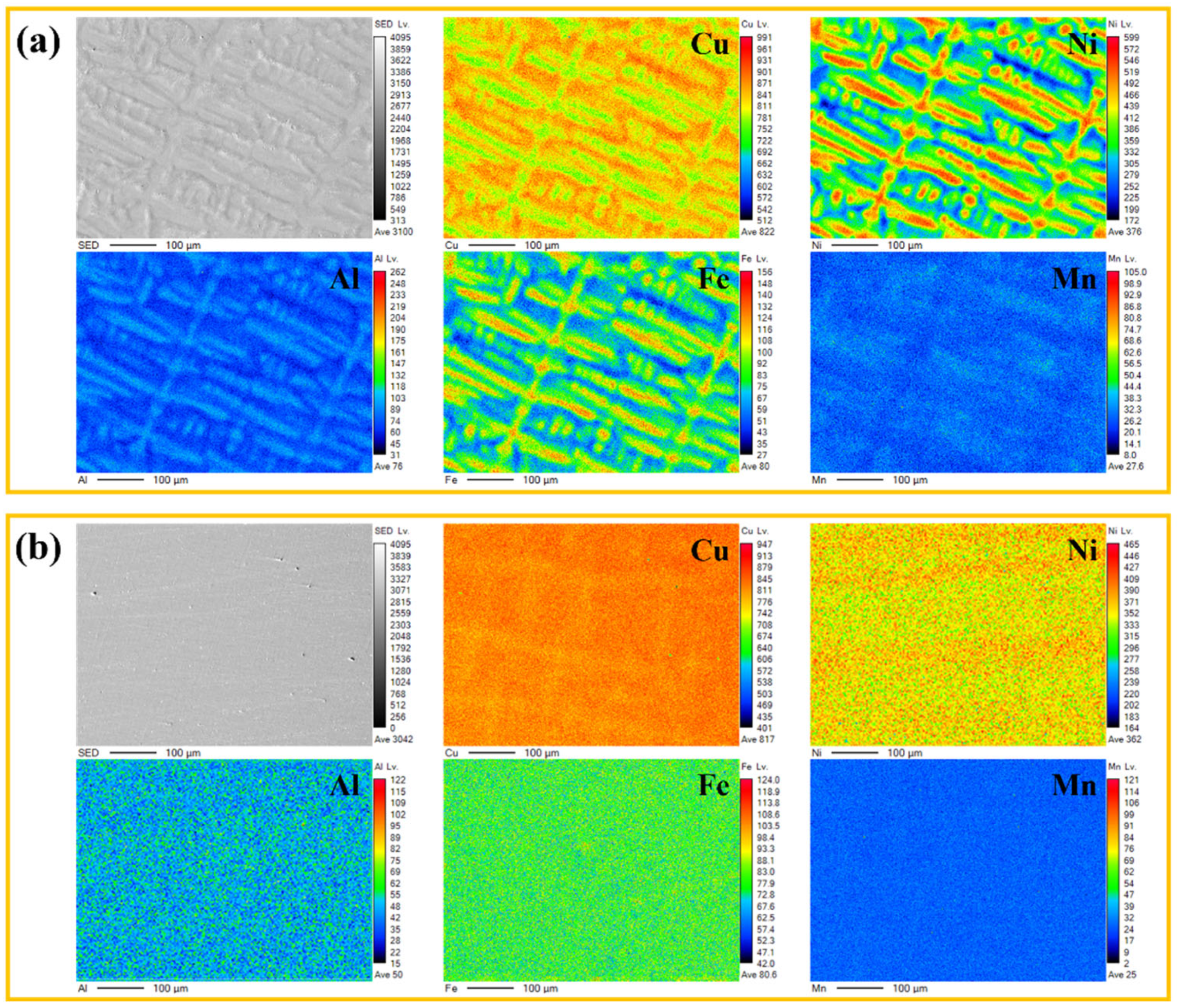
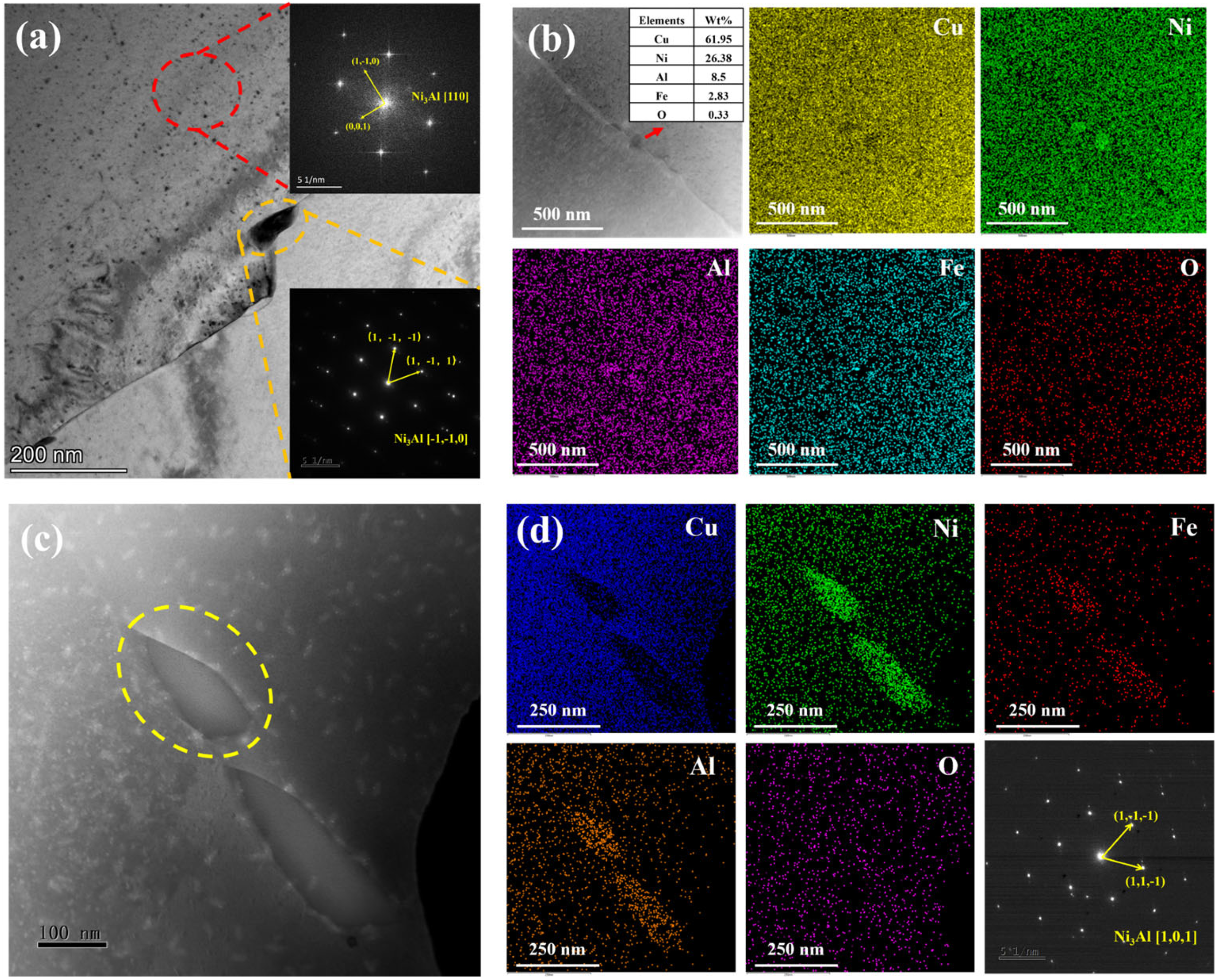
3.1. Microstructure
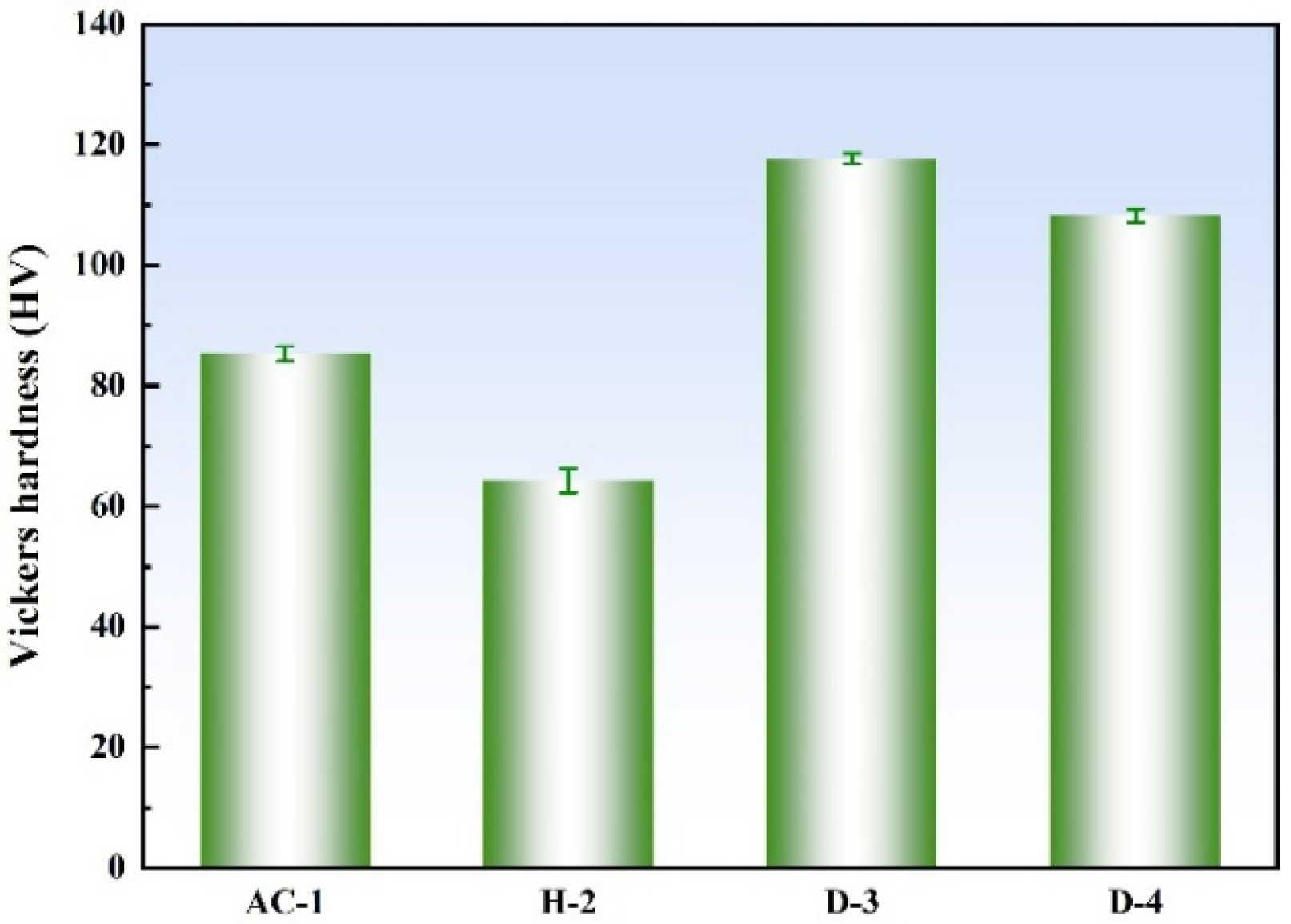
3.2. Mechanical Properties
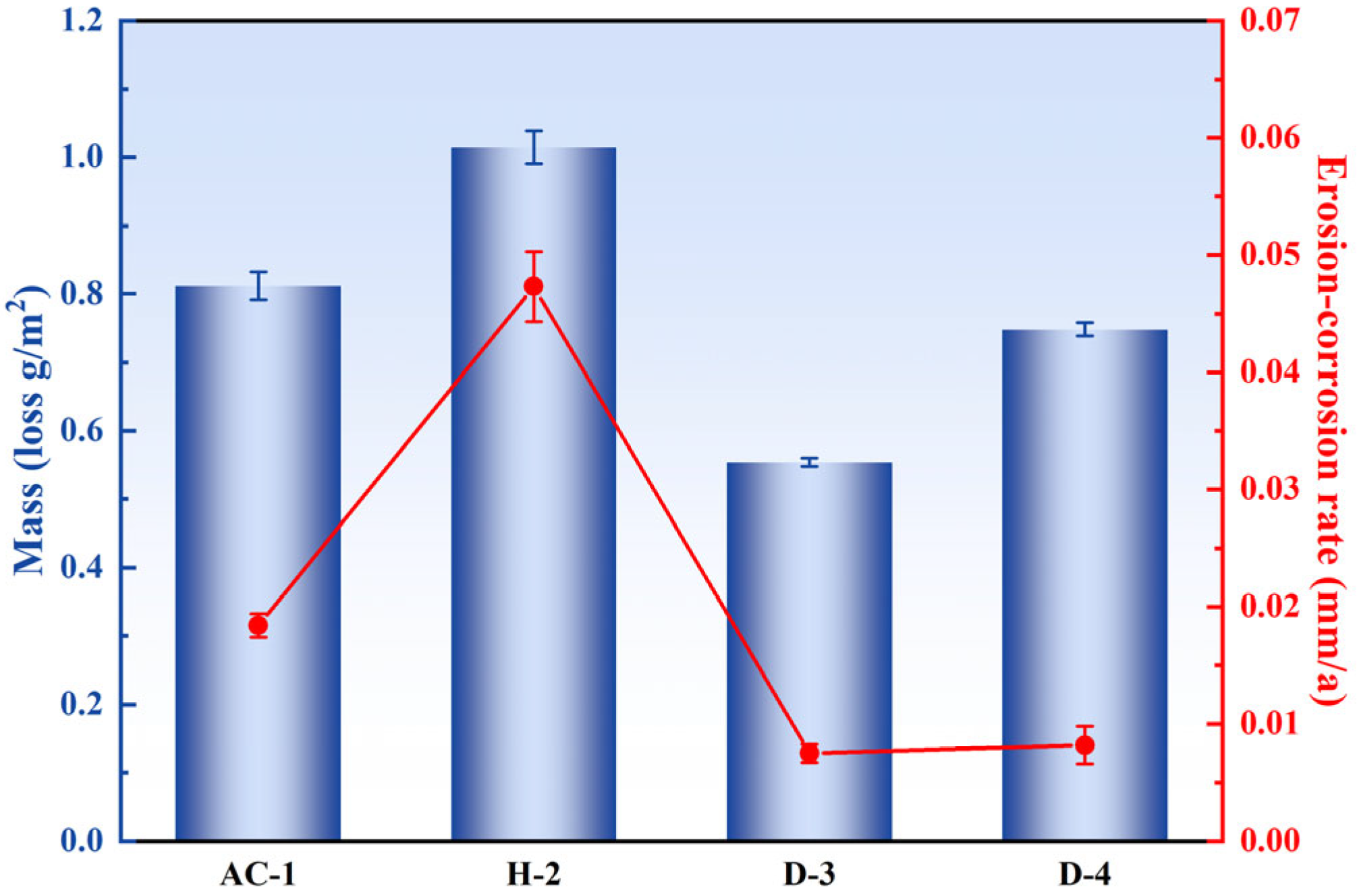
3.3. Corrosion Resistance
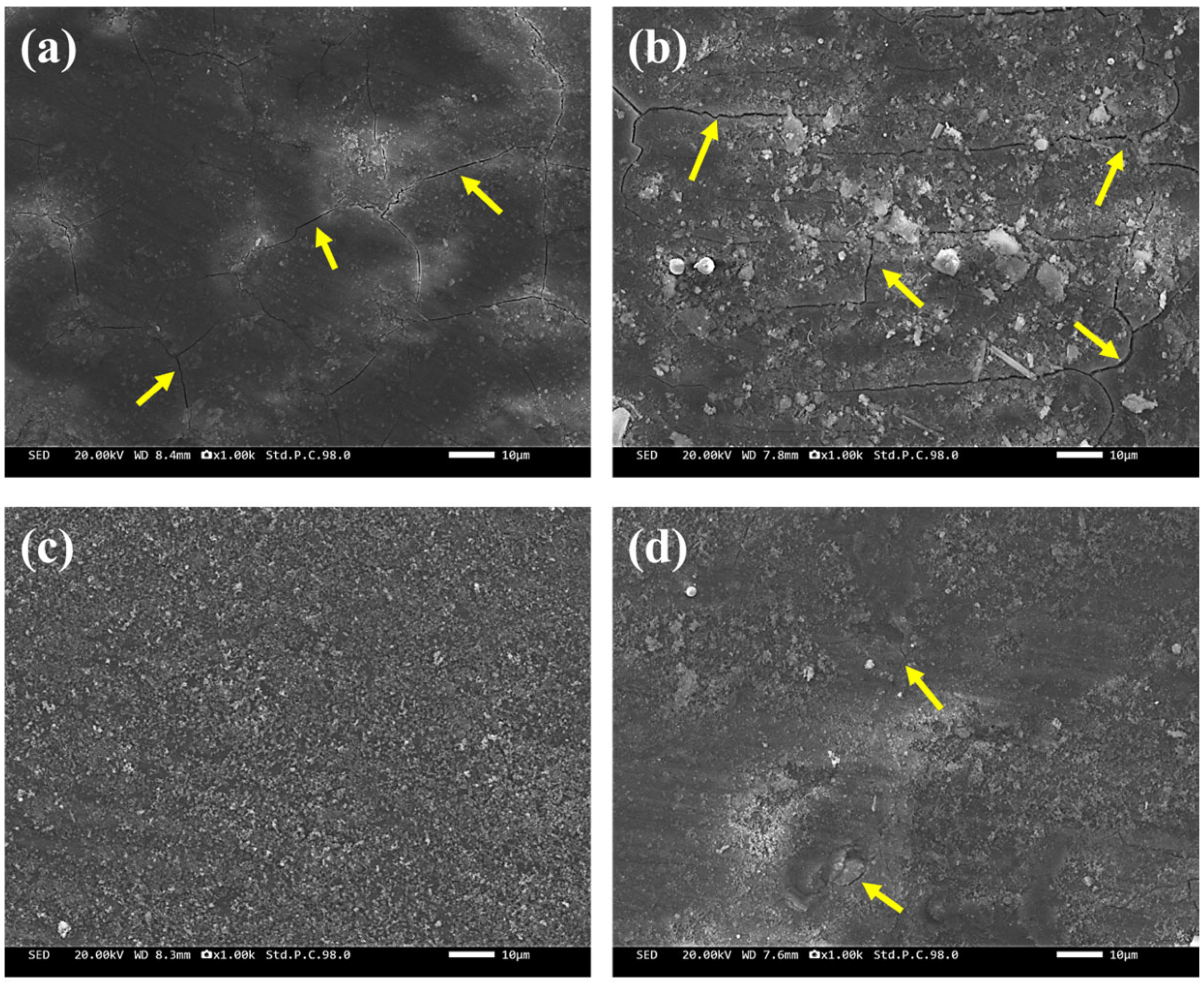
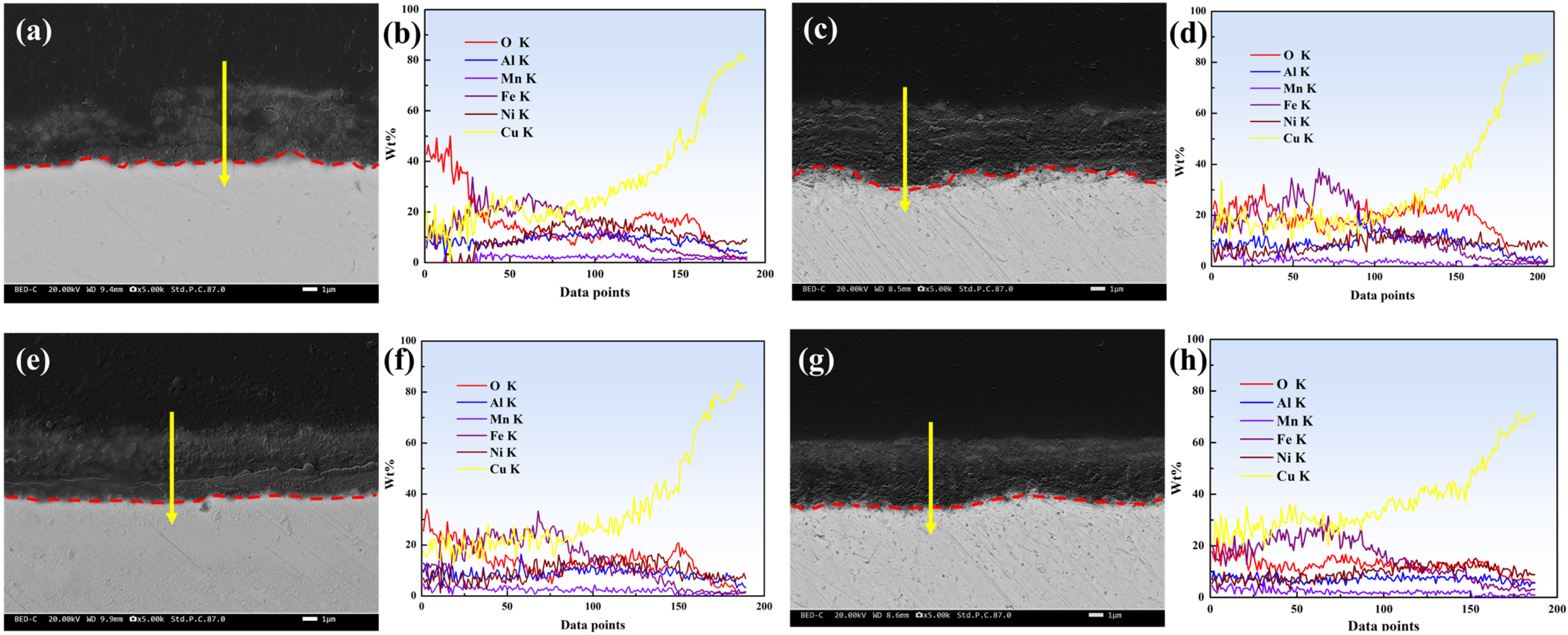
3.4. Corrosion Product Film
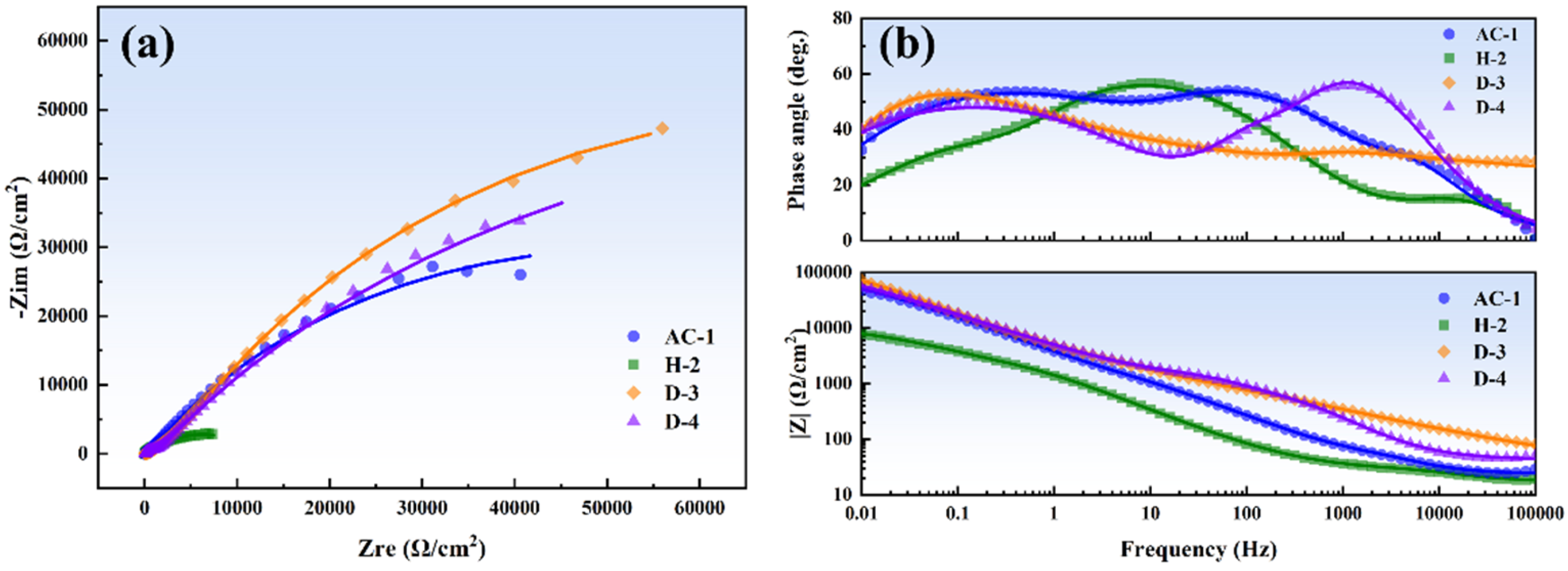
3.5. EIS Analysis
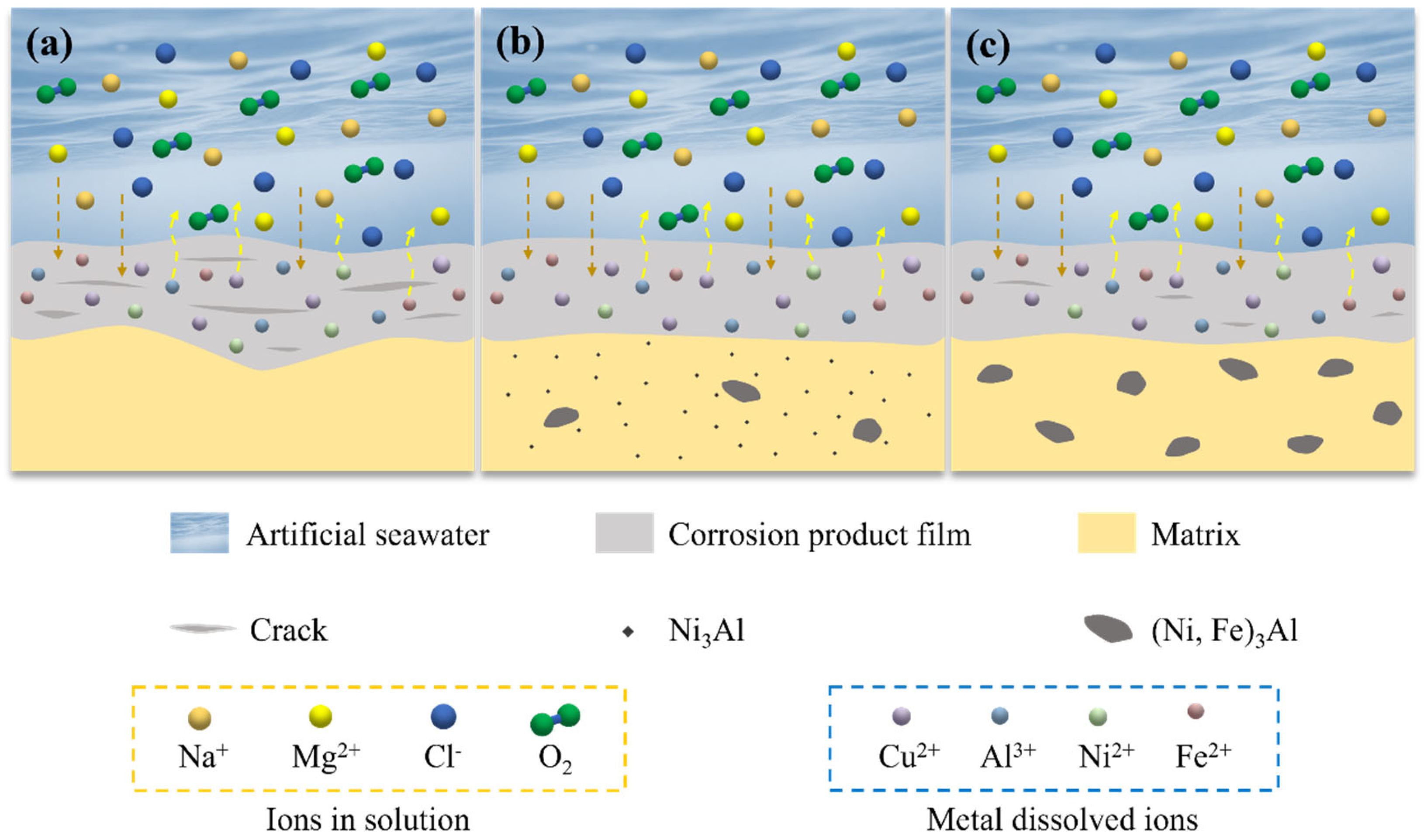
3.6. Mechanism of Corrosion Product Film Formation
4. Conclusions
- The D-3 specimen, aged at 500 °C, exhibited the highest hardness (118 HV5), primarily due to the fine and uniformly dispersed Ni3Al precipitates in the matrix. These precipitates significantly enhanced the mechanical properties of the alloy through dispersion strengthening.
- The D-3 specimen had the lowest erosion–corrosion rate of 0.0075 mm/a in artificial seawater. This was attributed to the fine and dispersed Ni3Al precipitates, which not only homogenized the micro-galvanic effect and reduced localized corrosion but also promoted the formation of a dense Cu2O oxide layer that effectively blocked the penetration of corrosive species.
- The corrosion product film of the D-3 specimen was dense and featured a flat interface with the matrix, demonstrating the best protective performance. EIS analysis indicated that the D-3 specimen had the highest charge transfer resistance and corrosion product film impedance, further confirming its excellent corrosion resistance.
- Deformation, followed by aging at 500 °C, is the optimal heat treatment process for Cu-10Ni-3Al-1.8Fe-0.8Mn alloy, significantly enhancing its mechanical properties and resistance to erosion–corrosion. This treatment is suitable for high-demand applications in marine environments.
- This study can deepen the understanding of the relationship between the microstructure of copper–nickel alloys and the erosion–corrosion behavior. Long-term exposure experiments in real marine environments could be carried out in the future to assess the corrosion resistance and mechanical properties of copper–nickel alloys under real conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fateh, A.; Aliofkhazraei, M.; Rezvanian, A. Review of corrosive environments for copper and its corrosion inhibitors. Arab. J. Chem. 2020, 13, 481–544. [Google Scholar]
- Antonijevic, M.; Petrovic, M. Copper corrosion inhibitors. A review. Int. J. Electrochem. Sci. 2008, 3, 1–28. [Google Scholar] [CrossRef]
- Mioč, E.K.; Gretić, Z.H.; Ćurković, H.O. Modification of cupronickel alloy surface with octadecylphosphonic acid self-assembled films for improved corrosion resistance. Corros. Sci. 2018, 134, 189–198. [Google Scholar] [CrossRef]
- Chandra, K.; Kain, V.; Dey, G.; Shetty, P.; Kishan, R. Failure analysis of cupronickel evaporator tubes of a chilling plant. Eng. Fail. Anal. 2010, 17, 587–593. [Google Scholar]
- She, X.; Peng, J.; Qiang, Y.; Zhou, Y.; Zhang, S. Recent advances in protective technologies against copper corrosion. J. Mater. Sci. Technol. 2024, 201, 75–94. [Google Scholar]
- Vrsalović, L.; Ivanić, I.; Kožuh, S.; Kosec, B.; Bizjak, M.; Kovač, J.; Gabor, U.; Gojić, M. Influence of heat treatment on the corrosion properties of CuAlMn shape memory alloys. Corros. Rev. 2019, 37, 579–589. [Google Scholar]
- Robin, A.; Martinez, G.A.S.; Suzuki, P.A. Effect of cold-working process on corrosion behavior of copper. Mater. Des. 2012, 34, 319–324. [Google Scholar] [CrossRef]
- Ma, A.; Jiang, S.; Zheng, Y.; Yao, Z.; Ke, W.; Xia, S. Correlation between microstructure and corrosion behavior of two 90Cu10Ni alloy tubes. Acta Metall. Sin. Engl. Lett. 2014, 27, 730–738. [Google Scholar]
- Powell, C.A.; Michels, H.T. Copper-nickel alloys for seawater corrosion resistance and anti-fouling—A state of the art review. In Proceedings of the CORROSION 2000, Orlando, FL, USA, 26–31 March 2000. [Google Scholar]
- Macdonald, D.; Syrett, B.; Wing, S. The corrosion of copper-nickel alloys 706 and 715 in flowing sea water. I—Effect of oxygen. Corrosion 1978, 34, 289–301. [Google Scholar]
- Ding, P.; Lanford, W.; Hymes, S.; Murarka, S. Effects of the addition of small amounts of Al to copper: Corrosion, resistivity, adhesion, morphology, and diffusion. J. Appl. Phys. 1994, 75, 3627–3631. [Google Scholar]
- Li, Q.; Zhang, Y.; Cheng, Y.; Zuo, X.; Wang, Y.; Yuan, X.; Huang, H. Effect of temperature on the corrosion behavior and corrosion resistance of copper–aluminum laminated composite plate. Materials 2022, 15, 1621. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, J.; Liu, Y.; Gong, S.; Dai, Y.; Zhao, G.; Xie, G.; Peng, L.; Li, Z. Effects of Al addition on corrosion behavior and mechanical property of high-strength and high-elasticity Cu-20Ni-20Mn-0.3Nb-0.3Cr-0.3Zr alloy. Mater. Charact. 2020, 167, 110476. [Google Scholar]
- Cairs, J. Ferrous sulfate treatment to prevent corrosion of condenser and exchange tubes. In Technical Bulletin TB/7; Yorkshire Imperial Metals Ltd.: Leeds, UK, 1990. [Google Scholar]
- Shao, G.; Gao, Y.; Wu, J.; Liu, P.; Zhang, K.; Li, W.; Ma, F.; Zhou, H.; Chen, X. Effect of Fe/Mn content on mechanical and corrosion properties of 90/10 copper–nickel alloy. Mater. Corros. 2022, 73, 1085–1098. [Google Scholar]
- Saud, S.N.; Hamzah, E.; Abubakar, T.; Bakhsheshi-Rad, H.; Zamri, M.; Tanemura, M. Effects of Mn additions on the structure, mechanical properties, and corrosion behavior of Cu-Al-Ni shape memory alloys. J. Mater. Eng. Perform. 2014, 23, 3620–3629. [Google Scholar]
- Yang, R.; Wen, J.; Zhou, Y.; Song, K.; Song, Z. Effect of Al element on the microstructure and properties of Cu-Ni-Fe-Mn alloys. Materials 2018, 11, 1777. [Google Scholar] [CrossRef]
- Wood, R.J. Erosion–corrosion interactions and their effect on marine and offshore materials. Wear 2006, 261, 1012–1023. [Google Scholar]
- Wang, W.; Zhang, W.; Liu, Y.; Zou, J.; Mi, X.; Li, D.; Li, K.; Huang, G. Tailoring microstructure of a nickel aluminium bronze by hot extrusion and its impact on mechanical and corrosion behaviour. Corros. Sci. 2023, 215, 111049. [Google Scholar]
- Meng, F.; Lu, X.; Liu, Y.; Qi, Y. First-principles study on the effect and magnetism of iron segregation in Cu grain boundary. J. Mater. Sci. 2017, 52, 4309–4322. [Google Scholar]
- Kuzmina, M.; Ponge, D.; Raabe, D. Grain boundary segregation engineering and austenite reversion turn embrittlement into toughness: Example of a 9 wt.% medium Mn steel. Acta Mater. 2015, 86, 182–192. [Google Scholar]
- Ye, X.-P.; Li, Y.-L.; Weng, J.-D.; Cai, L.-C.; Liu, C.-L. Research status on strengthening mechanism of particle-reinforced metal matrix composites. J. Mater. Eng. 2018, 46, 28–37. [Google Scholar]
- Geng, T.; Xu, Z.; Lu, J.; Wang, J.; Wang, Y. Morphological evolution and anodic electrochemical behaviors of Ni-based single crystal superalloy and its recast layer in NaNO3 electrolyte. Electrochim. Acta 2023, 462, 142709. [Google Scholar]
- Hu, Y.; Li, X.; Wang, C.; Hou, Y.; Li, M.; Xue, S.; Zhang, S.; Dong, C.; Li, Z.; Zheng, Y. Linear evolution of the γ′-(Ni, Cu)3Al phase composition in Cu-based high-temperature alloys. J. Mater. Sci. 2024, 59, 11998–12010. [Google Scholar]
- Liu, C.; Feng, H.; Yang, Y.; Zhang, E.; Ding, J.; Dai, J.; Xia, X.; Tang, Y.; Li, C.; Chen, X. Short-term corrosion behavior of polycrystalline Ni3Al-based superalloy in sulfur-containing atmosphere. Intermetallics 2022, 142, 107446. [Google Scholar]
- Han, S.; Lei, X.; Xia, W.; Guo, J.; Yang, R.; Liu, J.; Yao, W.; Wang, N. Optimizing the electrochemical corrosion performance of laser cladded Ni-based single crystal superalloy via manipulation of γ′(Ni3Al) phase. Surf. Coat. Technol. 2023, 467, 129700. [Google Scholar]
- Zhu, X.; Lei, T. Characteristics and formation of corrosion product films of 70Cu–30Ni alloy in seawater. Corros. Sci. 2002, 44, 67–79. [Google Scholar]
- Prakashaiah, B.; Kumara, D.V.; Pandith, A.A.; Shetty, A.N.; Rani, B.A. Corrosion inhibition of 2024-T3 aluminum alloy in 3.5% NaCl by thiosemicarbazone derivatives. Corros. Sci. 2018, 136, 326–338. [Google Scholar]
- North, R.; Pryor, M. The influence of corrosion product structure on the corrosion rate of Cu-Ni alloys. Corros. Sci. 1970, 10, 297–311. [Google Scholar]
| Ni | Al | Fe | Mn | Cu | |
|---|---|---|---|---|---|
| Cu-10Ni-3Al-1.8Fe-0.8Mn | 9.98 | 2.97 | 1.78 | 0.78 | Bal. |
| Specimen | Heat Treatment |
|---|---|
| AC-1 | As-cast |
| H-2 | Homogenization (950 °C/4 h) |
| D-3 | Deformation–aging (500 °C/0.5 h) |
| D-4 | Deformation–aging (750 °C/0.5 h) |
| Specimen | AC-1 | H-2 | D-3 | D-4 |
|---|---|---|---|---|
| Rs (Ω·cm2) | 23.49 | 1.77 | 10.56 | 42.24 |
| Rct (Ω·cm2) | 441 | 9 | 1146 | 249 |
| Qct × 10−5 (F·cm−2) | 7.83 | 0.40 | 11.50 | 0.17 |
| n1 | 0.78 | 0.69 | 0.99 | 0.90 |
| Rf1 (Ω·cm2) | 12.35 | 33.25 | 1339 | 1117 |
| Q1 × 10−5 (F·cm−2) | 3.22 | 24.43 | 0.79 | 7.40 |
| n2 | 0.77 | 0.61 | 0.95 | 0.82 |
| Rf2 × 103 (Ω·cm2) | 104.62 | 10.42 | 298.50 | 175.91 |
| Q2 × 10−5 (F·cm−2) | 8.22 | 38.53 | 16.18 | 0.52 |
| n3 | 0.66 | 0.62 | 0.92 | 0.79 |
| Rt × 103 (Ω·cm2) | 105.07 | 10.46 | 300.99 | 177.28 |
| ∑χ2 × 10−3 | 1.47 | 0.65 | 0.22 | 1.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Zhao, Y.; Cao, Y.; Huang, L.; Chu, H.; Wang, H.; Yue, D.; Zhang, W. Microstructural Optimization and Erosion–Corrosion Resistance of Cu-10Ni-3Al-1.8Fe-0.8Mn Alloy via Tailored Heat Treatment. Materials 2025, 18, 1511. https://doi.org/10.3390/ma18071511
Yuan Y, Zhao Y, Cao Y, Huang L, Chu H, Wang H, Yue D, Zhang W. Microstructural Optimization and Erosion–Corrosion Resistance of Cu-10Ni-3Al-1.8Fe-0.8Mn Alloy via Tailored Heat Treatment. Materials. 2025; 18(7):1511. https://doi.org/10.3390/ma18071511
Chicago/Turabian StyleYuan, Yi, Yizhi Zhao, Yicheng Cao, Lue Huang, Hao Chu, Hongqian Wang, Dongyan Yue, and Wenjing Zhang. 2025. "Microstructural Optimization and Erosion–Corrosion Resistance of Cu-10Ni-3Al-1.8Fe-0.8Mn Alloy via Tailored Heat Treatment" Materials 18, no. 7: 1511. https://doi.org/10.3390/ma18071511
APA StyleYuan, Y., Zhao, Y., Cao, Y., Huang, L., Chu, H., Wang, H., Yue, D., & Zhang, W. (2025). Microstructural Optimization and Erosion–Corrosion Resistance of Cu-10Ni-3Al-1.8Fe-0.8Mn Alloy via Tailored Heat Treatment. Materials, 18(7), 1511. https://doi.org/10.3390/ma18071511





