Corrosion Behavior and Surface Characterization of Medium-Entropy Alloy Under Different Media Conditions
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, P.J.; Li, R.G.; Li, Y.; Zhong, Y.B.; Ren, W.L.; Shen, Z.; Zheng, T.X.; Peng, J.C.; Liang, X.; Hu, P.F.; et al. Hierarchical crack buffering triples ductility in eutectic herringbone high-entropy alloys. Science 2021, 373, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.P.; Yang, Y.J.; Duan, S.G.; Dong, Y.; Li, C.Q.; Zhang, Z.R. Rapid Design, Microstructures, and Properties of Low-Cost Co-Free Al-Cr-Fe-Ni Eutectic Medium Entropy Alloys. Materials 2023, 16, 56. [Google Scholar] [CrossRef]
- Wang, Y.F.; Ma, X.L.; Guo, F.J.; Zhao, Z.F.; Huang, C.X.; Zhu, Y.T.; Wei, Y.G. Strong and ductile CrCoNi medium-entropy alloy via dispersed heterostructure. Mater. Des. 2023, 225, 111593. [Google Scholar] [CrossRef]
- Ma, Y.; Yuan, F.P.; Yang, M.X.; Jiang, P.; Ma, E.; Wu, X.L. Dynamic shear deformation of a CrCoNi medium-entropy alloy with heterogeneous grain structures. Acta Mater. 2018, 148, 407–418. [Google Scholar] [CrossRef]
- Shuang, S.; Lyu, G.J.; Chung, D.; Wang, X.Z.; Gao, X.; Mao, H.H.; Li, W.P.; He, Q.F.; Guo, B.S.; Zhong, X.Y.; et al. Unusually high corrosion resistance in MoxCrNiCo medium entropy alloy enhanced by acidity in aqueous solution. J. Mater. Sci. Technol. 2023, 139, 29–68. [Google Scholar] [CrossRef]
- Wetzel, A.; Au, M.; Dietrich, P.M.; Radnik, J.; Ozcan, O.; Witt, J. The comparison of the corrosion behavior of the CrCoNi medium entropy alloy and CrMnFeCoNi high entropy alloy. Appl. Surf. Sci. 2022, 601, 154171. [Google Scholar] [CrossRef]
- Wang, D.P.; Meng, H.; Wang, J.B.; Wang, Z.J.; Ye, Y.; Dong, Z.Z.; Wu, Y.C.; Wang, Y.X. Corrosion performance of a high-strength FeNiCrAl medium-entropy alloy compared with 304 stainless steel in KOH solution. Appl. Surf. Sci. 2024, 678, 161069. [Google Scholar] [CrossRef]
- Wan, X.L.; Lan, A.D.; Zhang, M.; Jin, X.; Yang, H.J.; Qiao, J.W. Corrosion and passive behavior of Al0.8CrFeNi2.2 eutectic high entropy alloy in different media. J. Alloys Compd. 2023, 944, 169217. [Google Scholar] [CrossRef]
- Cui, P.C.; Bao, Z.J.; Liu, Y.; Zhou, F.; Lai, Z.H.; Zhou, Y.; Zhu, J.C. Corrosion behavior and mechanism of dual phase Fe1.125Ni1.06CrAl high entropy alloy. Corros. Sci. 2022, 201, 110276. [Google Scholar] [CrossRef]
- Yen, C.C.; Lu, H.N.; Tsai, M.H.; Wu, B.W.; Lo, Y.C.; Wang, C.C.; Chang, S.Y.; Yen, S.K. Corrosion mechanism of annealed equiatomic AlCoCrFeNi tri-phase high entropy alloy in 0.5 M H2SO4 aerated aqueous solution. Corros. Sci. 2019, 157, 462–471. [Google Scholar] [CrossRef]
- Shi, Y.Z.; Yang, B.; Xie, X.; Brechtl, J.; Dahmen, K.A.; Liaw, P.K. Corrosion of AlxCoCrFeNi high-entropy alloys: Al-content and potential scan-rate dependent pitting behavior. Corros. Sci. 2017, 119, 33–45. [Google Scholar] [CrossRef]
- Li, M.J.; Chen, Q.J.; Cui, X.; Peng, X.Y.; Huang, G.S. Evaluation of corrosion resistance of the single-phase light refractory high entropy alloy TiCrVNb0.5Al0.5 in chloride environment. J. Alloys Compd. 2021, 867, 158278. [Google Scholar] [CrossRef]
- Sünbül, S.E.; İçïn, K.; Şeren, F.Z.; Şahin, Ö.; Çakil, D.D.; Sezer, R.; Öztürk, S. Determination of structural, tribological, isothermal oxidation and corrosion properties of Al–Co–Cr–Fe–Ni–Ti–Cu high-entropy alloy. Vacuum 2021, 187, 110072. [Google Scholar] [CrossRef]
- Liu, J.J.; Zhao, Y.C.; Hu, R.N.; Zhang, M.Y.; Ding, Y.T. Effects of Cu and Ag elements on corrosion resistance of Dual-Phase Fe-Based medium-entropy alloys. Materials 2023, 16, 3242. [Google Scholar] [CrossRef]
- Han, Z.H.; Guo, C.H.; Huang, C.D.; Fan, X.Y.; Zhang, J.Y.; Liu, G.; Wang, H.Y.; Wei, R. Corrosion resistant body-centered cubic VNbTa refractory medium-entropy alloy. Corros. Sci. 2024, 229, 111885. [Google Scholar] [CrossRef]
- Fu, Y.; Li, J.; Luo, H.; Du, C.W.; Li, X.G. Recent advances on environmental corrosion behavior and mechanism of high-entropy alloys. J. Mater. Sci. Technol. 2021, 80, 217–233. [Google Scholar] [CrossRef]
- Yan, X.L.; Guo, H.; Yang, W.; Pang, S.J.; Wang, Q.; Liu, Y.; Liaw, P.K.; Zhang, T. Al0.3CrxFeCoNi high-entropy alloys with high corrosion resistance and good mechanical properties. J. Alloys Compd. 2021, 860, 158436. [Google Scholar] [CrossRef]
- Guo, Z.H.; Liu, M.; Ma, Y.; Yu, H.; Jing, S.R.; Yan, Y. Hydrogen embrittlement and corrosion resistance of NiCoCr-based equimolar face-centered cubic medium-/high-entropy alloys. Corros. Sci. 2025, 245, 112700. [Google Scholar] [CrossRef]
- Chai, W.K.; Lu, T.; Pan, Y. Corrosion behaviors of FeCoNiCrx(x = 0, 0.5, 1.0) multi-principal element alloys: Role of Cr-induced segregation. Intermetallics 2020, 116, 106654. [Google Scholar] [CrossRef]
- Tsau, C.H.; Lin, S.X.; Fang, C.H. Microstructures and corrosion behaviors of FeCoNi and CrFeCoNi equimolar alloys. Mater. Chem. Phys. 2017, 186, 534–540. [Google Scholar] [CrossRef]
- Wang, N.R.; Zhang, Y.J.; Cai, L.; Huang, Q.K.; Zhang, Z.Y.; Ma, W.S.; Wu, H.; Wang, Y. Tailoring recrystallization for optimum mechanical combination in Ni-rich medium-entropy alloy via simplified thermomechanical treatment. J. Alloys Compd. 2024, 994, 174685. [Google Scholar] [CrossRef]
- Yao, Y.H.; Jin, Y.H.; Gao, W.; Liang, X.Y.; Chen, J.; Zhu, S.D. Corrosion behavior of AlFeCrCoNiZrx high-entropy alloys in 0.5 M sulfuric acid solution. Metals 2021, 11, 1471. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, H.; Chen, X.R.; Prabhakar, J.M.; Wang, X.F.; Cheng, H.X.; Du, C.W.; Hu, S.Q.; Li, X.G. The corrosion behavior and passive film properties of the cast and annealed AlCoCrFeNi2.1 eutectic high-entropy alloy in sulfuric acid solution. Corros. Sci. 2024, 240, 112456. [Google Scholar] [CrossRef]
- Flitt, H.J.; Schweinsberg, D.P. Evaluation of corrosion rate from polarisation curves not exhibiting a Tafel region. Corros. Sci. 2005, 47, 3034–3052. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, G.H.; Fan, X.H.; Jin, J.; Zhang, L.; Du, Y.X. Corrosion behavior and surface characterization of an equiatomic CoCrFeMoNi high-entropy alloy under various pH conditions. J. Alloys Compd. 2022, 900, 163432. [Google Scholar]
- Gao, M.H.; Zhang, S.D.; Yang, B.J.; Wang, J.Q. Influence of yttrium on surface chemistry and stability of passive film in Al based binary metallic glasses. Appl. Surf. Sci. 2018, 457, 536–547. [Google Scholar] [CrossRef]
- Fu, Y.; Dai, C.D.; Luo, H.; Li, D.Y.; Du, C.W.; Li, X.G. The corrosion behavior and film properties of Al-containing high-entropy alloys in acidic solutions. Appl. Surf. Sci. 2021, 560, 149854. [Google Scholar]
- Ha, H.Y.; Jang, M.H.; Lee, T.H. Influences of Mn in solid solution on the pitting corrosion behaviour of Fe-23 wt%Cr-based alloys. Electrochim. Acta. 2016, 191, 864–875. [Google Scholar]
- Marcelin, S.; Pébère, N.; Régnier, S. Electrochemical characterisation of a martensitic stainless steel in a neutral chloride solution. Electrochim. Acta. 2013, 87, 32–40. [Google Scholar] [CrossRef]
- Ray, M.; Singh, V.B. Effect of sulfuric acid on corrosion and passivation of 316 SS in organic solution. J. Electrochem. Soc. 2011, 158, 359. [Google Scholar] [CrossRef]
- Mert, B.D.; Yüce, A.O.; Kardas, G.; Yazıcı, B. Inhibition effect of 2-amino-4-methylpyridine on mild steel corrosion: Experimental and theoretical investigation. Corros. Sci. 2014, 85, 287–295. [Google Scholar] [CrossRef]
- Hsu, K.M.; Chen, S.H.; Lin, C.S. Microstructure and corrosion behavior of FeCrNiCoMnx (x = 1.0, 0.6, 0.3, 0) high entropy alloys in 0.5 M H2SO4. Corros. Sci. 2021, 190, 109694. [Google Scholar] [CrossRef]
- Musiani, M.; Orazem, M.E.; Pébère, N.; Tribollet, B.; Vivier, V. Constant-Phase-Element behavior caused by coupled resistivity and permittivity distributions in films. J. Electrochem. Soc. 2011, 158, C424–C428. [Google Scholar] [CrossRef]
- Orazem, M.E.; Frateur, I.; Tribollet, B.; Vivier, V.; Marcelin, S.; Pebere, N.; Bunge, A.L.; White, E.A.; Riemer, D.P.; Musiani, M. Dielectric properties of materials showing constant-phase-element (CPE) impedance response. J. Electrochem. Soc. 2013, 160, C215–C225. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, Z.; Zhang, L. Effect of high temperature on the corrosion behavior and passive film composition of 316 L stainless steel in high H2S-containing environments. Corros. Sci. 2020, 174, 108844. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, J.; Zhang, M.; Wang, X.; Gong, P.; Zhang, J.; Liu, J. Effect of the grain size on the corrosion behavior of CoCrFeMnNi HEAs in a 0.5 M H2SO4 solution. J. Alloys Compd. 2021, 858, 157712. [Google Scholar] [CrossRef]
- Xing, B.W.; Ding, Q.; Jin, B.Q.; Zuo, X.J.; Zhang, N.N.; Yin, S. Corrosion resistance and passivation behavior of CoCrFeNi-TiAl high-entropy alloy coatings in acidic solutions. J. Therm Spray Tech. 2022, 31, 1673–1682. [Google Scholar] [CrossRef]
- Dai, C.D.; Zhao, T.L.; Du, C.W.; Liu, Z.Y.; Zhang, D.W. Effect of molybdenum content on the microstructure and corrosion behavior of FeCoCrNiMox high-entropy alloys. J. Mater. Sci. Technol. 2020, 46, 64–73. [Google Scholar] [CrossRef]
- He, R.; Yang, L.; Zhang, Y.; Jiang, D.C.; Lee, S.H.; Horta, S.; Liang, Z.F.; Lu, X.; Moghaddam, A.O.; Li, J.S.; et al. A 3d-4d-5d high entropy alloy as a bifunctional oxygen catalyst for robust aqueous Zinc–Air batteries. Adv. Mater. 2023, 35, 2303719. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Thomas, S.; Gibson, M.A.; Fraser, H.L.; Pohl, K.; Birbilis, N. Microstructure and corrosion properties of the low-density single-phase compositionally complex alloy AlTiVCr. Corros. Sci. 2018, 133, 386–396. [Google Scholar] [CrossRef]
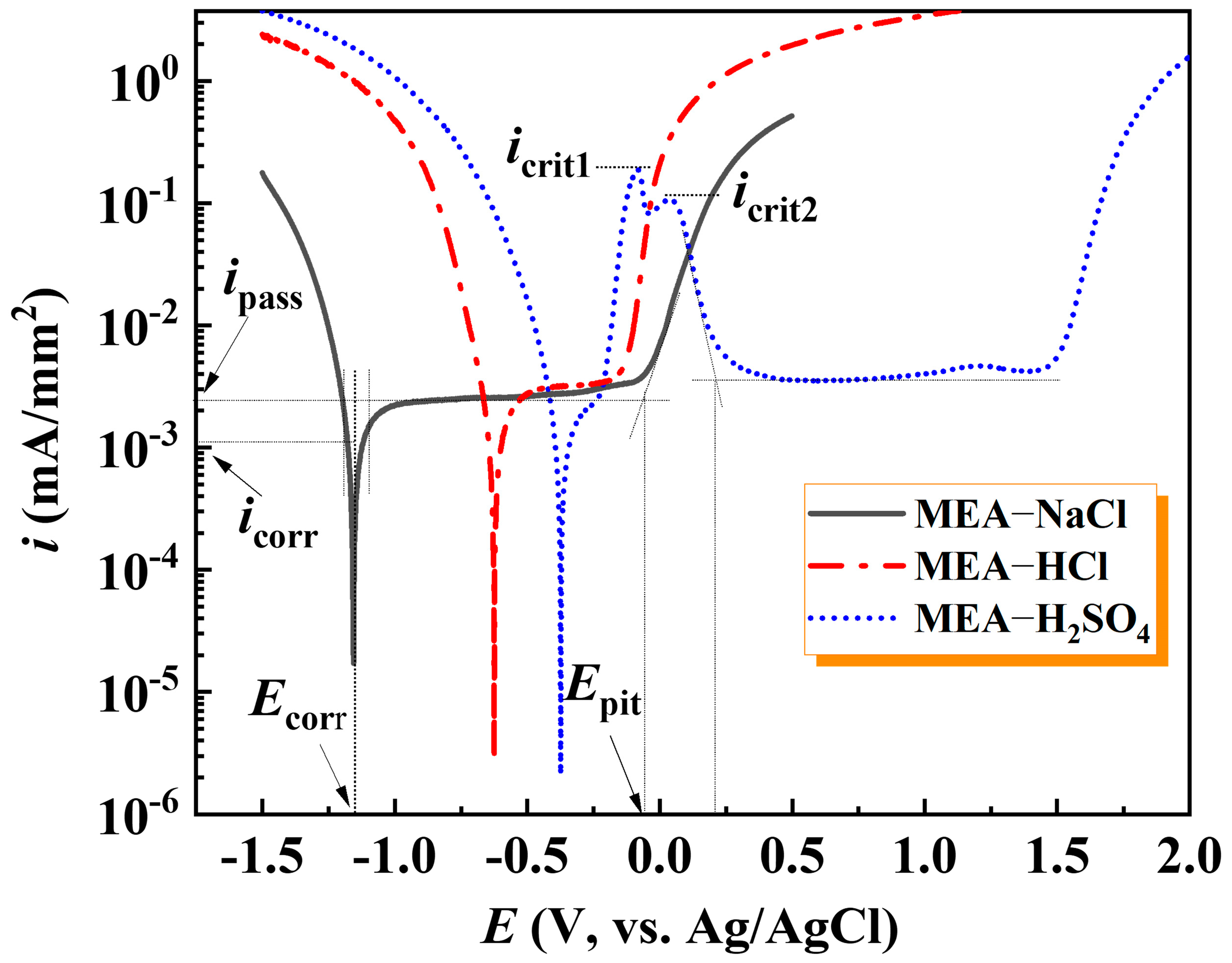
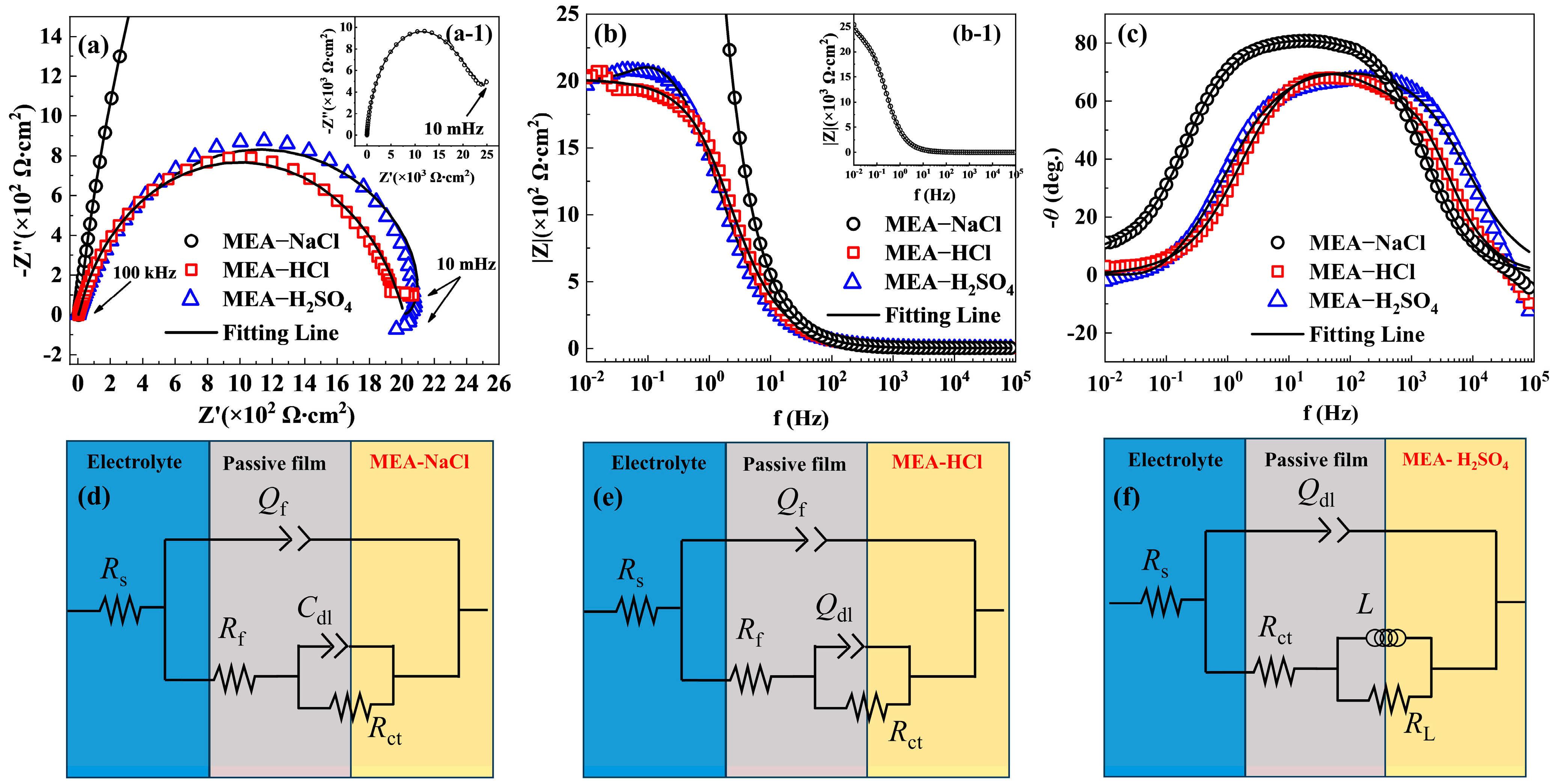

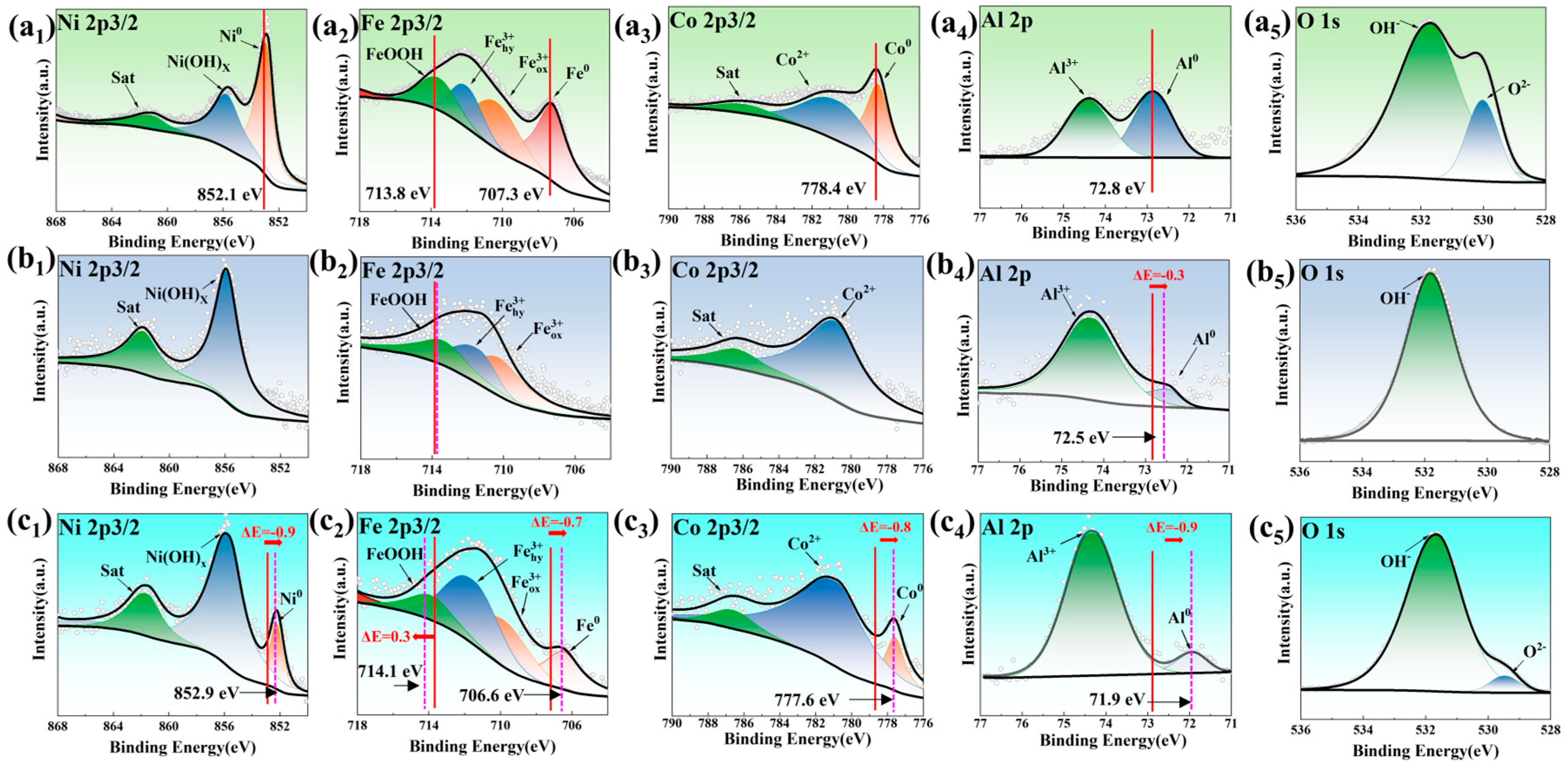
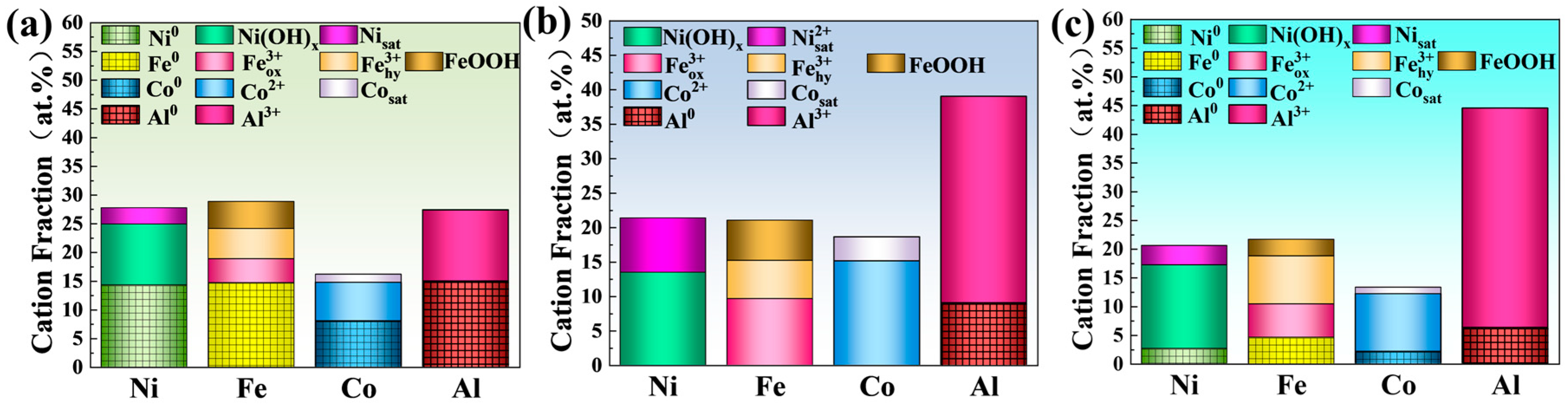
| Samples | Ecorr (V, vs. Ag/AgCl) | Epit (V, vs. Ag/AgCl) | ∆E (V, vs. Ag/AgCl) | icorr × 10−4 (mA/mm2) | ipass × 10−3 (mA/mm2) |
|---|---|---|---|---|---|
| MEA-NaCl | −1.16 | −0.07 | 1.09 | 13.99 | 2.50 |
| MEA-HCl | −0.58 | −0.15 | 0.43 | 14.73 | 2.98 |
| MEA-H2SO4 | −0.37 | 1.50 | 1.87 | 15.35 | 3.84 |
| Samples | Rs (Ω cm2) | Qf × 10−5 (Ω−1 cm−2 Sn) | n1 | Rf (Ω cm2) | Rct (Ω cm2) | Qdl × 10−6 (Ω−1 cm−2 Sn) | n2 | L (H) | RL (Ω cm2) | Cdl × 10−3 (F cm−2) | d (nm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MEA-NaCl | 5.03 | 1.88 | 0.90 | 21,940 | 5051 | - | - | - | - | 1.92 | 2.93 |
| MEA-HCl | 3.83 | 1.35 | 0.95 | 38.59 | 1972 | 4.33 | 0.77 | - | - | - | 2.37 |
| MEA-H2SO4 | 1.06 | - | - | - | 2026 | 11.1 | 0.81 | 604 | 249.7 | - | 1.58 |
| Regions | Ni | Fe | Co | Al | O | Cl |
|---|---|---|---|---|---|---|
| Nominal Component (at.%) | 40 | 30 | 20 | 10 | - | - |
| I | 34.17 | 24.32 | 14.01 | 11.69 | 15.81 | - |
| II | 18.89 | 14.84 | 12.03 | 3.24 | 39.88 | 11.12 |
| III | 25.58 | 21.31 | 14.71 | 5.27 | 27.97 | 5.16 |
| IV | 33.63 | 28.20 | 18.29 | 7.37 | 12.51 | - |
| Samples | Ni (mg/L) | Fe (mg/L) | Co (mg/L) | Al (mg/L) | ||||
|---|---|---|---|---|---|---|---|---|
| Passive Region | Transpassive Region | Passive Region | Transpassive Region | Passive Region | Transpassive Region | Passive Region | Transpassive Region | |
| MEA-NaCl | 0.77 | 53.23 | 0.26 | 48.44 | 0.71 | 47.55 | 2.50 | 13.85 |
| MEA-HCl | 3.95 | 22.80 | 6.10 | 25.68 | 3.48 | 19.98 | 57.70 | 19.31 |
| MEA-H2SO4 | 2.37 | 112.98 | 3.57 | 119.16 | 2.27 | 100.57 | 24.60 | 65.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ye, S.; Min, Q.; Li, C.; Li, D.; Cao, B.; Ma, W.; Zhao, K.; Wang, Y.; Zhang, Z. Corrosion Behavior and Surface Characterization of Medium-Entropy Alloy Under Different Media Conditions. Materials 2025, 18, 977. https://doi.org/10.3390/ma18050977
Zhang Y, Ye S, Min Q, Li C, Li D, Cao B, Ma W, Zhao K, Wang Y, Zhang Z. Corrosion Behavior and Surface Characterization of Medium-Entropy Alloy Under Different Media Conditions. Materials. 2025; 18(5):977. https://doi.org/10.3390/ma18050977
Chicago/Turabian StyleZhang, Yingjie, Shuyang Ye, Qifan Min, Changlong Li, Delong Li, Bosheng Cao, Wensheng Ma, Kaimin Zhao, Yan Wang, and Zhonghua Zhang. 2025. "Corrosion Behavior and Surface Characterization of Medium-Entropy Alloy Under Different Media Conditions" Materials 18, no. 5: 977. https://doi.org/10.3390/ma18050977
APA StyleZhang, Y., Ye, S., Min, Q., Li, C., Li, D., Cao, B., Ma, W., Zhao, K., Wang, Y., & Zhang, Z. (2025). Corrosion Behavior and Surface Characterization of Medium-Entropy Alloy Under Different Media Conditions. Materials, 18(5), 977. https://doi.org/10.3390/ma18050977







