Isothermal Oxidation Behavior of Nickel Base Single Crystal DD6 Film-Cooling Blades at 1050 °C
Abstract
1. Introduction
2. Materials and Methods
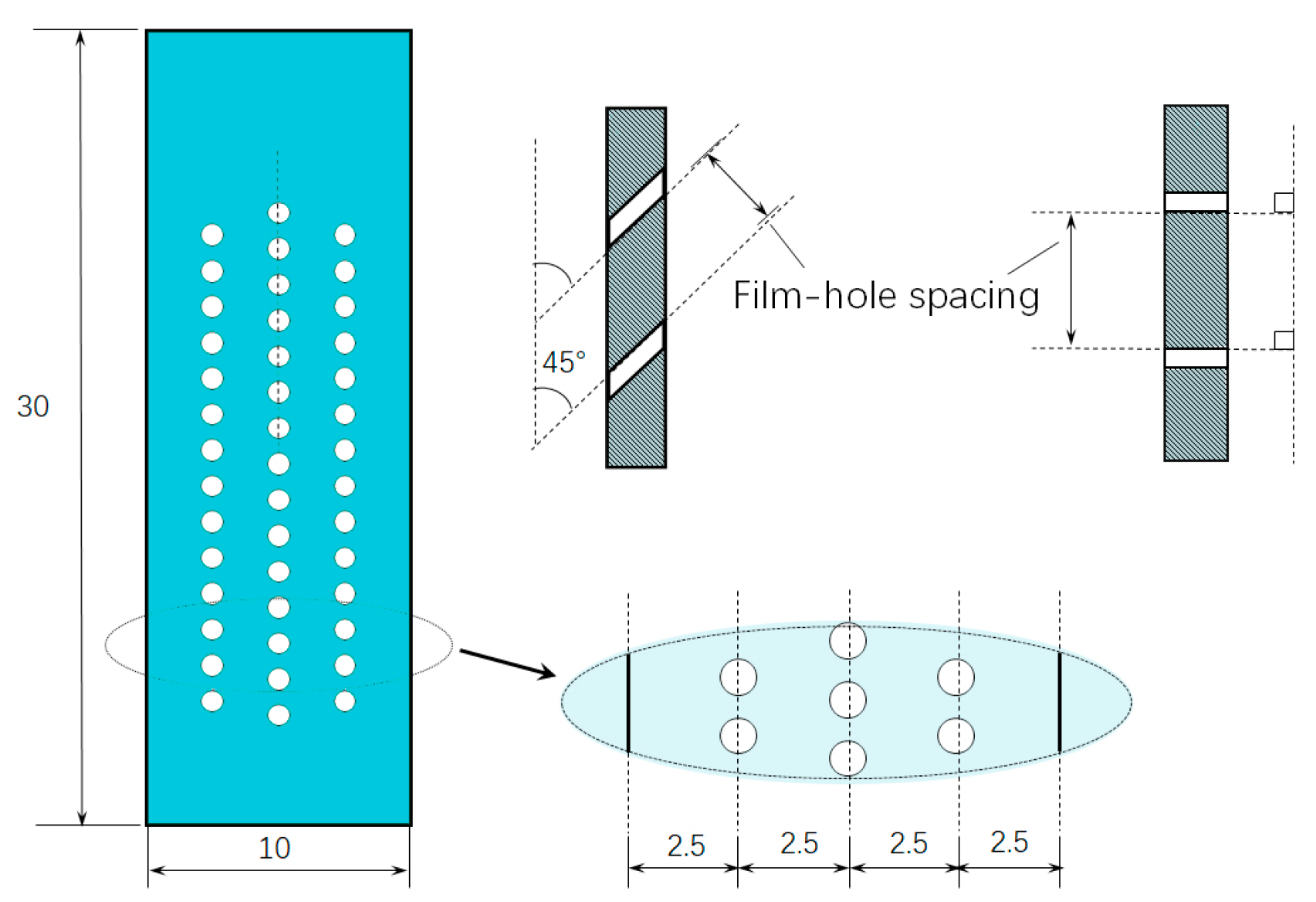
2.1. Materials
2.2. Isothermal Oxidation Test
2.3. Phase Analysis and Morphology Observation
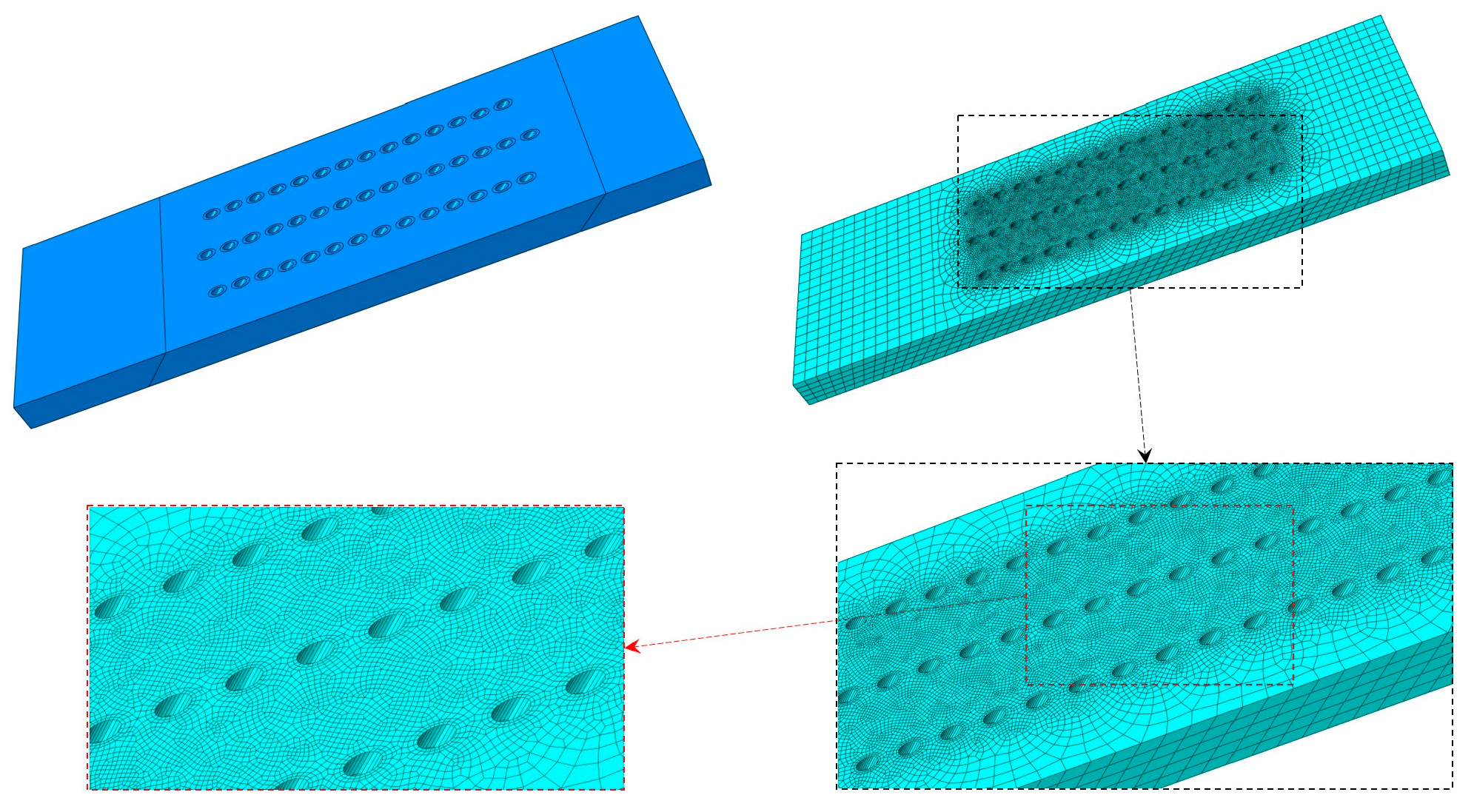
2.4. Numerical Simulation
3. Experimental Results
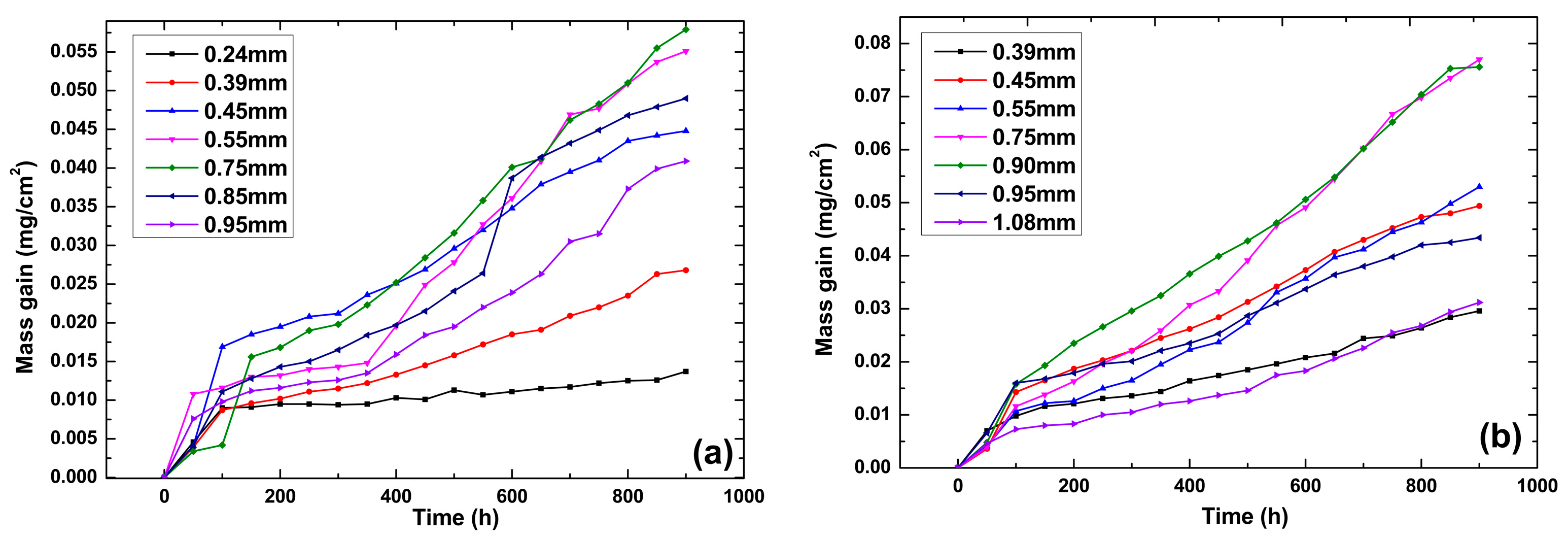
3.1. Oxidation Kinetics
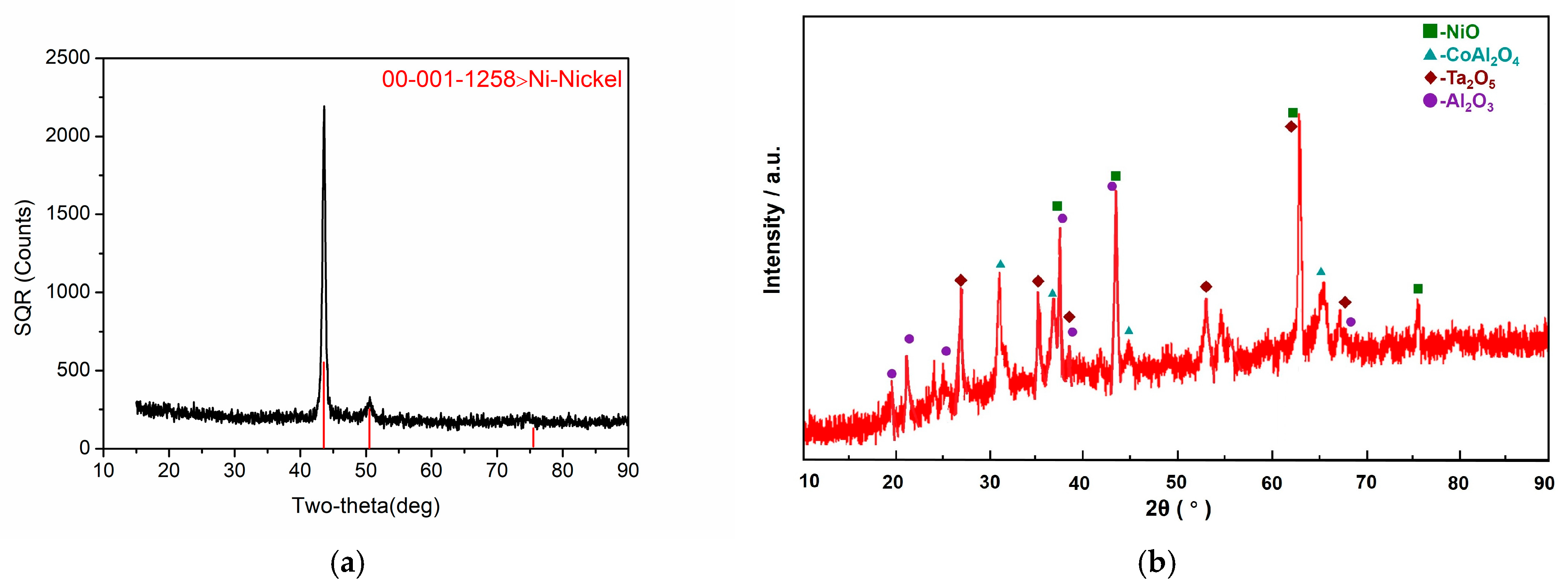
3.2. Phase Analysis
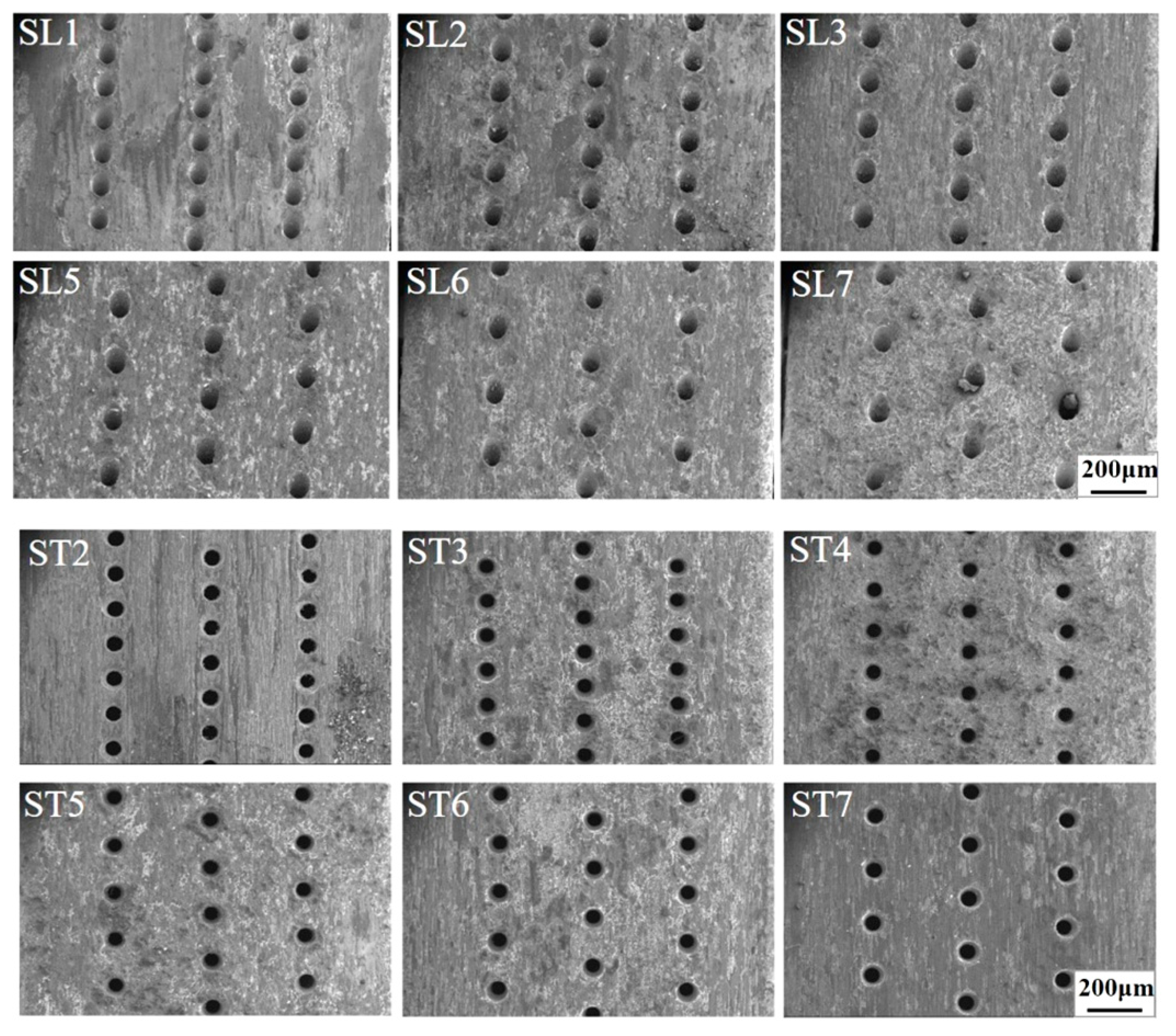
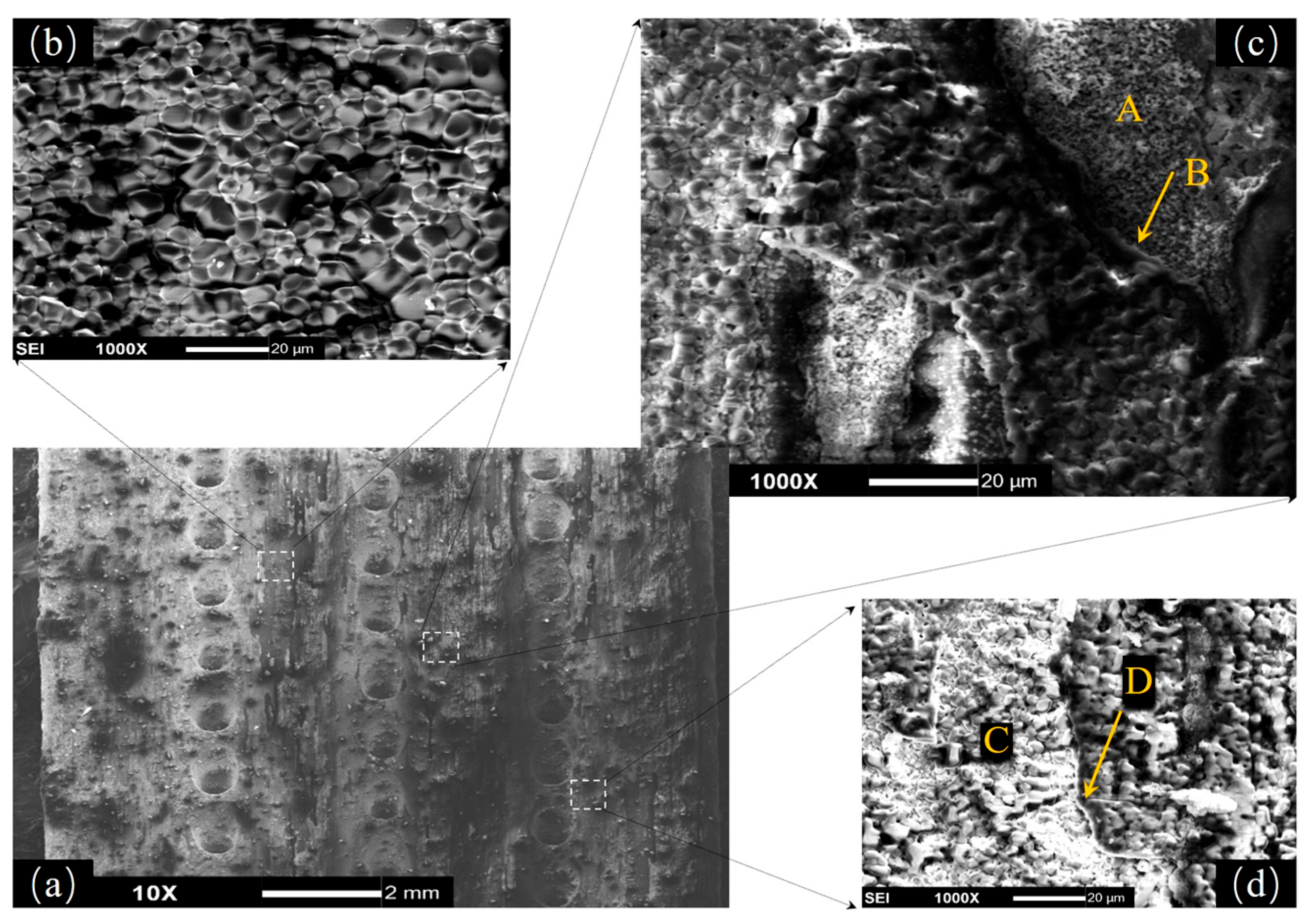
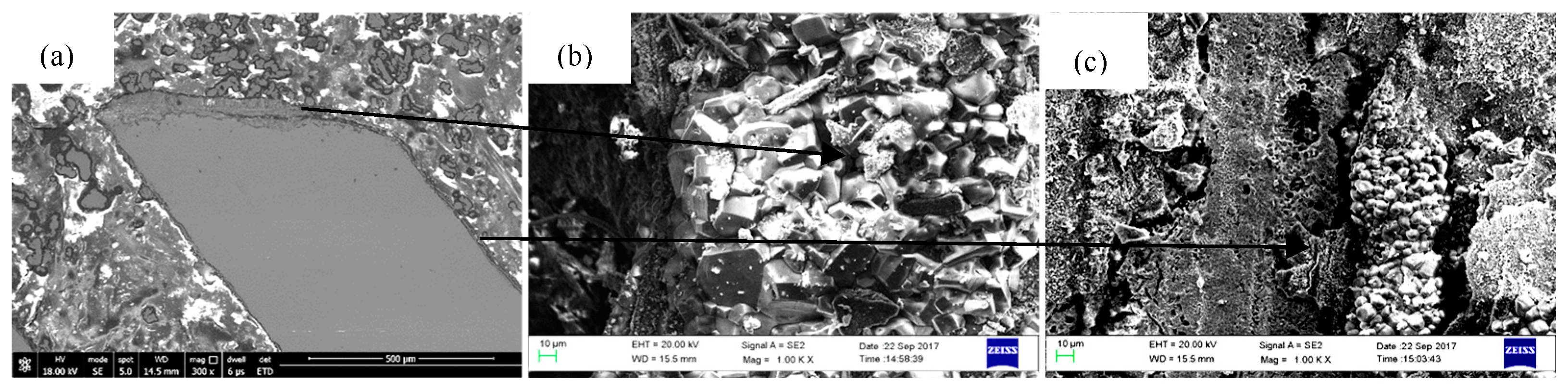
3.3. Morphologies and Microstructures
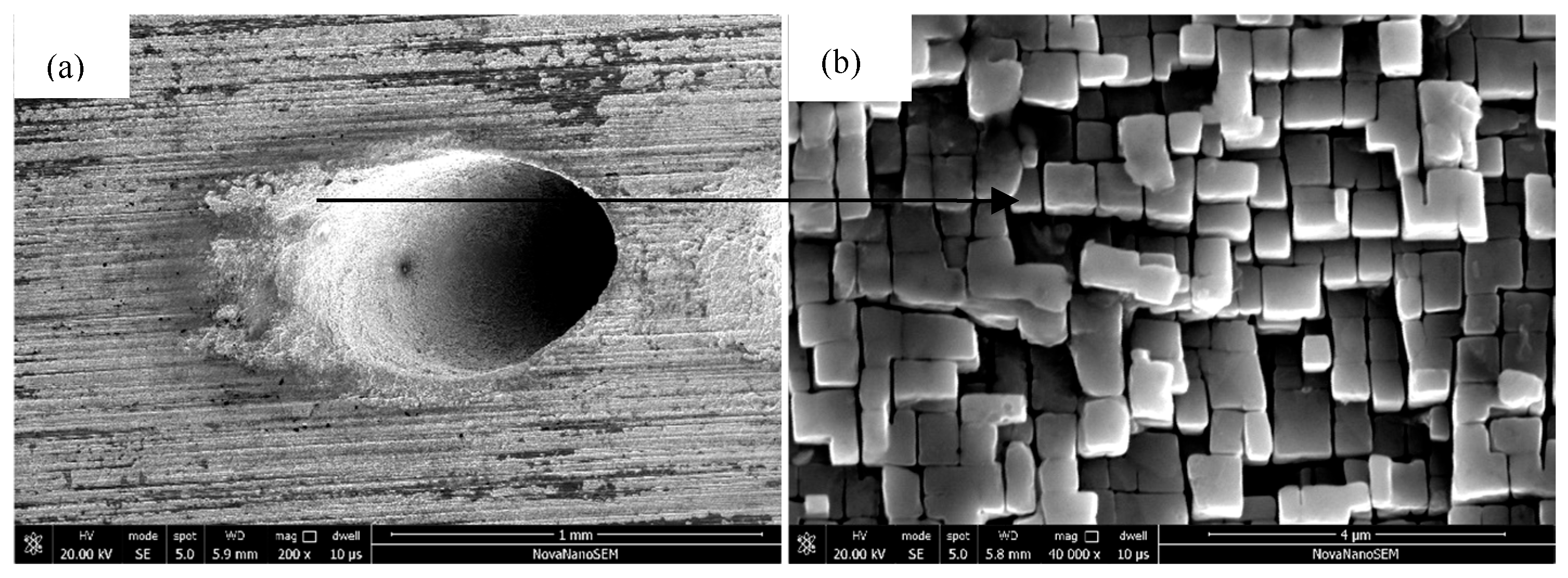
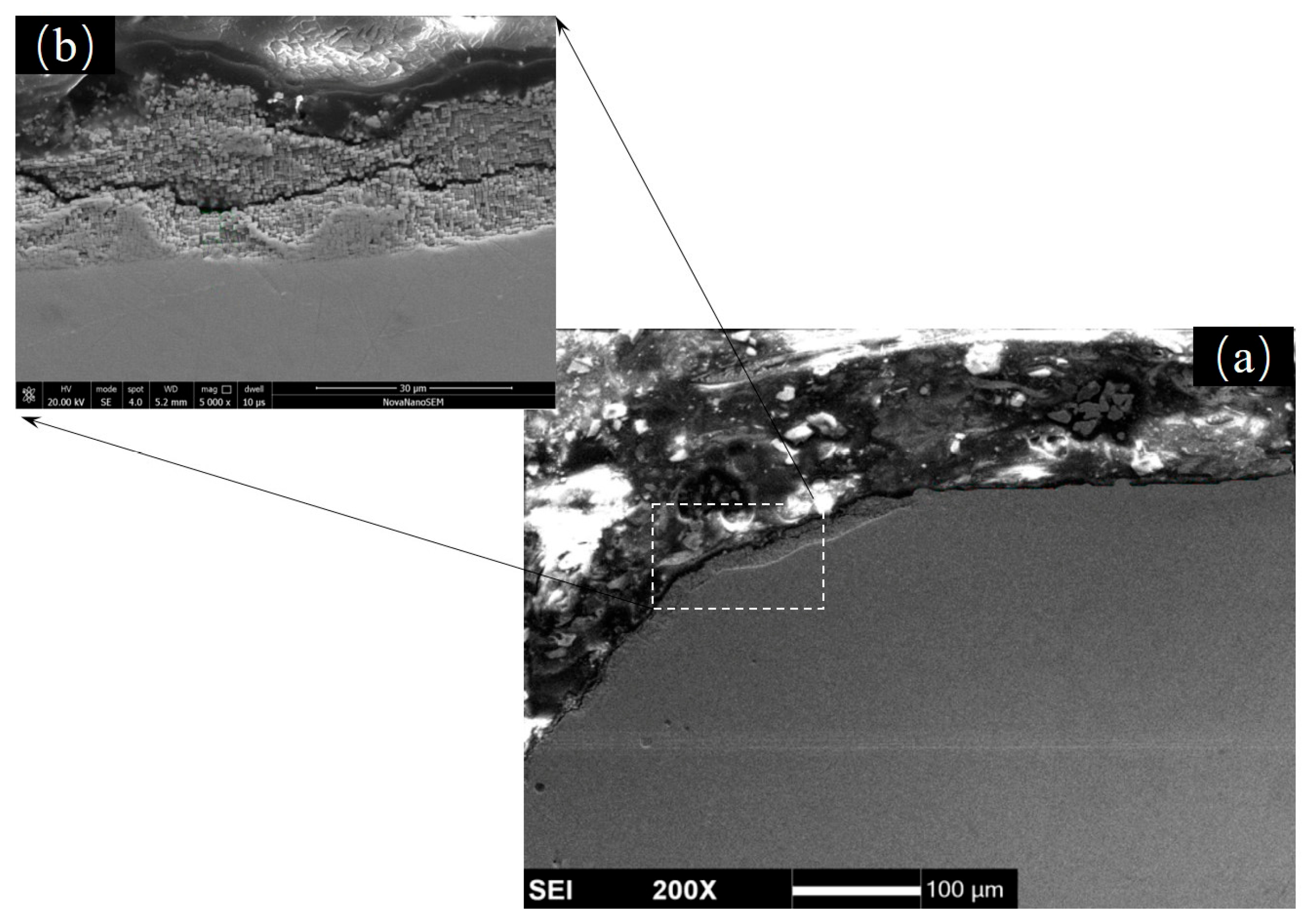
3.4. Thin-Wall Effect of Oxidative Damage on the Film Holes
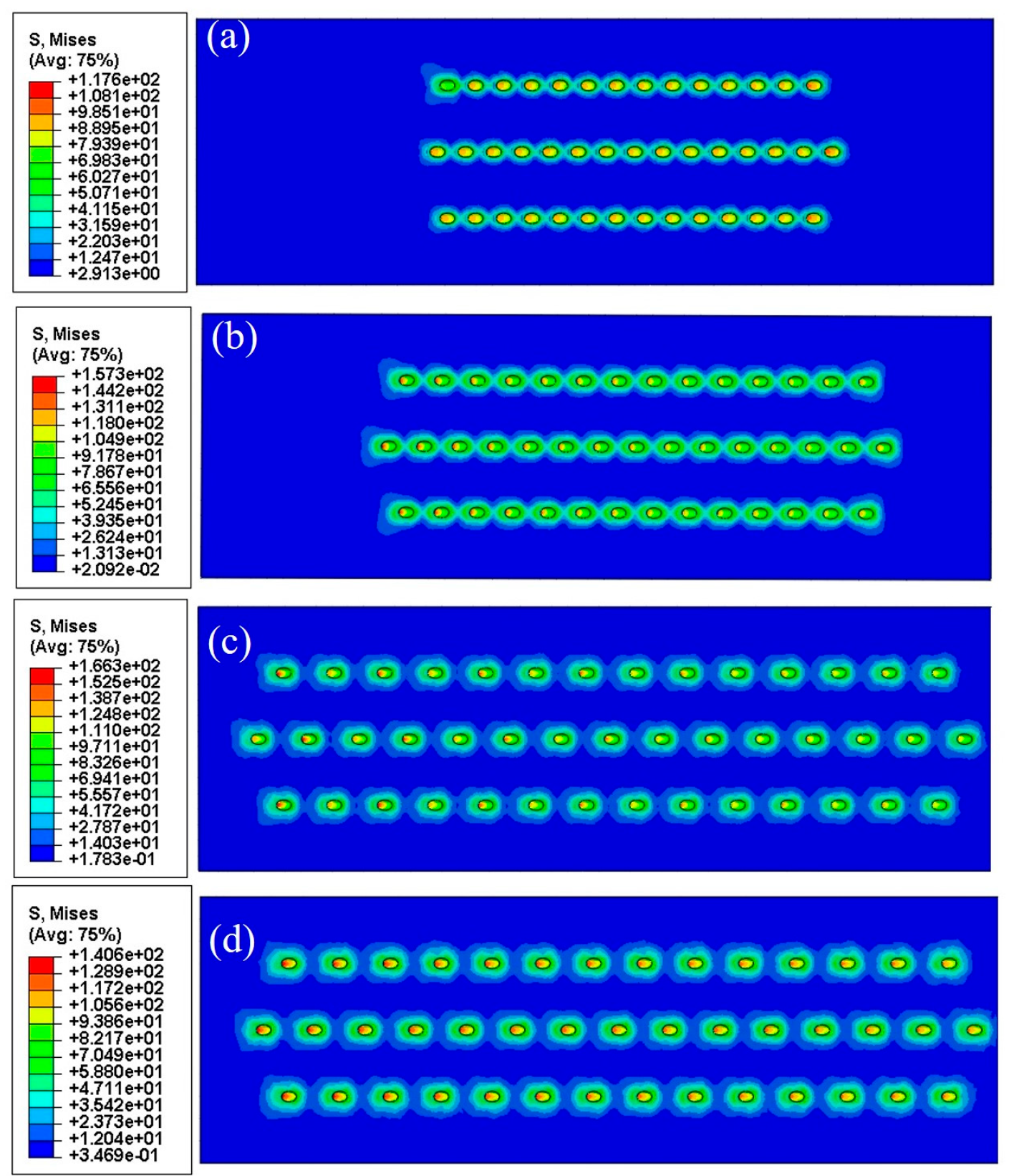
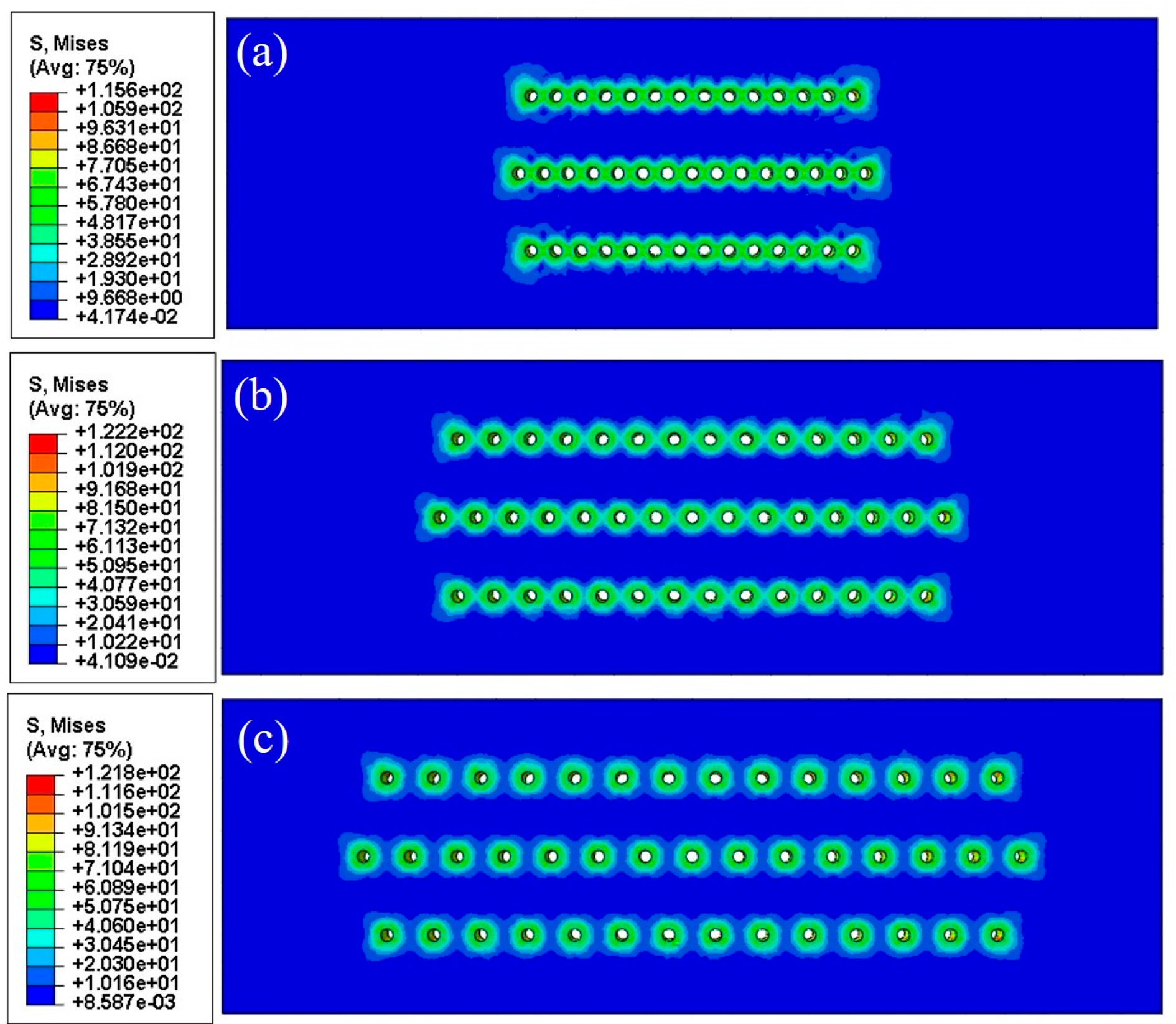
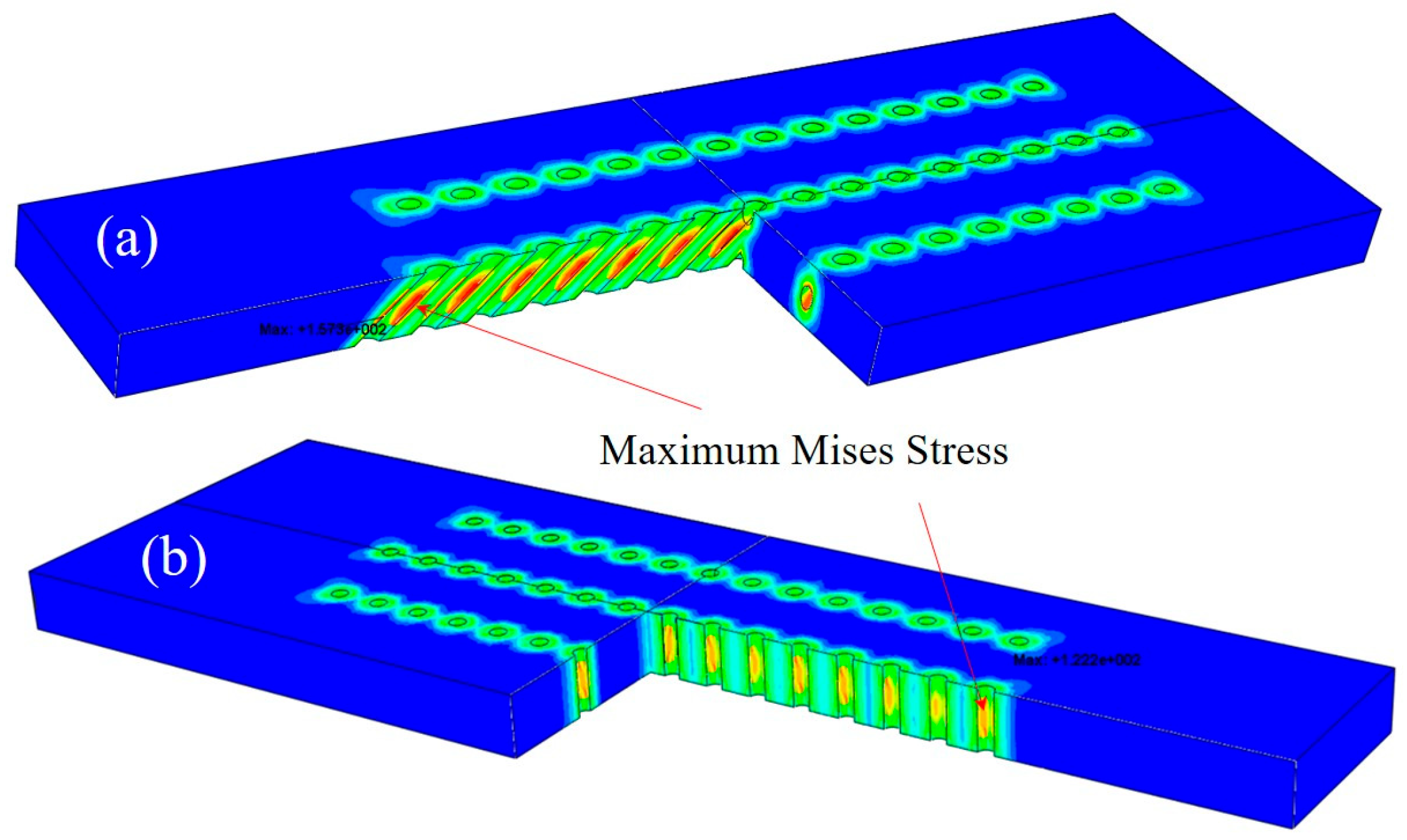
3.5. Numerical Results
4. Discussion
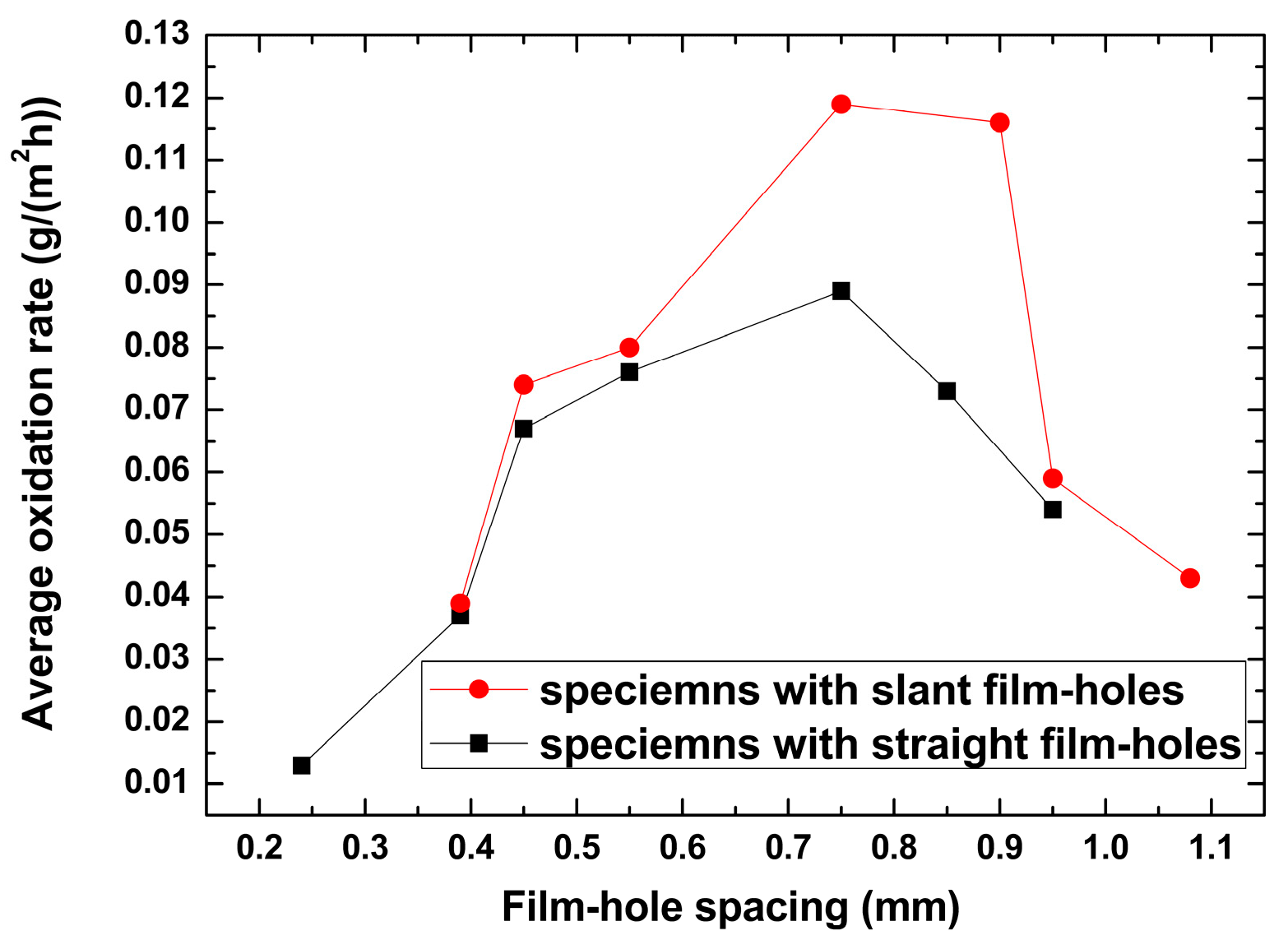
4.1. Oxidation Kinetics of Specimens with Different Film Hole Spacings
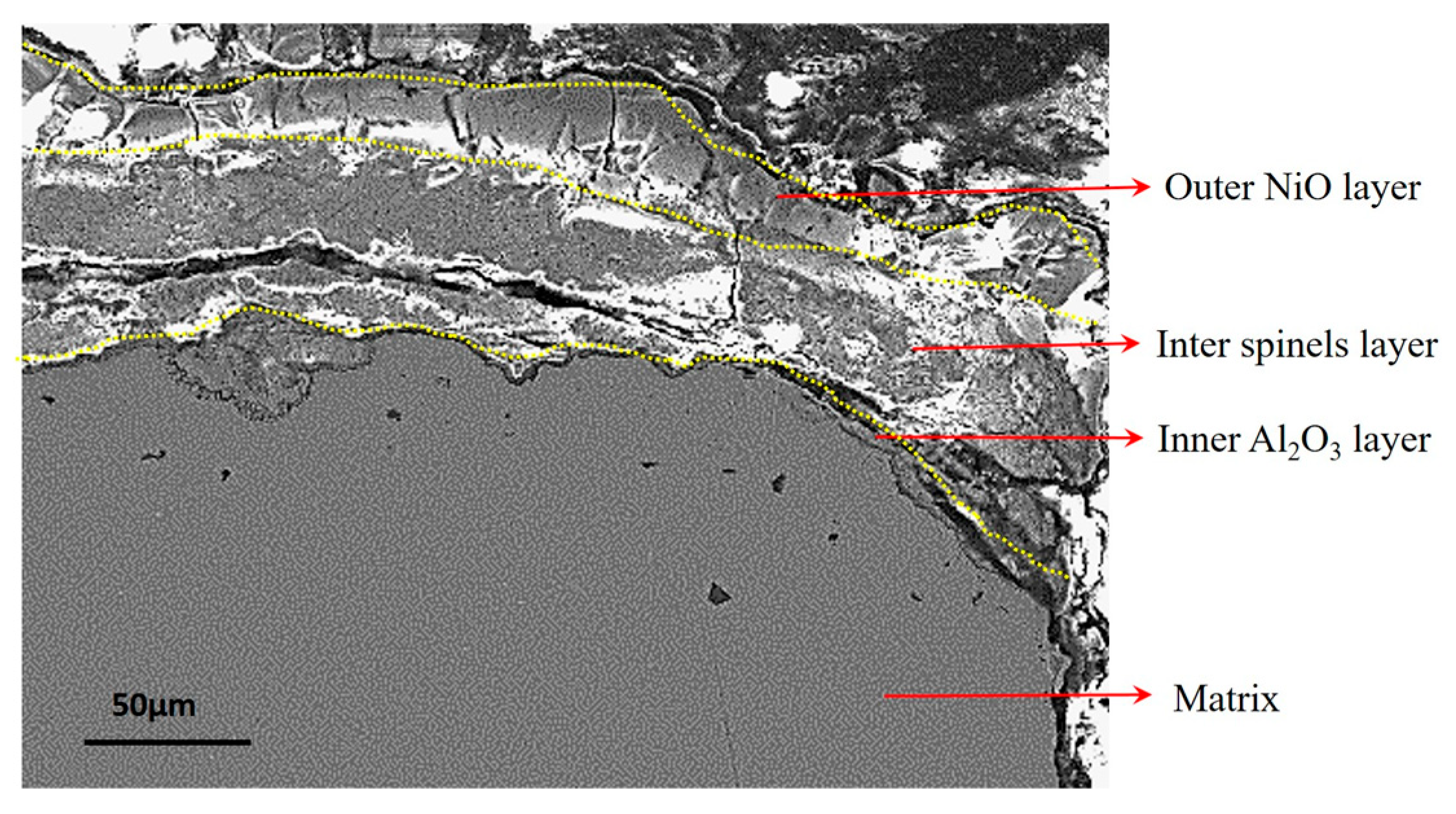
4.2. Oxide Layers and Oxidation Affected Zone
4.3. Growth Stress in Oxide Layers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pint, B.A.; DiStefano, J.R.; Wright, I.G. Oxidation resistance: One barrier to moving beyond Ni-base superalloys. Mater. Sci. Eng. A 2006, 415, 255–263. [Google Scholar] [CrossRef]
- Sato, A.; Chiu, Y.L.; Reed, R.C. Oxidation of nickel-based single-crystal superalloys for industrial gas turbine applications. Acta Mater. 2011, 59, 225–240. [Google Scholar] [CrossRef]
- Shi, Z.; Li, J.; Liu, S. Isothermal oxidation behavior of single crystal superalloy DD6. Trans. Nonferrous Met. Soc. China 2012, 22, 534–538. [Google Scholar] [CrossRef]
- Bensch, M.; Sato, A.; Warnkenb, N.; Affeldt, E.; Reed, R.C.; Glatzel, U. Modelling of High Temperature Oxidation of Alumina-Forming Single-Crystal Nickel-Base Superalloys. Acta Mater. 2012, 60, 5468–5480. [Google Scholar] [CrossRef]
- Hu, C.Y.; Liu, X.L.; Liu, C.K.; Tao, C. Thin-wall effect of oxidative damage on film holes of single crystal superalloy. Fail. Anal. Prev. 2023, 18, 8–13. [Google Scholar]
- Liu, X.L.; Wu, X.C.; Li, Z.; Hu, C.; Chen, X.; Liu, C. Muti-angle characterization methods and damage mechanism of single crystal superalloy caused by EDM drilling. Fail. Anal. Prev. 2023, 18, 21–28. [Google Scholar]
- Younes, C.M.; Allen, G.C.; Nicholson, J.A. High temperature oxidation behaviour of single crystal superalloys RR3000 and CMSX-4. Corros. Eng. Sci. Technol. 2007, 42, 80–88. [Google Scholar] [CrossRef]
- Saltykov, P.; Fabrichnaya, O.; Golczewski, J.; Aldinger, F. Thermodynamic modeling of oxidation of Al–Cr–Ni alloys. J. Alloys Compd. 2004, 381, 99–113. [Google Scholar] [CrossRef]
- Feng, Q.; Tryon, B.; Carroll, L.J.; Pollock, T.M. Cyclic oxidation of Ru-containing single crystal superalloys at 1100 °C. Mater. Sci. Eng. A 2007, 458, 184–194. [Google Scholar] [CrossRef]
- Wang, X.; Szpunar, J.A. Effects of grain sizes on the oxidation behavior of Ni-based alloy 230 and N. J. Alloys Compd. 2018, 752, 40–52. [Google Scholar] [CrossRef]
- Wu, Y.; Narita, T. Oxidation behavior of the single crystal Ni-based superalloy at 900 °C in air and water vapor. Surf. Coatings Technol. 2007, 202, 140–145. [Google Scholar] [CrossRef]
- Karabela, A.; Zhao, L.G.; Tong, J.; Simms, N.J.; Nicholls, J.R.; Hardy, M.C. Effects of cyclic stress and temperature on oxidation damage of a nickel-based superalloy. Mater. Sci. Eng. A 2011, 528, 6194–6202. [Google Scholar] [CrossRef]
- Krewinkel, R. A review of gas turbine effusion cooling studies. Int. J. Heat Mass Transf. 2013, 66, 706–722. [Google Scholar] [CrossRef]
- Lukas, P.; Preclik, P.; Cadek, J. Notch effcets on creep behavior of CMSX-4 superalloy single crystal. Mater. Sci. Eng. A 2001, 298, 84–89. [Google Scholar] [CrossRef]
- Raffaitin, A.; Monceau, D.; Andrieu, E.; Crabos, F. Cyclic oxidation of coated and uncoated single-crystal nickel-based superalloy MC2 analyzed by continuous thermogravimetry analysis. Acta Mater. 2006, 54, 4473–4487. [Google Scholar] [CrossRef]
- Nunez, J.E.; Glinka, G. Analysis of non-localized creep induced strains and stresses in notches. Eng. Frac. Mech. 2004, 71, 1791–1803. [Google Scholar] [CrossRef]
- Hopgood, A.A.; Martin, J.W. The creep behavior of anickel-based single-crystal superalloy. Mater. Sci. Eng. A 1986, 82, 27–36. [Google Scholar] [CrossRef]
- Akhtar, A.; Hook, M.S.; Reed, R.C. On the oxidation of the third-generation single crystal superalloy CMSX-10. Metall. Mater. Trans. A 2005, 36, 3001–3017. [Google Scholar] [CrossRef]
- Bensch, M.; Preußner, J.; Hüttner, R.; Obigodi, G.; Virtanen, S.; Gabel, J.; Glatzel, U. Modelling and analysis of the oxidation influence on creep behaviour of thin-walled structures of the single-crystal nickel-base superalloy René N5 at 980 °C. Acta Mater. 2010, 58, 1607–1617. [Google Scholar] [CrossRef]
- Bensch, M.; Konrad, C.H.; Fleischmann, E.; Rae, C.M.F.; Glatzel, U. Influence of oxidation on near-surface γ′ fraction and resulting creep behaviour of single crystal Ni-base superalloy M247LC SX. Mater. Sci. Eng. A 2013, 577, 179–188. [Google Scholar] [CrossRef]
- Stringer, J. Stress generation and relief in growing oxide films. Corros. Sci. 1970, 10, 513–543. [Google Scholar] [CrossRef]
- Krishnamurthy, R.; Srolovitz, D.J. Stress distributions in growing oxide films. Acta Mater. 2003, 51, 2171–2190. [Google Scholar] [CrossRef]
| Co | W | Ta | Al | Cr | Re | Mo | Ni |
|---|---|---|---|---|---|---|---|
| 9 | 8 | 7.5 | 5.6 | 4.3 | 2 | 2 | Balance |
| Specimen No. | Drilling Angles to the Longitudinal Axis (°) | Film Hole Spacing (mm) | Specimen No. | Drilling Angles to the Longitudinal Axis (°) | Film Hole Spacing (mm) |
|---|---|---|---|---|---|
| SL1 | 45 | 0.24 | ST1 | 90 | 0.39 |
| SL2 | 45 | 0.39 | ST2 | 90 | 0.45 |
| SL3 | 45 | 0.45 | ST3 | 90 | 0.55 |
| SL4 | 45 | 0.55 | ST4 | 90 | 0.75 |
| SL5 | 45 | 0.75 | ST5 | 90 | 0.9 |
| SL6 | 45 | 0.85 | ST6 | 90 | 0.95 |
| SL7 | 45 | 0.95 | ST7 | 90 | 1.08 |
| Material Model | Young’s Modulus (GPa) | Poisson’s Ratio | Thermal Expansion Coefficient (10−6) | |
|---|---|---|---|---|
| Matrix | Isotropic | 200.0 | 0.30 | 1.0 |
| Orthotropic ([001]) | 80.5 | 0.39 | 1.0 | |
| Oxide layer | Isotropic | 200.0 | 0.30 | 5.0 |
| Orthotropic ([001]) | 80.5 | 0.39 | 5.0 | |
| Specimens | Film Hole Spacing (mm) | Maximum Mises Stress of FEM Models (MPa) | |
|---|---|---|---|
| With Isotropic Materials | With Orthotropic Materials | ||
| With slanted film holes | 0.35 | 135.9 | 117.6 |
| 0.55 | 173.5 | 157.3 | |
| 0.75 | 187.3 | 166.3 | |
| 0.95 | 154.6 | 140.6 | |
| With straight film holes | 0.4 | 147.7 | 115.6 |
| 0.75 | 153.6 | 122.2 | |
| 1.1 | 151.7 | 121.8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Liu, X.; Liu, C.; Sun, W.; Tao, C. Isothermal Oxidation Behavior of Nickel Base Single Crystal DD6 Film-Cooling Blades at 1050 °C. Materials 2025, 18, 1498. https://doi.org/10.3390/ma18071498
Hu C, Liu X, Liu C, Sun W, Tao C. Isothermal Oxidation Behavior of Nickel Base Single Crystal DD6 Film-Cooling Blades at 1050 °C. Materials. 2025; 18(7):1498. https://doi.org/10.3390/ma18071498
Chicago/Turabian StyleHu, Chunyan, Xinling Liu, Changkui Liu, Weikang Sun, and Chunhu Tao. 2025. "Isothermal Oxidation Behavior of Nickel Base Single Crystal DD6 Film-Cooling Blades at 1050 °C" Materials 18, no. 7: 1498. https://doi.org/10.3390/ma18071498
APA StyleHu, C., Liu, X., Liu, C., Sun, W., & Tao, C. (2025). Isothermal Oxidation Behavior of Nickel Base Single Crystal DD6 Film-Cooling Blades at 1050 °C. Materials, 18(7), 1498. https://doi.org/10.3390/ma18071498






