High-Temperature Wetting Behavior and Adhesion Mechanism of Cryolite-Based Molten Salt on SiC Refractory Substrate
Highlights
- The wetting behaviors of molten aluminum slag droplets on SiC plates in various Al2O3 contents were investigated.
- Al2O3 enhances both surface tensions of the slag, thereby increasing the adhesion work between the slag and SiC.
- Revealing the interfacial microstructure evolution and the interaction mechanism between Na3AlF6-Al2O3-CaF2 slag and SiC.
- Providing a theoretical basis for the guidance of developing, designing, and applying inner wall materials for vacuum ladles.
Abstract
1. Introduction
2. Experimental and Thermodynamic Calculations
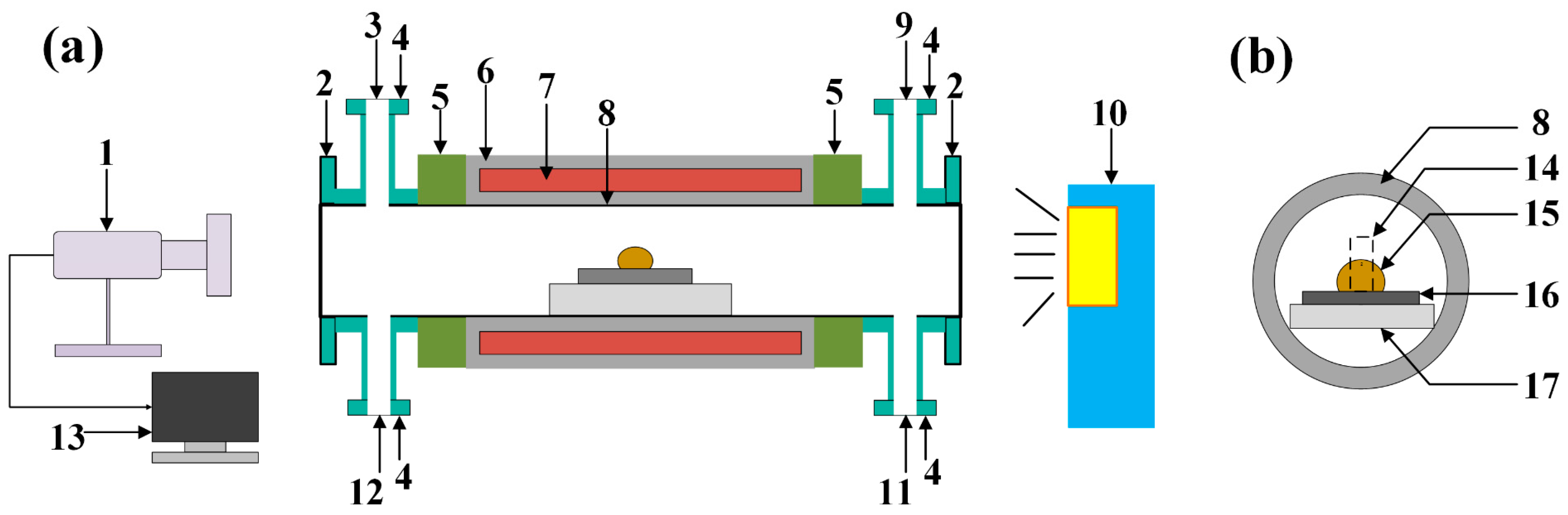
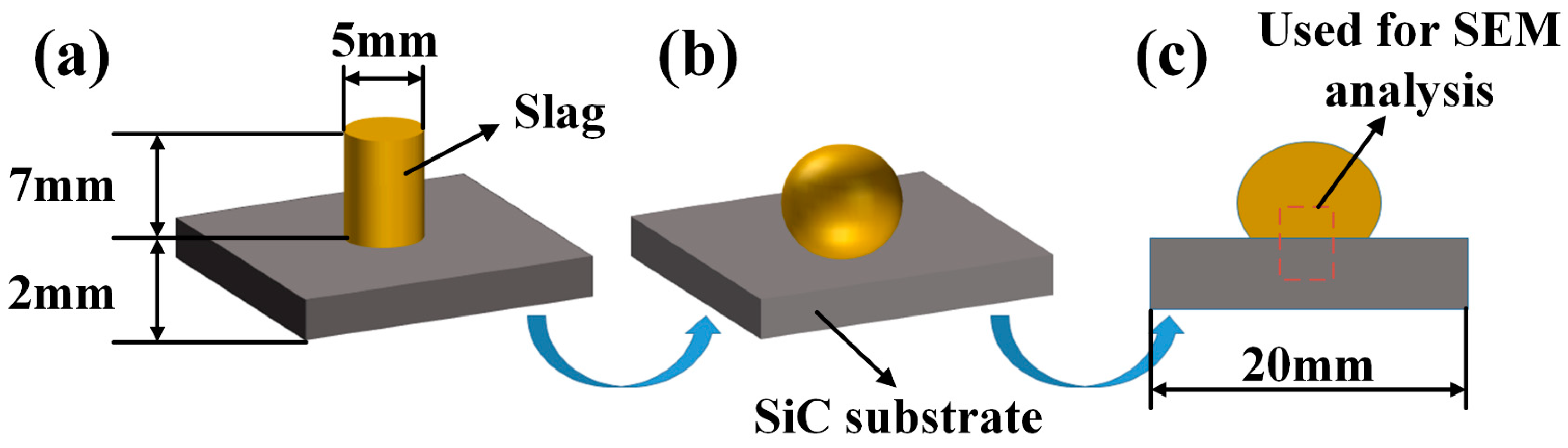
2.1. Preparation of the Aluminum Slag and SiC Substrate
2.2. Experimental Methods and Thermodynamic Simulation Calculations
3. Results and Discussion
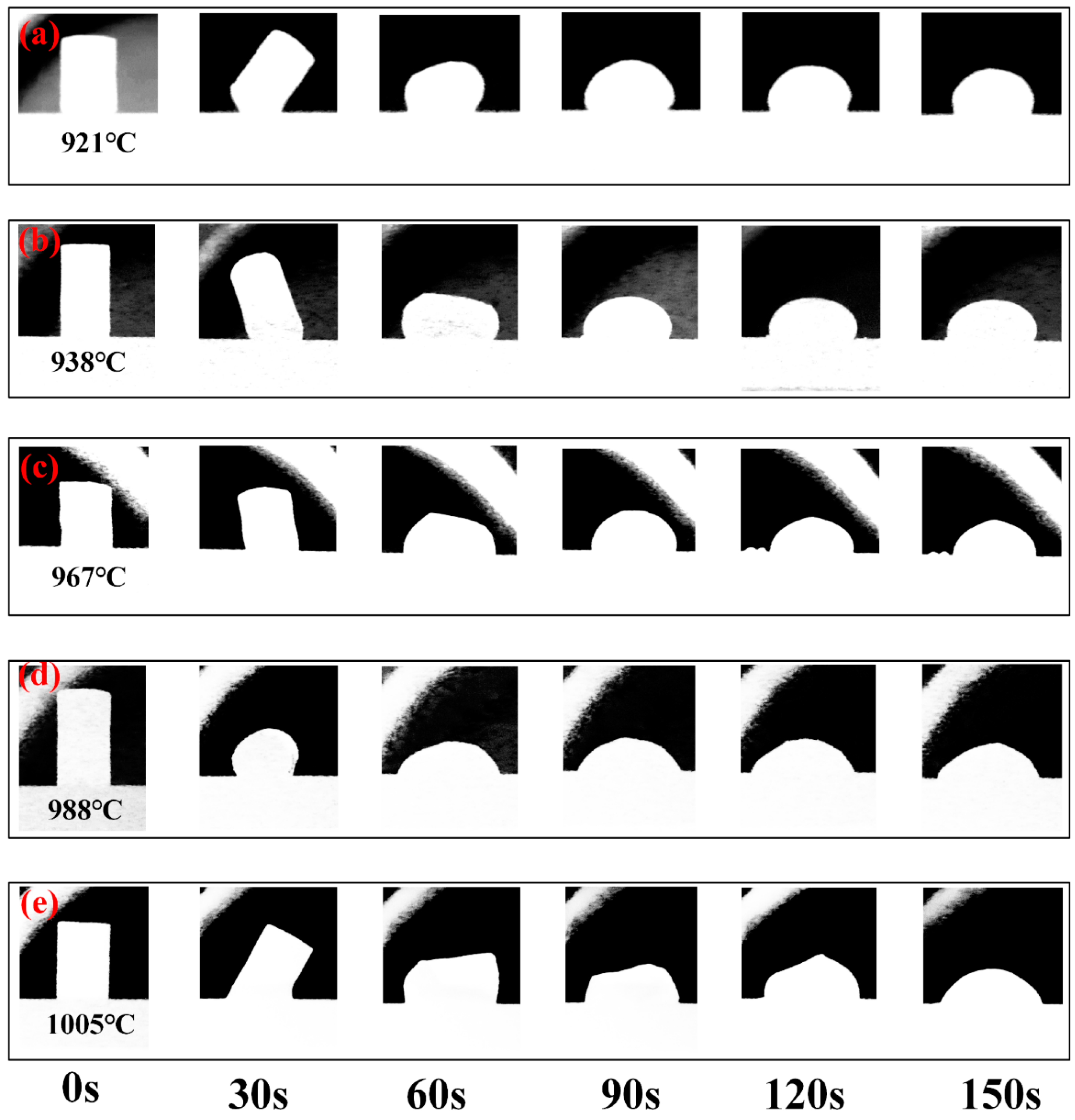
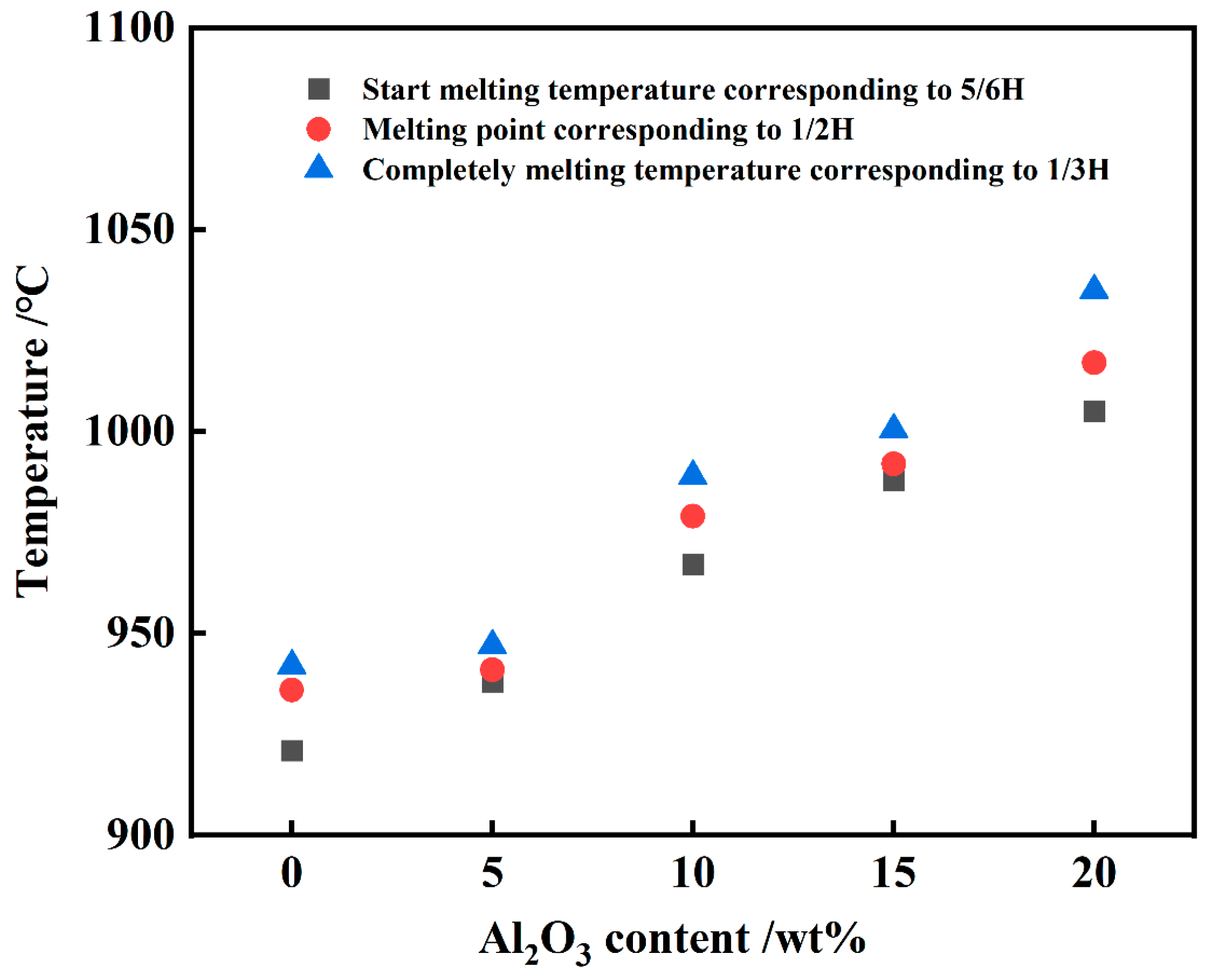
3.1. Aluminum Slag Melting and the Change of Droplet Morphology
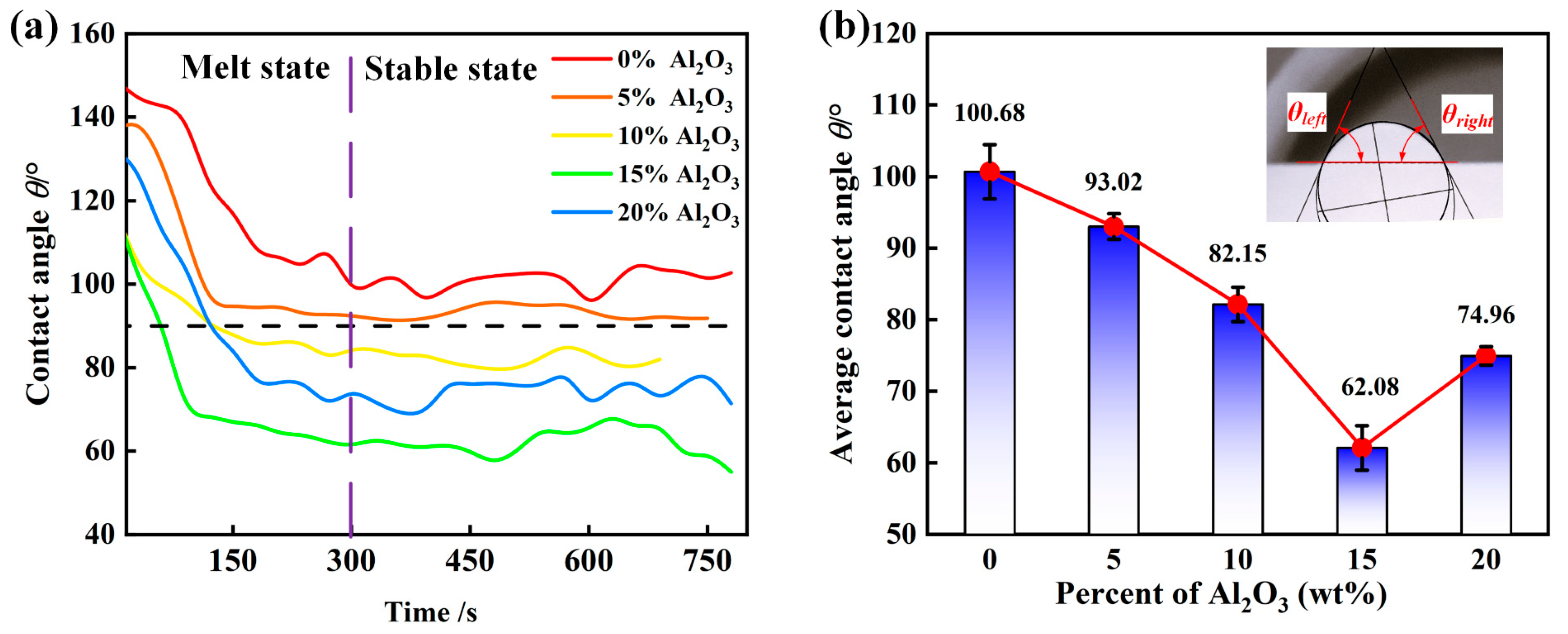
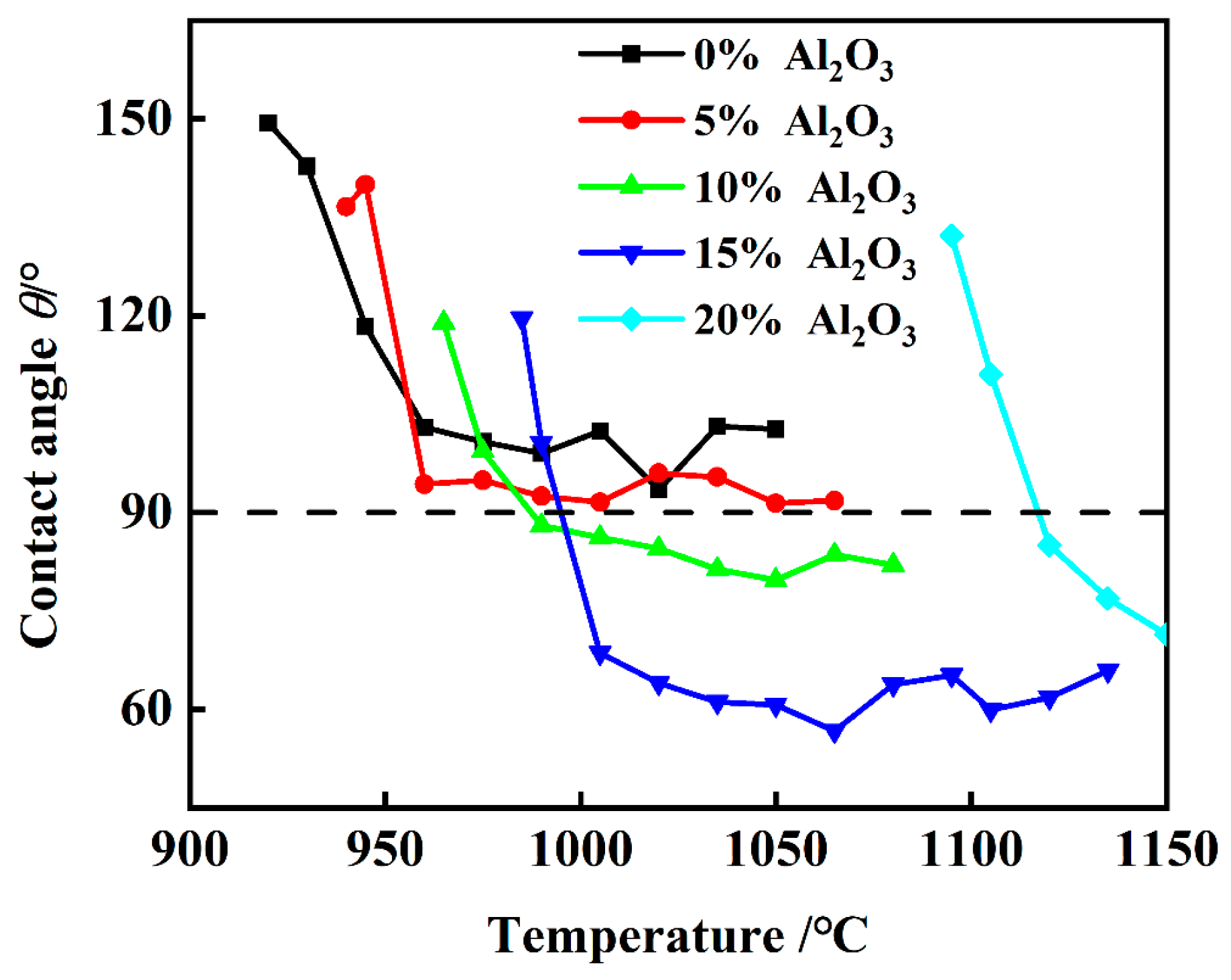
3.2. The Apparent Contact Angle Between the Molten Slag and the SiC Substrate
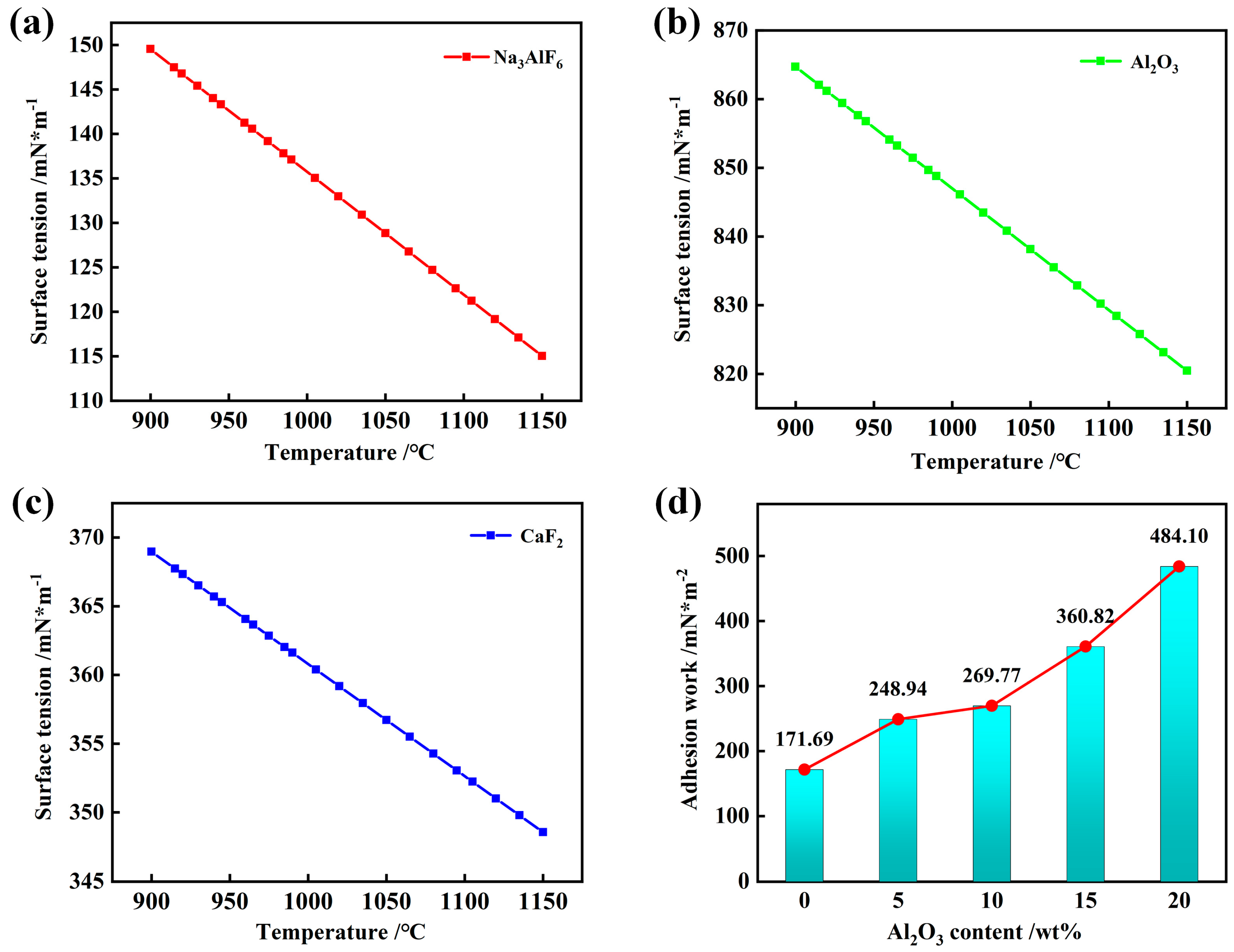
3.3. Effect of Al2O3 Content on Surface Tension and Interfacial Properties Between Slag and Silicon Carbide Substrate
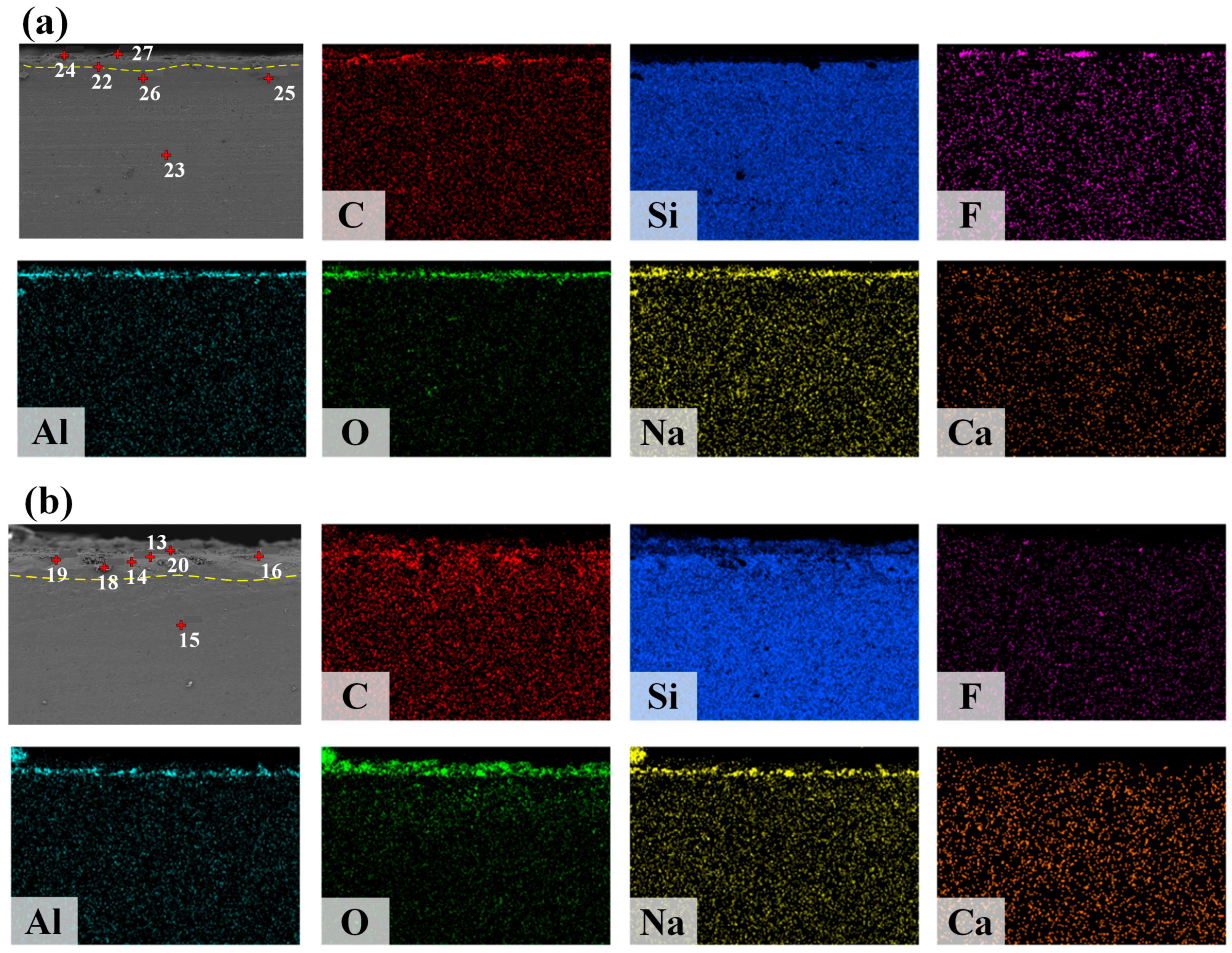
3.4. Analysis of the Microstructure and Interfacial Behavior of the Interface Between Slag and Refractory Materials
4. Conclusions
- (1)
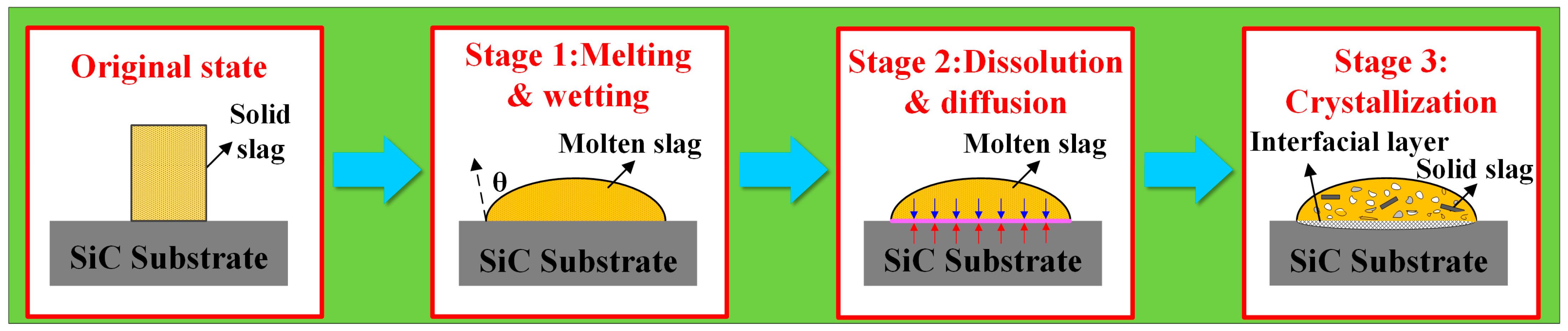
- The experimental wetting process is summarized in three stages: melting and wetting, dissolution and diffusion, and crystallization. Two primary factors that influence substrate adhesion are considered: good wettability and the dissolution of Al2O3 in the slag. The interaction between the slag and the SiC substrate is described as non-reactive wetting, and the results demonstrate that the melting temperature of the Na3AlF6-Al2O3-CaF2 slag increases significantly with the addition of Al2O3. This increase in temperature leads to enhanced mobility of the elements within the slag, facilitating improved mass transfer and diffusion of molecules or ions. Furthermore, as the Al2O3 content increases, the contact angle between the cryolite-based molten salt and SiC decreases, reducing the height of the resulting slag layer and gradually increases the wetting radius, thus rendering the slag more effective at wetting and spreading on the substrate.
- (2)
- Theoretical calculations indicate that Al2O3 enhances both surface tensions of the slag, thereby increasing the adhesion work between the slag and SiC. The initial spreading of the slag on the SiC substrate is primarily due to the reduction in the surface tension. The penetration depth of the slag into the SiC substrate gradually increased with increasing Al2O3 content, resulting in more severe penetration into the SiC refractory material. At high temperatures, the liquid slag phase formed within the refractory entered the SiC pores, promoting the penetration of the slag.
- (3)
- In summary, this study provides a comprehensive analysis of the wettability and adhesion mechanisms of aluminum slag on SiC-based refractory materials. Increasing the Al2O3 content in the slag can reduce its wettability on SiC, thereby minimizing slag adherence to the ladle lining. Material Selection: Using SiC-based refractory materials with optimized Al2O3 content can improve the ladle lining’s resistance to slag penetration and mechanical erosion. Surface Treatment: Applying surface treatments or coatings that further reduce the wettability of the slag on SiC can enhance the durability of the ladle lining. These results have important implications for optimizing the design of vacuum ladles and other industrial equipment used in aluminum electrolysis. While our study provides valuable insights into the interactions between aluminum slag and SiC-based refractory materials, several avenues for future research remain. For instance, further investigations could explore the effects of other slag components, such as CaF2 and Na3AlF6, on wetting and adhesion behavior. Additionally, the impact of operational parameters, such as temperature and applied voltage, on these interactions can be studied in more detail. Long-term durability tests under realistic industrial conditions would also be beneficial for assessing the performance of optimized refractory materials over an extended period.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, K.; Gong, P.; Xin, X.; Tian, Z.; Lai, Y. Purifying Spent Carbon Anode (SCA) from Aluminum Reduction Industry by Alkali Fusion Method to Apply for Li-Ion Batteries Anodes: From Waste to Resource. J. Taiwan Inst. Chem. E 2020, 116, 121–127. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, D.; Zhao, Z. Progress in Application of Energy-Saving Measures in Aluminum Reduction Cells. JOM 2019, 71, 2420–2429. [Google Scholar] [CrossRef]
- Lavoie, P.; Taylor, M.P.; Metson, J.B. A Review of Alumina Feeding and Dissolution Factors in Aluminum Reduction Cells. Met. Mater. Trans. B 2016, 47, 2690–2696. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, L.; Xiao, L.; Huo, X.; Guo, M.; Zhang, M. Effect of MgO on Corrosion Mechanism under LF Refining Slag for MgAlON–MgO Composites Synthesized from Spent MgO–C Brick: Wetting Behaviour, Reaction Process and Infiltration Mechanism. J. Alloys Compd. 2023, 963, 171278. [Google Scholar] [CrossRef]
- Liu, Z.; Pan, S.; Zhang, R.; Gao, Y.; Gao, W.; Wang, X.; Wei, S.; Wen, T.; Ma, B.; Yu, J. Wetting and Corrosion Behavior of V– and Ti–Containing Slag on Oxidation Layer of MgO–C Refractory. J. Aust. Ceram. Soc. 2024, 60, 777–789. [Google Scholar] [CrossRef]
- Cheng, W. Study on the Wetting Characteristics of Liquid-Al/ SiO2 Interface with Si Content in Liquid-Al. Mater. Today Commun. 2024, 41, 110722. [Google Scholar] [CrossRef]
- Cheng, W.; Rui, Z.; Sun, H.; Lyu, X.; Mei, C.; Dong, Y. Interfacial Wetting Characteristics of Na3AlF6-Al2O3-CaF2 Slag with SiC: Experiment and Molecular Dynamics Simulation. Ceram. Int. 2025, in press. [CrossRef]
- Ji, S.; Zhang, Z.; Wang, F. Overview of High Voltage Sic Power Semiconductor Devices: Development and Application. Trans. Electr. Mach. Syst. 2017, 1, 254–264. [Google Scholar] [CrossRef]
- Park, J.; Lee, K.; Pak, J.-J.; Chung, Y. Initial Wetting and Spreading Phenomena of a CaO–SiO2 Liquid Slag on MgO Substrates. ISIJ Int. 2014, 54, 2059–2063. [Google Scholar] [CrossRef]
- Shen, P.; Zhang, L.; Wang, Y.; Sridhar, S.; Wang, Q. Wettability between Molten Slag and Dolomitic Refractory. Ceram. Int. 2016, 42, 16040–16048. [Google Scholar] [CrossRef]
- Yu, B.; Lv, X.; Xiang, S.; Bai, C.; Yin, J. Wetting Behavior of Calcium Ferrite Melts on Sintered MgO. ISIJ Int. 2015, 55, 1558–1564. [Google Scholar] [CrossRef]
- Kudyba, A.; Akhtar, S.; Johansen, I.; Safarian, J. Valorization of Aluminum Dross with Copper via High Temperature Melting to Produce Al-Cu Alloys. Materials 2021, 14, 4117. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Z.; Liu, C.; Yu, C.; Deng, C.; Ying, Z.; Ma, B. Enhancing the Comprehensive Performance of MgO–MgAlON Composite Refractories Synthesized from Natural Minerals. Ceram. Int. 2024, 50, 30068–30077. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, H.; Wang, Q.; Li, G. Effect of Applied Voltage on Wetting and Corrosion of Corundum Refractory by CaO–SiO2–MgO Molten Slag. Ceram. Int. 2022, 48, 9753–9764. [Google Scholar] [CrossRef]
- Monaghan, B.J.; Abdeyazdan, H.; Dogan, N.; Rhamdhani, M.A.; Longbottom, R.J.; Chapman, M.W. Effect of Slag Composition on Wettability of Oxide Inclusions. ISIJ Int. 2015, 55, 1834–1840. [Google Scholar] [CrossRef]
- Ze, X.R.; Liang, Z.J.; Yu, W.Z.; Xin, J.K. Influence of Cr2O3 and B2O3 on Viscosity and Structure of High Alumina Slag. Steel Res. Int. 2017, 88, 1600241. [Google Scholar]
- Wang, Z.; Shu, Q.; Chou, K. Viscosity of Fluoride-Free Mold Fluxes Containing B2 O3 and TiO2. Steel Res. Int. 2013, 84, 766–776. [Google Scholar] [CrossRef]
- Lee, J.; Hoai, L.T.; Choe, J.; Park, J.H. Density Measurements of CaO–MnO–SiO2 Slags. ISIJ Int. 2012, 52, 2145–2148. [Google Scholar] [CrossRef]
- Kim, J.B.; Sohn, I. Influence of TiO2/SiO2 and MnO on the Viscosity and Structure in the TiO2–MnO–SiO2 Welding Flux System. J. Non-Cryst. Solids 2013, 379, 235–243. [Google Scholar] [CrossRef]
- Du, C.K.; Yang, J.; Zhao, X.Z.; Shi, Y.J.; Gao, X.D. Viscosity and Desulfurization Behavior of Blast Furnace Slag with High Al2O3 Content. J. Iron. Steel Res. 2013, 25, 19–22. [Google Scholar] [CrossRef]
- Deng, Y.-C.; Wu, S.-L.; Jiang, Y.-J.; Jia, S.-Q. Study on Visco sity of the La2O3-SiO2-Al2O3 Slag System. Met. Mater. Trans. B 2016, 47, 2433–2439. [Google Scholar] [CrossRef]
- Zuo, H.-B.; Wang, C.; Xu, C.-F.; Zhang, J.-L.; Zhang, T. Effects of MnO on Slag Viscosity and Wetting Behaviour between Slag and Refractory. Ironmak. Steelmak. 2016, 43, 56–63. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, J.; Fan, X.; Zheng, W.; Zhao, Y. Effect of MnO on High-Alumina Slag Viscosity and Corrosion Behavior of Refractory in Slags. ISIJ Int. 2017, 57, 1887–1894. [Google Scholar] [CrossRef]
- Yoon, T.; Lee, K.; Lee, B.; Chung, Y. Wetting, Spreading and Penetration Phenomena of Slags on MgAl2O4 Spinel Refractories. ISIJ Int. 2017, 57, 1327–1333. [Google Scholar] [CrossRef]
- Sahajwalla, V.; Khanna, R.; Mehta, A.S. Influence of Chemical Compositions of Slag and Graphite on the Phenomena Occurring in the Graphite/Slag Interfacial Region. Met. Mater. Trans. B 2004, 35, 75–83. [Google Scholar] [CrossRef]
- Jung, E.J.; Kim, W.; Sohn, I.; Min, D.J. A Study on the Interfacial Tension between Solid Iron and CaO-SiO2-MO System. J. Mater. Sci. 2010, 45, 2023–2029. [Google Scholar]
- Yang, W.; Yu, X.; Dongxu, W.; Changqing, D.; Yongping, Y.; Xianbin, X.; Qiang, L.; Ying, Z. Effect of Sodium Oxides in Ash Composition on Ash Fusibility. Energy Fuels 2016, 30, 1437–1444. [Google Scholar]
- Liu, J.; Ren, B.; Liu, W.; Feng, J.; Ma, Q.; Chen, H.; Li, B.; Chen, J.; Yin, S. Effect of Slag Basicity on the Interfacial Wetting Behavior between CA6 Refractories and CaO–SiO2–MgO–Al2O3 Based Slag. Ceram. Int. 2023, 49, 22068–22075. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, E.; Xu, L.; Yang, T.; Hou, X.; He, Z.; Liang, T. Synthesis of CA6/AlON Composites with Enhanced Slag Resistance. Int. J. Miner. Metall. Mater. 2023, 30, 756–765. [Google Scholar] [CrossRef]
- Yuan, Z.; Wu, Y.; Zhao, H.; Matsuura, H.; Tsukihashi, F. Wettability between Molten Slag and MgO–C Refractories for the Slag Splashing Process. ISIJ Int. 2013, 53, 598–602. [Google Scholar] [CrossRef]
- Yu, B.; Lv, X.; Xiang, S.; Bai, C.; Yin, J. Wetting Behavior of Al2O3 Substrate by Calcium Ferrite Series Melts. ISIJ Int. 2015, 55, 483–490. [Google Scholar] [CrossRef]
- Zhao, F.; Bian, Z.; Zhao, H.; Chen, D.; Yuan, Z.; Zhen, Y.; Wang, L.; Qi, T. Wettability and Corrosion Behavior between Alkaline Slag from Sodium Smelting of Vanadium–Titanium Magnetite and Refractory Substrates. J. Iron Steel Res. Int. 2024, 31, 1399–1410. [Google Scholar] [CrossRef]
- Kaptay, G. On the Temperature Dependence of Surface Tension: Historical Perspective on the Eötvös Equation of Capillarity, Celebrating His 175th Anniversary. Adv. Colloid. Interfac. 2024, 332, 103275. [Google Scholar] [CrossRef]
- Kaptay, G. The Chemical (Not Mechanical) Paradigm of Thermodynamics of Colloid and Interface Science. Adv. Colloid. Interfac. 2018, 256, 163–192. [Google Scholar] [CrossRef]
- Kaptay, G. A Coherent Set of Model Equations for Various Surface and Interface Energies in Systems with Liquid and Solid Metals and Alloys. Adv. Colloid. Interfac. 2020, 283, 102212. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jinguu, S.; Nishimura, S.; Oyama, Y.; Terashima, K. Density and Surface Tension of Molten Calcium Fluoride. J. Cryst. Growth 2002, 240, 445–453. [Google Scholar] [CrossRef]
- Schmetterer, C.; Masset, P.J. Determination of FeO Containing Liquid Slag Surface Tensions Using the Sessile Drop Method. In 2nd International Symposium on High-Temperature Metallurgical Processing; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Eustathopoulos, N.; Nicholas, M.G.; Drevet, B. Wettability at High Temperatures, 1st ed.; Pergamon Materials Series; Pergamon: Amsterdam, The Netherlands; New York, NY, USA, 1999; ISBN 978-0-08-042146-9. [Google Scholar]
- Wu, X.; Hu, X.; Xu, J.; Su, L.; Zhang, J. Wetting and Interfacial Tension of Molten CaO–Al2O3–MgO–FeO Slag and BN Substrate. J. Mater. Res. Technol. 2022, 16, 764–772. [Google Scholar] [CrossRef]
- Yuan, H.; Dan, Z.; Wang, Q.; He, S. Contact Angle and Adhesion of CaO-SiO2- and CaO-Al2O3-Based Mold Slags on Solid Steel of Various Compositions. J. Mater. Res. Technol. 2020, 9, 7828–7837. [Google Scholar] [CrossRef]

| Chemical | Specification |
|---|---|
| Na3AlF6 | ≤0.2% |
| Al2O3 | 94 HRA |
| CaF2 | 0.14 |
| Sample Slag | Na3AlF6 | Al2O3 | CaF2 |
|---|---|---|---|
| 1# | 95 | 0 | 5 |
| 2# | 90 | 5 | 5 |
| 3# | 85 | 10 | 5 |
| 4# | 80 | 15 | 5 |
| 5# | 75 | 20 | 5 |
| Component | SiC | SiO | Fe2O3 | Free Silicon Content |
|---|---|---|---|---|
| Content/mass% | 99.45 | 0.02 | 0.01 | ≤0.1 |
| Properties | Silicon Carbide Substrate |
|---|---|
| Apparent porosity | ≤0.2% |
| Rockwell hardness | 94 HRA |
| Poisson’s ratio | 0.14 |
| Chemical | Surface Tension (mN/m) |
|---|---|
| Na3AlF6 | 273.74–0.138 T |
| Al2O3 | 1024–0.177 T |
| CaF2 | 442.4–0.0816 T |
| Sample | Point | Element Content/wt% | ||||||
|---|---|---|---|---|---|---|---|---|
| C | O | F | Na | Al | Si | Ca | ||
| 5 | 22 | 41.47 | 4.81 | 0.31 | 0.34 | 0.34 | 52.61 | 0.12 |
| 23 | 34.11 | 1.16 | 0.16 | 0.09 | 0 | 64.48 | 0 | |
| 24 | 48.22 | 23.24 | 1.43 | 2.93 | 7.88 | 15.13 | 1.17 | |
| 25 | 66.05 | 1.3 | 0.04 | 0.11 | 0 | 32.42 | 0.08 | |
| 26 | 37.54 | 1.22 | 0.17 | 0 | 0 | 61.03 | 0.03 | |
| 27 | 33.3 | 30.19 | 0.49 | 8.61 | 12.64 | 13.66 | 1.12 | |
| 20 | 13 | 49.38 | 1.84 | 0.04 | 0 | 0 | 48.69 | 0.05 |
| 14 | 35.24 | 1.29 | 0.09 | 0.01 | 0 | 63.37 | 0 | |
| 15 | 36.32 | 2.34 | 0.11 | 0.04 | 0 | 61.08 | 0.1 | |
| 16 | 29.74 | 1.73 | 0.19 | 0.06 | 0 | 68.1 | 0.19 | |
| 18 | 76.01 | 8.86 | 0.43 | 0.17 | 0 | 14.1 | 0.43 | |
| 19 | 50 | 1.88 | 0.09 | 0 | 0 | 47.95 | 0.08 | |
| 20 | 11.17 | 42.93 | 0.06 | 12.31 | 13.76 | 19.68 | 0.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Cheng, W.; Rui, Z.; Sun, H.; Lyu, X.; Dong, Y. High-Temperature Wetting Behavior and Adhesion Mechanism of Cryolite-Based Molten Salt on SiC Refractory Substrate. Materials 2025, 18, 1428. https://doi.org/10.3390/ma18071428
Feng Y, Cheng W, Rui Z, Sun H, Lyu X, Dong Y. High-Temperature Wetting Behavior and Adhesion Mechanism of Cryolite-Based Molten Salt on SiC Refractory Substrate. Materials. 2025; 18(7):1428. https://doi.org/10.3390/ma18071428
Chicago/Turabian StyleFeng, Yuxi, Wandong Cheng, Zhiyuan Rui, Haobo Sun, Xin Lyu, and Yun Dong. 2025. "High-Temperature Wetting Behavior and Adhesion Mechanism of Cryolite-Based Molten Salt on SiC Refractory Substrate" Materials 18, no. 7: 1428. https://doi.org/10.3390/ma18071428
APA StyleFeng, Y., Cheng, W., Rui, Z., Sun, H., Lyu, X., & Dong, Y. (2025). High-Temperature Wetting Behavior and Adhesion Mechanism of Cryolite-Based Molten Salt on SiC Refractory Substrate. Materials, 18(7), 1428. https://doi.org/10.3390/ma18071428






