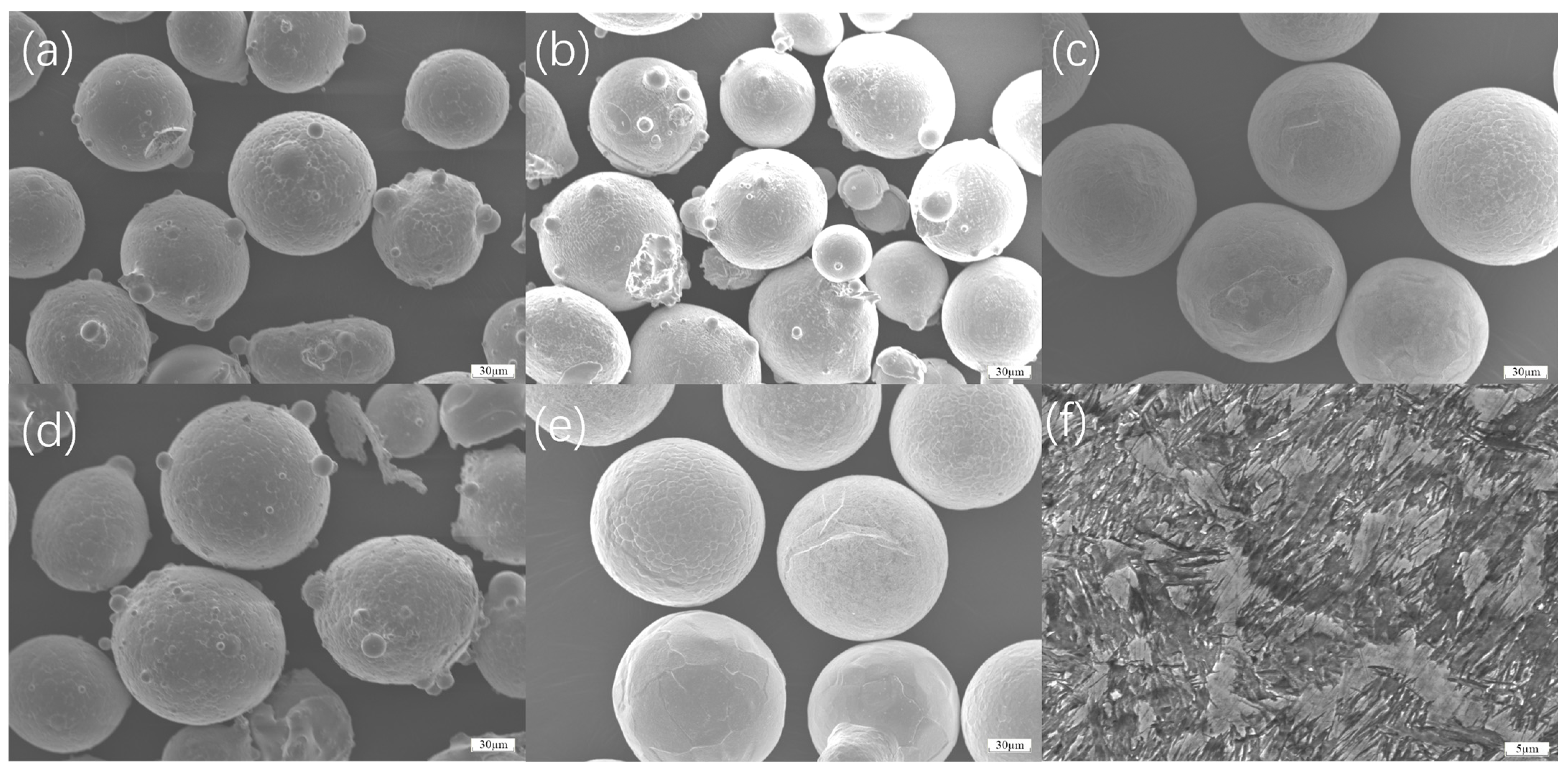
As shown in
Figure 4, cracks appeared in the heat-affected zone when CoCrFeNiAl high-entropy alloy was directly laser cladded onto H13 steel. The specific process parameters are shown in
Table 2. This is due to the significant difference in the coefficients of thermal expansion between H13 steel and CoCrFeNiAl high-entropy alloy. During the rapid thermal cycling in the laser-cladding process, the cooling phase causes the two materials to contract at different rates, resulting in the cladded layer experiencing intense tensile stress from the substrate, while the substrate is subjected to compressive stress from the cladded layer [
18]. The thermal stress is concentrated in the interface region between the cladded layer and the substrate, where the microstructure and properties are relatively weak. When the stress exceeds the critical value, microcracks begin to nucleate and propagate, severely compromising the integrity of the cladded layer and reducing the bonding strength [
19].
Nickel-based alloys have emerged as the preferred materials for transition coatings between CoCrFeNiAl high-entropy alloys and H13 steel substrates, owing to their optimal thermal expansion coefficient matching, excellent chemical compatibility, favorable wettability, and superior mechanical properties [
20]. Specifically, Inconel 718, Inconel 625, Hastelloy X, FGH4096, and FGH4169, as representative nickel-based superalloys, demonstrate well-matched thermal expansion coefficients with both the high-entropy alloy coating and H13 steel substrate. This compatibility significantly reduces thermal stress and minimizes the risk of cracking in the heat-affected zone during laser-cladding processes [
21]. Regarding chemical compatibility, these alloys exhibit remarkable stability against interfacial reactions with both CoCrFeNiAl high-entropy alloys [
22] and H13 tool steels [
23] at elevated processing temperatures. Furthermore, each alloy possesses distinct strengthening mechanisms (e.g., solid solution strengthening, precipitation hardening) that ensure exceptional mechanical performance under service conditions.
As shown in
Figure 5a, in GH4169, the distribution of Ni elements is not uniform, and there is obvious aggregation at the bonding place between the transition coating and the working coating, which is due to the temperature gradient at the bonding place, the difference in atomic diffusion rate, and the different physicochemical properties of different coating materials in the process of laser melting and cladding, leading to the preferential enrichment and formation of aggregation of Ni atoms in this region [
25]. As shown in
Figure 5b, in FGH4096, it is obvious that the iron elements in the substrate and the working coating cannot bond at the transition coating because the crystal structure and lattice constants of the transition coating do not match with those of the substrate and the working coating, and the weak inter-elemental interactions prevent the iron atoms from diffusing and bonding effectively across the interface [
26].
As shown in
Figure 5c, in Hastelloy X, the diffusion of iron is not uniform, which is due to the uneven distribution of the local temperature field during the fusion coating process, the existence of stress concentration areas within the coating, and the difference in the diffusion activation energy between the elements, which makes the diffusion of iron atoms in different positions at different rates; the chromium element of the transition coating is not bonded to the substrate because chromium and the substrate surface of the oxide layer, impurities, or other elements to form a stable This is because chromium and the substrate surface oxide layer, impurities or other elements to form a stable compound, preventing the formation of effective chemical bonding between chromium atoms and substrate atoms [
27].
As shown in
Figure 5d, Inconel 625 elements diffuse relatively uniformly due to their stable chemical composition, relatively homogeneous crystal structure, and similar diffusion coefficients and migration ability of atoms during the laser-cladding process, which allows for a more uniform distribution of elements within the coating [
28]. As shown in
Figure 5e, Inconel 718’s elemental diffusion uniformity is due to its good thermal stability and organizational uniformity. In the high-temperature environment generated by laser cladding, the diffusion of atoms is the more consistent driving force, and the stress distribution within the coating is uniform, which is conducive to the uniform diffusion of the elements in the entire range of coatings and the formation of stable bonding [
29].
3.1. Three-Point Bending Test
The bond strength of five transition coatings (Inconel 718, Inconel 625, Hastelloy X, FGH4096, and FGH4169) between H13 steel and CoCrFeNiAl can be determined by a three-point bending test. This test is used to assess the first crack initiation in the tensile region of the case-hardened layer and the delamination between the case-hardened layer and the substrate [
30]. The applied load corresponding to crack initiation can be easily determined from the load–deflection curve [
31]. According to the basic beam theory, the cohesive strength of the bond between the transition coating and the substrate is proportional to the critical load. Therefore, this approach can be used to investigate the bond strength of different coating materials and their effect on crack propagation, and thus further investigate the role of these transition coatings on the surface toughness and durability of the material [
32]. Therefore, the three-point bending test allows an in-depth analysis of their bonding properties under extreme operating conditions and the effect on the overall mechanical properties of the material.
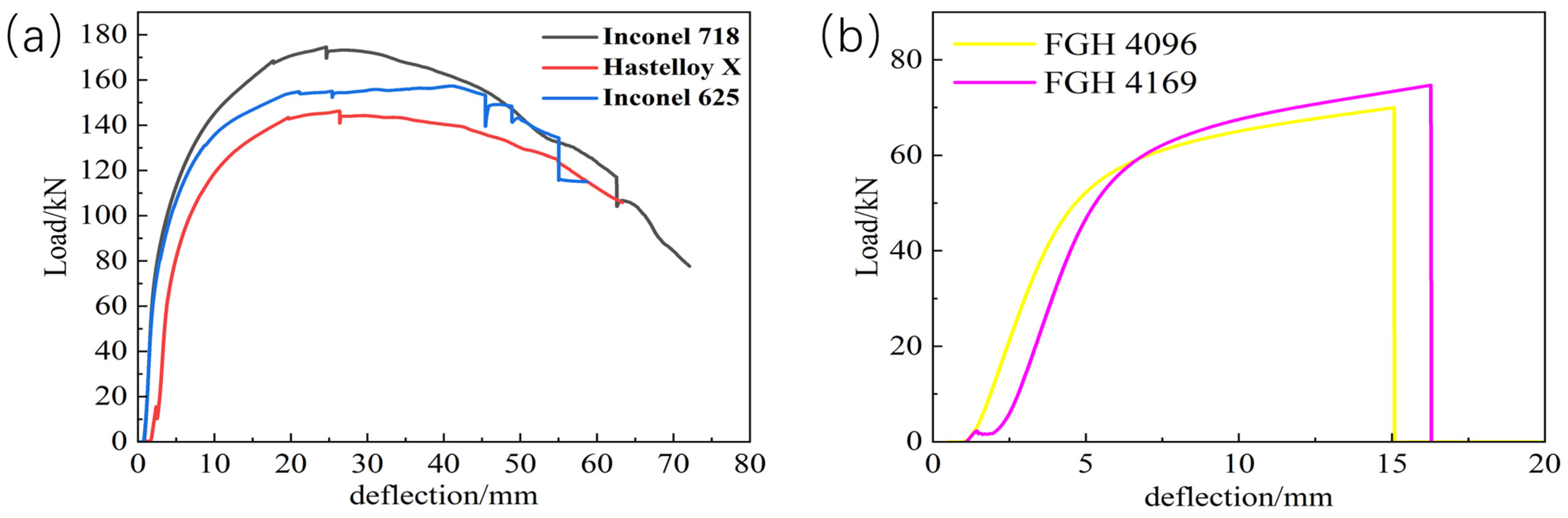
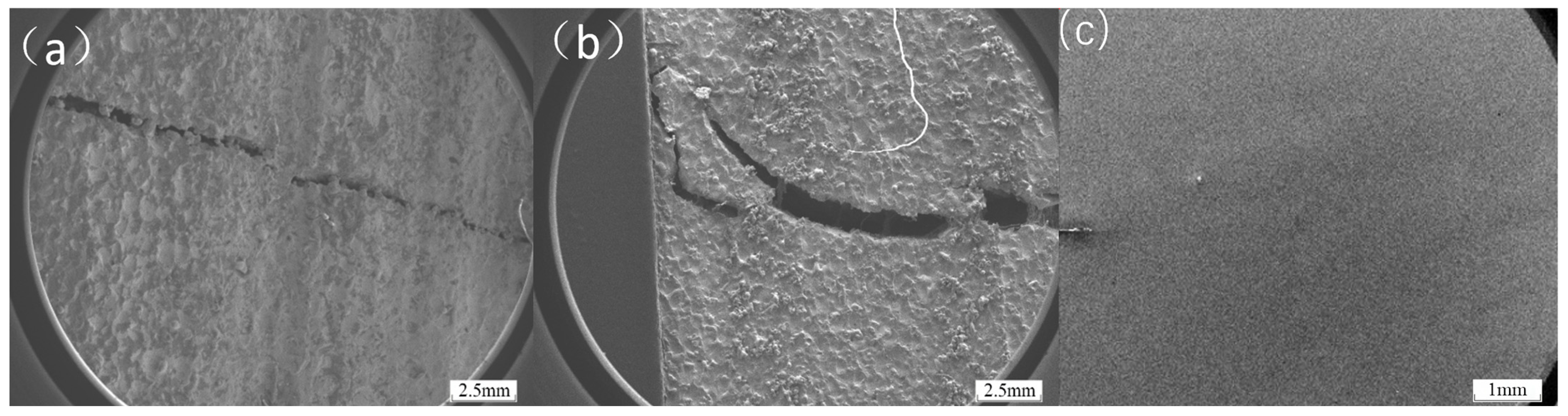
In the three-point bending test, loads were applied to transition-coated samples of Inconel 718, Inconel 625, Hastelloy X, FGH4096, and FGH4169. As shown in
Figure 6b, the FGH4096 and FGH4169 coatings were the first to experience problems as the load increased. The load–deflection curves in the three-point bending tests reflect the changes in the mechanical behavior of the materials when subjected to stresses and provide important clues for analyzing the causes of cracking of the fusion-coated layers [
32]. At the initial stage of the test, as the load increases gradually, the deflection increases slowly, and the load–deflection curve usually shows a steady upward trend. This indicates that at lower stress levels, the fused cladding and the substrate can deform synergistically, and the material is in the elastic deformation stage. However, when the load reaches a certain level, due to the insufficient diffusion of certain elements, the number of chemical bonds at the interface is limited, and effective stress transfer and dissipation cannot be realized. As the load increases further, the bonding interface cannot withstand higher tensile stresses, resulting in atomic bond breakage in localized regions and microcracks begin to sprout [
28]. As the load continues to increase, these cracks will gradually expand, causing the slope of the load–deflection curve to decrease. As a result, the stiffness of the material gradually decreases, eventually leading to cracking of the fusion cladding.
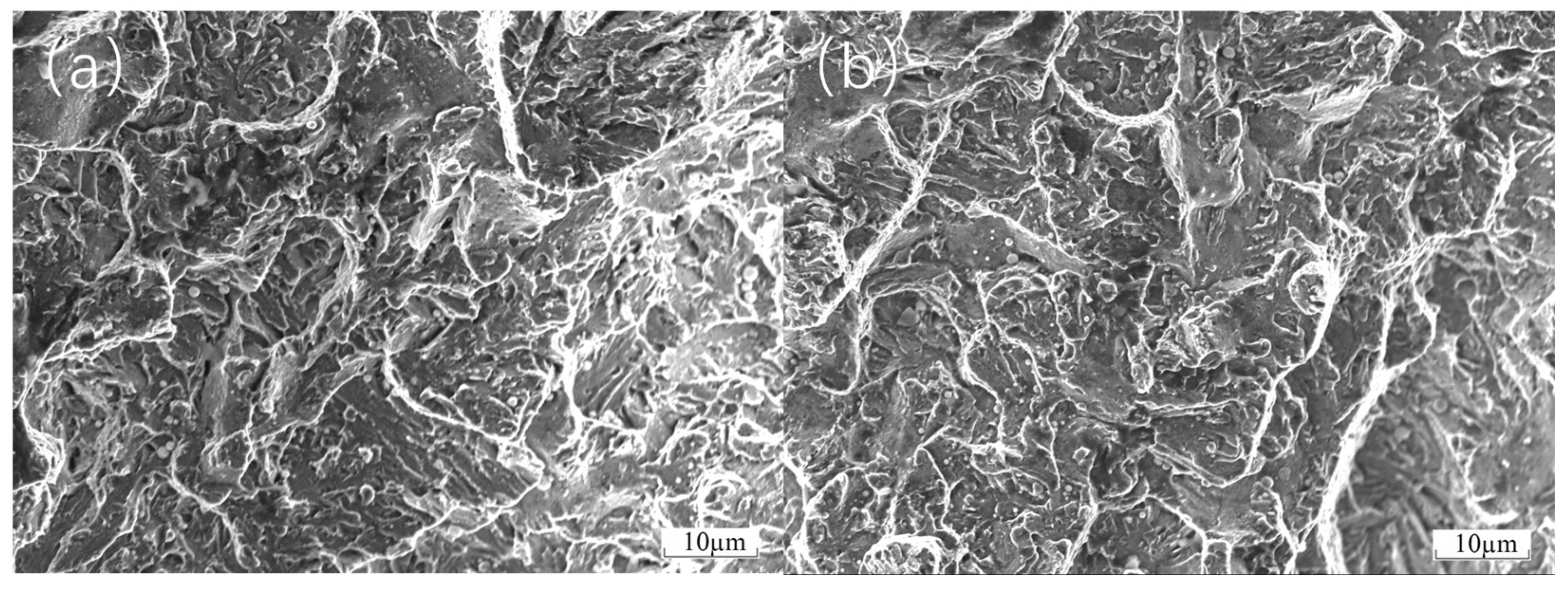
As shown in
Figure 7, during three-point bending tests of FGH4096 and FGH4169 transition coatings, when a mixed brittle–tough fracture occurs, the fracture surfaces show complex and recognizable features under the electron microscope. In the localized area of the fracture, the characteristics of brittle fracture can be observed with a relatively smooth fracture surface and clear grain boundaries. This is due to rapid crack initiation at certain high-stress concentration points and subsequent crack extension along grain boundaries or disintegration surfaces, leaving these regions without significant plastic deformation. However, the adjacent regions show signs of ductile fracture with the presence of some tough nests. These tough nests were formed during plastic deformation due to nucleation, growth, and polymerization of micropores, which suggests that the material in these regions experienced a considerable degree of plastic flow with more active dislocation motion prior to fracture. In addition, some tear ribs can be observed in the transition region from brittle to ductile fracture. These tear ribs are the result of plastic deformation competing with brittle cracking during crack extension. As the material undergoes some degree of plastic stretching, the cracks also expand forward under the applied stress. The combination of these two processes leads to the formation of tear ribs. Together, these features clearly reveal the microscopic nature of mixed brittle–tough fracture in three-point bending tests.
As shown in
Figure 8, when observing the Inconel 625 and Hastelloy X transition coatings using a scanning electron microscope, significantly different phenomena were observed. For both Inconel 625 and Hastelloy X transition coatings, clear cracks were detected at the interface between the substrate and the working coating. This is due to the complex stress distribution generated within the cladding layer during the three-point bending process under the applied external load. Although the similar thermal expansion coefficients help reduce thermal stress effects, the mechanical stress induced by the external load cannot be ignored. During bending, different parts of the cladding layer experience varying amounts and directions of tensile and compressive stresses. When the localized stress concentration reaches the material’s yield strength, plastic deformation begins to accumulate. Once the stress exceeds the material’s fracture strength, cracks initiate. In contrast, for the Inconel 718 transition coating, no similar cracks at the interface between the substrate and the working coating were observed in the electron microscope images. This is most likely due to the excellent compatibility of Inconel 718, as its thermal expansion coefficient is better matched with both the substrate and the working coating. This results in relatively low thermal stress. Additionally, during the preparation process, good element diffusion and chemical bonding at the interface contribute to a more stable overall structure, effectively preventing crack formation even under the same observation conditions.
3.2. Hot Tensile Test
Laser-cladding technology has great potential in the research of material surface modification and property optimization. In this paper, an innovative coating system was constructed with H13 steel as the substrate, CoCrFeNiAl as the working coating, and Inconel 718, Inconel 625, Hastelloy X, FGH4096, and FGH4169 as the transition coatings. In order to investigate the mechanical properties and interfacial bonding characteristics of the system under thermal conditions, thermal tensile tests were conducted on the laser-melted specimens, aiming to reveal the behavior mechanism of the coatings under thermal stress and their interaction with the substrate.
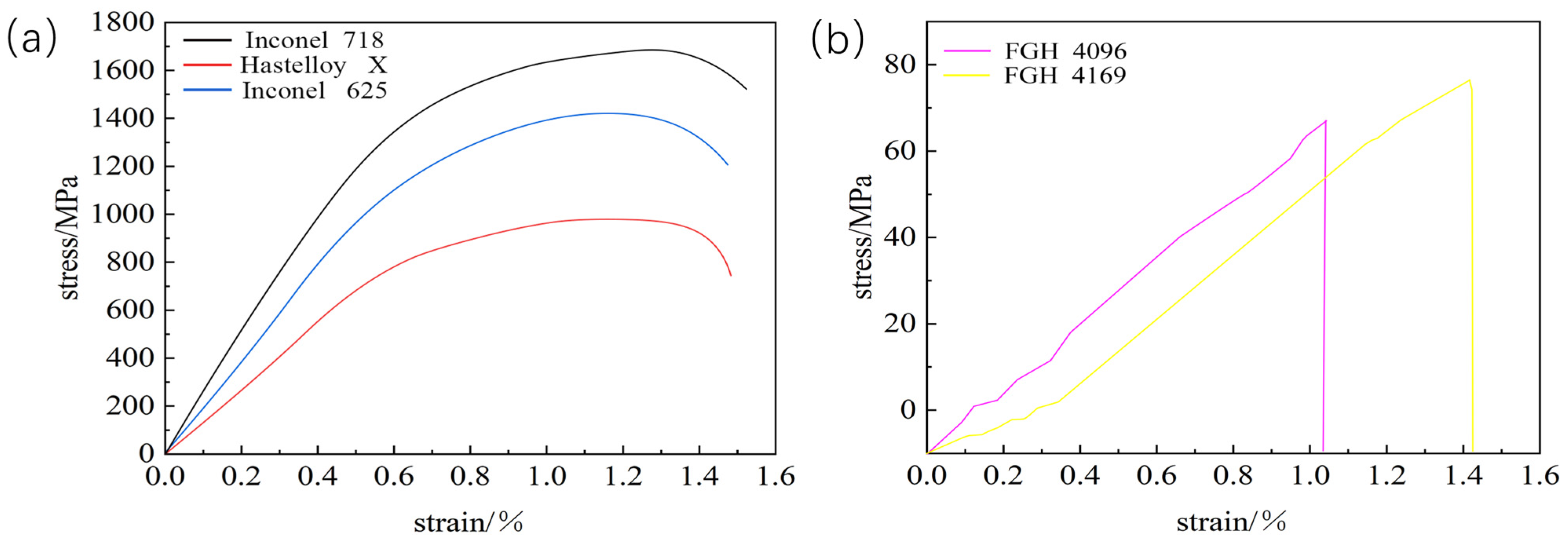
Figure 9 shows the thermal tensile curves of these five transition layer materials, which visualize the stress–strain relationship. The curve of Inconel 718 is at a high position with a remarkable stress level, indicating a high yield strength. During hot tension, it needs to withstand a large amount of stress before significant plastic deformation occurs, and it has a strong ability to resist plastic deformation in practical applications. As the strain increases, the stress first rises rapidly and then slows down, which is due to work hardening. At high temperatures, strengthening mechanisms such as dislocation motion effectively enhance its strength and deformation-resistance ability. The stress level of Inconel 625 is lower than that of Inconel 718, and its yield strength is relatively low. Under the same hot-tensile conditions, it enters the obvious plastic-deformation stage earlier. Its stress–strain curve is similar to that of Inconel 718. Although there is work hardening, the degree is weaker, resulting in a slightly weaker yield strength and plastic-deformation-resistance ability. The stress level of Hastelloy X is even lower, with a weak yield strength and poor deformation-resistance ability during hot tension. It shows obvious plastic deformation at an earlier stage. The overall curve is relatively flat, indicating that the work-hardening effect at high temperatures is weak, and it cannot significantly increase its strength through work-hardening like Inconel 718, resulting in poor yield strength. The overall stress levels and yield strengths of FGH 4096 and FGH 4169 are both low. In the initial stage, the stress–strain relationship is approximately linear, conforming to Hooke’s law, and the materials are in the elastic-deformation stage. As the strain increases, they enter the plastic-deformation stage and undergo irreversible deformation. Under the same strain, the stress of FGH 4096 is slightly higher, with a slightly stronger yield strength and plastic-deformation-resistance ability.
It is worth noting that there are differences in the bonding strengths between these five materials and the substrate. Even if the materials themselves have not reached their yield strengths, due to insufficient bonding strength, stress concentration may occur at the interface when subjected to external forces. Once the degree of stress concentration exceeds the bearing capacity of the bonding strength, cracks will initiate, affecting the overall performance and structural stability of the materials. Therefore, the differences in mechanical behaviors reflected by the hot-tensile curves, combined with the consideration of bonding strengths, are of great significance for studying the performance of transition layer materials under actual high-temperature working conditions. This can provide references for rational material selection, optimization of the laser-cladding process, and improvement of the overall material performance, ensuring the reliability and stability of materials in practical applications.
Even though the thermal expansion coefficients of the different materials are not significantly different, the earliest fracture of the FGH4096 and FGH4169 transition coatings during thermal tensile testing may still be attributed to the critical factor of low interfacial bonding strength. On one hand, the inherent chemical activity of Hastelloy X and the FGH series coatings is relatively low. During laser cladding, they are less likely to undergo strong chemical reactions with the adjacent CoCrFeNiAl and the H13 substrate to form stable chemical bonds [
29]. As a result, the bonding at the interface relies more on weaker physical adsorption, which is highly susceptible to failure under external forces during thermal tensile testing. Consequently, when external forces are applied and the interface is subjected to stress, cracks initiate and propagate rapidly in the interfacial regions of the FGH4096 and FGH4169 coatings due to the poor bonding strength, ultimately leading to their premature fracture. As shown in
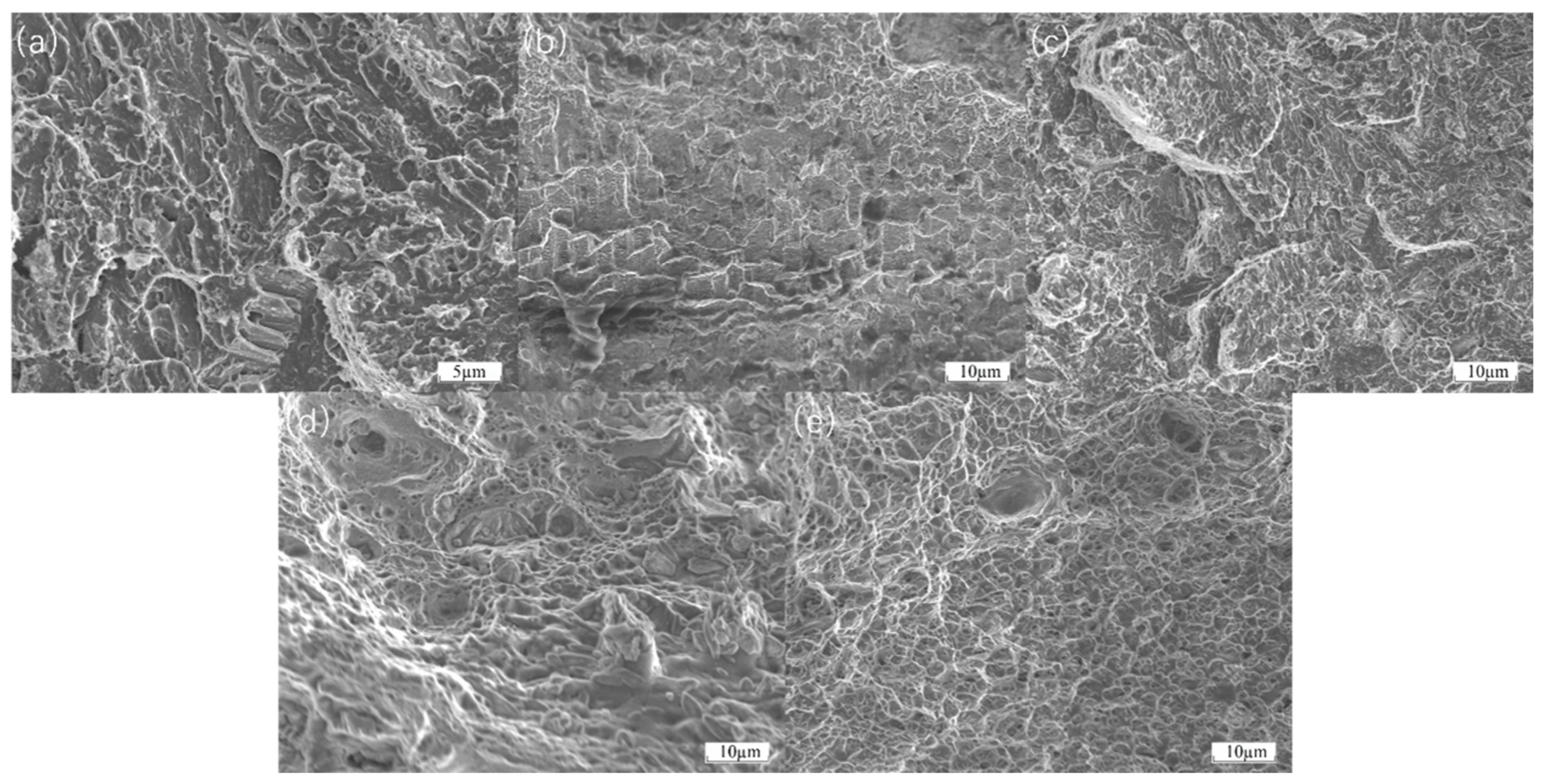
Figure 10a,b, the fracture surfaces of FGH4096 and FGH4169 exhibit complex morphological features after thermal tensile testing. In some localized areas, relatively flat fracture surfaces are observed, indicating the presence of brittle fracture components. Cracks may propagate rapidly along grain boundaries or cleavage planes, leaving insufficient time for significant plastic deformation in these regions. Simultaneously, dimple structures are observed in other areas of the fracture surfaces. These dimples are formed due to the nucleation, growth, and coalescence of microvoids during plastic deformation, suggesting that the material did not undergo complete brittle fracture but experienced some degree of plastic flow before failure. Additionally, tear ridges are visible on the fracture surfaces, resulting from the competition between plastic deformation and brittle cracking during crack propagation, reflecting the complex micromechanical behavior of the material during thermal tensile fracture.
The earliest cracking of Hastelloy X as a transition coating and the delayed cracking of Inconel 718 can be explained in terms of bonding strength and yield strength. From the initial stage of the stress–strain curve, at lower stress levels, the material undergoes elastic deformation, where stress and strain exhibit a linear relationship. During this stage, atomic bonds within the material undergo minor elastic deformation under stress, but the overall structure remains stable. As temperature increases and stress continues to rise, reaching the material’s yield strength, a distinct inflection point appears on the stress–strain curve. For the cladding materials, their yield strength during thermal tensile testing may be influenced by various factors. On one hand, if there are issues with the bonding between the cladding layer and the substrate, such as insufficient chemical bonding or the presence of microdefects, stress concentration occurs at the interface under the combined effects of thermal and tensile stresses. This stress concentration causes the local stress to far exceed the material’s macroscopic yield strength, leading to plastic deformation at lower macroscopic stress levels, manifesting as a reduction in yield strength. As stress continues to increase, the material enters the plastic deformation stage, where strain increases rapidly with stress. During this process, if the cladding layer contains inhomogeneous microstructures, such as uneven distribution of second-phase particles or the presence of pores and other defects, these areas become stress concentration points, further reducing the effective load-bearing area of the material. When the stress reaches the material’s tensile strength, defects within the material rapidly propagate, leading to crack initiation and coalescence, ultimately causing material fracture. As shown in
Figure 10c–e, the fracture surfaces of Hastelloy X, Inconel 625, and Inconel 718 exhibit distinct characteristics after thermal tensile testing. The fracture surface of Hastelloy X may display relatively smooth regions due to weak interfacial bonding, alongside irregular micro-roughness resulting from some degree of plastic deformation. The fracture surface of Inconel 625 shows evident signs of plastic deformation, such as dimples of varying sizes and depths, indicating dislocation motion and material elongation before fracture. However, localized stress concentrations may also lead to the formation of fine cracks. The fracture surface of Inconel 718 is relatively uniform, with well-distributed dimples and no significant cracking at grain boundaries caused by thermal or mechanical stress concentrations, demonstrating its superior fracture resistance.
Figure 11 illustrates the microstructure of the BCC-phase CoCrFeNiAl. The image clearly reveals rich microstructural features on the material surface. Numerous dimple structures are distributed throughout the field of view, interwoven with each other, indicating that the material underwent significant plastic deformation under certain conditions. The formation of dimples is attributed to the nucleation, growth, and coalescence of microvoids within the material, which are typical microscopic indicators of ductile fracture. Additionally, tear ridges are observed interspersed among the dimples, further demonstrating localized tearing and slip phenomena during the deformation process. This image shows the ductile fracture surface of H13 after thermal tensile testing. The fracture surface exhibits typical ductile fracture characteristics, with numerous dimples of varying sizes distributed uniformly across the surface. The presence of these dimples indicates that the material underwent substantial plastic deformation before fracture. The formation of dimples is due to the nucleation, growth, and coalescence of microvoids within the material, which are key microscopic features of ductile fracture. The presence of these dimples suggests that the H13 material absorbed significant energy through plastic deformation during thermal tensile testing, delaying crack propagation and ultimately leading to ductile fracture. Furthermore, tear ridges are visible on the fracture surface, indicating that the material experienced considerable plastic deformation, with relative sliding and tearing occurring between different parts of the material. Overall, the fracture morphology fully demonstrates the excellent ductility of H13 under thermal tensile conditions, with its microstructural characteristics consistent with the mechanisms of ductile fracture.