Interplay Between Composition and Cycling Performance of Pre-Lithiated SiOx-Si-C Composite Anodes for Lithium–Sulfur Full Cells
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation and Electrochemical Characterization of the Electrodes
3. Results and Discussion
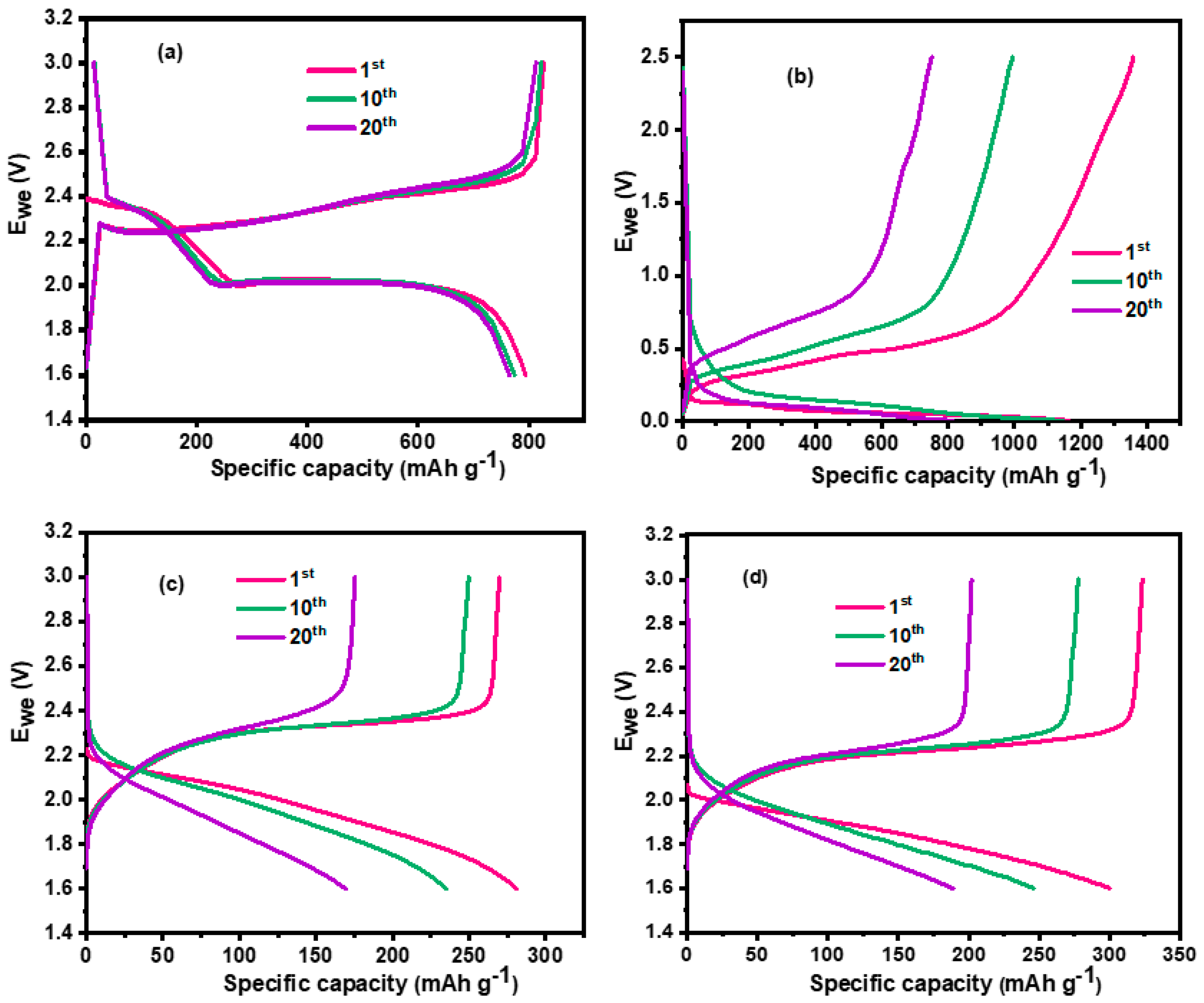
3.1. Charge–Discharge Studies
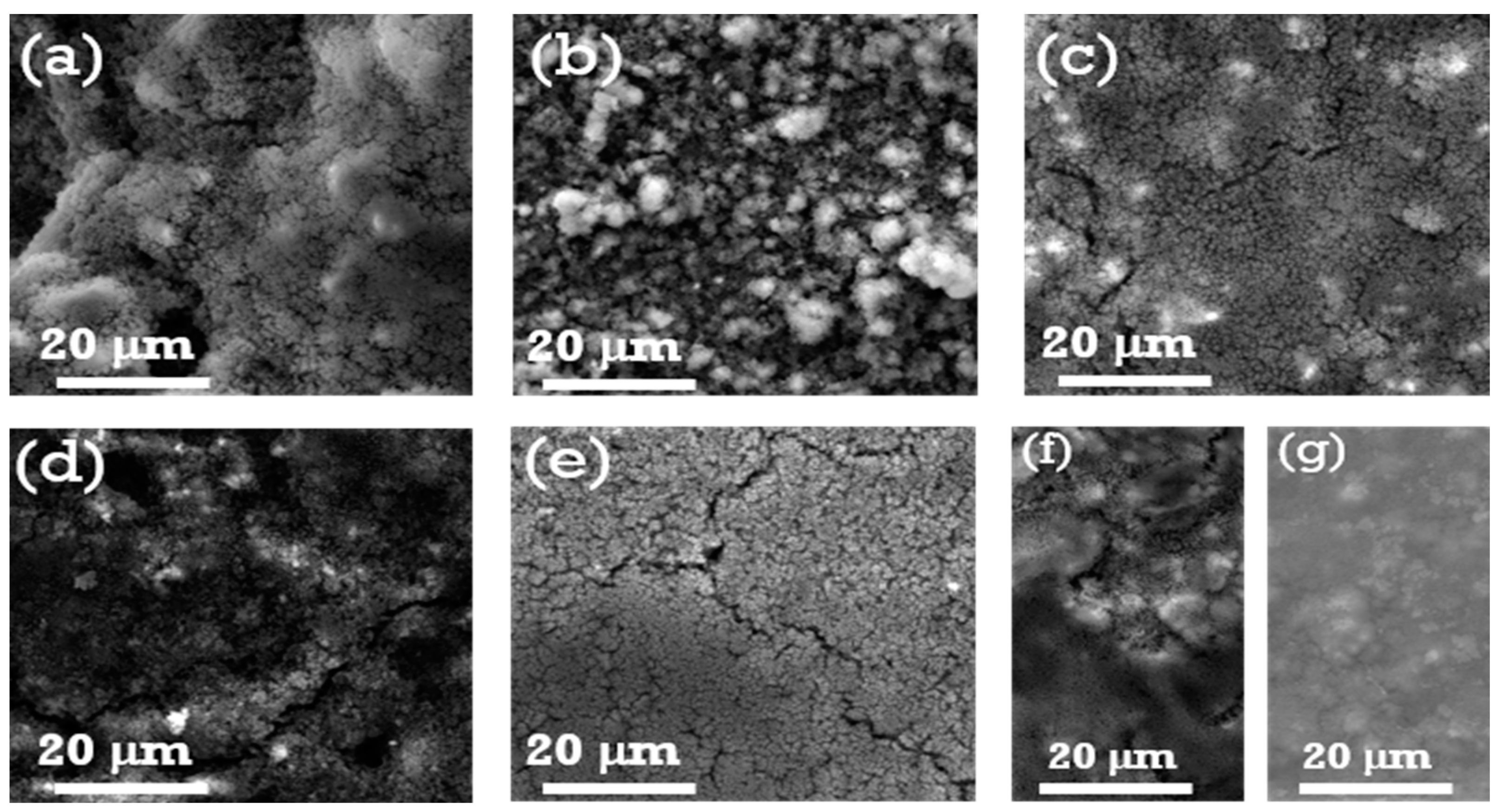
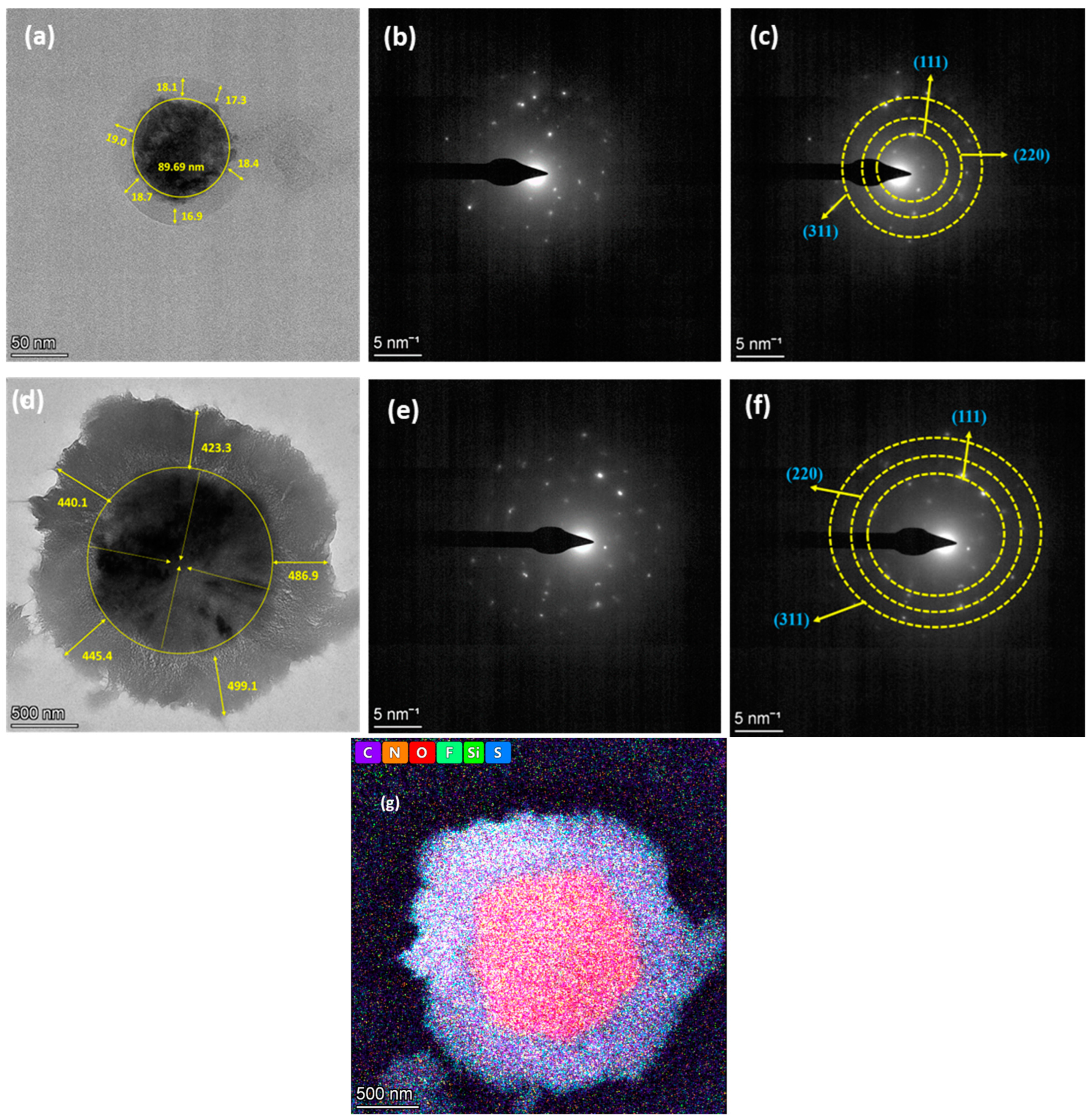
3.2. Morphological Characterization
3.3. Cyclic Voltammetry Studies
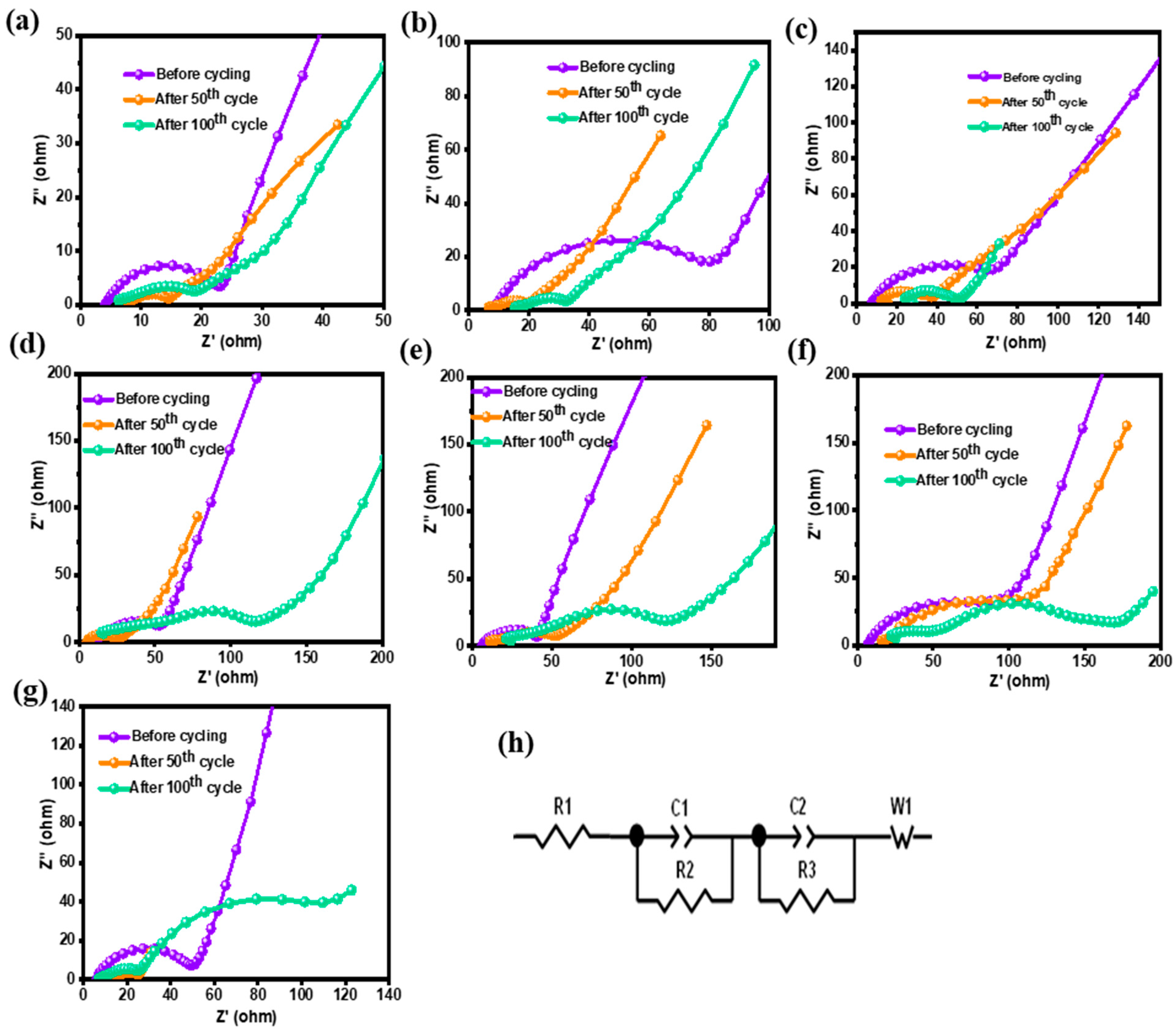
3.4. Impedance Analysis
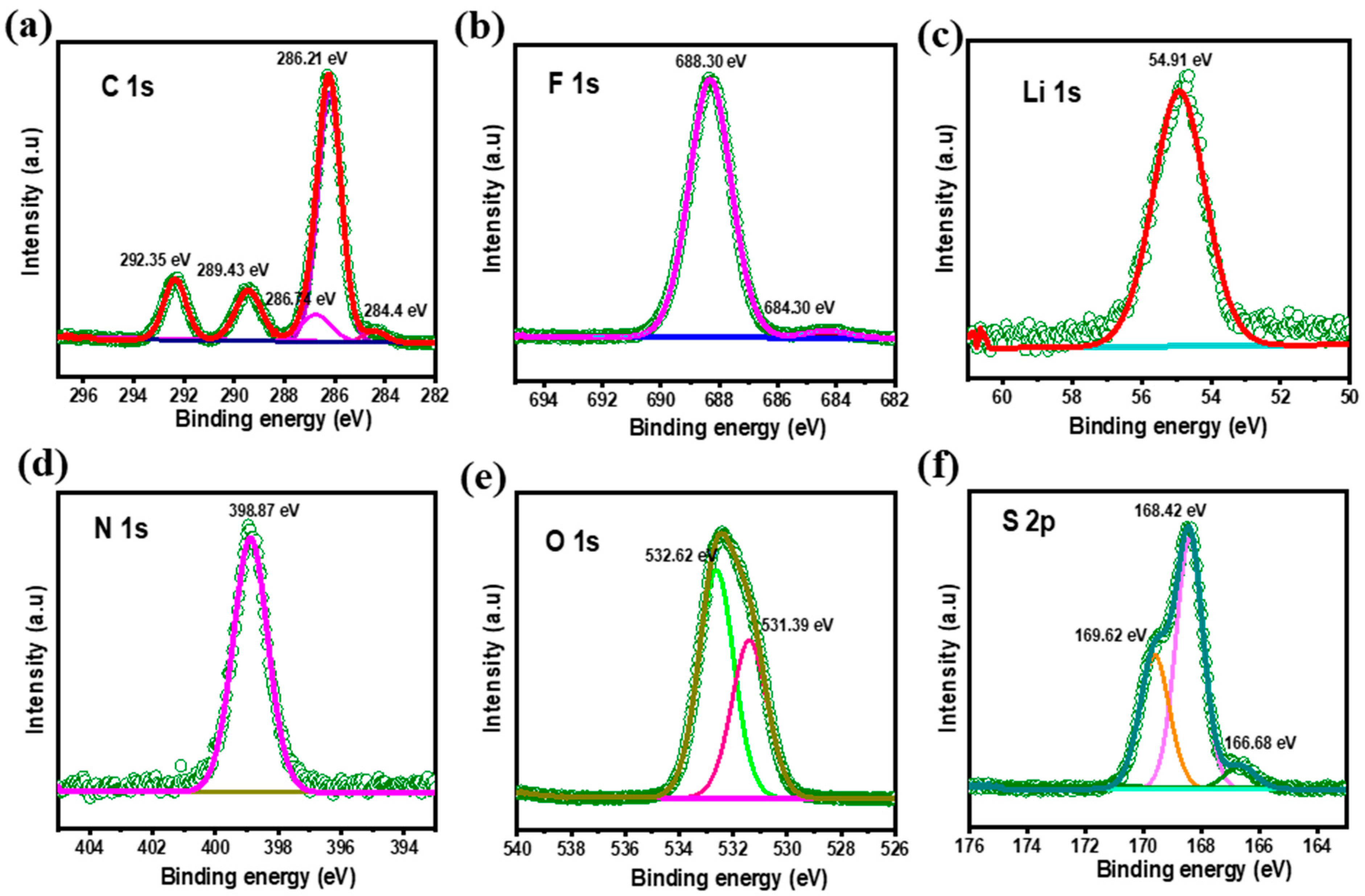
3.5. XPS Studies
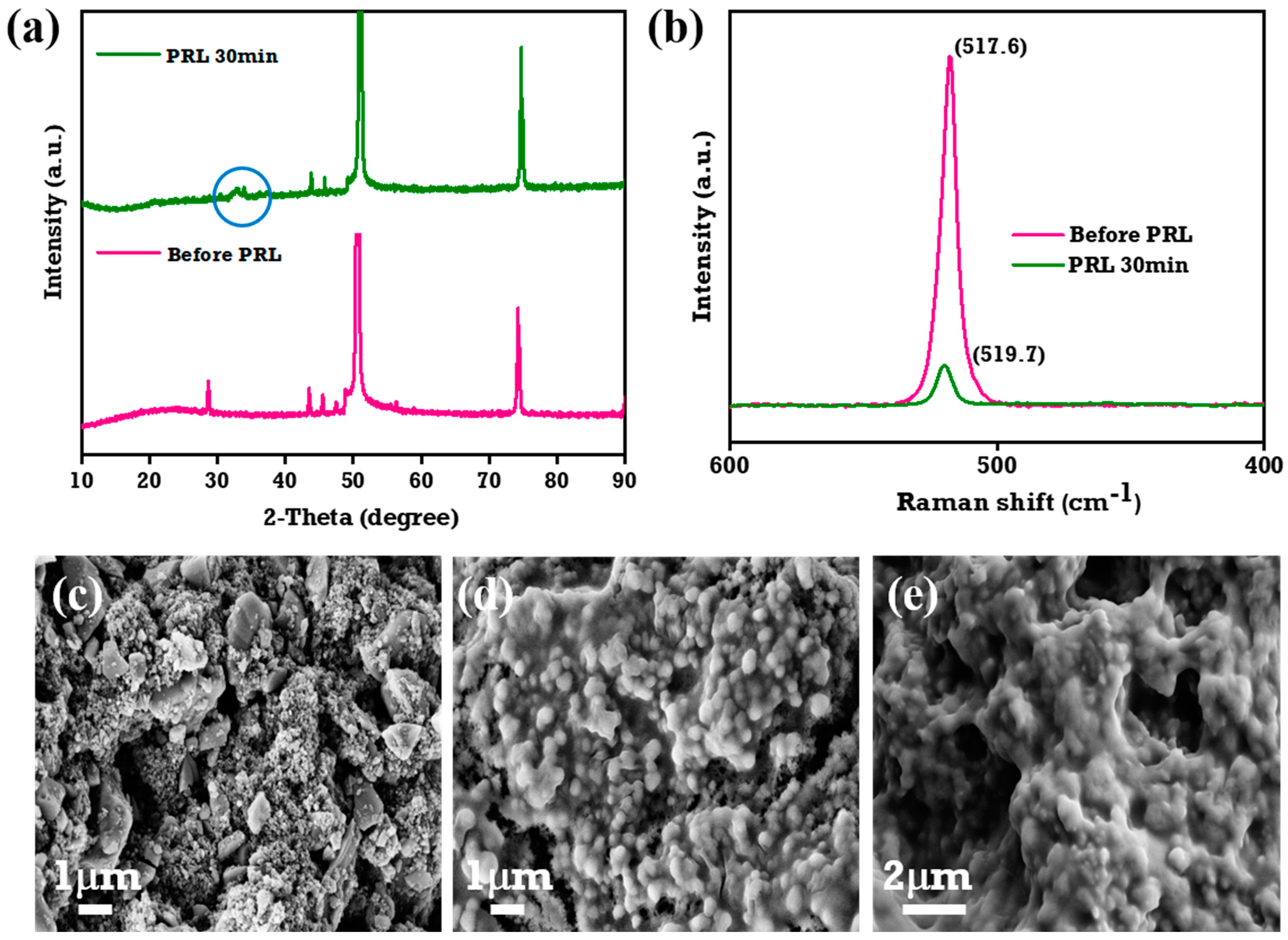
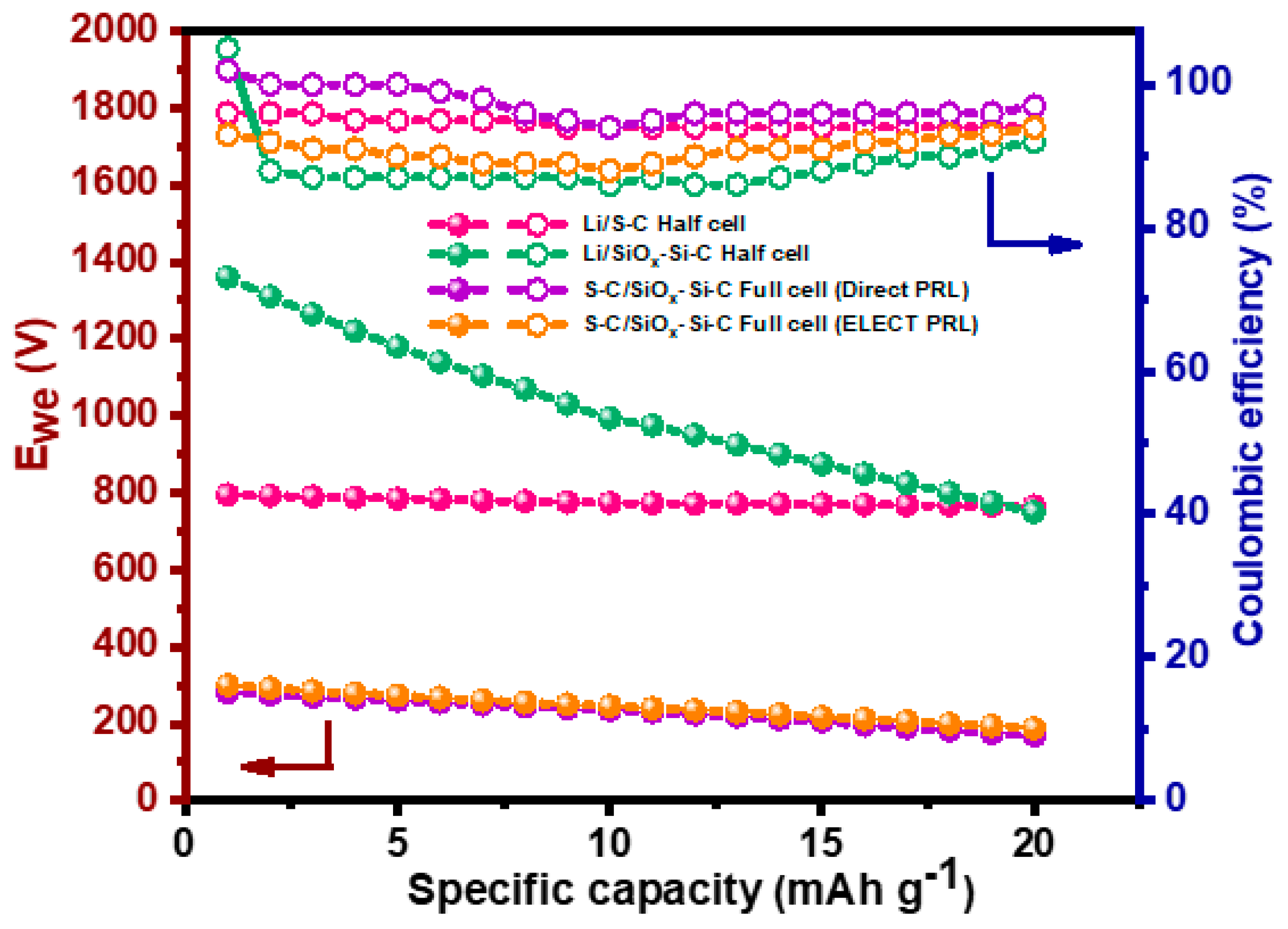
3.6. Pre-Lithiation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Yoo, H.D.; Markevich, E.; Salitra, G.; Sharon, D.; Aurbach, D. On the challenge of developing advanced technologies for electrochemical energy storage and conversion. Mater. Today 2014, 17, 110–121. [Google Scholar] [CrossRef]
- Cleaver, T.; Kovacik, P.; Marinescu, M.; Zhang, T.; Offer, G. Perspective-Commercializing lithium sulfur batteries: Are we doing the right research? J. Electrochem. Soc. 2018, 165, A6029–A6033. [Google Scholar] [CrossRef]
- Ji, X.; Nazar, L.F. Advances in Li-S batteries. J. Mater. Chem. 2010, 20, 9821–9826. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, G.; Cui, Y. Nanostructured sulfur cathodes. Chem. Soc. Rev. 2013, 42, 3018–3032. [Google Scholar] [CrossRef]
- Suriyakumar, S.; Stephan, A.M. Mitigation of polysulfide shuttling by interlayer/permselective separators in lithium-sulfur batteries. ACS Appl. Energy Mater. 2020, 3, 8095–8129. [Google Scholar] [CrossRef]
- Rosenman, A.; Markevich, E.; Salitra, G.; Aurbach, D.; Garsuch, A.; Chesneau, F.F. Review on Li-sulfur battery systems: An integral perspective. Adv. Energy Mater. 2015, 5, 1500212. [Google Scholar] [CrossRef]
- Zhao, X.; Kim, D.-S.; Ahn, H.-J.; Kim, K.-W.; Cho, K.-K.; Ahn, J.-H. Infiltrating sulfur into a highly porous carbon sphere as cathode material for lithium–sulfur batteries. Mater. Res. Bull. 2014, 58, 204–207. [Google Scholar] [CrossRef]
- Wu, H.; Chan, G.; Choi, J.W.; Ryu, I.; Yao, Y.; McDowell, M.T.; Lee, S.W.; Jackson, A.; Yang, Y.; Hu, L.; et al. Stable cycling of double-walled silicon nanotube battery anodes through solid-electrolyte interphase control. Nat. Nanotechnol. 2012, 7, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Han, B.; Choo, J.; Cho, J. Three-dimensional porous silicon particles for use in high-performance lithium secondary batteries. Angew. Chem. Int. Ed. 2008, 47, 10151–10154. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Xu, W.; Lv, D.; Xiao, J.; Zhang, J.-G. Anodes for rechargeable lithium-sulfur batteries. Adv. Energy Mater. 2015, 5, 1402273. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Muller-Buschbaum, P.; Cheng, Y.-J. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Jin, Y.; Li, S.; Kushima, A.; Zheng, X.; Sun, Y.; Xie, J.; Sun, J.; Xue, W.; Zhou, G.; Wu, J. Self-healing SEI enables full-cell cycling of a silicon-majority anode with a coulombic efficiency exceeding 99.9%. Energy Environ. Sci. 2017, 10, 580–592. [Google Scholar] [CrossRef]
- Xu, Q.; Li, J.-Y.; Sun, J.-K.; Yin, Y.-X.; Wan, L.-J.; Guo, Y.-G. Watermelon-inspired Si/C microspheres with hierarchical buffer structures for densely compacted lithium-ion battery anodes. Adv. Energy Mater. 2017, 7, 1601481. [Google Scholar] [CrossRef]
- Zhou, X.; Yin, Y.X.; Wan, L.J.; Guo, Y.G. Facile synthesis of silicon nanoparticles inserted into graphene sheets as improved anode materials for lithium-ion batteries. Chem. Commun. 2012, 48, 2198–2200. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Ernst, E.M.; Yoo, S.; Sandhage, K. Syntheses of porous self-supporting metal-nanoparticle assemblies with 3D morphologies inherited from Biosilica templates (diatom frustules). Adv. Mater. 2009, 21, 474–478. [Google Scholar] [CrossRef]
- Dalavi, S.; Guduru, P.; Lucht, B.L. Performance enhancing electrolyte additives for lithium-ion batteries with silicon anodes. J. Electrochem. Soc. 2012, 159, A642–A646. [Google Scholar] [CrossRef]
- Higgins, T.M.; Park, S.-H.; King, P.J.; Zhang, C.J.; McEvoy, N.; Berner, N.C.; Daly, D.; Shmeliov, A.; Khan, U.; Duesberg, G.; et al. A commercial conducting polymer as both binder and conductive additive for silicon nanoparticle-based lithium-ion battery negative electrodes. ACS Nano 2016, 10, 3702–3713. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yu, Q.; Zhao, Y.; He, R.; Xu, M.; Feng, S.; Li, S.; Zhou, L.; Mai, L. Silicon oxides: A promising family of anode materials for lithium-ion batteries. Chem. Soc. Rev. 2019, 48, 285–309. [Google Scholar] [CrossRef]
- Choi, I.; Lee, M.J.; Oh, S.M.; Kim, J.J. Fading mechanisms of carbon-coated and disproportionated Si/SiOx negative electrode (Si/SiOx/C) in Li-ion secondary batteries: Dynamics and component analysis by TEM. Electrochim. Acta 2012, 85, 369–376. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, Y.; Zhou, J.; Lin, C.; Lv, F.; Wang, K.; Feng, J.; Xu, Z.; Li, J.; Guo, S. Hollow Si/SiOx nanosphere/nitrogen-doped carbon superstructure with a double shell and void for high-rate and long-life lithium-ion storage. J. Mater. Chem. A 2018, 6, 8039–8046. [Google Scholar] [CrossRef]
- Chen, T.; Wu, J.; Zhang, Q.; Su, X. Recent advancement of SiOx based anodes for lithium-ion batteries. J. Power Sources 2017, 363, 126–144. [Google Scholar] [CrossRef]
- Choi, N.-S.; Yew, K.H.; Lee, K.Y.; Sung, M.; Kim, H.; Kim, S.-S. Effect of fluoroethylene carbonate additive on interfacial properties of silicon thin-film electrode. J. Power Sources 2006, 161, 1254–1259. [Google Scholar] [CrossRef]
- Tang, J.; Hou, L.; Hu, T.; Fan, S.; Zhou, X.; Yang, J. Influence of oxygen content on the electrochemical behavior of SiOx@C anodes for Li-ion battery. Compos. Commun. 2021, 23, 100544. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, J.; Liu, L.; Si, W.; Oswald, S.; Xi, L.; Kundu, M.; Ma, G.; Gemming, T.; Baunack, S.; et al. Hierarchically designed SiOx/SiOy bilayer nanomembranes as stable anodes for lithium ion Batteries. Adv. Mater. 2014, 26, 4527–4532. [Google Scholar] [CrossRef]
- Sun, M.; Ma, J.; Xu, M.; Yang, H.; Zhang, J.; Wang, C. A low-cost SiOx/C@Graphite composite derived from oat husk as an advanced anode for high-performance lithium-ion Batteries. ACS Omega 2022, 7, 15123–15131. [Google Scholar] [CrossRef] [PubMed]
- Hagen, M.; González, E.Q.; Dörfler, S.; Fahrer, G.; Tübke, J.; Hoffmann, M.J.; Althues, H.; Speck, R.; Krampfert, M.; Kaskel, S.; et al. Studies on preventing Li dendrite formation in Li-S batteries by using pre-lithiated Si microwire anodes. J. Power Sources 2014, 248, 1058–1066. [Google Scholar] [CrossRef]
- Rahman, I.A.; Padavettan, V. Synthesis of Silica nanoparticles by sol-gel: Size-dependent properties, surface modification, and applications in silica-polymer nanocompositesa review. J. Nanomater. 2012, 2012, 132424. [Google Scholar] [CrossRef]
- Liu, Y.; Pelster, T.; Lee, T.-T.; Wang, Y.; Luo, G. Study on the three-stage growth of silica nanoparticles prepared by the drop-by-drop precipitation method. Powder Technol. 2022, 397, 117115. [Google Scholar] [CrossRef]
- Qi, D.; Lin, C.; Zhao, H.; Liu, H.; Lü, T. Size regulation and prediction of the SiO2 nanoparticles prepared via Stöber process. J. Dispersion Sci. Technol. 2017, 38, 70–74. [Google Scholar] [CrossRef]
- Sathya, S.; Soosaimanickam, C.; Bella, F.; Yoo, D.J.; Stephan, A.M. Cycling performance of SiOx-Si-C composite anode with different blend ratios of PAA-CMC as binder for lithium sulfur batteries. J. Polym. Res. 2024, 31, 205. [Google Scholar] [CrossRef]
- Sathya, S.; Angulakshmi, N.; Ahn, J.-H.; Kathiresan, M.; Stephan, A.M. Influence of additives on the electrochemical and interfacial properties of SiOx-based anode materials for lithium–sulfur batteries. Langmuir 2022, 38, 2423–2434. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wang, D.; Liu, T.; Zhu, J.; Lang, X. A three dimensional SiOx/C@RGO nanocomposite as a high energy anode material for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 3521–3527. [Google Scholar] [CrossRef]
- Pollak, E.; Salitra, G.; Baranchugov, V.; Aurbach, D. In situ conductivity, impedance spectroscopy, and ex situ Raman spectra of amorphous silicon during the insertion/extraction of lithium. J. Phys. Chem. C 2007, 111, 11437–11444. [Google Scholar] [CrossRef]
- Netz, A.; Huggins, R.A.; Weppner, W. The formation and properties of amorphous silicon as negative electrode reactant in lithium systems. J. Power Sources 2003, 119–121, 95–100. [Google Scholar] [CrossRef]
- Minakshi, M.; Mitchell, D.R.G.; Munnangi, A.R.; Barlow, A.J.; Fichtner, M. New insights into the electrochemistry of magnesium molybdate hierarchical architectures for high performance sodium devices. Nanoscale 2018, 10, 13277–13288. [Google Scholar] [CrossRef]
- Sharma, P.; Minakshi Sundaram, M.; Watcharatharapong, T.; Laird, D.; Euchner, H.; Ahuja, R. Zn metal atom doping on the surface plane of one-dimesional NiMoO4 nanorods with improved redox chemistry. ACS Appl. Mater. Interfaces 2020, 12, 44815–44829. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.W.; Celio, H.; Webb, L.J.; Stevenson, K.J. Examining solid electrolyte interphase formation on crystalline silicon electrodes: Influence of electrochemical preparation and ambient exposure conditions. J. Phys. Chem. C 2012, 116, 19737–19747. [Google Scholar] [CrossRef]
- Etacheri, V.; Haik, O.; Goffer, Y.; Roberts, G.A.; Stefan, I.C.; Fasching, R.; Aurbach, D. Effect of fluoroethylene carbonate (FEC) on the performance and surface chemistry of Si-nanowire Li-ion battery anodes. Langmuir 2012, 28, 965–976. [Google Scholar] [CrossRef]
- Yamin, H.; Peled, E. Electrochemistry of a nonaqueous lithium/sulfur cell. J. Power Sources 1983, 9, 281–287. [Google Scholar] [CrossRef]
- Ruffo, R.; Hong, S.S.; Chan, C.K.; Huggins, R.A.; Cui, Y. Impedance analysis of silicon nanowire lithium ion battery anodes. J. Phys. Chem. C 2009, 113, 11390–11398. [Google Scholar] [CrossRef]
- Chakrapani, V.; Rusli, F.; Filler, M.A.; Kohl, P.A. Quaternary ammonium ionic liquid electrolyte for a silicon nanowire-based lithium ion battery. J. Phys. Chem. C 2011, 115, 22048–22053. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, S.; Qiu, X.; Zhu, W.; Chen, L. Preparation and characterization of silicon-based three-dimensional cellular anode for lithium-ion battery. Electrochem. Commun. 2007, 9, 930–934. [Google Scholar] [CrossRef]
- Eriksson, T.; Andersson, A.M.; Gejke, C.; Gustafsson, T.; Thomas, J.O. Influence of temperature of the interface chemistry of LixMn2O4 electrodes. Langmuir 2002, 18, 3609–3619. [Google Scholar] [CrossRef]
- Jia, H.; Zou, L.; Gao, P.; Cao, X.; Zhao, W.; He, Y.; Engelhard, M.H.; Burton, S.D.; Wang, H.; Ren, X.; et al. High-performance silicon anodes enabled by nonflammable localized high-concentration electrolytes. Adv. Energy Mater. 2019, 9, 1900784. [Google Scholar] [CrossRef]
- Diao, Y.; Xie, K.; Xiong, S.; Hong, X. Insights into Li-S battery cathode capacity fading mechanisms: Irreversible oxidation of active mass during cycling. J. Electrochem. Soc. 2012, 159, A1816–A1821. [Google Scholar] [CrossRef]
- Jiang, L.; Park-Lee, K.J.; Clinton, R.M.; Tang, Z.; Breedveld, V.; Hess, D.W. Mechanical durability of liquid repellent coatings. Surf. Coat. Technol. 2017, 328, 182–191. [Google Scholar] [CrossRef]
- Gnanaraj, J.S.; Levi, M.D.; Levi, E.; Salitra, G.; Aurbach, D.; Fischer, J.E.; Claye, A. Comparison between the electrochemical behavior of disordered carbons and graphite electrodes in connection with their structure. J. Electrochem. Soc. 2001, 148, A525–A536. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Dornbusch, M. Chapter IV. In Corrosion Analysis; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: New York, NY, USA, 2018. [Google Scholar]
- Marinaro, M.; Weinberger, M.; Mehrens, M. Toward pre-lithiated high areal capacity silicon anodes for lithium-ion batteries. Electrochim. Acta 2016, 206, 99–107. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Z.; Wang, C. Effect of Pre-lithiation degrees of mesocarbon microbeads anode on the electrochemical performance of lithium-ion capacitors. Electrochim. Acta 2014, 125, 22–28. [Google Scholar] [CrossRef]
- Kim, H.S.; Jeoung, T.-G.; Kim, Y.-T. Electrochemical properties of lithium sulfur battery with silicon anodes lithiated by direct contact method. J. Electrochem. Sci. Technol. 2016, 7, 228–233. [Google Scholar] [CrossRef]
- Holtstiege, F.; Bärmann, P.; Nölle, R.; Winter, M.; Placke, T. Pre-lithiation strategies for rechargeable energy storage technologies: Concepts, promises and challenges. Batteries 2018, 4, 4. [Google Scholar] [CrossRef]
- Domi, Y.; Usui, H.; Iwanari, D.; Sakaguchi, H. Effect of Mechanical pre-lithiation on electrochemical performance of silicon negative electrode for lithium-ion batteries. J. Electrochem. Soc. 2017, 164, A1651–A1654. [Google Scholar] [CrossRef]
- Chang, S.; Moon, J.; Cho, K.; Cho, M. Multiscale analysis of prelithiated silicon nanowire for Li-ion battery. Comput. Mater. Sci. 2015, 98, 99–104. [Google Scholar] [CrossRef]
- Shellikeri, A.; Watson, V.G.; Adams, D.L.; Kalu, E.E.; Read, J.A.; Jow, T.R.; Zheng, J.P. Pre-lithiation of carbon anodes using different lithium–sources. ECS Trans. 2017, 77, 293–303. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, Y.; Yang, L.; Zhu, W.; Liu, W.; Yang, Y.; Liu, K. Sustainable silicon anodes facilitated via a double-layer interface engineering: Inner SiOx combined with outer nitrogen and boron co-doped carbon. Carbon Energy 2022, 4, 399–410. [Google Scholar] [CrossRef]
- Jiang, R.; Yuan, H.; Wei, X.; Wang, H.; Shin, H.J.; Lan, J.; Yu, Y.; Yang, Y. Constructing robust and freestanding MXene/Si@C core–shell nanofibers via coaxial electrospinning for high performance Li-ion batteries. Mater. Chem. Front. 2021, 5, 8218–8228. [Google Scholar] [CrossRef]


| Formulation | SiOx (wt.%) | Si (wt.%) | Graphitic Carbon (wt.%) |
|---|---|---|---|
| a | 85 | 0.0 | 10.0 |
| b | 75 | 10 | 10.0 |
| c | 70 | 12.5 | 12.5 |
| d | 65 | 20 | 10.0 |
| e | 10 | 75 | 10.0 |
| f | 0.0 | 85 | 10.0 |
| g | 10 | 10 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sathya, S.; Kumar, R.S.; Garcia-Ballesteros, S.; Bella, F.; Yoo, D.J.; Stephan, A.M. Interplay Between Composition and Cycling Performance of Pre-Lithiated SiOx-Si-C Composite Anodes for Lithium–Sulfur Full Cells. Materials 2025, 18, 1053. https://doi.org/10.3390/ma18051053
Sathya S, Kumar RS, Garcia-Ballesteros S, Bella F, Yoo DJ, Stephan AM. Interplay Between Composition and Cycling Performance of Pre-Lithiated SiOx-Si-C Composite Anodes for Lithium–Sulfur Full Cells. Materials. 2025; 18(5):1053. https://doi.org/10.3390/ma18051053
Chicago/Turabian StyleSathya, Swamickan, Ramasamy Santhosh Kumar, Sara Garcia-Ballesteros, Federico Bella, Dong Jin Yoo, and Arul Manuel Stephan. 2025. "Interplay Between Composition and Cycling Performance of Pre-Lithiated SiOx-Si-C Composite Anodes for Lithium–Sulfur Full Cells" Materials 18, no. 5: 1053. https://doi.org/10.3390/ma18051053
APA StyleSathya, S., Kumar, R. S., Garcia-Ballesteros, S., Bella, F., Yoo, D. J., & Stephan, A. M. (2025). Interplay Between Composition and Cycling Performance of Pre-Lithiated SiOx-Si-C Composite Anodes for Lithium–Sulfur Full Cells. Materials, 18(5), 1053. https://doi.org/10.3390/ma18051053








