Construction of Antibacterial MoS2-ACF Phenotype Switcher for Bidirectionally Regulating Inflammation–Proliferation Transition in Wound Healing
Highlights
- MoS2 nanoparticles were anchored onto ACF fiber to form MAPS, which promotes electron transfer;
- Efficient electron transfer improved the photothermal and antioxidant properties of MAPS;
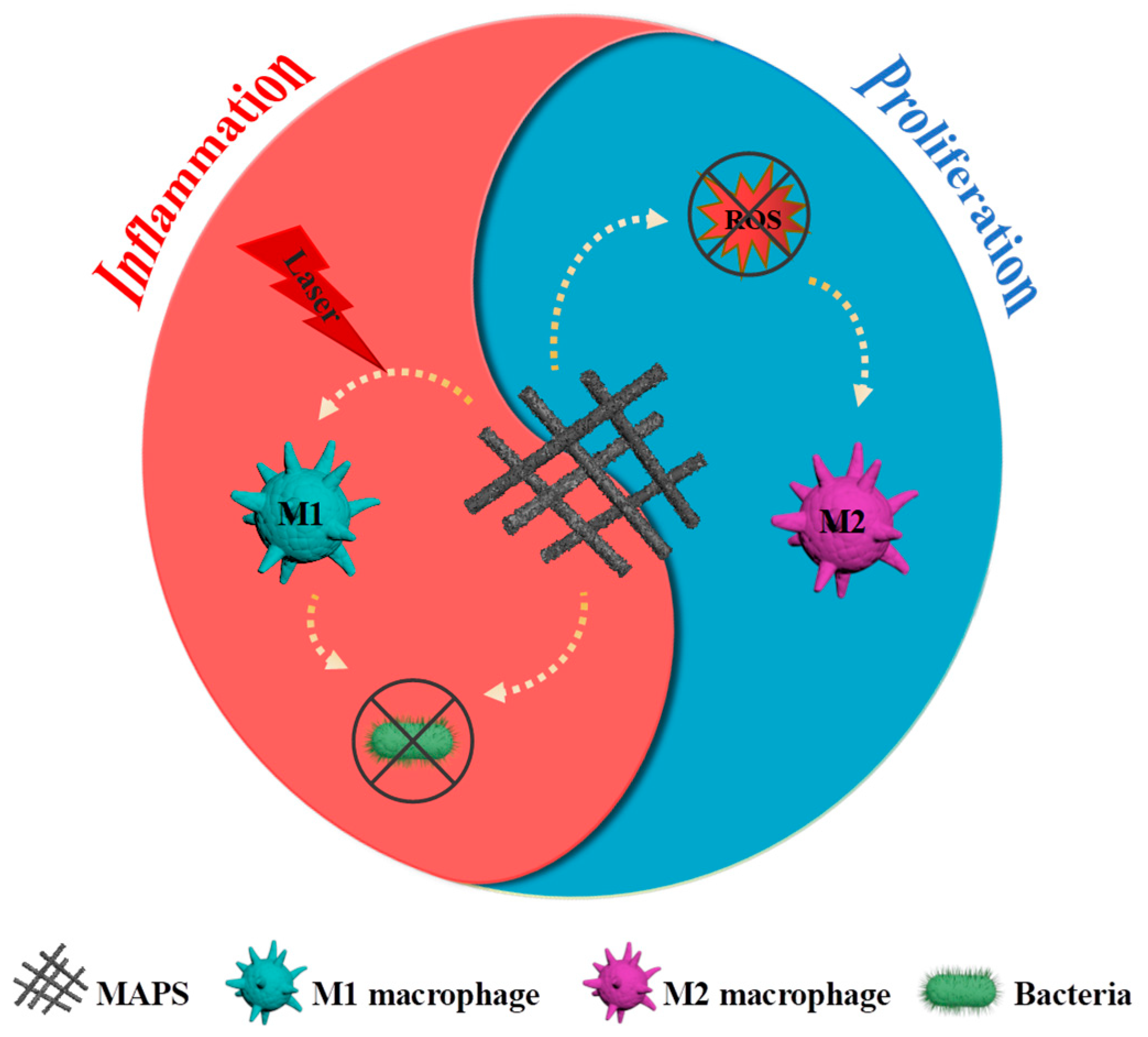
- MAPS reprogramed macrophages from M2 to M1 under an NIR laser due to enhanced photothermal property;
- MAPS reprogramed macrophages from M1 to M2 due to enhanced antioxidant property;
- MAPS could accelerate wound healing through promoting inflammation and proliferation phases.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Characterization of Materials
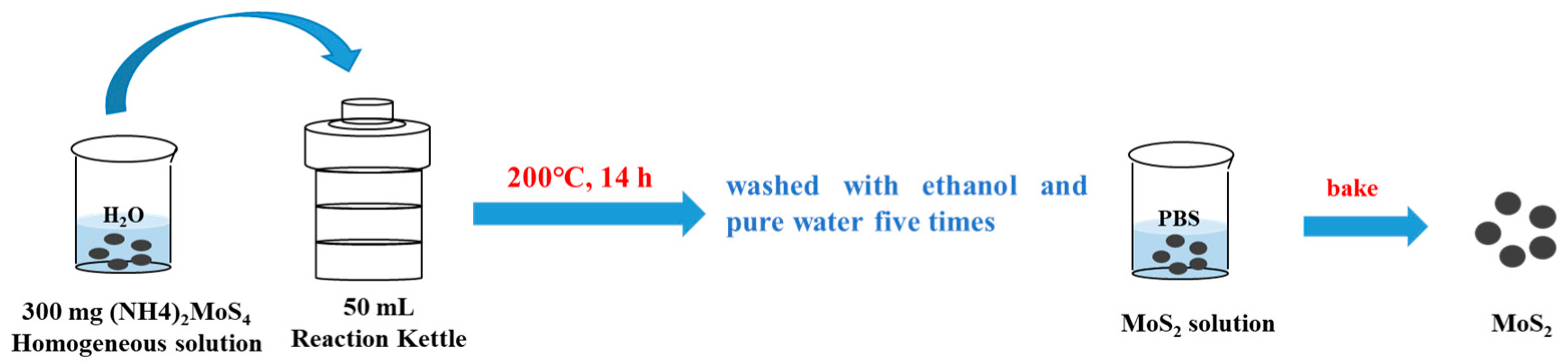
2.3. Preparation of MoS2 Particles
2.4. Preparation of MAPS
2.5. Photothermal Performance Evaluation
2.6. SOD and CAT Enzyme Activity Evaluation
2.7. Cell Culture
2.8. Cell Viability Assay Assessed by MTT
2.9. In Vitro Effect of MAPS on Macrophage Phenotype Modulation with NIR Laser Irradiation
2.10. In Vitro Assessment of Cytokines Produced by Macrophages
2.11. In Vitro Antibacterial Activity Assessment
2.12. Cell Proliferation Assessment
2.13. In Vivo Wound Healing Effect
2.14. Statistical Analysis
3. Results
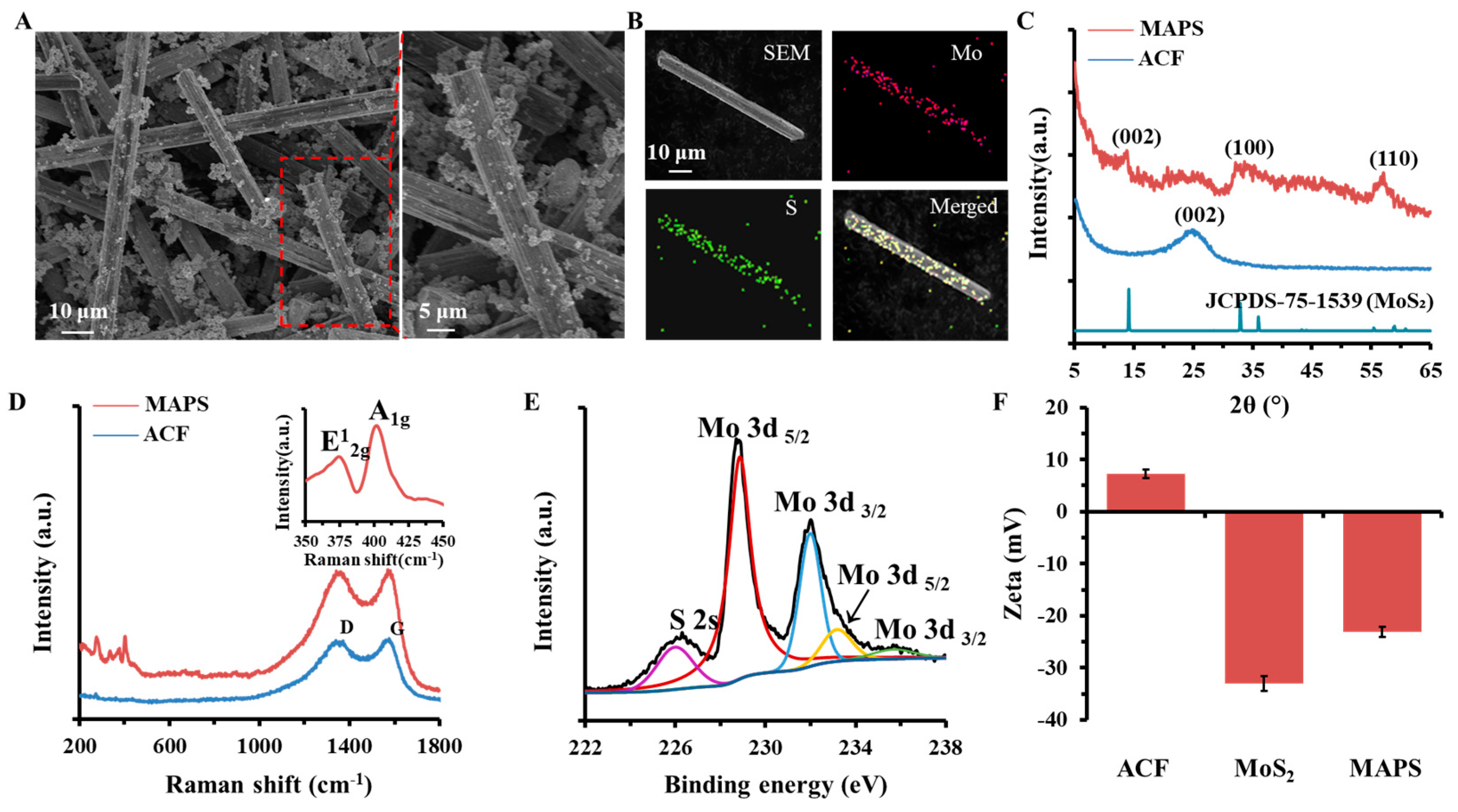
3.1. Physicochemical Structure Characterization of MAPS
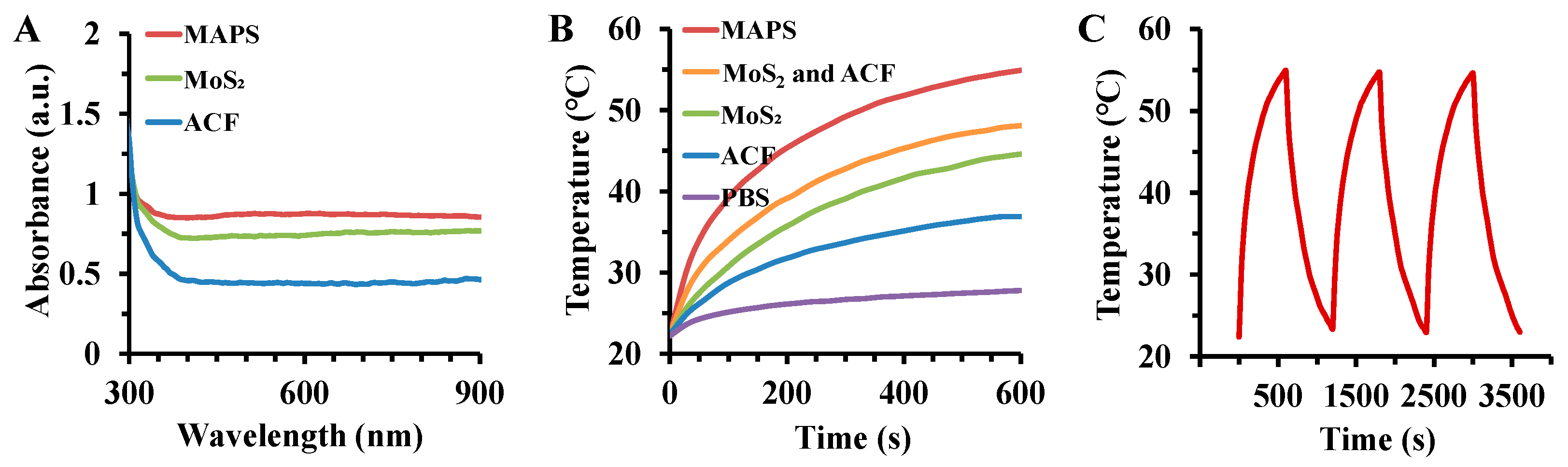
3.2. Photothermal Performance of MAPS
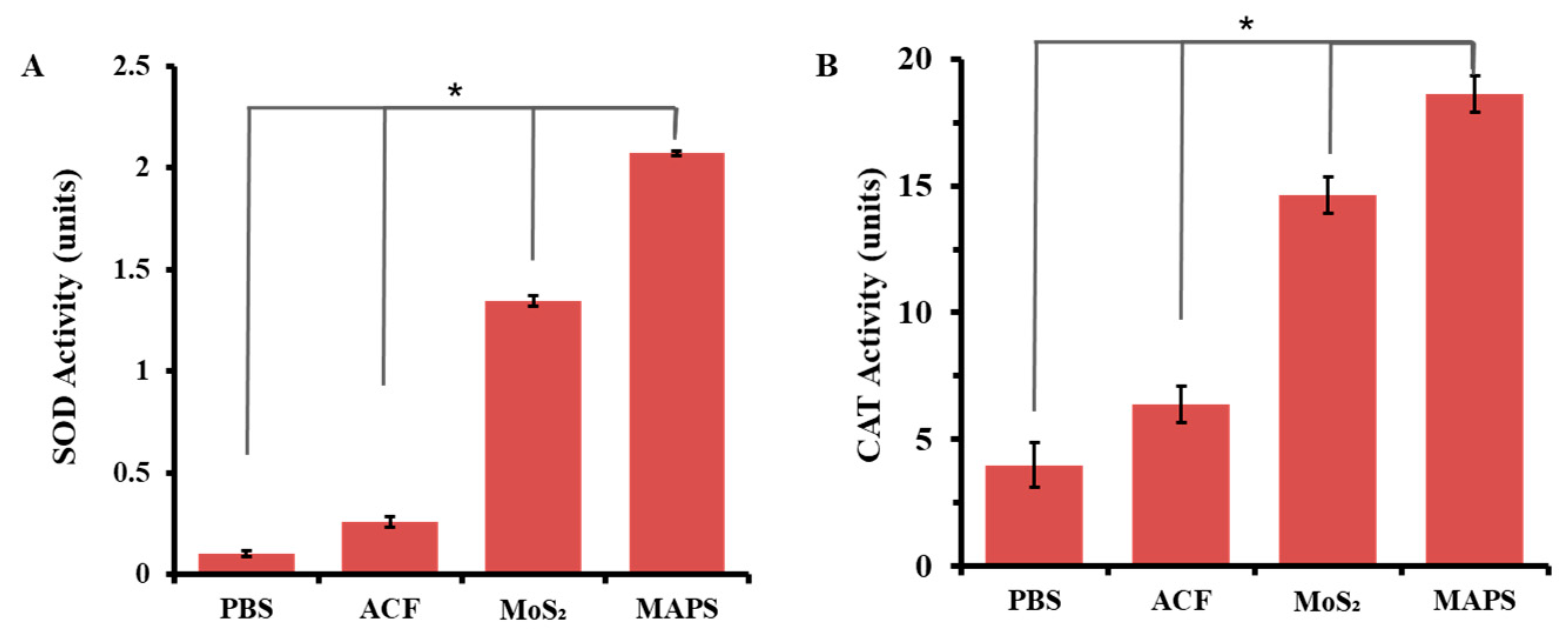
3.3. Antioxidant Enzyme-Mimicking Activity of MAPS
3.4. In Vitro Promotion of Inflammatory Processing by MAPS-Treated Macrophages with NIR Laser Irradiation
3.5. In Vitro Modulation of Wound Healing Phase Transition by MAPS
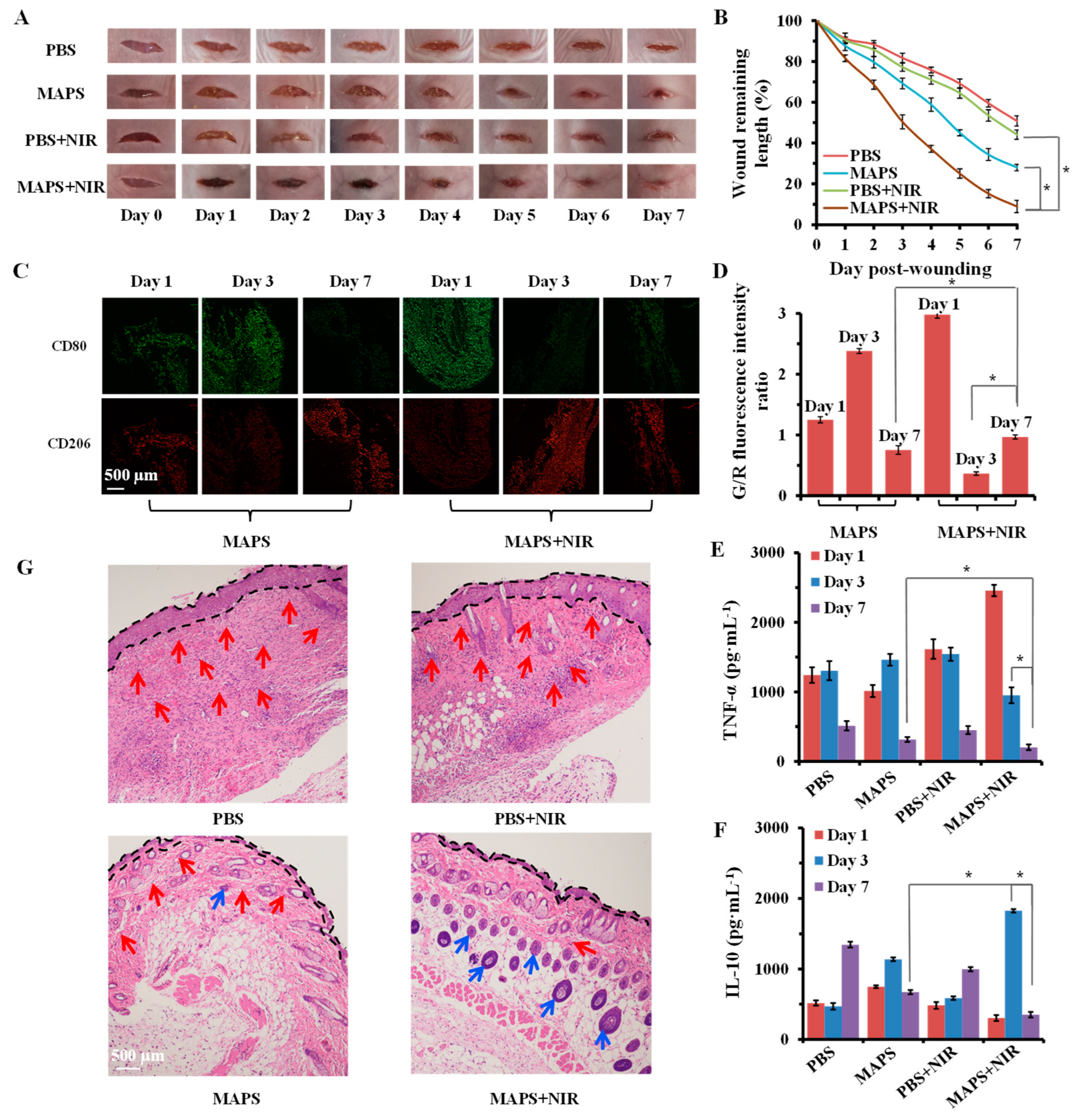
3.6. In Vivo Wound Healing by MAPS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef]
- Landen, N.X.; Li, D.; Stahle, M. Transition From Inflammation to Proliferation: A Critical Step During Wound Healing. Cell Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef] [PubMed]
- Ridiandries, A.; Bursill, C.; Tan, J. Broad-Spectrum Inhibition of the CC-Chemokine Class Improves Wound Healing and Wound Angiogenesis. Int. J. Mol. Sci. 2017, 18, 155. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.E.; Garcia, A.J. Macrophage Phenotypes in Tissue Repair and the Foreign Body Response: Implications for Biomaterial-Based Regenerative Medicine Strategies. Acta Biomater. 2021, 133, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, F.; Ju, Y.; Hong, J.; Ding, Y. Gold Nanomaterial Engineering for Macrophage-Mediated Inflammation and Tumor Treatment. Adv. Healthc. Mater. 2021, 10, e2000818. [Google Scholar] [CrossRef]
- Mustoe, T.A.; O’Shaughnessy, K.; Kloeters, O. Chronic Wound Pathogenesis and Current Treatment Strategies: A Uunifying Hypothesis. Plast. Reconstr. Surg. 2006, 117, 35S–41S. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.P.; Kirsner, R.S. Angiogenesis in Wound Repair: Angiogenic Growth Factors and the Extracellular Matrix. Microsc. Res. Tech. 2003, 60, 107–114. [Google Scholar] [CrossRef]
- Li, D.; Zhang, M.; Xu, F.; Chen, Y.; Chen, B.; Chang, Y.; Zhong, H.; Jin, H.; Huang, Y. Biomimetic Albumin-Modified Gold Nanorods for Photothermo-Chemotherapy and Macrophage Polarization Modulation. Acta Pharm. Sin. B 2018, 8, 74–84. [Google Scholar] [CrossRef]
- Im, N.R.; Yang, T.D.; Park, K.; Lee, J.H.; Lee, J.; Hyuck Kim, Y.; Lee, J.S.; Kim, B.; Jung, K.Y.; Choi, Y.; et al. Application of M1 Macrophage As a Live Vector in Delivering Nanoparticles for in vivo Photothermal Treatment. J. Adv. Res. 2021, 31, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wang, S.Y.; Kwak, G.; Yang, Y.; Kwon, I.C.; Kim, S.H. Exosome-Guided Phenotypic Switch of M1 to M2 Macrophages for Cutaneous Wound Healing. Adv. Sci. 2019, 6, 1900513. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, X.; Li, X.; Li, W.; Li, C.; Zhou, Y.; Wang, L.; Dong, B. A Versatile Nanocomposite Based on Nanoceria for Antibacterial Enhancement and Protection from aPDT-aggravated Inflammation via Modulation of Macrophage Polarization. Biomaterials 2021, 268, 120614. [Google Scholar] [CrossRef]
- Xiao, S.; Lu, Y.; Feng, M.; Dong, M.; Cao, Z.; Zhang, X.; Chen, Y.; Liu, J. Multifunctional FeS2 Theranostic Nanoparticles for Photothermal-Enhanced Chemodynamic/Photodynamic Cancer Therapy and Photoacoustic Imaging. Chem. Eng. J. 2020, 396, 125294. [Google Scholar] [CrossRef]
- Leng, F.; Liu, Y.; Li, G.; Lai, W.; Zhang, Q.; Liu, W.; Hu, C.; Li, P.; Sheng, F.; Huang, J.; et al. Cu2−xSe Nanoparticles (Cu2−xSe NPs) Mediated Neurotoxicity via Oxidative Stress Damage in PC-12 Cells and BALB/c mice. RSC Adv. 2019, 9, 36558–36569. [Google Scholar] [CrossRef]
- Wang, J.; Chang, Y.; Luo, H.; Jiang, W.; Xu, L.; Chen, T.; Zhu, X. Designing Immunogenic Nanotherapeutics for Photothermal-Triggered Immunotherapy Involving Reprogramming Immunosuppression and Activating Systemic Antitumor Responses. Biomaterials 2020, 255, 120153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, W.; Mohammadniaei, M.; Zheng, T.; Zhang, Q.; Ashley, J.; Liu, S.; Sun, Y.; Tang, B.Z. Upregulating Aggregation-Induced-Emission Nanoparticles with Blood-Tumor-Barrier Permeability for Precise Photothermal Eradication of Brain Tumors and Induction of Local Immune Responses. Adv. Mater. 2021, 33, e2008802. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Qin, Y.; Liu, Y.; Liu, T.; Liu, C.; Han, T.; Chen, Y.; Ma, C.; Li, X. Fever-Inducible Lipid Nanocomposite for Boosting Cancer Therapy through Synergistic Engineering of a Tumor Microenvironment. ACS Appl. Mater. Interfaces 2020, 12, 32301–32311. [Google Scholar] [CrossRef]
- Tan, H.Y.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The Reactive Oxygen Species in Macrophage Polarization: Reflecting Its Dual Role in Progression and Treatment of Human Diseases. Oxid. Med. Cell Longev. 2016, 2016, 2795090. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Wan, Y.; Qi, M.; Chen, Q.; Sun, Y.; Sun, X.; Fang, J.; Fu, L.; Xu, L.; et al. Quercetin-Loaded Ceria Nanocomposite Potentiate Dual-Directional Immunoregulation via Macrophage Polarization against Periodontal Inflammation. Small 2021, 17, e2101505. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, L.; Wang, Z.; Liu, P.; Liu, X.; Ding, J.; Zhou, W. Targeted Silver Nanoparticles for Rheumatoid Arthritis Therapy via Macrophage Apoptosis and Re-polarization. Biomaterials 2021, 264, 120390. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, Z.; Luo, Y.; Ning, T.; Liu, P.; Chen, Q.; Chu, Y.; Guo, Q.; Zhang, Y.; Zhou, W.; et al. Macrophage-Disguised Manganese Dioxide Nanoparticles for Neuroprotection by Reducing Oxidative Stress and Modulating Inflammatory Microenvironment in Acute Ischemic Stroke. Adv. Sci. 2021, 8, e2101526. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.Y.; Song, S.Y.; Go, S.H.; Sohn, H.S.; Baik, S.; Soh, M.; Kim, K.; Kim, D.; Kim, H.C.; et al. Synergistic Oxygen Generation and Reactive Oxygen Species Scavenging by Manganese Ferrite/Ceria Co-decorated Nanoparticles for Rheumatoid Arthritis Treatment. ACS Nano 2019, 13, 3206–3217. [Google Scholar] [CrossRef] [PubMed]
- Magazzù, A.; Marcuello, C. Investigation of Soft Matter Nanomechanics by Atomic Force Microscopy and Optical Tweezers: A Comprehensive Review. Nanomaterials 2023, 13, 963. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Tsuji, T. Mechanical and Immunological Regulation in Wound Healing and Skin Reconstruction. Int. J. Mol. Sci. 2021, 22, 5474. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Yu, M.; Hartono, S.B.; Yang, J.; Xu, H.; Zhang, H.; Zhang, J.; Zou, J.; Dexter, A.; Gu, W.; et al. Nanoparticles Mimicking Viral Surface Topography for Enhanced Cellular Delivery. Adv. Mater. 2013, 25, 6233–6237. [Google Scholar] [CrossRef]
- Song, H.; Ahmad Nor, Y.; Yu, M.; Yang, Y.; Zhang, J.; Zhang, H.; Xu, C.; Mitter, N.; Yu, C. Silica Nanopollens Enhance Adhesion for Long-Term Bacterial Inhibition. J. Am. Chem. Soc. 2016, 138, 6455–6462. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Cheng, Y.; Feng, Y.; Jian, H.; Wang, L.; Ma, X.; Li, X.; Zhang, H. Resonance Energy Transfer-Promoted Photothermal and Photodynamic Performance of Gold-Copper Sulfide Yolk-Shell Nanoparticles for Chemophototherapy of Cancer. Nano Lett. 2018, 18, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chang, Y.; Feng, Y.; Li, X.; Cheng, Y.; Jian, H.; Ma, X.; Zheng, R.; Wu, X.; Xu, K.; et al. Nitric Oxide Stimulated Programmable Drug Release of Nanosystem for Multidrug Resistance Cancer Therapy. Nano Lett. 2019, 19, 6800–6811. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Yeh, C.L.; Lin, J.H.; Yang, J.S.; Ko, T.H.; Lin, Y.H. Development of Fibroblast Culture in Three-Dimensional Activated Carbon Fiber-Based Scaffold for Wound Healing. J. Mater. Sci. Mater. Med. 2012, 23, 1465–1478. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Lin, J.H.; Wang, S.H.; Ko, T.H.; Tseng, G.C. Evaluation of Silver-Containing Activated Carbon Fiber for Wound Healing Study: In vitro and in vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Yu, J.; Lv, F.; Yan, L.; Zheng, L.R.; Gu, Z.; Zhao, Y. Functionalized Nano-MoS2 with Peroxidase Catalytic and Near-Infrared Photothermal Activities for Safe and Synergetic Wound Antibacterial Applications. ACS Nano 2016, 10, 11000–11011. [Google Scholar] [CrossRef] [PubMed]
- Berthet, M.; Gauthier, Y.; Lacroix, C.; Verrier, B.; Monge, C. Nanoparticle-Based Dressing: The Future of Wound Treatment? Trends Biotechnol. 2017, 35, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Yang, R. Two-Dimensional Nanomaterials with Enzyme-Like Properties for Biomedical Applications. Front. Chem. 2020, 8, 565940. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Xin, S.; Liang, H.W.; Song, L.T.; Yu, S.H. Carbon Nanofibers Decorated with Molybdenum Disulfide Nanosheets: Synergistic Lithium Storage and Enhanced Electrochemical Performance. Angew. Chem. Int. Ed. Engl. 2014, 53, 11552–11556. [Google Scholar] [CrossRef]
- GB/T 32993-2016; Determination of Volume Resistivity of Carbon Fibre. National Standard Committee on Glass Fiber Standardization: Beijing, China, 2016.
- Wang, S.; Li, K.; Chen, Y.; Chen, H.; Ma, M.; Feng, J.; Zhao, Q.; Shi, J. Biocompatible PEGylated MoS2 Nanosheets: Controllable Bottom-up Synthesis and Highly Efficient Photothermal Regression of Tumor. Biomaterials 2015, 39, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chatti, M.; Ge, Y.; Wang, C.; Chao, Y.; Simonov, A.N.; Wallace, G.G. Binder-Free Electrodes Derived from Interlayer-Expanded MoS2 Nanosheets on Carbon Cloth with a 3D Porous Structure for Lithium Storage. ChemElectroChem 2019, 6, 2338–2343. [Google Scholar] [CrossRef]
- Xian, Z.; Zhang, L.; Yu, Y.; Lin, B.; Wang, Y.; Guo, M.; Cao, Y. Nanozyme Based on CoFe2O4 Modified with MoS2 for Colorimetric Determination of Cysteine and Glutathione. Mikrochim. Acta 2021, 188, 65. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, S.; Wang, X.; Cai, C.; Chen, G.; Ma, L. Nitroxide-Modified Protein-Incorporated Nanoflowers with Dual Enzyme-Like Activities. Int. J. Nanomed. 2020, 15, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Ouyang, Z.; Tan, Y.; Wu, H.; Liu, Y. Activating Macrophages for Enhanced Osteogenic and Bactericidal Performance by Cu ion Release from Micro/Nano-topographical Coating on a Titanium Substrate. Acta Biomater. 2019, 100, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Cheng, Y.; Jian, H.; Feng, Y.; Chang, Y.; Zheng, R.; Wu, X.; Wang, L.; Li, X.; Zhang, H. Hollow, Rough, and Nitric Oxide-Releasing Cerium Oxide Nanoparticles for Promoting Multiple Stages of Wound Healing. Adv. Healthc. Mater. 2019, 8, e1900256. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Han, X.; Zheng, R.; Cheng, H.; Yan, J.; Wu, X.; Hu, Y.; Li, B.; Wang, Z.; Li, X.; et al. Neutrophil Mediated Postoperative Photoimmunotherapy against Melanoma Skin Cancer. Nanoscale 2021, 13, 14825–14836. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Dai, K.; Yu, Y.; Wang, J.; Liu, C. Sulfated Chitosan Rescues Dysfunctional Macrophages and Accelerates Wound Healing in Diabetic Mice. Acta Biomater. 2020, 117, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, H.; Ji, H.; Zhang, K.; Wang, D. Featuring Surface Sodium Storage Properties of Confined MoS2/Bacterial Cellulose-Derived Carbon Nanofibers Anode. Appl. Surf. Sci. 2020, 530, 147261. [Google Scholar] [CrossRef]
- Cc, S.; Anusri, A.; Levna, C.; Pm, A.; Lekha, D. MoS2 Nanoparticles Induce Behavioral Alteration and Oxidative Stress Mediated Cellular Toxicity in the Social Insect Oecophylla Smaragdina (Asian weaver ant). J. Hazard. Mater. 2020, 385, 121624. [Google Scholar] [CrossRef]
- Li, Y.; Fu, R.; Duan, Z.; Zhu, C.; Fan, D. Adaptive Hydrogels Based on Nanozyme with Dual-Enhanced Triple Enzyme-Like Activities for Wound Disinfection and Mimicking Antioxidant Defense System. Adv. Healthc. Mater. 2021, 2021, e2101849. [Google Scholar] [CrossRef]
- Zhao, C.; Yu, C.; Zhang, M.; Sun, Q.; Li, S.; Norouzi Banis, M.; Han, X.; Dong, Q.; Yang, J.; Wang, G.; et al. Enhanced Sodium Storage Capability Enabled by Super Wide-Interlayer-Spacing MoS2 Integrated on Carbon Fibers. Nano Energy 2017, 41, 66–74. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, R.; Jiao, W.; Ding, G.; Hao, L.; Yang, F.; He, X. MoS2 Graphene Fiber Based Gas Sensing Devices. Carbon 2015, 95, 34–41. [Google Scholar] [CrossRef]
- Feito, M.J.; Diez-Orejas, R.; Cicuendez, M.; Casarrubios, L.; Rojo, J.M.; Portoles, M.T. Characterization of M1 and M2 Polarization Phenotypes in Peritoneal Macrophages after Treatment with Graphene Oxide Nanosheets. Colloids Surf. B Biointerfaces 2019, 176, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Lescoat, A.; Ballerie, A.; Jouneau, S.; Fardel, O.; Vernhet, L.; Jego, P.; Lecureur, V. M1/M2 Polarisation State of M-CSF Blood-Derived Macrophages in Systemic Sclerosis. Ann. Rheum. Dis. 2019, 78, e127. [Google Scholar] [CrossRef]
- Park, J.; Pramanick, S.; Kim, J.; Lee, J.; Kim, W.J. Nitric oxide-Activatable Gold Nanoparticles for Specific Targeting and Photo-thermal Ablation of Macrophages. Chem Commun. 2017, 53, 11229–11232. [Google Scholar] [CrossRef]
- Ran, X.; Du, Y.; Wang, Z.; Wang, H.; Pu, F.; Ren, J.; Qu, X. Hyaluronic Acid-Templated Ag Nanoparticles/Graphene Oxide Composites for Synergistic Therapy of Bacteria Infection. ACS Appl. Mater. Interfaces 2017, 9, 19717–19724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shi, S.; Wang, Y.; Yu, S.; Zhu, W.; Zhang, X.; Zhang, D.; Yang, B.; Wang, X.; Wang, J. Versatile Molybdenum Disulfide Based Antibacterial Composites for in vitro Enhanced Sterilization and in vivo Focal Infection Therapy. Nanoscale 2016, 8, 11642–11648. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Liu, X.; Cui, Z.; Chen, D.F.; Li, Z.; Liang, Y.; Zhu, S.; Wu, S. The Rapid Photoresponsive Bacteria-Killing of Cu-doped MoS2. Biomater. Sci. 2020, 8, 4216–4224. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, M.; Li, D.; Wu, Y.; Li, B.; Han, X.; Yan, J.; Shang, L.; Zhang, H.; Li, X. Construction of Antibacterial MoS2-ACF Phenotype Switcher for Bidirectionally Regulating Inflammation–Proliferation Transition in Wound Healing. Materials 2025, 18, 963. https://doi.org/10.3390/ma18050963
Mao M, Li D, Wu Y, Li B, Han X, Yan J, Shang L, Zhang H, Li X. Construction of Antibacterial MoS2-ACF Phenotype Switcher for Bidirectionally Regulating Inflammation–Proliferation Transition in Wound Healing. Materials. 2025; 18(5):963. https://doi.org/10.3390/ma18050963
Chicago/Turabian StyleMao, Mengxin, Diyi Li, Yunyun Wu, Bing Li, Xiaoqing Han, Jiao Yan, Lei Shang, Haiyuan Zhang, and Xi Li. 2025. "Construction of Antibacterial MoS2-ACF Phenotype Switcher for Bidirectionally Regulating Inflammation–Proliferation Transition in Wound Healing" Materials 18, no. 5: 963. https://doi.org/10.3390/ma18050963
APA StyleMao, M., Li, D., Wu, Y., Li, B., Han, X., Yan, J., Shang, L., Zhang, H., & Li, X. (2025). Construction of Antibacterial MoS2-ACF Phenotype Switcher for Bidirectionally Regulating Inflammation–Proliferation Transition in Wound Healing. Materials, 18(5), 963. https://doi.org/10.3390/ma18050963






