Effect of Tribocorrosion on Mechanical Behavior of Titanium Dental Implants: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Implantoplasty Procedure
2.2. Bacterial Culture
2.3. Corrosion Assays
2.4. Fatigue Tests
2.5. Statistical Analysis
3. Results
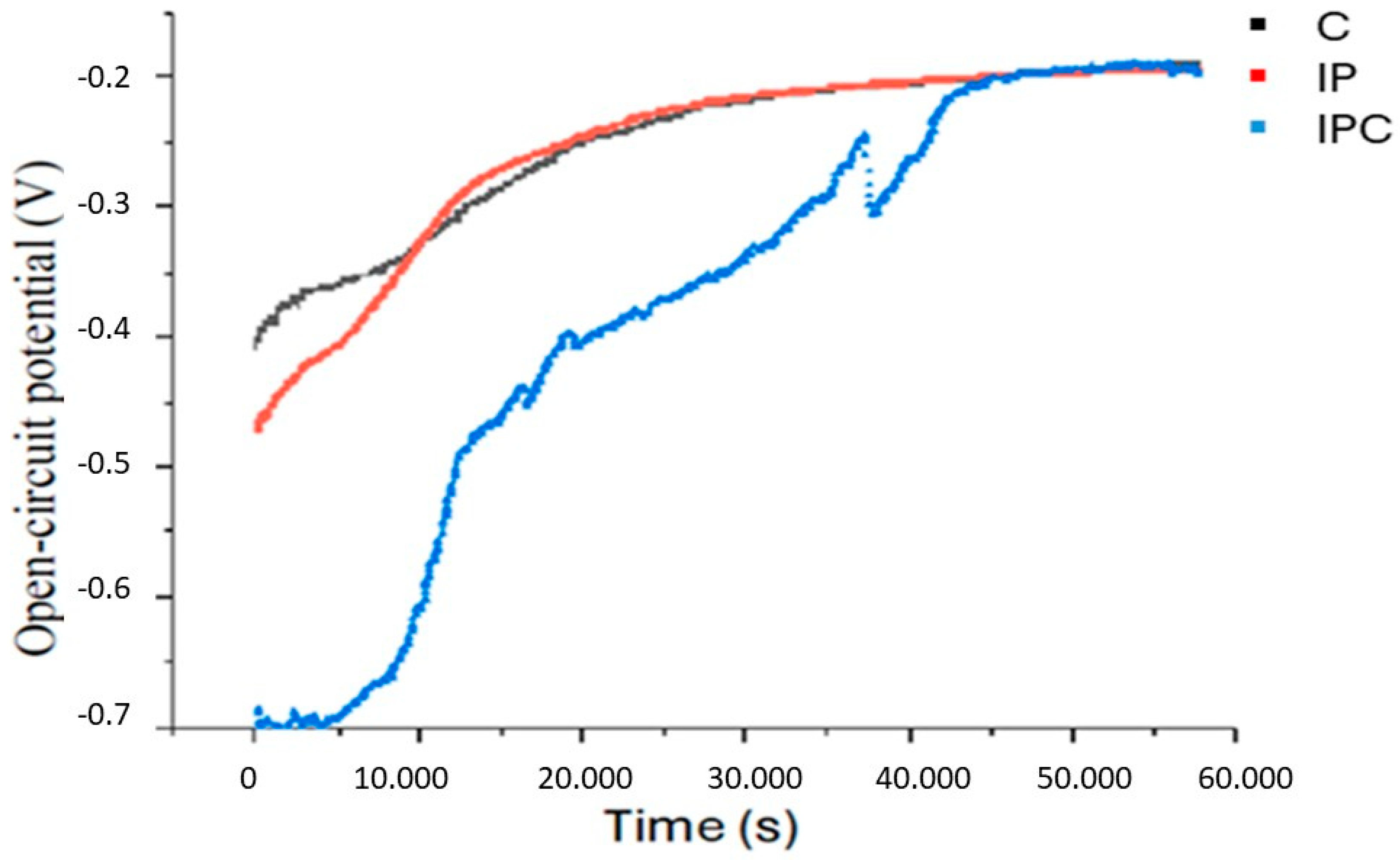
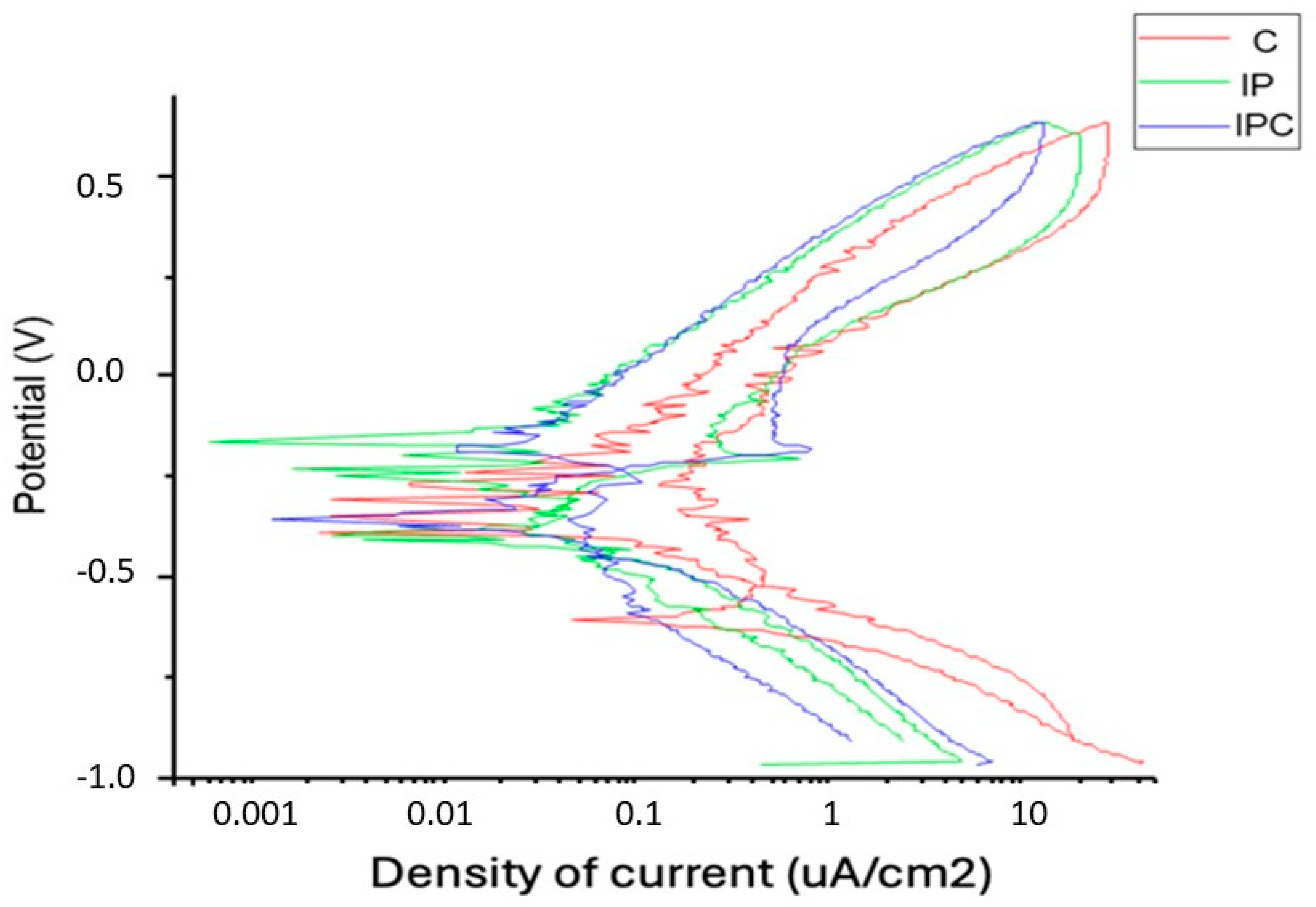
3.1. Corrosion Assays
3.2. Fatigue Tests
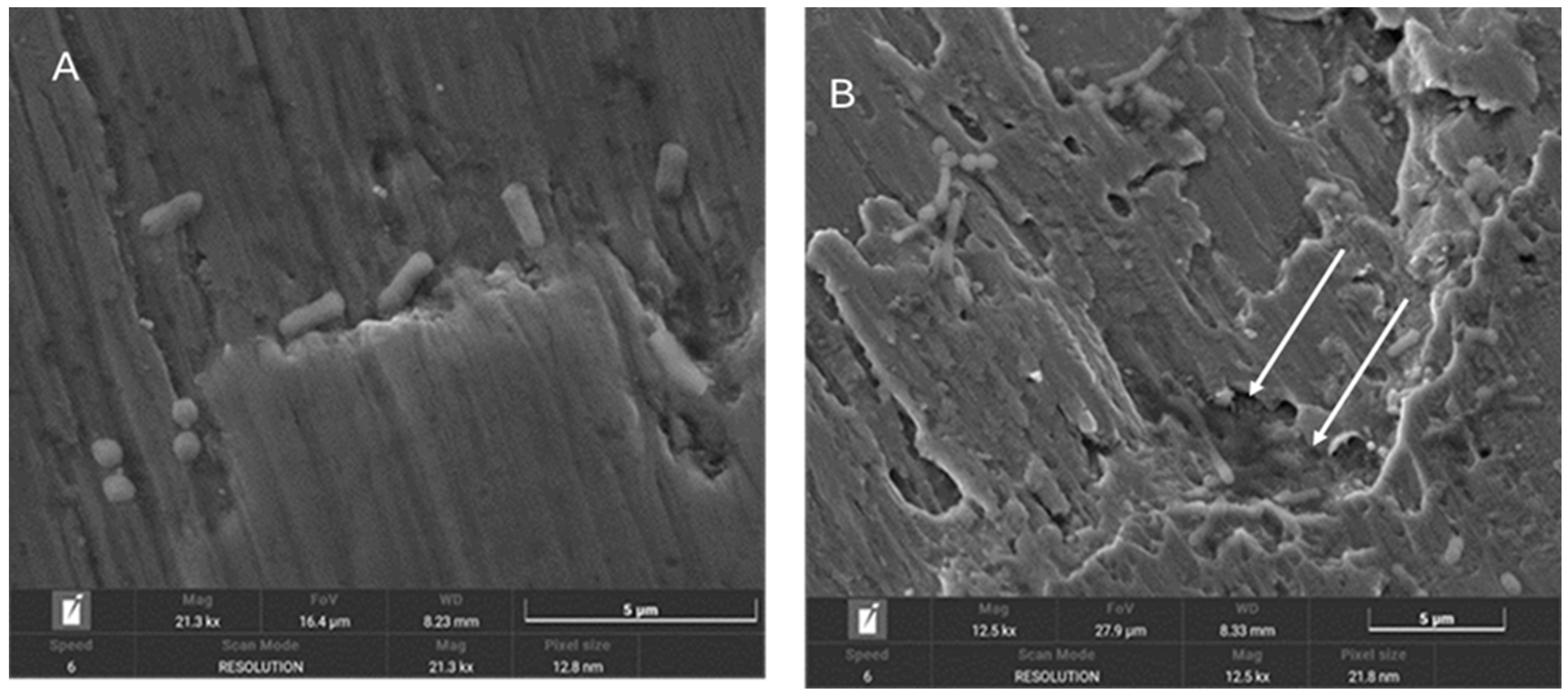
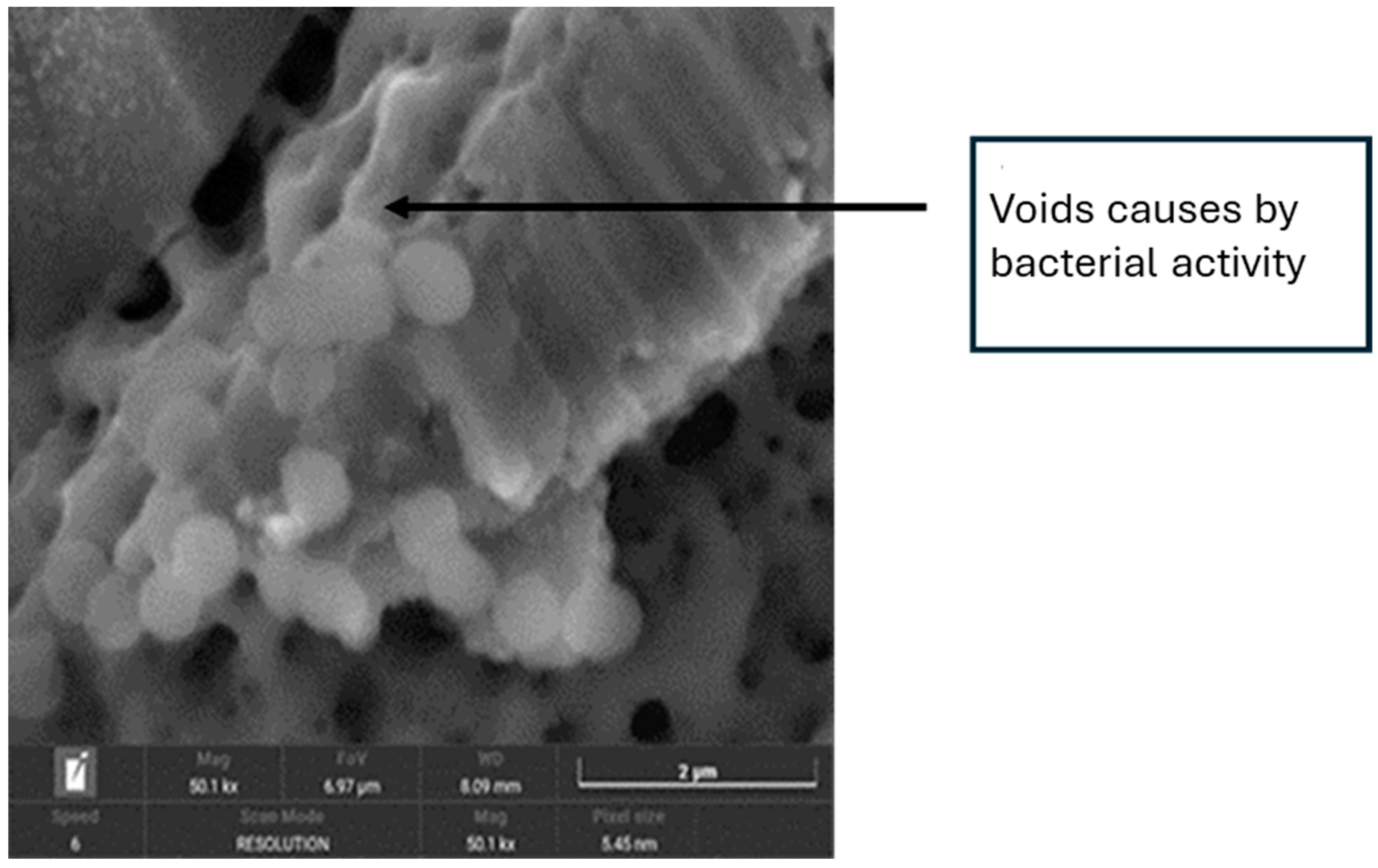
3.3. SEM Images Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berbel, L.O.; Banczek, E.P.; Karousis, I.K.; Kotsakis, G.A.; Costa, I. Determinants of corrosion resistance of Ti-6Al-4V alloy dental implants in an In Vitro model of peri-implant inflammation. PLoS ONE 2019, 14, e0210530. [Google Scholar]
- Gaur, S.; Agnihotri, R.; Albin, S. Bio-Tribocorrosion of titanium dental implants and its toxicological implications: A scoping review. Sci. World J. 2022, 2022, 4498613. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.N.Q.; Dotta, T.C.; Galo, R.; Soares, M.E.C.; Pedrazzi, V. Effect of tribocorrosion on surface-treated titanium alloy implants: A systematic review with meta-analysis. J. Mech. Behav. Biomed. Mater. 2023, 145, 106008. [Google Scholar] [CrossRef]
- Gil, F.J.; Espinar, E.; Llamas, J.M.; Sevilla, P. Fatigue life of bioactive titanium dental implants treated by means of grit-blasting and thermo-chemical treatment. Clin. Implant. Dent. Relat. Res. 2014, 16, 273–281. [Google Scholar] [CrossRef]
- Gil, F.J.; Herrero-Climent, M.; Lázaro, P.; Rios, J.V. Implant-abutment connections: Influence of the design on the microgap and their fatigue and fracture behavior of dental implants. J. Mater. Sci. Mater. Med. 2014, 25, 1825–1830. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.A.; Gargallo, J.; Altuna, P.; Herrero-Climent, M.; Gil, F.J. Fatigue of narrow dental implants: Influence of the hardening method. Materials 2020, 13, 1429. [Google Scholar] [CrossRef] [PubMed]
- Lozano, P.; Peña, M.; Herrero-Climent, M.; Rios-Santos, J.V.; Rios-Carrasco, B.; Brizuela, A.; Gil, J. Corrosion behavior of titanium dental implants with implantoplasty. Materials 2022, 15, 1563. [Google Scholar] [CrossRef]
- Rodrigues, D.C.; Valderrama, P.; Wilson, T.G.; Palmer, K.; Thomas, A.; Sridhar, S.; Adapalli, A.; Burbano, M.; Wadhwani, C. Titanium corrosion mechanisms in the oral environment: A retrieval study. Materials 2013, 6, 5258–5274. [Google Scholar] [CrossRef]
- Gil, F.J.; Rodriguez, A.; Espinar, E.; Llamas, J.M.; Padullés, E.; Juárez, A. Effect of oral bacteria on the mechanical behavior of titanium dental implants. Int. J. Oral Maxillofac. Implants. 2012, 27, 64–68. [Google Scholar]
- Bürgers, R.; Gerlach, T.; Hahnel, S.; Schwarz, F.; Handel, G.; Gosau, M. In vivo and in vitro biofilm formation on two different titanium implant surfaces. Clin. Oral Implant. Res. 2010, 21, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Pozhitkov, A.E.; Daubert, D.; Donimirski, A.B.; Goodgion, D.; Vagin, M.Y.; Leroux, B.G.; Hunter, C.M.; Flemmig, T.F.; Noble, P.A.; Bryers, J.D. Interruption of electrical conductivity of titanium dental implants suggests a path towards elimination of corrosion. PLoS ONE 2015, 10, e140393. [Google Scholar] [CrossRef]
- Daubert, D.; Pozhitkov, A.; McLean, J.; Kotsakis, G. Titanium as a modifier of the peri-implant microbiome structure. Clin. Implant. Dent. Relat. Res. 2018, 20, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.C.M.; Ponthiaux, P.; Henriques, M.; Oliveira, R.; Teughels, W.; Celis, J.P.; Rocha, A.L. Corrosion behavior of titanium in the presence of Streptococcus mutans. J. Dent. 2013, 41, 528–534. [Google Scholar] [CrossRef]
- Ramanauskaite, A.; Schwarz, F. Current concepts for the treatment of peri-implant disease. Int. J. Prosthodont. 2023, 37, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; John, G.; Becker, J. The influence of implantoplasty on the diameter, chemical surface composition, and biocompatibility of titanium implants. Clin. Oral. Investig. 2017, 21, 2355–2361. [Google Scholar] [CrossRef] [PubMed]
- Burgueño-Barris, G.; Camps-Font, O.; Figueiredo, R.; Valmaseda-Castellón, E. The influence of implantoplasty on surface roughness, biofilm formation, and biocompatibility of titanium implants: A systematic review. Int. J. Oral Maxillofac. Implant. 2021, 36, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Ramel, C.F.; Lüssi, A.; Özcan, M.; Jung, R.E.; Hämmerle, C.H.F.; Thoma, D.S. Surface roughness of dental implants and treatment time using six different implantoplasty procedures. Clin. Oral Implant. Res. 2016, 27, 776–781. [Google Scholar] [CrossRef]
- Sivolella, S.; Brunello, G.; Michelon, F.; Concheri, G.; Graiff, L.; Meneghello, R. Implantoplasty: Carbide burs vs dia-mond sonic tips. An in vitro study. Clin. Oral Implant. Res. 2021, 32, 324–336. [Google Scholar] [CrossRef]
- Camps-Font, O.; Toledano-Serrabona, J.; Juiz-Camps, A.; Gil, J.; Sánchez-Garcés, M.A.; Figueiredo, R.; Gay-Escoda, C.; Valmaseda-Castellón, E. Effect of implantoplasty on roughness, fatigue and corrosion behavior of narrow diameter dental implants. J. Funct. Biomater. 2023, 14, 61. [Google Scholar] [CrossRef]
- Stavropoulos, A.; Bertl, K.; Eren, S.; Gotfredsen, K. Mechanical and biological complications after implantoplasty—A systematic review. Clin. Oral Implant. Res. 2019, 30, 833–848. [Google Scholar] [CrossRef]
- Stavropoulos, A.; Bertl, K.; Isidor, F.; von Steyern, P.V. Implantoplasty and the risk of fracture of narrow implants with advanced bone loss: A laboratory study. Clin. Oral Implant. Res. 2023, 34, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Costa-Berenguer, X.; García-García, M.; Sánchez-Torres, A.; Sanz-Alonso, M.; Figueiredo, R.; Valmaseda-Castellón, E. Effect of implantoplasty on fracture resistance and surface roughness of standard diameter dental implants. Clin. Oral Implant. Res. 2018, 29, 46–54. [Google Scholar] [CrossRef]
- Toledano-Serrabona, J.; Sánchez-Garcés, M.A.; Gay-Escoda, C.; Valmaseda-Castellón, E.; Camps-Font, O.; Verdeguer, P.; Molmeneu, M.; Gil, F.J. Mechanical properties and corrosion behavior of Ti6Al4V particles obtained by implantoplasty: An in vitro study. Part II. Materials 2021, 14, 6519. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Serrabona, J.; Camps-Font, O.; Moraes, D.P.; Corte-Rodríguez, M.; Montes-Bayón, M.; Valmaseda-Castellón, E.; Gay-Escoda, C.; Sánchez-Garcés, M.Á. Ion release and local effects of titanium metal particles from dental implants: An experimental study in rats. J. Periodontol. 2023, 94, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Suárez-López del Amo, F.; Garaicoa-Pazmiño, C.; Fretwurst, T.; Castilho, R.M.; Squarize, C.H. Dental implants-associated release of titanium particles: A systematic review. Clin. Oral Implant. Res. 2018, 29, 1085–1100. [Google Scholar] [CrossRef]
- Fonseca, D.; de Tapia, B.; Pons, R.; Aparicio, C.; Guerra, F.; Messias, A.; Gil, J. The effect of implantoplasty on the fatigue behavior and corrosion resistance in titanium dental implants. Materials 2024, 17, 2944. [Google Scholar] [CrossRef]
- Chun Giok, K.; Menon, R.K. The microbiome of peri-implantitis: A systematic review of next-generation sequencing studies. Antibiotics 2023, 12, 1610. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Guillem-Marti, J.; Sevilla, P.; Manero, J.M.; Gil, F.J.; Rodriguez, D. Anhydride-functional silane immobilized onto titanium surfaces induces osteoblast cell differentiation and reduces bacterial adhesion and biofilm formation. Mater. Sci. Eng. C. Mater. Biol. Appl. 2016, 59, 524–532. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- ASTM G5-14e1; Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements. ASTM International: West Conshohocken, PA, USA, 2014.
- ISO 14801:2016; Dentistry–Implants–Dynamic Loading Test for Endosseous Dental Implants. ISO: Geneva, Switzerland, 2016.
- Sridhar, S.; Wang, F.; Wilson, T.G.; Valderrama, P.; Palmer, K.; Rodrigues, D.C. Multifaceted roles of environmental factors toward dental implant performance: Observations from clinical retrievals and in vitro testing. Dent. Mater. 2018, 34, e265–e279. [Google Scholar] [CrossRef]
- Safioti, L.M.; Kotsakis, G.A.; Pozhitkov, A.E.; Chung, W.O.; Daubert, D.M. Increased levels of dissolved titanium are associated with peri-implantitis—A cross-sectional study. J. Periodontol. 2017, 88, 436–442. [Google Scholar] [CrossRef]
- Hoyos-Nogués, M.; Velasco, F.; Ginebra, M.P.; Manero, J.M.; Gil, F.J.; Mas-Moruno, C. Regenerating bone via multifunctional coatings: The blending of cell integration and bacterial inhibition properties on the surface of biomaterials. ACS Appl. Mater. Interfaces 2017, 9, 21618–21630. [Google Scholar] [CrossRef] [PubMed]
- Yaegaki, K.; Sanada, K. Volatile sulfur compounds in mouth air from clinically healthy subjects and patients with perio-dontal disease. J. Periodontal. Res. 1992, 27, 233–238. [Google Scholar] [CrossRef]
- Al-Hity, R.R.; Kappert, H.F.; Viennot, S.; Dalard, F.; Grosgogeat, B. Corrosion resistance measurements of dental alloys, are they correlated? Dent. Mater. 2007, 23, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, C.; Gil, F.J.; Fonseca, C.; Barbosa, M.; Planell, J.A. Corrosion behaviour of commercially pure titanium shot blasted with different materials and sizes of shot particles for dental implant applications. Biomaterials 2003, 24, 263–273. [Google Scholar] [CrossRef]
- Fukushima, A.; Mayanagi, G.; Nakajo, K.; Sasaki, K.; Takahashi, N. Microbiologically induced corrosive properties of the titanium surface. RSC Adv. 2016, 6, 48283–48293. [Google Scholar] [CrossRef]
- Bertl, K.; Isidor, F.; von Steyern, P.V.; Stavropoulos, A. Does implantoplasty affect the failure strength of narrow and regular diameter implants? A laboratory study. Clin. Oral Investig. 2021, 25, 2203–2211. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Peng, X.; Zhou, X.; Li, M.; Ren, B.; Cheng, L. Influence of bio-aging on corrosion behavior of different implant materials. Clin. Implant. Dent. Relat. Res. 2019, 21, 1225–1234. [Google Scholar] [CrossRef]
- Rodrigues, D.C.; Sridhar, S.; Gindri, I.M.; Siddiqui, D.A.; Valderrama, P.; Wilson, T.G.; Chung, K.-H.; Wadhwani, C. Spectroscopic and microscopic investigation of the effects of bacteria on dental implant surfaces. RSC Adv. 2016, 6, 48283–48293. [Google Scholar] [CrossRef]
- Gil, F.J.; Planell, J.A.; Padrós, A.; Aparicio, C. The effect of shot blasting and heat treatment on the fatigue behavior of titanium for dental implant applications. Dent. Mater. 2007, 23, 486–491. [Google Scholar]
- Velasco, E.; Monsalve-Guil, L.; Jimenez, A.; Ortiz, I.; Moreno-Muñoz, J.; Nuñez-Marquez, E.; Pegueroles, M.; Pérez, R.A.; Gil, F.J. Importance of the roughness and residual stresses of dental implants on fatigue and osseointegration behavior. In vivo study in rabbits. J. Oral Impl. 2016, 42, 469–476. [Google Scholar] [CrossRef]
- Brizuela-Velasco, A.; Álvarez-Arenal, Á.; Gil-Mur, F.J.; Herrero-Climent, M.; Chávarri-Prado, D.; Chento-Valiente, Y.; Dieguez-Pereira, M. Relationship between insertion torque and resonance frequency measurements, performed by resonance frequency analysis, in micromobility of dental implants: An in vitro study. Implant. Dent. 2015, 24, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Tribst, J.P.M.; Piva, A.M.D.O.D.; Shibli, J.A.; Borges, A.L.S.; Tango, R.N. Influence of implantoplasty on stress distribution of exposed implants at different bone insertion levels. Braz. Oral Res. 2017, 31, e96. [Google Scholar] [CrossRef] [PubMed]




| Grupo C | Grupo IP | Grupo IPC | p-Value | |
|---|---|---|---|---|
| EOCP (mV) | −137.7 ± 5.2 | −194.3 ± 3.6 | −198.23 ± 5.7 | p < 0.001 |
| ICORR (μA/cm2) | 0.025 ± 0.011 | 0.089 ± 0.022 | 0.122 ± 0.030 | p < 0.001 |
| ECORR (mV) | −380 ± 18 | −450 ± 40 | −495 3 ± 54 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vegas-Bustamante, E.; Sanmartí-García, G.; Gil, J.; Delgado-Garoña, L.; Figueiredo, R.; Camps-Font, O.; Sánchez-Garcés, M.Á.; Toledano-Serrabona, J. Effect of Tribocorrosion on Mechanical Behavior of Titanium Dental Implants: An In Vitro Study. Materials 2025, 18, 1136. https://doi.org/10.3390/ma18051136
Vegas-Bustamante E, Sanmartí-García G, Gil J, Delgado-Garoña L, Figueiredo R, Camps-Font O, Sánchez-Garcés MÁ, Toledano-Serrabona J. Effect of Tribocorrosion on Mechanical Behavior of Titanium Dental Implants: An In Vitro Study. Materials. 2025; 18(5):1136. https://doi.org/10.3390/ma18051136
Chicago/Turabian StyleVegas-Bustamante, Erika, Gemma Sanmartí-García, Javier Gil, Luis Delgado-Garoña, Rui Figueiredo, Octavi Camps-Font, Mª Ángeles Sánchez-Garcés, and Jorge Toledano-Serrabona. 2025. "Effect of Tribocorrosion on Mechanical Behavior of Titanium Dental Implants: An In Vitro Study" Materials 18, no. 5: 1136. https://doi.org/10.3390/ma18051136
APA StyleVegas-Bustamante, E., Sanmartí-García, G., Gil, J., Delgado-Garoña, L., Figueiredo, R., Camps-Font, O., Sánchez-Garcés, M. Á., & Toledano-Serrabona, J. (2025). Effect of Tribocorrosion on Mechanical Behavior of Titanium Dental Implants: An In Vitro Study. Materials, 18(5), 1136. https://doi.org/10.3390/ma18051136







