Performance of Sewer Concretes with Calcium Sulpho-Aluminate Cement and Portland Cement Blends: Field and Laboratory Studies
Abstract
1. Introduction
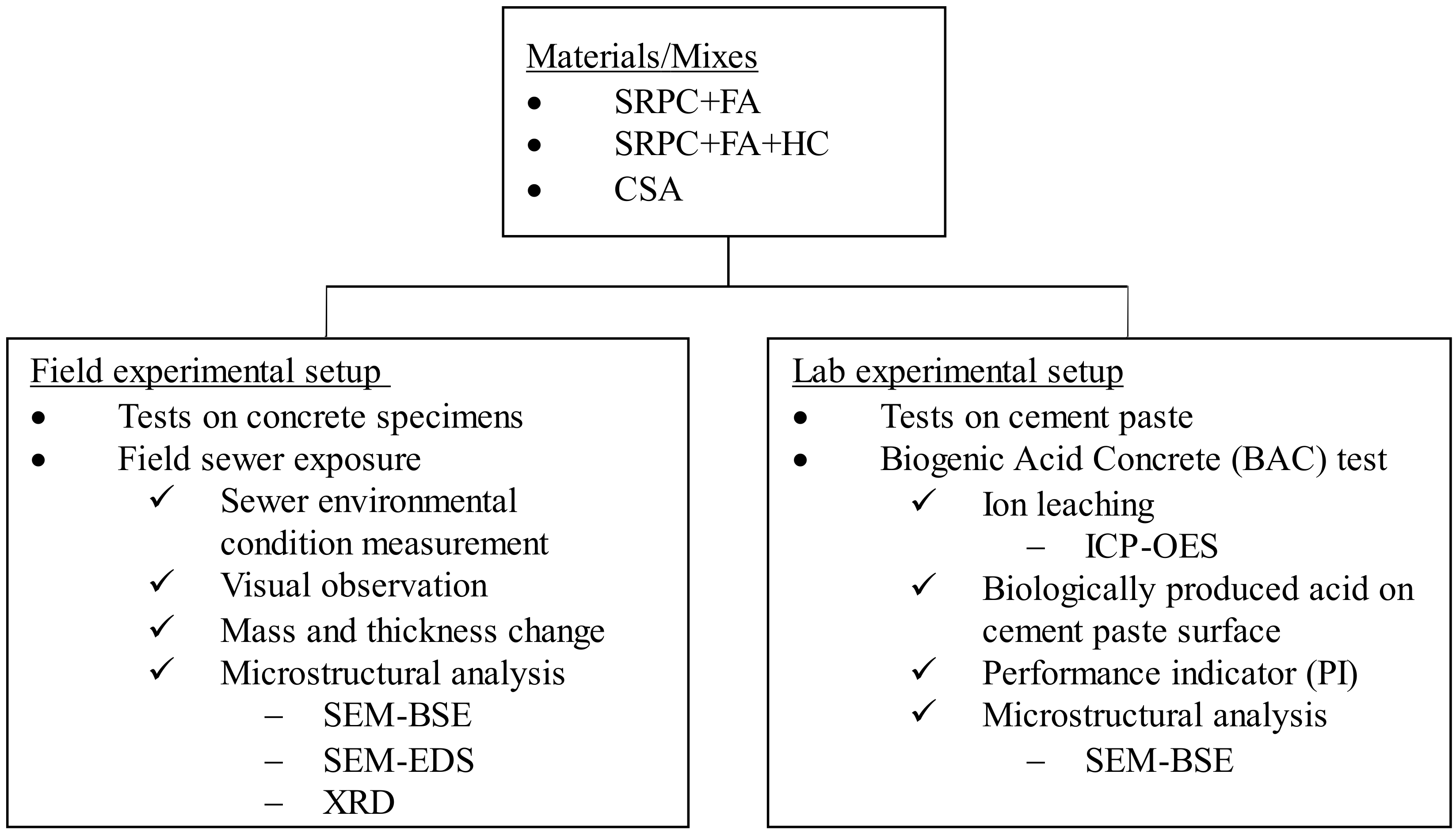
2. Materials and Methods
2.1. Materials
2.2. Field Experimental Setup
2.2.1. Concrete Mixes and Casting Procedures
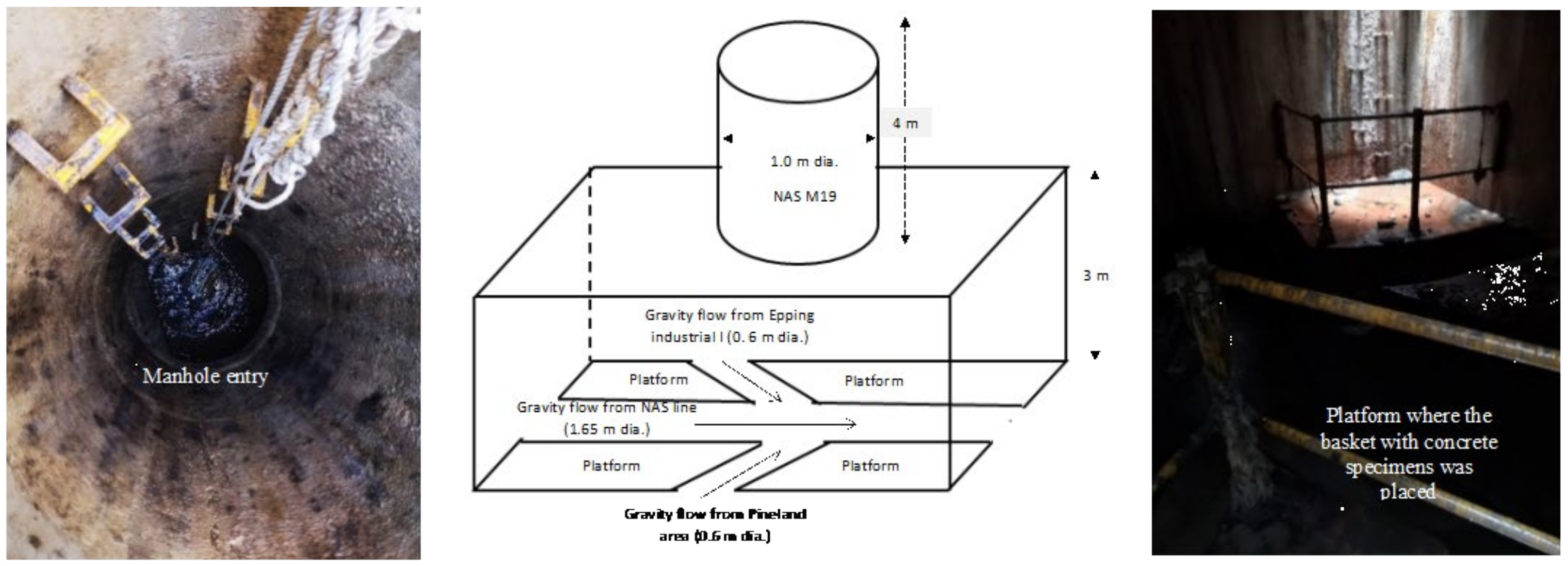
2.2.2. Sewer Site Background and Environmental Conditions
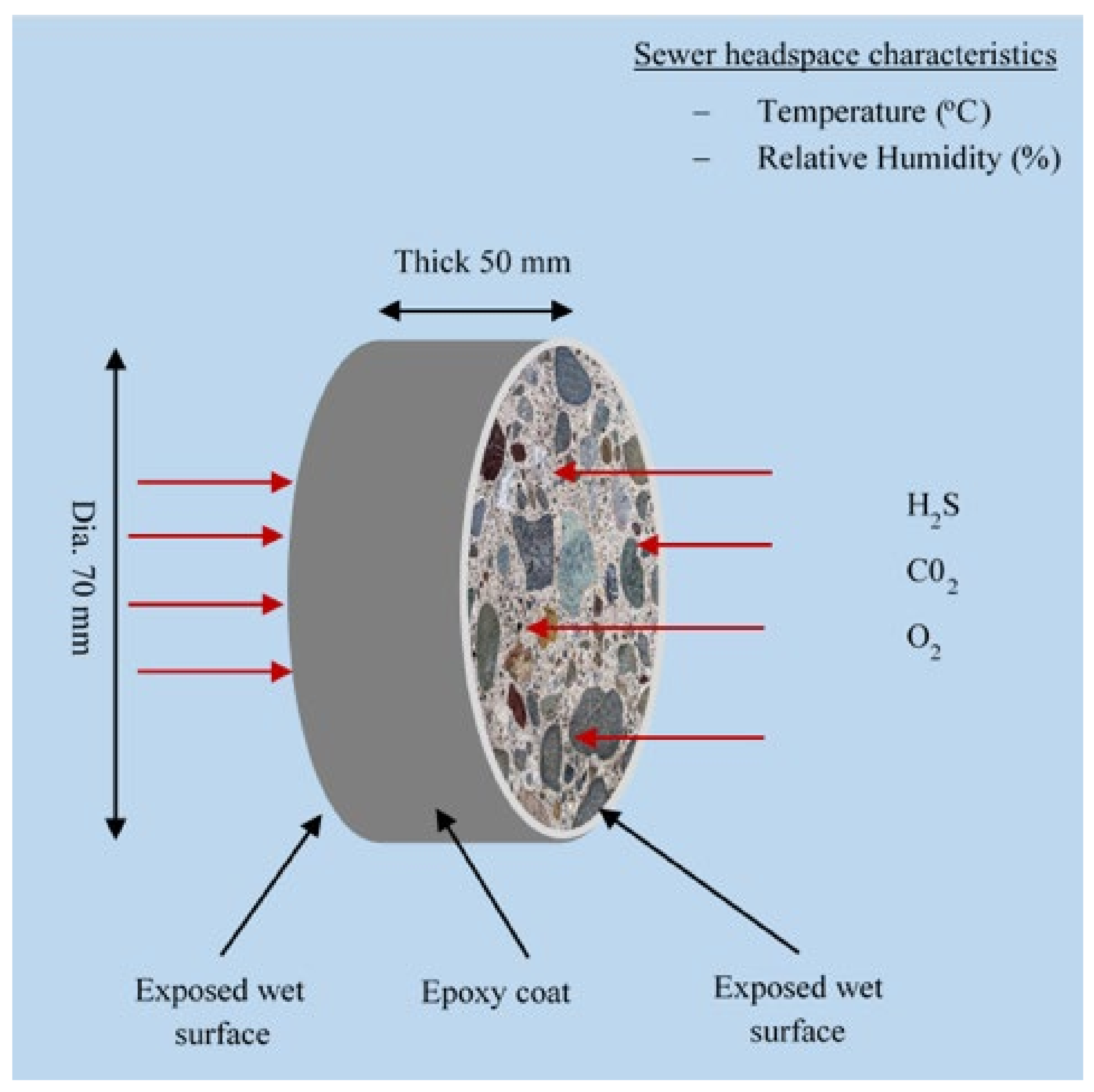
2.2.3. Concrete Specimen Preparation for Sewer Exposure
2.2.4. Concrete Deterioration Monitoring Strategy
2.2.5. Microstructural Analysis on Concrete Specimens Exposed on Site
2.3. Laboratory Experimental Setup
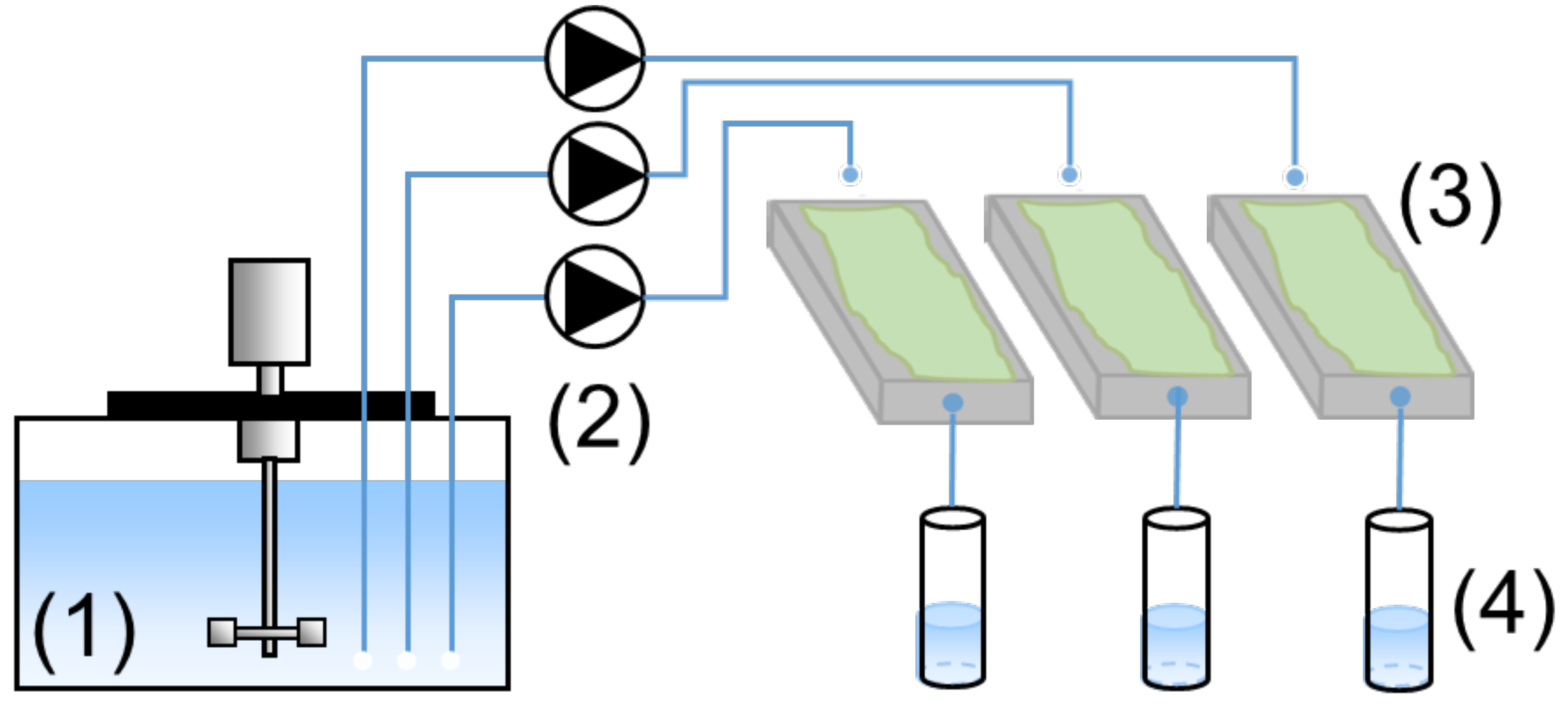
2.3.1. Biogenic Acid Concrete (BAC) Test
2.3.2. Cement Paste Preparation for Exposure to the BAC Test
2.3.3. Cement Paste Deterioration Monitoring Strategy
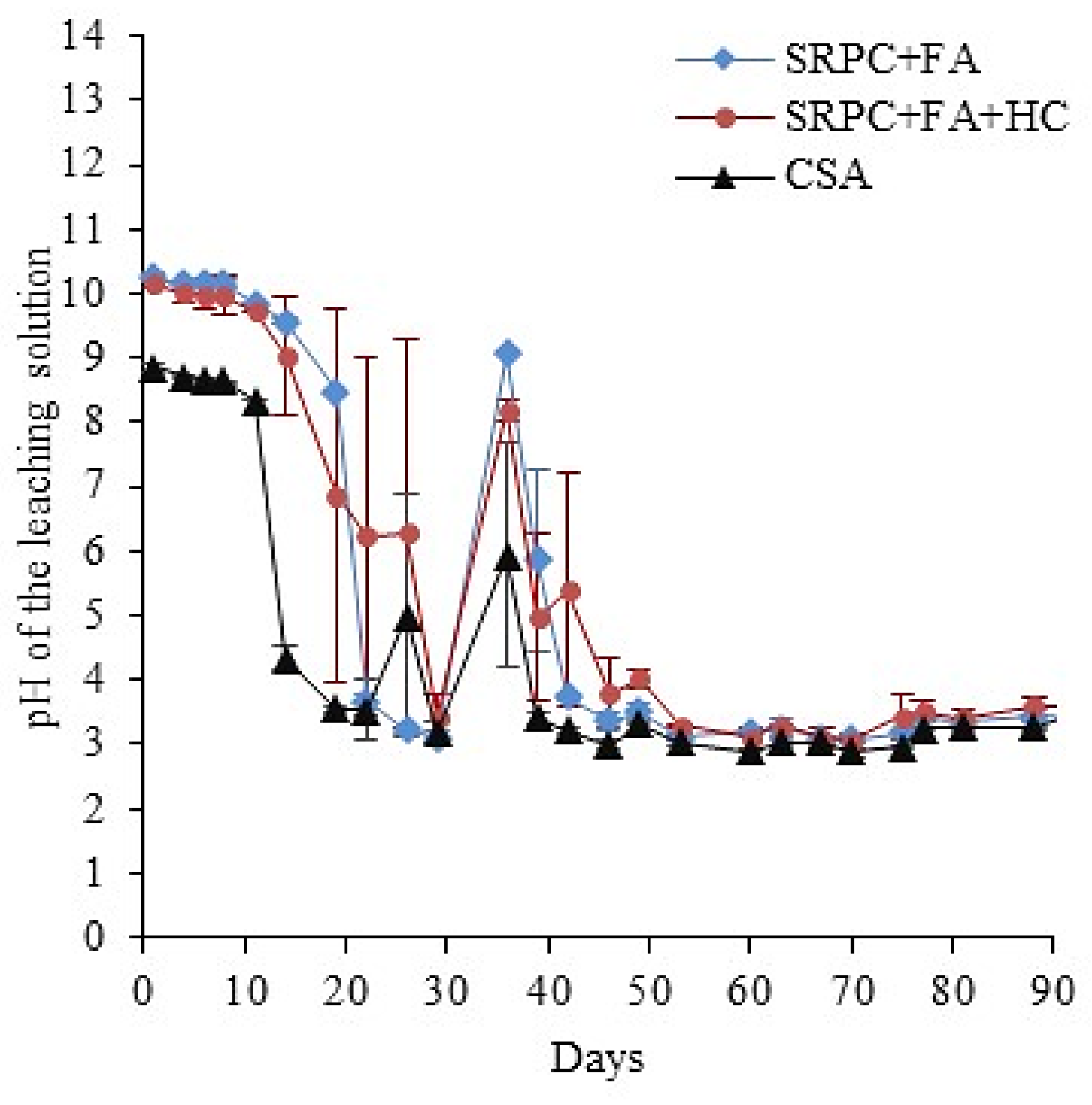
2.3.4. Analyses of the Leached Solutions
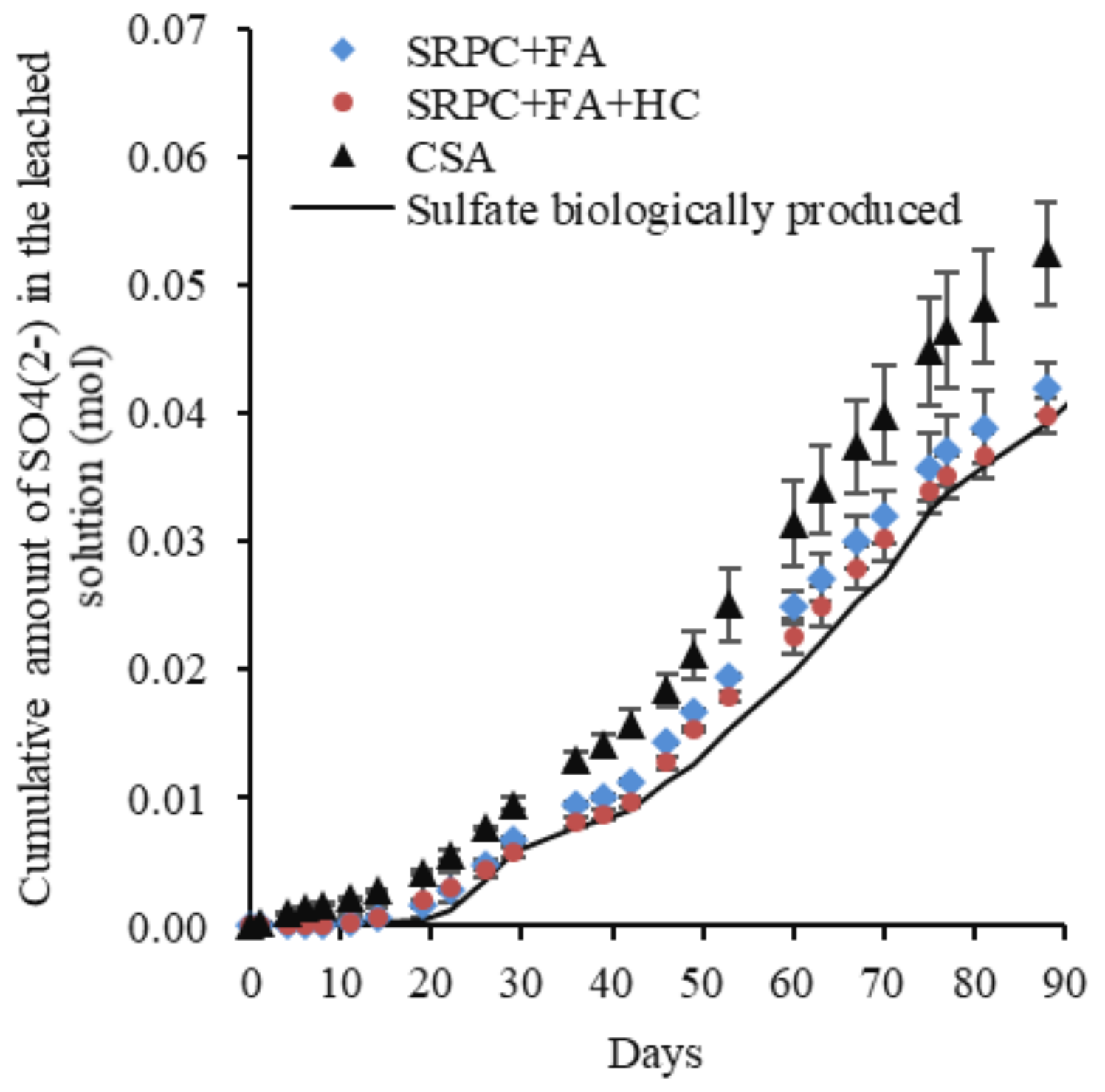
2.3.5. Calculation of the Biogenically Produced Acid on Cement Pastes Exposed to the BAC Test
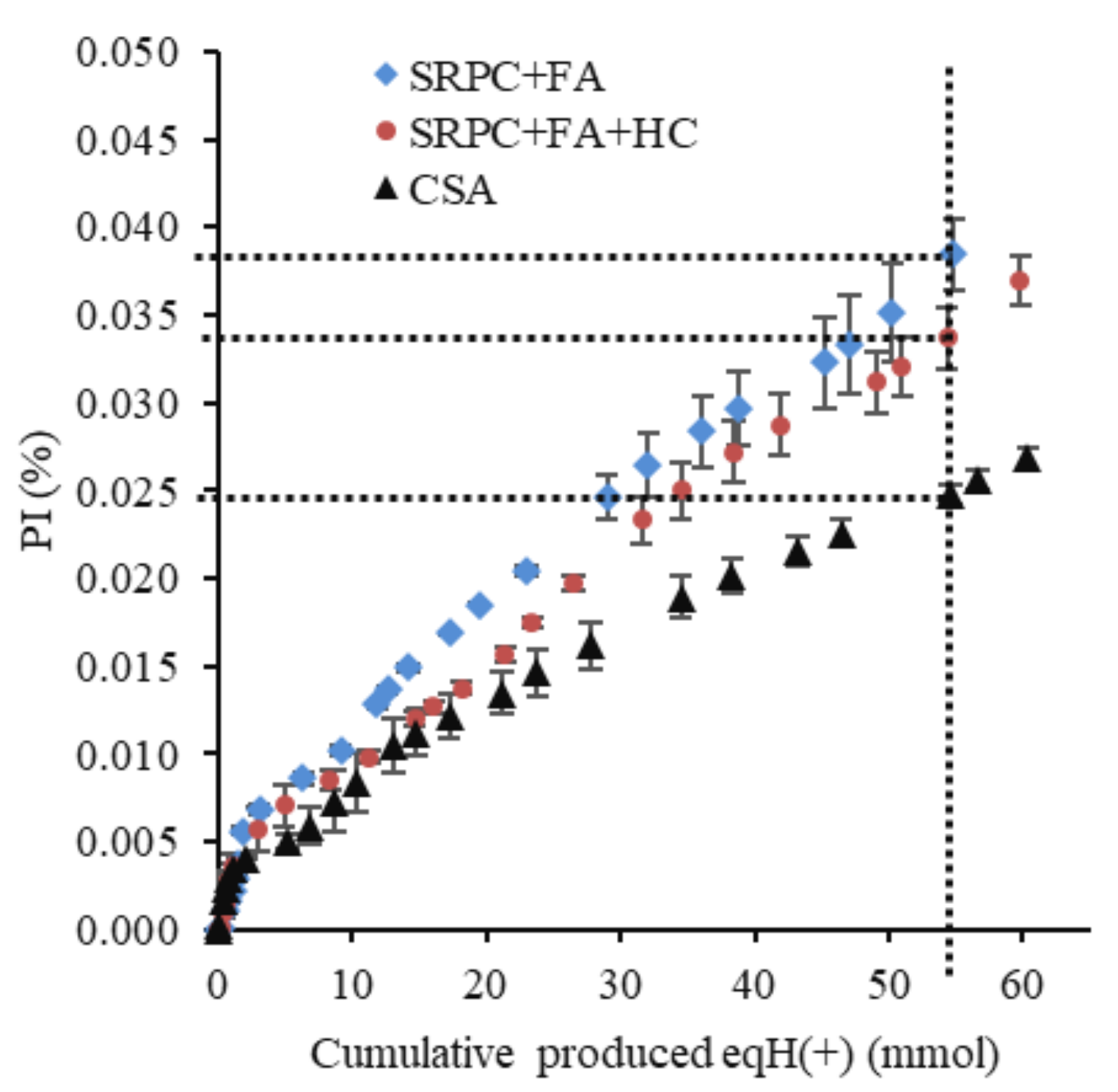
2.3.6. BAC Test Performance Indicator (PI)
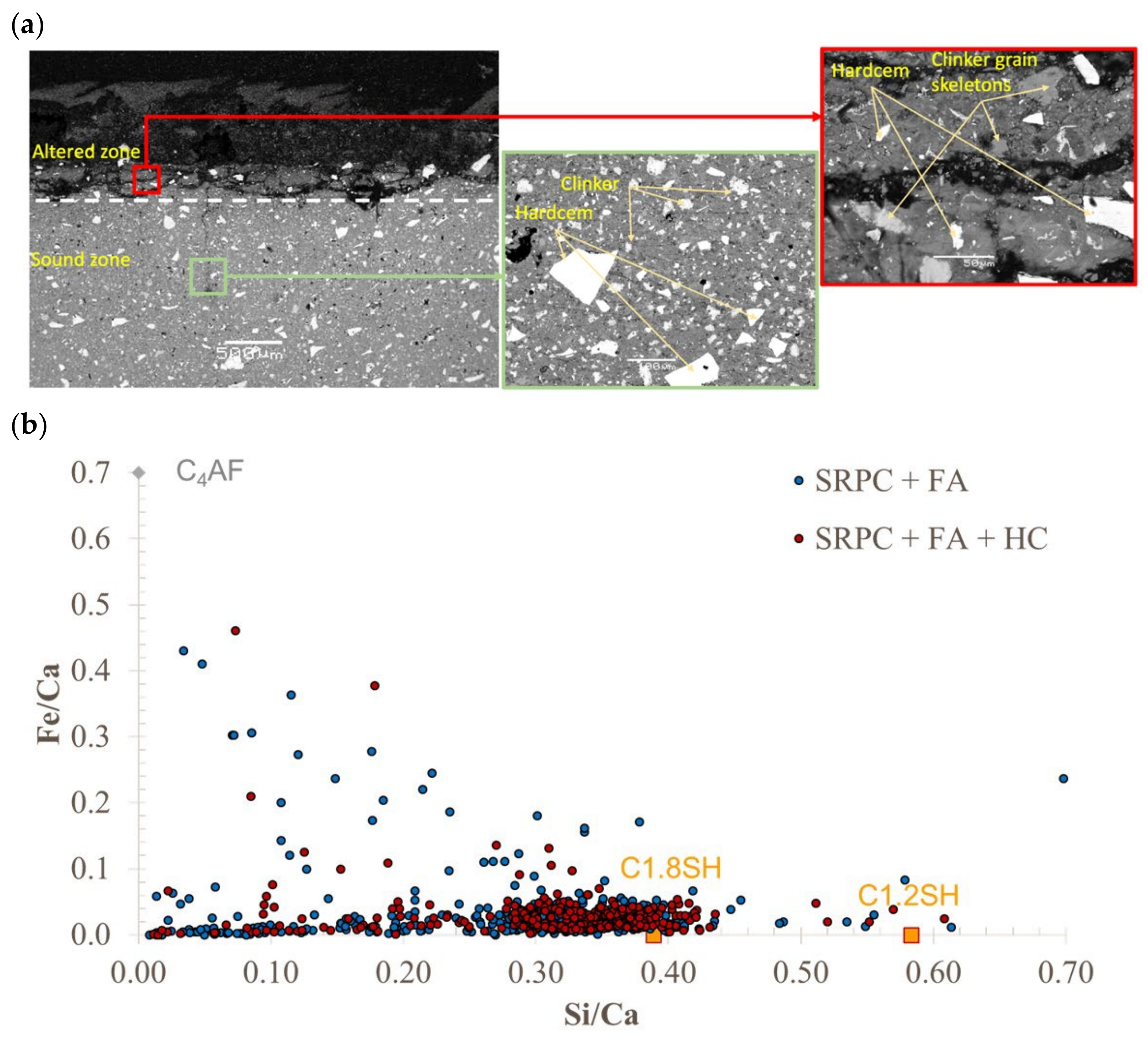
2.3.7. Cement Paste Microstructural Analysis
3. Results and Discussion
3.1. Field Sewer Conditions
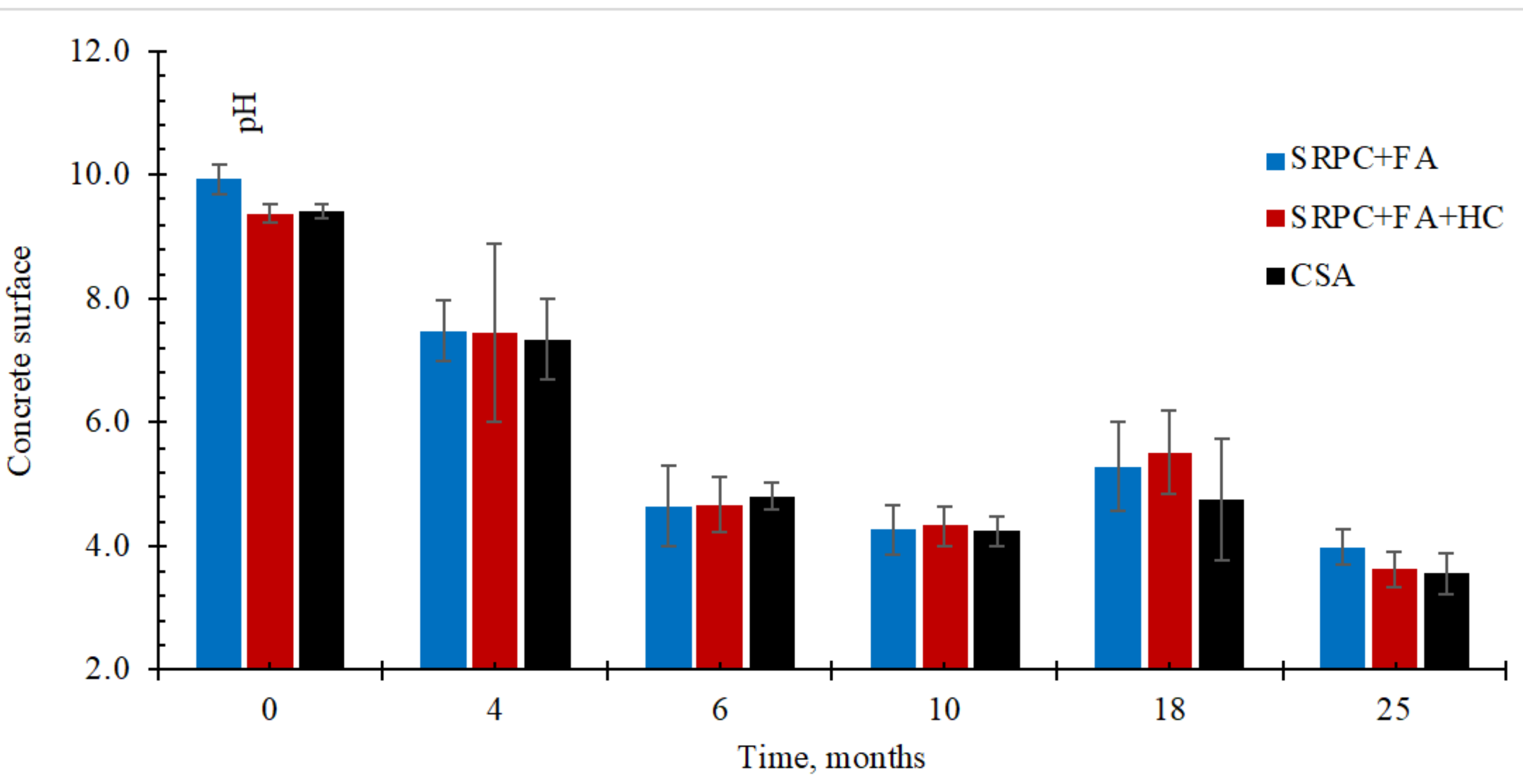
3.1.1. Sewer Environmental Condition
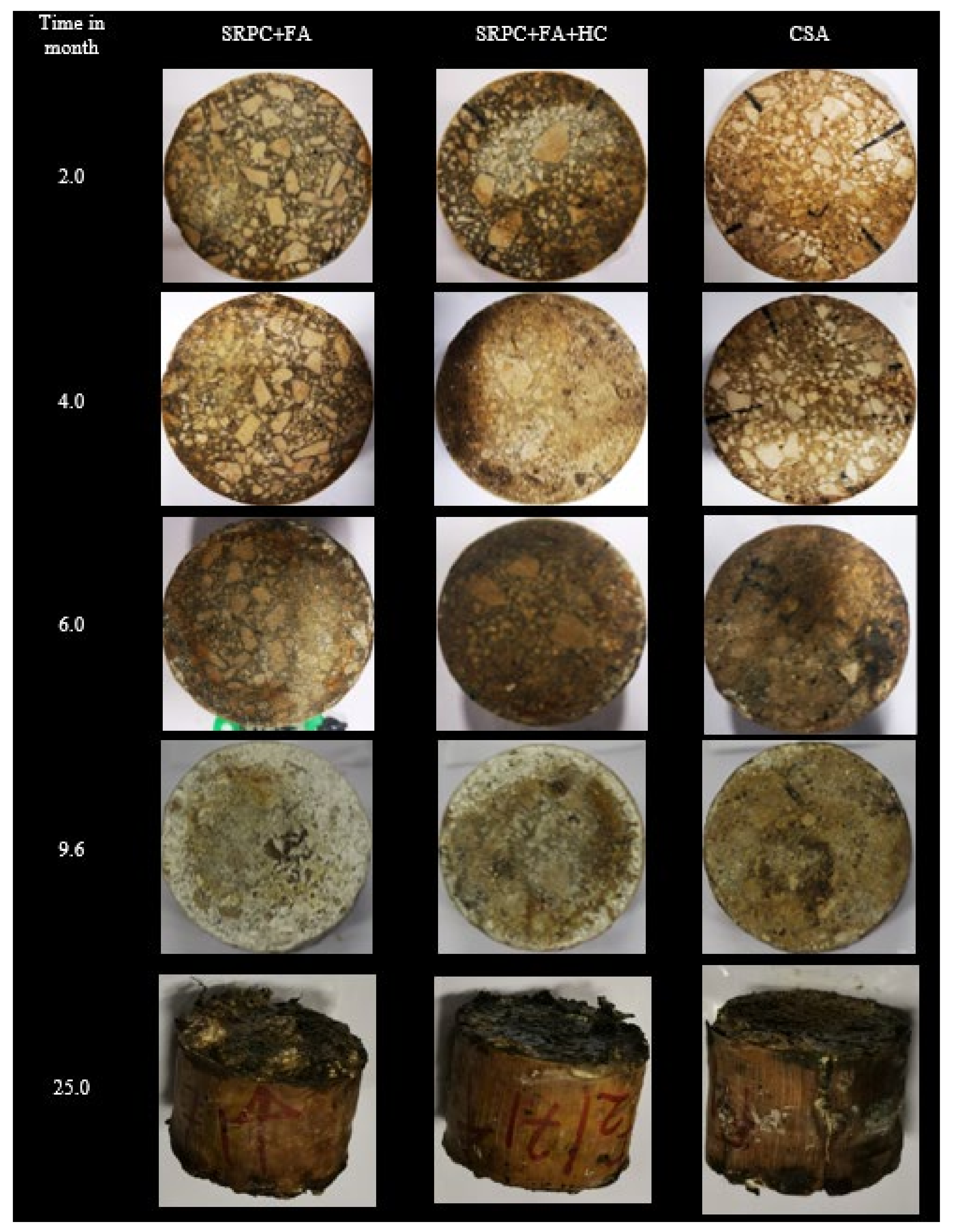
3.1.2. Visual Observations
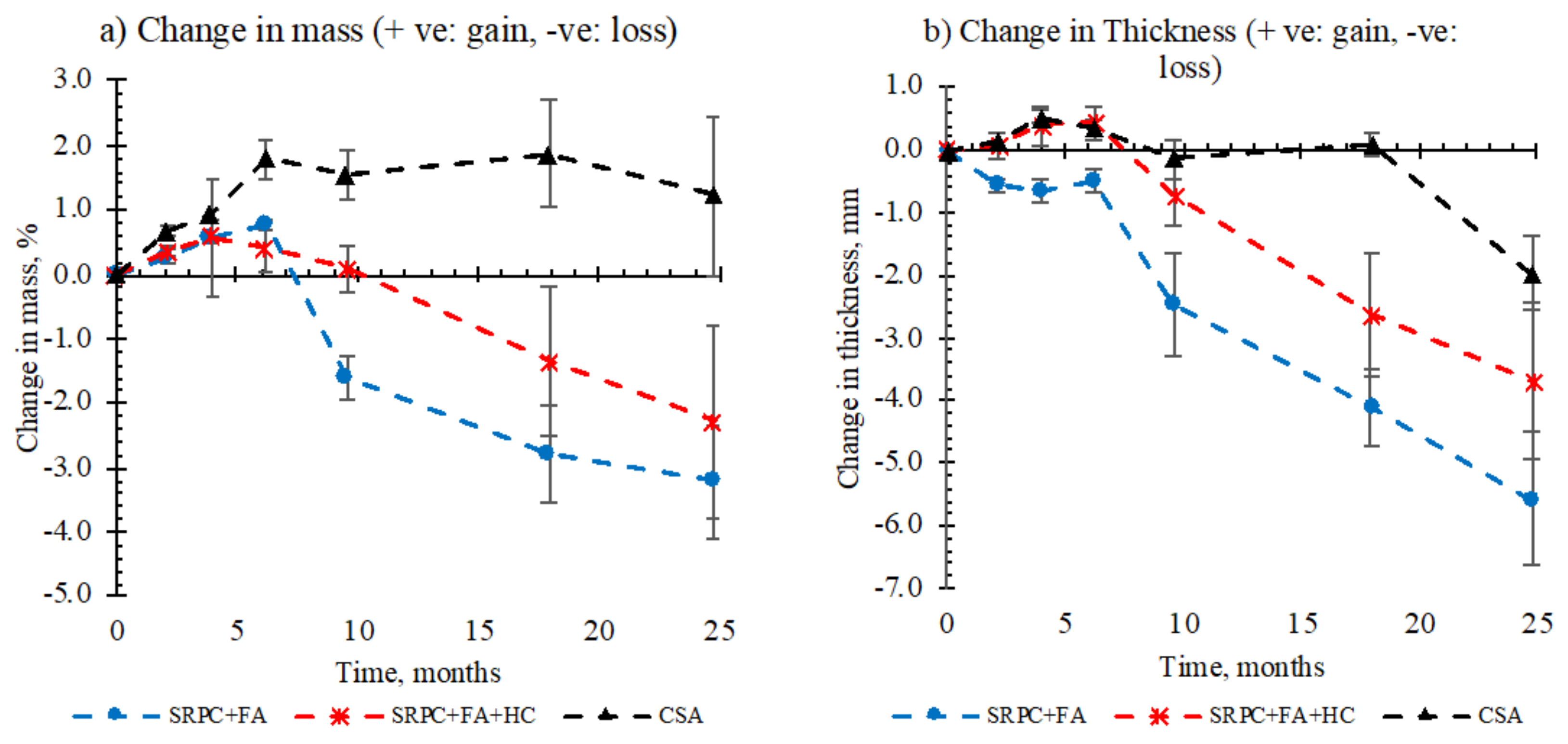
3.1.3. Mass and Thickness Changes
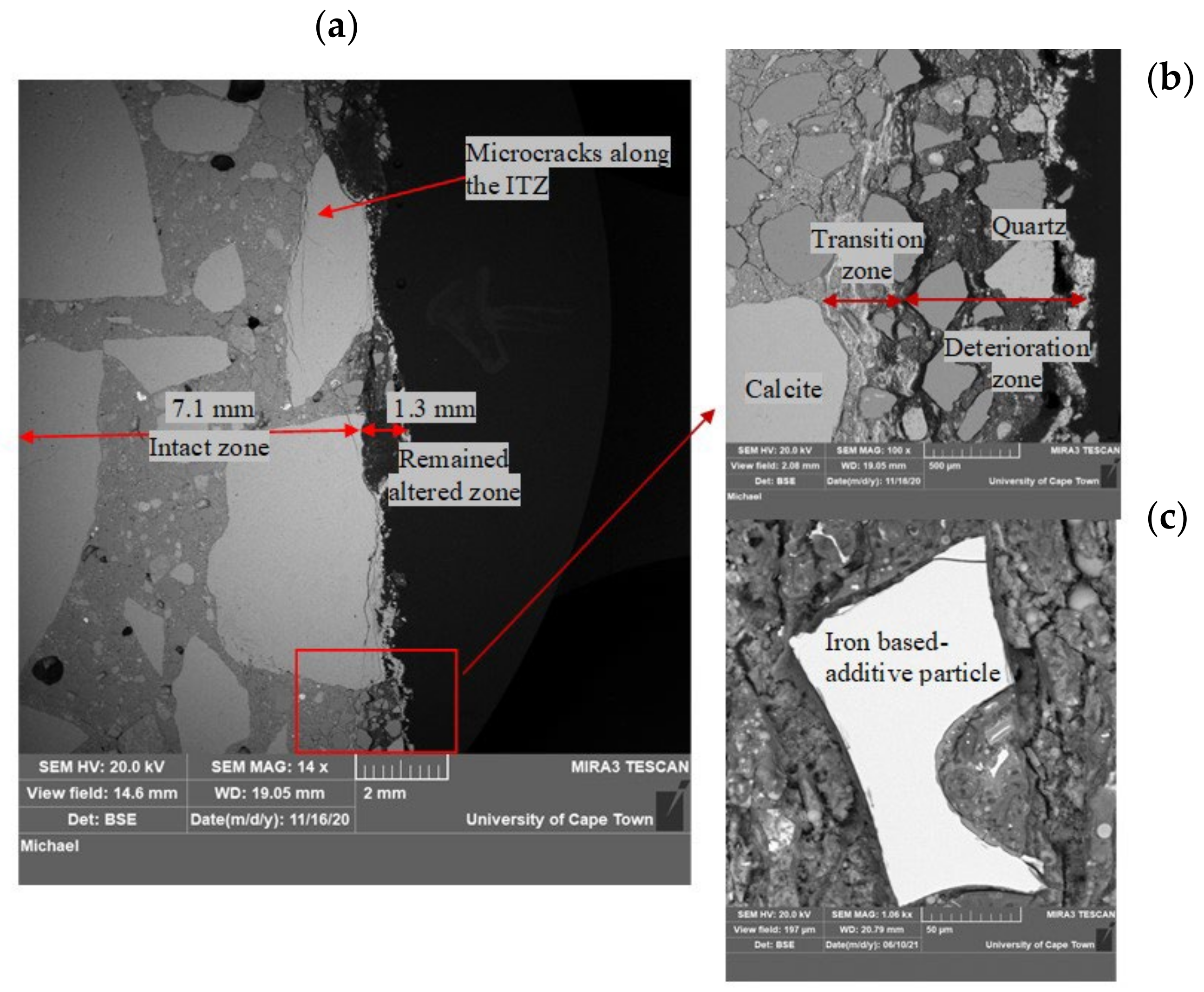
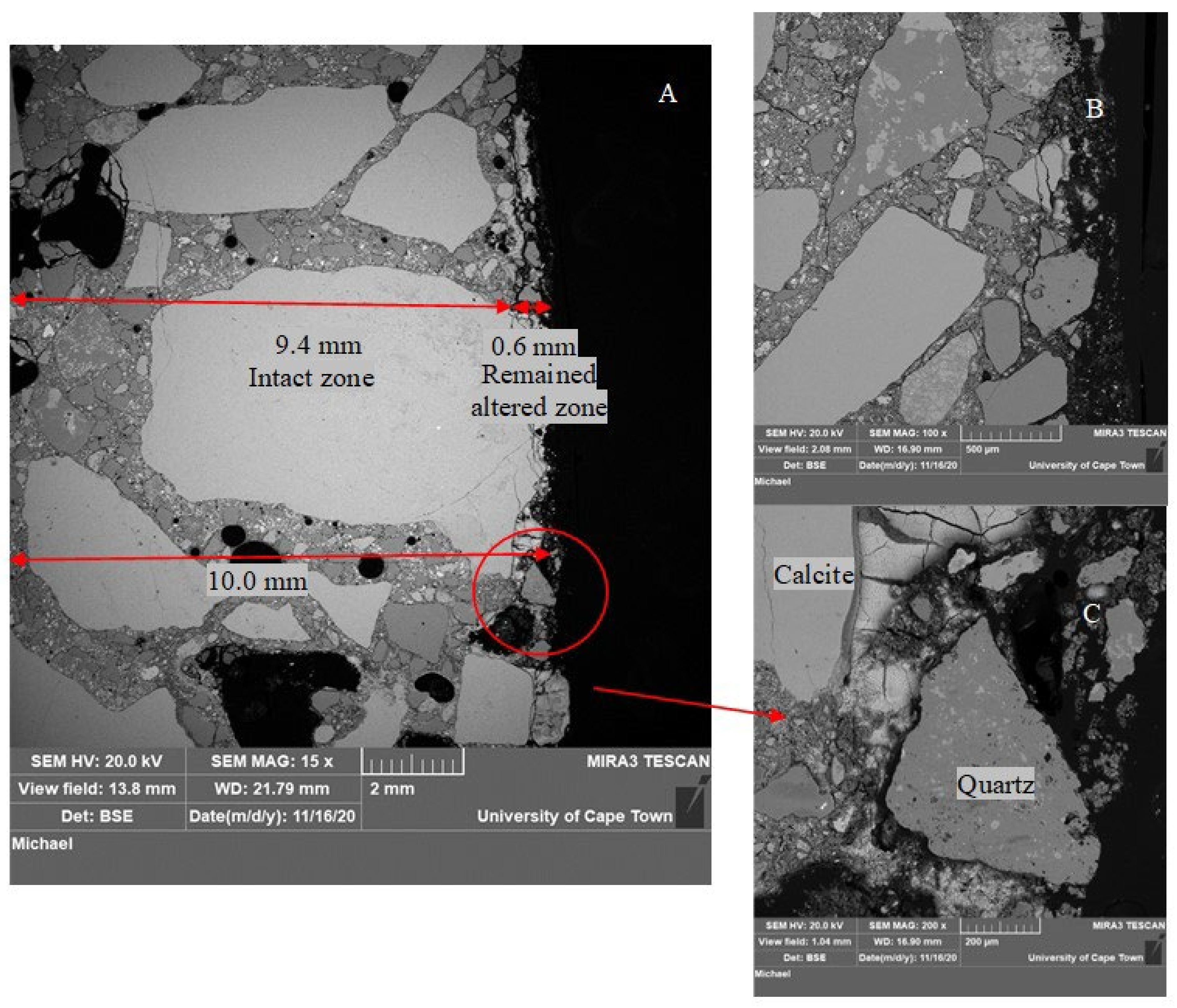
3.1.4. Concrete Microstructure Observations—SEM-BSE Images
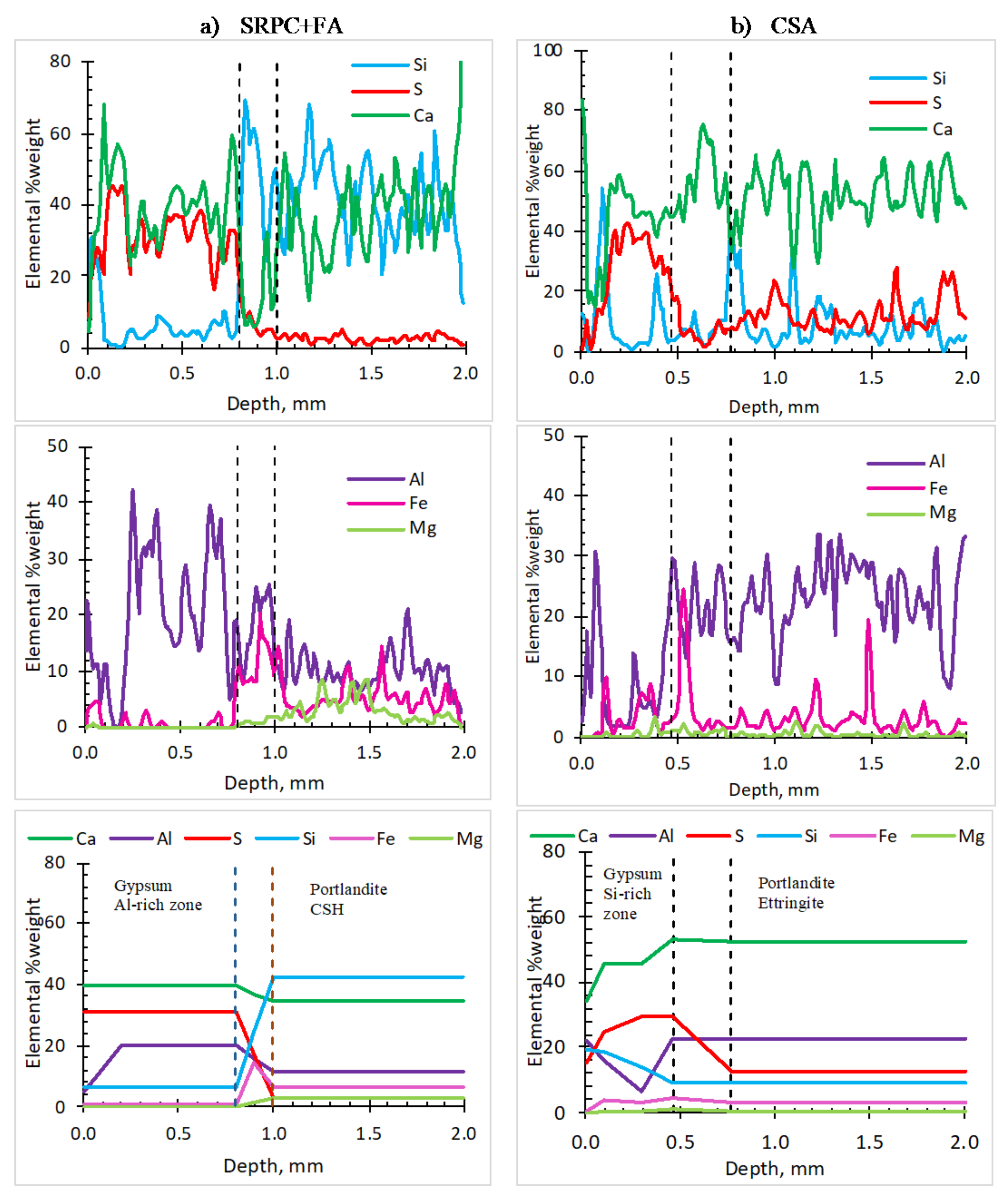
3.1.5. Profiles of Elements in the Deteriorated Concrete—SEM-EDS Analysis
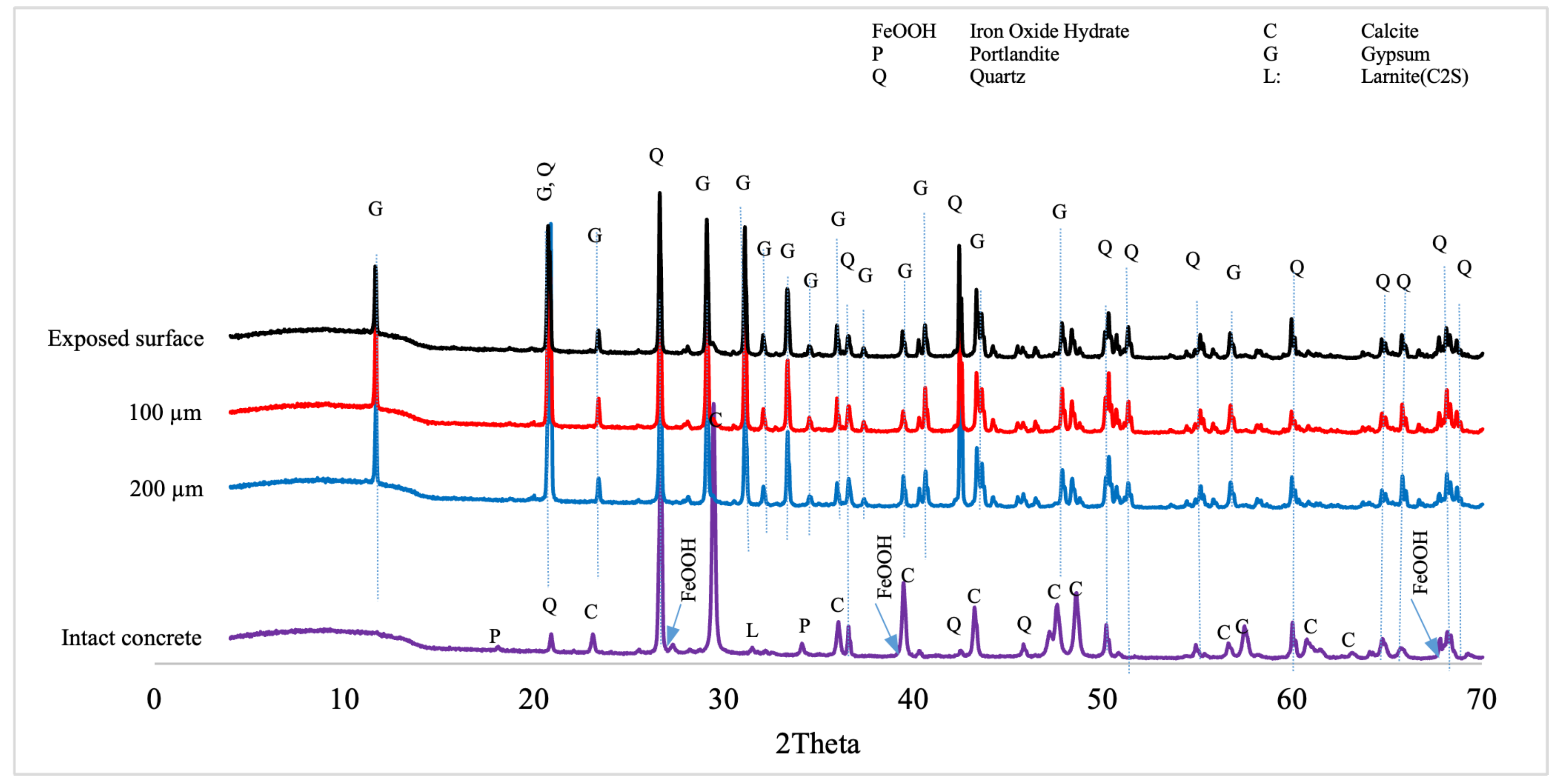
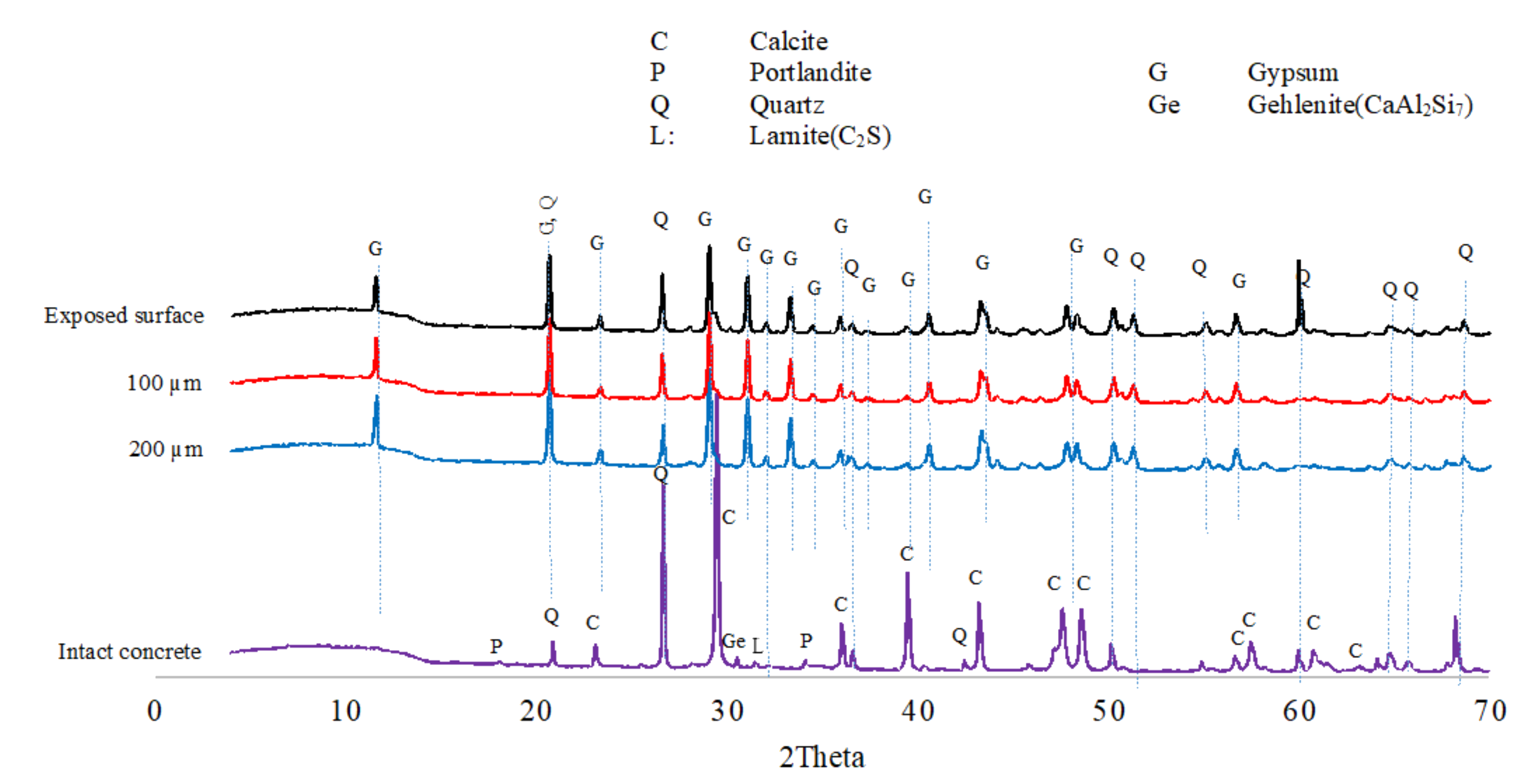
3.1.6. XRD Analyses
3.2. Laboratory Conditions
3.2.1. Microbial Activity
3.2.2. Evaluation of the Performance of Materials Using Cumulative Calcium and Aluminium in the Leached Solution
4. General Discussion
4.1. Performance of CSA and SRPC-Based Binder Systems Under Biogenic Acid Attack
4.2. Role of Iron-Based Additive (HC) in Concrete Under Biogenic Acid Attack
5. Conclusions
- (i)
- CSA cement had superior performance in the live sewer environment and also in laboratory conditions, compared with SRPC-based binder systems, when assessed using performance indicators of mass loss, thickness loss, and standardised cumulative leached calcium and aluminium in the laboratory test.
- (ii)
- Concrete specimens subjected to a live sewer environment initially experienced mass gain due to moisture absorption before they experienced mass loss due to deterioration. For concrete with CSA cement and a low water/binder ratio, sewer moisture likely activates hydration of the remaining un-hydrated cement within the microstructure, consequently causing mass gain, compensating for the mass loss due to thickness loss.
- (iii)
- The superior performance of CSA is associated with further ettringite formation due to sulphate penetration, which, at pH <10.7, dissolves to provide more CSA neutralisation capacity to acid penetration than in the SRPC-based system.
- (iv)
- The iron-based additive used in this study reduced the mass loss and thickness loss of SRPC-based concrete to a minor extent, due to its influence in improving concrete integrity by providing resistance against abrasion and erosion (i.e., sewer hydraulic erosion), but not due to its chemical influence, since it participates in neither hydration nor dissolution reactions. Questions remain as to whether this effect (a) is maintained over an extended period, and (b) whether it would be beneficial in the sewer tidal zone, where hydraulic erosion is a significant form of deterioration.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A




References
- Strande, L.; Evans, B.; von Sperling, M.; Bartram, J.; Harada, H.; Nakagiri, A.; Nguyen, V.A. Urban Sanitation: New Terminology for Globally Relevant Solutions? Environ. Sci. Technol. 2023, 57, 15771–15779. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Liu, X.; Zhang, L. Biogenic corrosion of cementitious composite in wastewater sewerage system—A review. Process Saf. Environ. Prot. 2022, 165, 545–585. [Google Scholar] [CrossRef]
- Goyns, A.M. Design Manual for Concrete Pipe Outfall Sewers, Pipes, Infrastructural Products and Engineering Solution Division. 2009. Available online: https://www.cma.org.za/Portals/0/Docs/pipes/design_manual_for_concrete_pipe_outfall_sewers_april_2009.pdf?ver=2014-04-21-170635-713 (accessed on 15 January 2022).
- Alexander, M.G.; Fourie, C. Performance of sewer pipe concrete mixtures with portland and calcium aluminate cements subject to mineral and biogenic acid attack. Mater. Struct. 2011, 44, 313–330. [Google Scholar] [CrossRef]
- Wells, T.; Melchers, R.E.; Bond, P. Factors involved in the long term corrosion of concrete sewers. In Proceedings of the 49th Annual Conference of the Australasian Corrosion Association, Coffs Harbour, Australia, 15–19 November 2009; pp. 345–356. [Google Scholar]
- Monteny, J.; Vincke, E.; Beeldens, A.; De Belie, N.; Taerwe, L.; Van Gemert, D.; Verstraete, W. Chemical, microbiological, and in situ test methods for biogenic sulfuric acid corrosion of concrete. Cem. Concr. Res. 2000, 30, 623–634. [Google Scholar] [CrossRef]
- Khan, H.A.; Castel, A.; Khan, M.S.H.; Mahmood, A.H. Durability of calcium aluminate and sulphate-resistant Portland cement-based mortars in aggressive sewer environment and sulphuric acid. Cem. Concr. Res. 2019, 124, 105852. [Google Scholar] [CrossRef]
- Wu, M.; Wang, T.; Wu, K.; Kan, L. Microbiologically induced corrosion of concrete in sewer structures: A review of the mechanisms and phenomena. Constr. Build. Mater. 2020, 239, 117813. [Google Scholar] [CrossRef]
- Kong, L.; Liu, C.; Cao, M.; Fang, J. Mechanism study of the role of biofilm played in sewage corrosion of mortar. Constr. Build. Mater. 2018, 164, 44–56. [Google Scholar] [CrossRef]
- Carrera, L.; Springer, F.; Lipeme-Kouyi, G.; Buffiere, P. A review of sulfide emissions in sewer networks: Overall approach and systemic modelling. Water Sci. Technol. 2016, 73, 1231–1242. [Google Scholar] [CrossRef]
- Vollertsen, J.; Nielsen, A.H.; Jensen, H.S.; Hvitved-Jacobsen, T. Modeling the Formation and Fate of Odorous Substances in Collection Systems. Water Environ. Res. 2008, 80, 118–126. [Google Scholar] [CrossRef]
- Okabe, S.; Odagiri, M.; Ito, T.; Satoh, H. Succession of sulfur-oxidizing bacteria in the microbial community on corroding concrete in sewer systems. Appl. Environ. Microbiol. 2007, 73, 971–980. [Google Scholar] [CrossRef]
- Davis, J.L.; Nica, D.; Shields, K.; Roberts, D.J. Analysis of concrete from corroded sewer pipe. Int. Biodeterior. Biodegrad. 1998, 42, 75–84. [Google Scholar] [CrossRef]
- Islander, R.L.; Devinny, J.S.; Mansfeld, F.; Postyn, A.; Shih, H. Microbial Ecology of crown corrosion in sewers. J. Environ. Eng. 1991, 117, 751–770. [Google Scholar] [CrossRef]
- Roberts, D.J.; Nica, D.; Zuo, G.; Davis, J.L. Quantifying microbially induced deterioration of concrete: Initial studies. Int. Biodeterior. Biodegrad. 2002, 49, 227–234. [Google Scholar] [CrossRef]
- Parande, A.K.; Ramsamy, P.L.; Ethirajan, S.; Rao, C.R.K.; Palanisamy, N. Deterioration of reinforced concrete in sewer environments. Proc. Inst. Civ. Eng. 2006, 159, 11–20. [Google Scholar] [CrossRef]
- Grandclerc, A.; Dangla, P.; Gueguen-Minerbe, M.; Chaussadent, T. Modelling of the sulfuric acid attack on different types of cementitious materials. Cem. Concr. Res. 2018, 105, 126–133. [Google Scholar] [CrossRef]
- Wells, T.; Melchers, R.E. Findings of a 4 years study of concrete sewer pipe corrosion. In Proceedings of the Annual Conference of the Australasian Corrosion Association 2014: Corrosion and Prevention 2014, Darwin, Australia, 21–24 September 2014; pp. 1–12. [Google Scholar]
- Bertron, A. Understanding interactions between cementitious materials and microorganisms: A key to sustainable and safe concrete structures in various contexts. Mater. Struct./Mater. Constr. 2014, 47, 1787–1806. [Google Scholar] [CrossRef]
- Voegel, C.; Bertron, A.; Erable, B. Biodeterioration of cementitious materials in biogas digester. Matériaux Tech. 2015, 103, 2–9. [Google Scholar] [CrossRef]
- Elmasry, M.; Hawari, A.; Zayed, T. Cost benefit analysis for failure of sewer pipelines. In Proceedings of the MATEC Web of Conferences, Sibiu, Romania, 7–9 June 2017; p. 120. [Google Scholar]
- Association of Metropolitan Water Agencies, Forecast_ Municipal Utilities Will Invest $532 Billion in Water Infrastructure by 2025, Association of Metropolitan Water Agencies. 2016. Available online: https://www.amwa.net/article/forecast-municipal-utilities-will-invest-532-billion-water-infrastructure-2025?utm_source=chatgpt.com (accessed on 1 February 2025).
- Pramanik, S.K.; Bhuiyan, M.; Robert, D.; Roychand, R.; Gao, L.; Cole, I.; Pramanik, B.K. Bio-corrosion in concrete sewer systems: Mechanisms and mitigation strategies. Sci. Total Environ. 2024, 921, 171231. [Google Scholar] [CrossRef]
- Berger, C.; Hetzel, F.; Falk, C.; Pinnekamp, J.; Roder, S.; Ruppelt, J. State of the Sewer System in Germany—Results of the DWA, KA Korrespondenz Abwasser. Abfall 2016, 63, 15–17. [Google Scholar]
- Fytianos, G.; Tziolas, E.; Papastergiadis, E.; Samaras, P. Least cost analysis for biocorrosion mitigation strategies in concrete sewers. Sustainability 2020, 12, 4578. [Google Scholar] [CrossRef]
- Xiaoqiang, H.; Hou, K.A.; Steiner, J.; Fraczek, J.A. Mahaney, Biogenic Sulfuric Acid Attack and Case Studies, n.d. Available online: www.concreteinternational.com (accessed on 1 February 2025).
- Tscheikner-Gratl, F.; Caradot, N.; Cherqui, F.; Leitão, J.P.; Ahmadi, M.; Langeveld, J.G.; Le Gat, Y.; Scholten, L.; Roghani, B.; Rodríguez, J.P.; et al. Sewer asset management—State of the art and research needs. Urban Water J. 2019, 16, 662–675. [Google Scholar] [CrossRef]
- De Muynck, W.; De Belie, N.; Verstraete, W. Effectiveness of admixtures, surface treatments and antimicrobial compounds against biogenic sulfuric acid corrosion of concrete. Cem. Concr. Compos. 2009, 31, 163–170. [Google Scholar] [CrossRef]
- Haile, T.; Nakhla, G.; Allouche, E. Evaluation of the resistance of mortars coated with silver-bearing zeolite to bacterial-induced corrosion. Corros. Sci. 2008, 50, 713–720. [Google Scholar] [CrossRef]
- Berndt, M.L. Evaluation of coatings, mortars and mix design for protection of concrete against sulphur oxidising bacteria. Constr. Build. Mater. 2011, 25, 3893–3902. [Google Scholar] [CrossRef]
- Noeiaghaei, T.; Mukherjee, A.; Dhami, N.; Chae, S.-R. Biogenic deterioration of concrete and its mitigation technologies. Constr. Build. Mater. 2017, 149, 575–586. [Google Scholar] [CrossRef]
- EPA 832-F-99-032; Collection Systems O&M Fact Sheet: Trenchless Sewer Rehabilitation. United States Environmental Protection Agency: Washington, DC, USA, 1999.
- Kung’u, F.T. Sanitary Sewer System Rehabilitation Techniques Vary, Wateronline 1999, 2–5. Available online: https://www.wateronline.com/doc/sanitary-sewer-system-rehabilitation-techniqu-0002 (accessed on 14 February 2018).
- Macphee, D.E.; Lachowski, E.E. Cement Components and Their Phase Relations. In Lea’s Chemistry of Cement and Concrete; Elsevier Ltd.: Amsterdam, The Netherlands, 2003; pp. 95–129. [Google Scholar] [CrossRef]
- Kiliswa, M.W.; Scrivener, K.L.; Alexander, M.G. The corrosion rate and microstructure of Portland cement and calcium aluminate cement-based concrete mixtures in outfall sewers: A comparative study. Cem. Concr. Res. 2019, 124, 105818. [Google Scholar] [CrossRef]
- Pacewska, B.; Wilińska, I.; Nowacka, M. Studies on the influence of different fly ashes and Portland cement on early hydration of calcium aluminate cement. J. Therm. Anal. Calorim. 2011, 106, 859–868. [Google Scholar] [CrossRef][Green Version]
- Odler, I. Chapter 4. Cements containing calcium sulfoaluminate. In Special Inorganic Cements, Modern Concrete Technology Series; CRC Press: Boca Raton, FL, USA, 2003; pp. 63–81. [Google Scholar]
- Habert, G. Assessing the environmental impact of conventional and ‘green’ cement production. In Eco-Efficient Construction and Building Materials: Life Cycle Assessment (LCA), Eco-Labelling and Case Studies; Woodhead Publishing Series; Woodhead: London, UK, 2014; pp. 199–238. [Google Scholar]
- Coppola, L.; Bellezze, T.; Belli, A.; Bignozzi, M.C.; Bolzoni, F.; Brenna, A.; Cabrini, M.; Candamano, S.; Cappai, M.; Caputo, D.; et al. Binders alternative to Portland cement and waste management for sustainable construction—Part 1. J. Appl. Biomater. Funct. Mater. 2018, 16, 207–221. [Google Scholar] [CrossRef]
- Péra, J.; Ambroise, J. New applications of calcium sulfoaluminate cement. Cem. Concr. Res. 2004, 34, 671–676. [Google Scholar] [CrossRef]
- Yang, Y.; Ji, T.; Lin, X.; Chen, C.; Yang, Z. Biogenic sulfuric acid corrosion resistance of new artificial reef concrete. Constr. Build. Mater. 2018, 158, 33–41. [Google Scholar] [CrossRef]
- Aïtcin, P.-C. Chapter 9. Special Portland cements and other types of hydraulic binder. In Binders for Durable and Sustainable Concrete; Taylor & Francis Group: Abingdon, UK, 2008; pp. 314–331. [Google Scholar]
- Kleib, J.; Aouad, G.; Louis, G.; Zakhour, M.; Boulos, M.; Rousselet, A.; Bulteel, D. The use of calcium sulfoaluminate cement to mitigate the alkali silica reaction in mortars. Constr. Build. Mater. 2018, 184, 295–303. [Google Scholar] [CrossRef]
- Alonso, M.C.; Calvo, J.L.G.; Hidalgo, A.; Luco, L.F. 10. Development and application of low-pH concretes for structural purposes in geological repository systems. In Geological Repository Systems for Safe Disposal of Spent Nuclear Fuels and Radioactive Waste; Elsevier: Amsterdam, The Netherlands, 2010; pp. 286–322. [Google Scholar] [CrossRef]
- Kalogridis, D.; Kostogloudis, G.C.; Ftikos, C.; Malami, C. A quantitative study of the influence of non-expansive sulfoaluminate cement on the corrosion of steel reinforcement. Cem. Concr. Res. 2000, 30, 1731–1740. [Google Scholar] [CrossRef]
- Kiliswa, M.W. Composition and Microstructure of Concrete Mixtures Subjected to Biogenic Acid Corrosion and Their Role in Corrosion Prediction of Concrete Outfall Sewers. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 2016. [Google Scholar]
- Jiang, G.; Wightman, E.; Donose, B.C.; Yuan, Z.; Bond, P.L.; Keller, J. The role of iron in sulfide-induced corrosion of sewer concrete. Water Res. 2014, 49, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Grengg, C.; Mittermayr, F.; Koraimann, G.; Konrad, F.; Szabó, M.; Demeny, A.; Dietzel, M. The decisive role of acidophilic bacteria in concrete sewer networks: A new model for fast progressing microbial concrete corrosion. Cem. Concr. Res. 2017, 101, 93–101. [Google Scholar] [CrossRef]
- Damion, T.; Chaunsali, P. Biogenic acid resistance of calcium sulfoaluminate cement: Revelations from a field study. Cem. Concr. Compos. 2024, 145, 105324. [Google Scholar] [CrossRef]
- Buvignier, A.; Peyre-Lavigne, M.; Robin, O.; Bounouba, M.; Patapy, C.; Bertron, A.; Paul, E. Influence of dissolved-aluminum concentration on sulfur-oxidizing bacterial activity in the biodeterioration of concrete. Appl. Environ. Microbiol. 2019, 85, e00302-19. [Google Scholar] [CrossRef]
- Erbektas, A.R.; Isgor, O.B.; Weiss, W.J. Comparison of chemical and biogenic acid attack on concrete. ACI Mater. J. 2020, 117, 255–264. [Google Scholar] [CrossRef]
- Buvignier, A.; Patapy, C.; Lavigne, M.P.; Paul, E.; Bertron, A. Resistance to biodeterioration of aluminium-rich binders in sewer network environment: Study of the possible bacteriostatic effect and role of phase reactivity. Cem. Concr. Res. 2019, 123, 105785. [Google Scholar] [CrossRef]
- Damion, T.; Cepuritis, R.; Chaunsali, P. Sulfuric acid and citric acid attack of calcium sulfoaluminate-based binders. Cem. Concr. Compos. 2022, 130, 104524. [Google Scholar] [CrossRef]
- Lavigne, M.P.; Bertron, A.; Botanch, C.; Auer, L.; Hernandez-Raquet, G.; Cockx, A.; Foussard, J.-N.; Escadeillas, G.; Paul, E. Innovative approach to simulating the biodeterioration of industrial cementitious products in sewer environment. Part II: Validation on CAC and BFSC linings. Cem. Concr. Res. 2016, 79, 409–418. [Google Scholar] [CrossRef]
- EN 197-1; Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements. British Standards Institute: London, UK, 2000.
- SANS 5863; Concrete Tests-Compressive Strength of Hardened Concrete. South African National Standards: Cape Town, South Africa, 2006.
- SANS 3001-CO3-1; Civil Engineering Test Methods Part CO3-1: Concrete Durability Index Testing—Preparation of Test Specimens. South African National Standards: Cape Town, South Africa, 2015.
- SANS 3001-CO3-2; Civil Engineering Test Methods Part CO3-2: Concrete Durability Index Testing—Oxygen Permeability Test. South African National Standards: Cape Town, South Africa, 2015.
- Alexander, M.G.; Ballim, Y.; Mackechnie, J.M.; Durability Index Testing Procedure Manual Ver. 4.5.1, Univeristy of Cape Town and University of Witwatersrand Johannesburg v. 2018. Available online: http://www.comsiru.uct.ac.za (accessed on 1 February 2025).
- Alexander, M.G.; Mackechnie, J.R.; Ballim, Y. Guide to the use of durability indexes for achieving durability in concrete structures. Res. Monogr. 1999, 36, 102. [Google Scholar]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials, CRC Press; Taylor & Francis Group, Boca Raton. 2016. Available online: www.crcpress.com (accessed on 1 February 2025).
- ASTM C1723; Standard Guide for Examination of Hardened Concrete Using Scanning Electron Microscopy. ASTM International: West Conshohocken, PA, USA, 2006; Volume 16, pp. 1–95. [CrossRef]
- Aboulela, A.; Lavigne, M.P.; Buvignier, A.; Fourré, M.; Schiettekatte, M.; Pons, T.; Patapy, C.; Robin, O.; Bounouba, M.; Paul, E.; et al. Laboratory test to evaluate the resistance of cementitious materials to biodeterioration in sewer network conditions. Materials 2021, 14, 686. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, M.P.; Bertron, A.; Auer, L.; Hernandez-Raquet, G.; Foussard, J.N.; Escadeillas, G.; Cockx, A.; Paul, E. An innovative approach to reproduce the biodeterioration of industrial cementitious products in a sewer environment. Part I: Test design. Cem. Concr. Res. 2015, 73, 246–256. [Google Scholar] [CrossRef]
- Aboulela, A.; Peyre-Lavigne, M.; Bertron, A. Investigation of test methods to qualify cementitious materials. In Proceedings of the RILEM TC 253-MCI Conference Microorganisms-Cementitious Materials Interactions PRO123, Toulouse, France, 25–26 June 2018; RILEM Edition. pp. 45–56. [Google Scholar]
- Aboulela, A. Study of the Resistance to Biodeterioration of Innovative and Low-Carbon Cementitious Materials for an Application in Sewerage Networks. Ph.D. Thesis, INSA, Toulouse, France, 2022. [Google Scholar]
- Miura, Y.; Kawaoi, A. Determination of Thiosulfate, Thiocyanate and Polythionates in a Mixture by Ion-Pair Chromatography with Ultraviolet Absorbance Detection. J. Chromatogr. A 2000, 884, 81–87. [Google Scholar]
- Gutiérrez-Padilla, M.G.D.; Bielefeldt, A.; Ovtchinnikov, S.; Hernandez, M.; Silverstein, J. Biogenic sulfuric acid attack on different types of commercially produced concrete sewer pipes. Cem. Concr. Res. 2010, 40, 293–301. [Google Scholar] [CrossRef]
- Joseph, A.P.; Keller, J.; Bustamante, H.; Bond, P.L. Surface neutralization and H2S oxidation at early stages of sewer corrosion: Influence of temperature, relative humidity and H2S concentration. Water Res. 2012, 46, 4235–4245. [Google Scholar] [CrossRef]
- Jiang, G.; Keller, J.; Bond, P.L. Determining the long-term effects of H2S concentration, relative humidity and air temperature on concrete sewer corrosion. Water Res. 2014, 65, 157–169. [Google Scholar] [CrossRef]
- Okabe, S.; Ito, T.; Satoh, H. Analyses of Spatial Distributions of Sulfate-Reducing Bacteria and Their Activity in Aerobic Wastewater Biofilms Analyses of Spatial Distributions of Sulfate-Reducing Bacteria and Their Activity in Aerobic Wastewater Biofilms. Appl. Environ. Microbiol. 1999, 65, 5107–5116. [Google Scholar] [CrossRef]
- Grengg, C.; Martin, D.; Bertron, A. Microbial Induced Acid Corrosion in Sewer Environments Statutory Declaration. Ph.D. Thesis, Graz University of Technology, Styria, Austria, 2017. [Google Scholar]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Gabrisovd, A.; Havlica, J.; Sahu, S. Stability of calcium sulphoaluminate hydrates in water solutions with various pH values. Cem. Concr. Res. 1991, 21, 1023–1027. [Google Scholar] [CrossRef]
- Girardi, F.; Di Maggio, R. Resistance of concrete mixtures to cyclic sulfuric acid exposure and mixed sulfates: Effect of the type of aggregate. Cem. Concr. Compos. 2011, 33, 276–285. [Google Scholar] [CrossRef]
- Bakera, A.T. Biogenic Acid Corrosion of Sewer Concretes with Different Binders: In-situ and Model Studies, with Advancement of the Life Factor Prediction Method. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 2023. [Google Scholar]
- Lee, Y.L.; Wang, W.H.; Lin, F.H.; Lin, C.P. Hydration behaviors of calcium silicate-based biomaterials. J. Formos. Med. Assoc. 2017, 116, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Aboulela, A.; Peyre-lavigne, M.; Patapy, C.; Bertron, A. Evaluation of the resistance of CAC and BFSC mortars to biodegradation: Laboratory test approach. MATEC Web Conf. 2018, 199, 02004. [Google Scholar] [CrossRef]
- Aboulela, A.; Peyre-Lavigne, M.; Huet, B.; Patapy, C.; Meulenyzer, S.; Bertron, A. Numerical modelling of sulfuric acid attack on OPC and CAC materials. In Proceedings of the 15th International Congress on the Chemistry of Cement, Prague, Czech Republic, 16–20 September 2019. [Google Scholar]
- Krishnan, S.; Bishnoi, S. Understanding the hydration of dolomite in cementitious systems with reactive aluminosilicates such as calcined clay. Cem. Concr. Res. 2018, 108, 116–128. [Google Scholar] [CrossRef]
- Beddoe, R.E.; Dorner, H.W. Modelling acid attack on concrete: Part I. The essential mechanisms. Cem. Concr. Res. 2005, 35, 2333–2339. [Google Scholar] [CrossRef]
- Hudon, E.; Mirza, S.; Frigon, D. Biodeterioration of Concrete Sewer Pipes: State of the Art and Research Needs. J. Pipeline Syst. Eng. Pract. 2011, 2, 42–52. [Google Scholar] [CrossRef]
- Warren, C.J.; Tteardon, E.J. The solubility of ettringite at 25 °C. Cem. Concr. Res. 1994, 24, 1515–1524. [Google Scholar] [CrossRef]
- Letourneux, R.; Scrivener, K. The resistance of Calcium Aluminate Cements to acid corrosion in wastewater applications. In Proceedings of the International Conference, University of Dundee, Scotland, UK, 8–10 September 1999; pp. 275–283. [Google Scholar]




| Reference | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | TiO2 | Others | LOI |
|---|---|---|---|---|---|---|---|---|---|
| SRPC | 67.95 | 21.45 | 3.14 | 2.17 | 0.95 | 1.85 | 0.14 | 0.68 | 1.63 |
| CSA * | 41.75 | 4.70 | 30.37 | 1.53 | 0.62 | 15.71 | 1.26 | 0.35 | 2.73 |
| HC # | 17.05 | 33.61 | 5.10 | 40.69 | 0.93 | 2.12 | 0.32 | 2.55 | −4.12 |
| FA | 4.32 | 53.72 | 32.94 | 3.23 | 1.07 | 0.00 | 1.71 | 1.94 | 1.03 |
| Anhydrite | Ye’elimite | Gehlenite | Perovskite | Calcite | Magnetite | Mayenite | C2Sα | Total | |
| 29.50 | 33.52 | 18.52 | 5.08 | 8.04 | 1.45 | 1.01 | 2.73 | 100 | |
| Mix Designation | SRPC + FA | SRPC + FA + HC | CSA | |
|---|---|---|---|---|
| Binder Systems | SRPC 80% + FA 20% | SRPC 80% + FA 20% + HC (11.4%) | 100% CSA | |
| w/b | 0.4 | |||
| Constituents | kg/m3 | |||
| Binder | 280 | 280 | 350 | |
| Fly Ash (FA) | 70 | 70 | 0 | |
| Iron-based additive (HC) a | 0 | 40 | 0 | |
| Water | 133 | 133 | 133 | |
| Fine aggregates | 0/1 mm Siliceous | 520 | 520 | 520 |
| 1.6/3 mm Calcite | 520 | 520 | 520 | |
| 3/6 mm Calcite | 343 | 343 | 343 | |
| Coarse aggregates | 6/11 mm Calcite | 516 | 516 | 516 |
| BASF-Glenium 7700 (% by mass, binder) | 0.64 | 0.64 | 1.08 | |
| % Total binder | 16 | 16 | 16 | |
| Theoretical density of concrete | 2383 | 2422 a | 2383 | |
| 28-day compressive strength (MPa) | 44.8 ± 11.0 | 43.8 ± 8.1 | 40.3 ± 3.9 | |
| 28-day saturated density (kg/m3) | 2379 | 2339 | 2320 | |
| OPI (log) | 10.7 | 10.6 | 10.7 | |
| WSI mm/h0.5 | 5.4 | 6.0 | 7.1 | |
| Water-penetrable porosity % | 8.1 | 8.4 | 6.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakera, A.T.; Aboulela, A.; Alexander, M.G.; Bertron, A.; Peyre Lavigne, M.; Meulenyzer, S.; Patapy, C. Performance of Sewer Concretes with Calcium Sulpho-Aluminate Cement and Portland Cement Blends: Field and Laboratory Studies. Materials 2025, 18, 1256. https://doi.org/10.3390/ma18061256
Bakera AT, Aboulela A, Alexander MG, Bertron A, Peyre Lavigne M, Meulenyzer S, Patapy C. Performance of Sewer Concretes with Calcium Sulpho-Aluminate Cement and Portland Cement Blends: Field and Laboratory Studies. Materials. 2025; 18(6):1256. https://doi.org/10.3390/ma18061256
Chicago/Turabian StyleBakera, Alice Titus, Amr Aboulela, Mark G. Alexander, Alexandra Bertron, Matthieu Peyre Lavigne, Samuel Meulenyzer, and Cédric Patapy. 2025. "Performance of Sewer Concretes with Calcium Sulpho-Aluminate Cement and Portland Cement Blends: Field and Laboratory Studies" Materials 18, no. 6: 1256. https://doi.org/10.3390/ma18061256
APA StyleBakera, A. T., Aboulela, A., Alexander, M. G., Bertron, A., Peyre Lavigne, M., Meulenyzer, S., & Patapy, C. (2025). Performance of Sewer Concretes with Calcium Sulpho-Aluminate Cement and Portland Cement Blends: Field and Laboratory Studies. Materials, 18(6), 1256. https://doi.org/10.3390/ma18061256








