Effects of Sand Type and Alkali Equivalent on Drying Shrinkage and Microstructure of Seawater–Sea Sand Alkali-Activated Slag Concrete
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Sand
2.1.2. Water
2.1.3. Slag
2.1.4. Activators and Retarders
2.2. Preparation Method
2.2.1. Cement Preparation Methods
2.2.2. Preparation Methods for SSAS Mortar
2.3. Testing and Analysis
2.3.1. Basic Performance Test
2.3.2. Drying Shrinkage and Mass Loss Test
2.3.3. XRD Test
2.3.4. TG Test
2.3.5. MIP Test
2.3.6. SEM Test
3. Results and Discussion
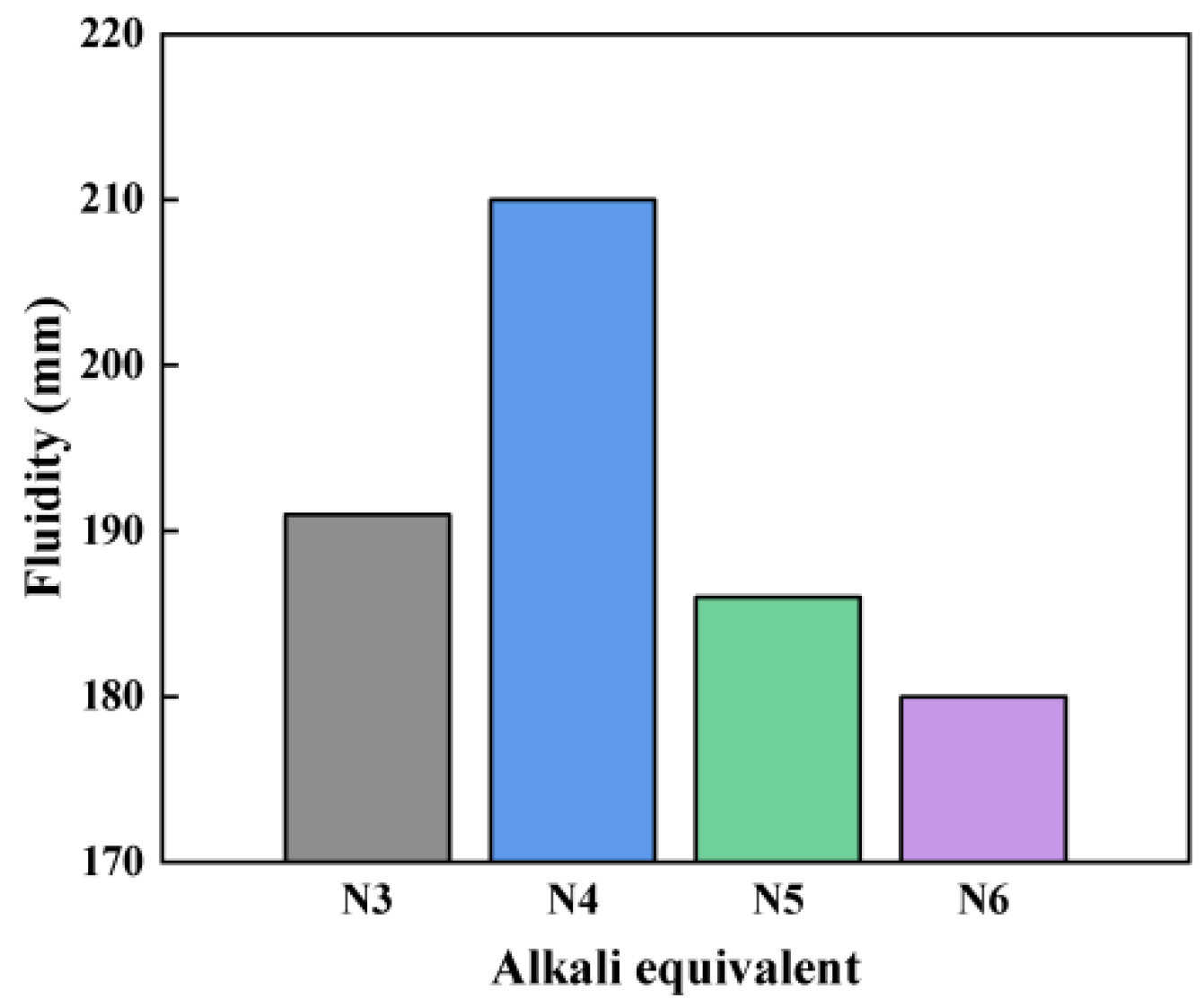
3.1. Flowability
3.1.1. Impact of Seawater and Sea Sand
3.1.2. Impact of Alkali Equivalent
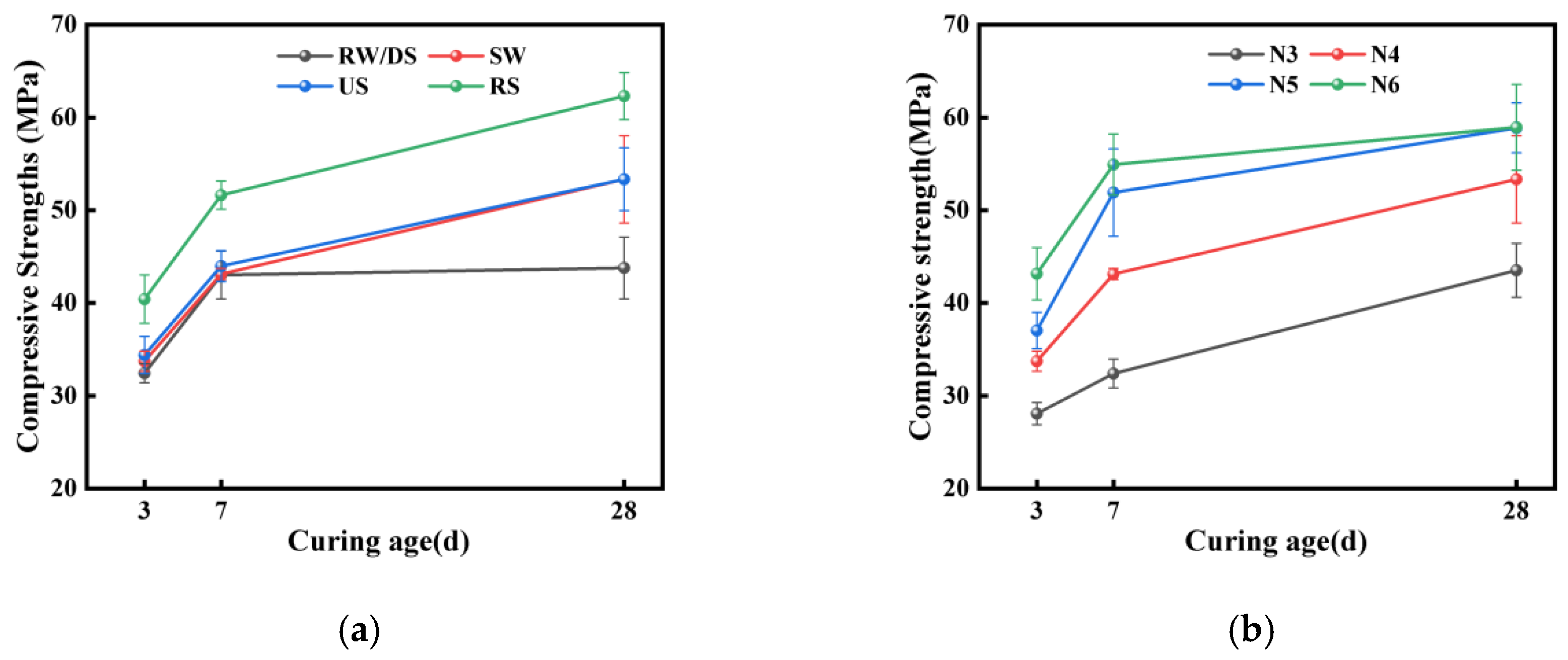
3.2. Mechanical Properties
3.2.1. Impact of Seawater and Sea Sand
3.2.2. Impact of Alkali Equivalent
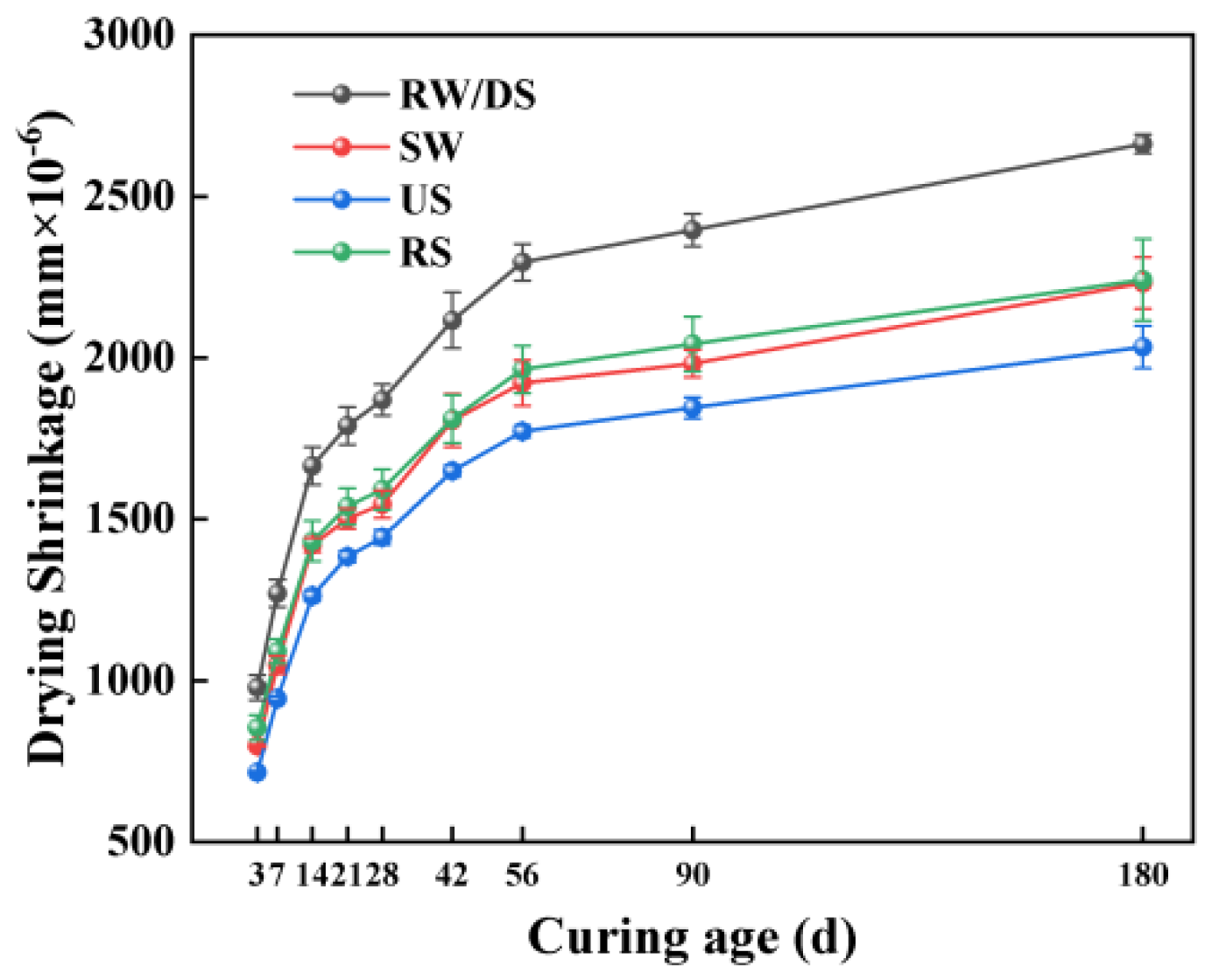
3.3. Drying Shrinkage Properties
3.3.1. Impact of Seawater and Sea Sand
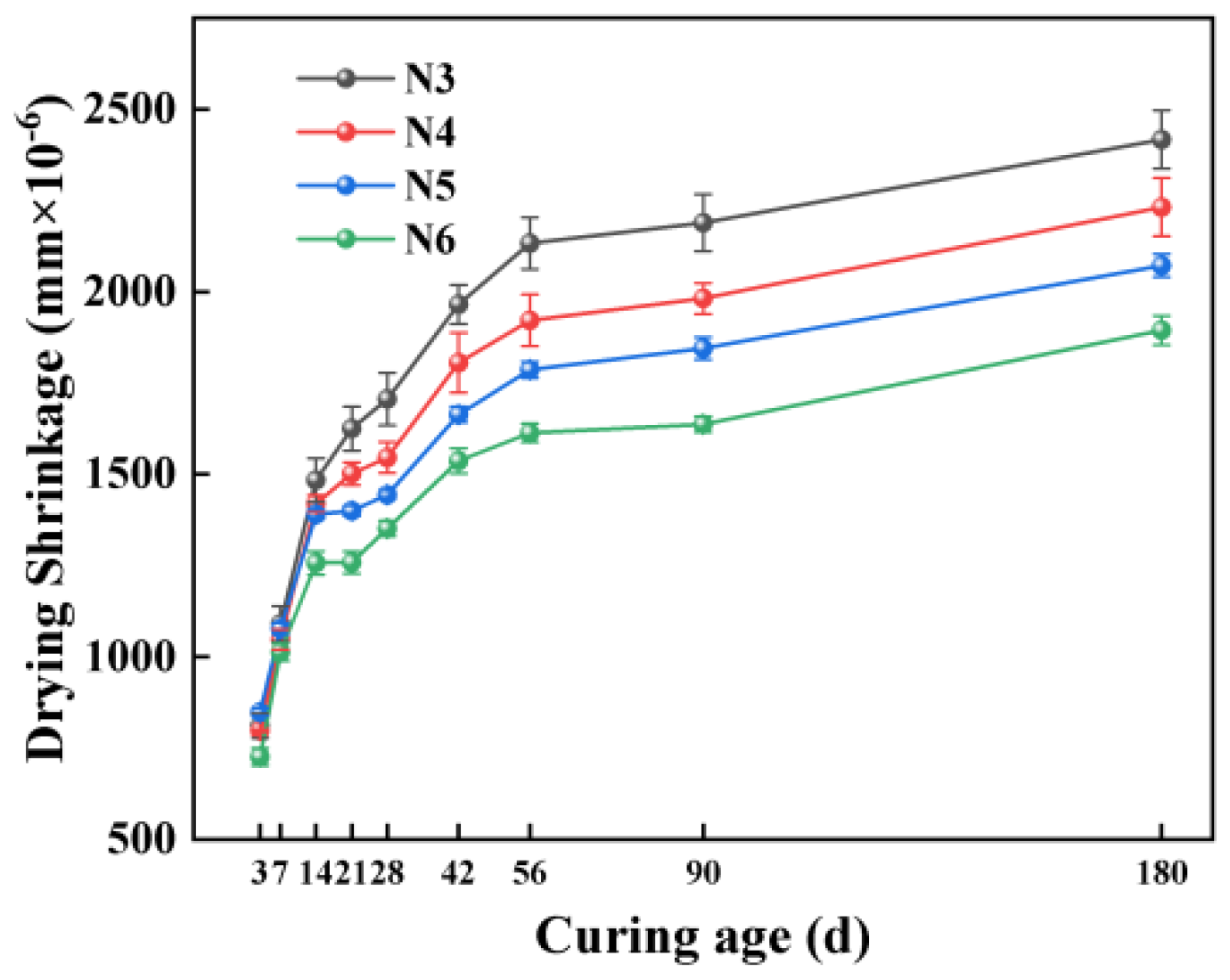
3.3.2. Impact of Alkali Equivalent
3.4. Mass Loss
3.4.1. Impact of Seawater and Sea Sand
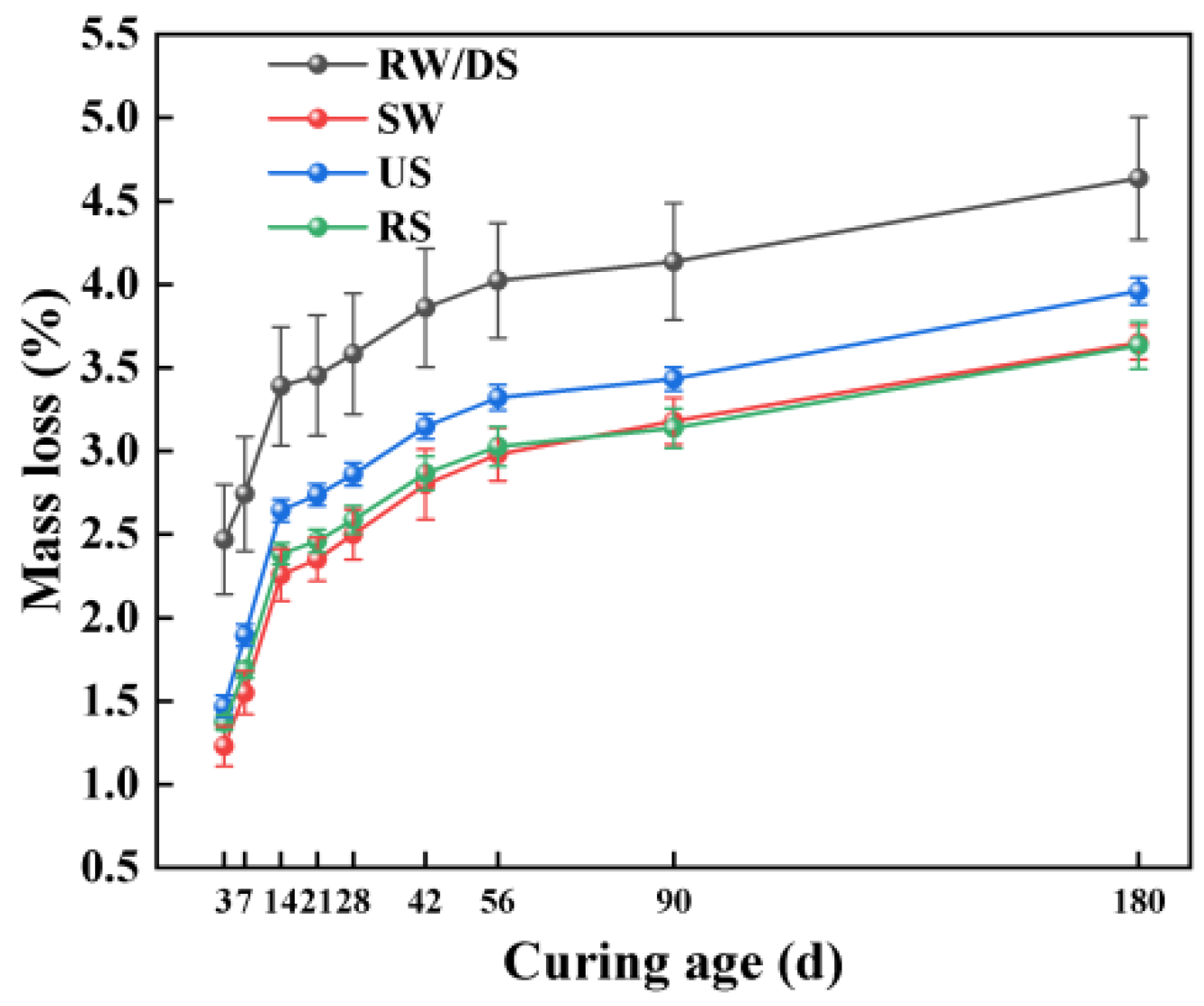
3.4.2. Impact of Alkali Equivalent
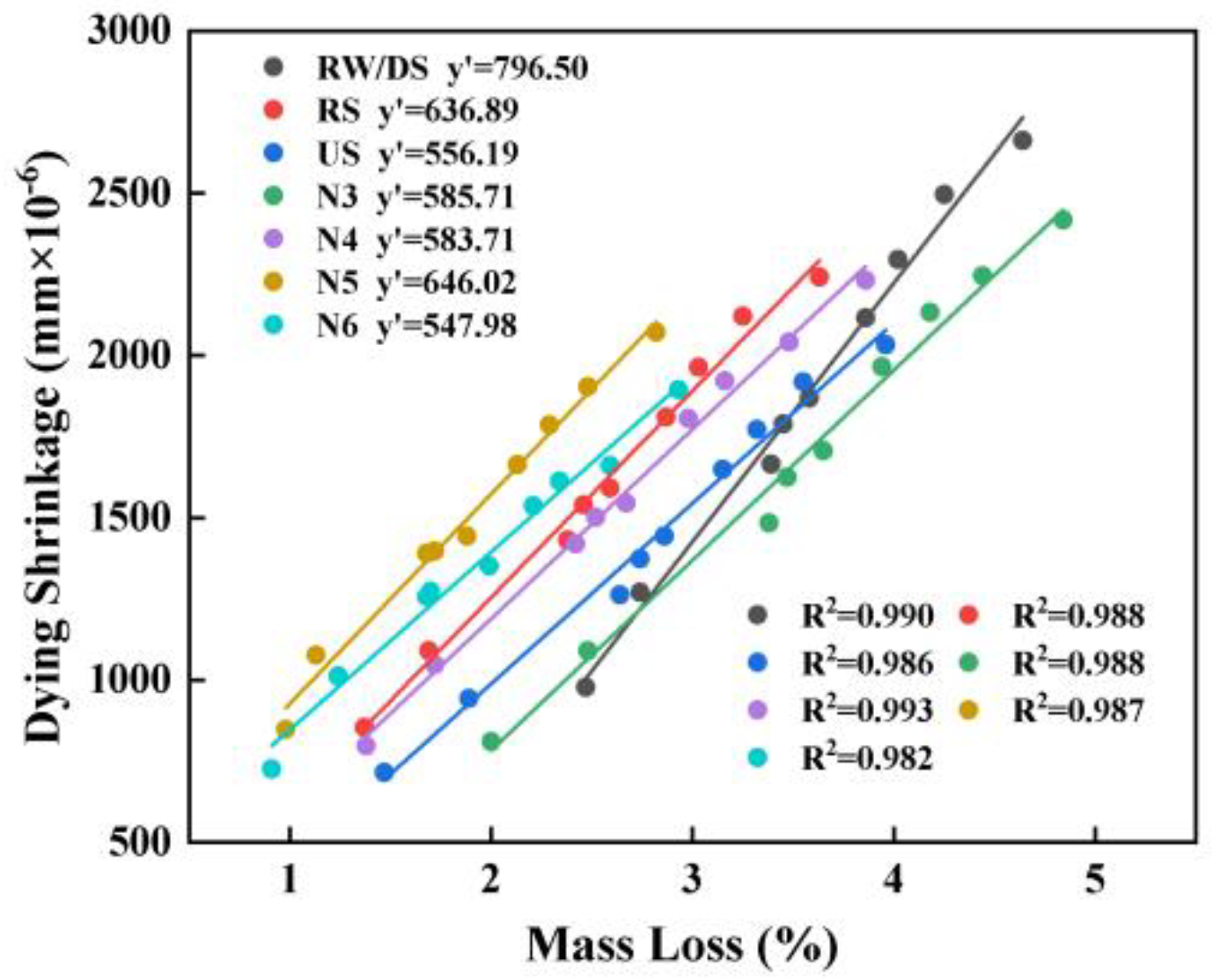
3.4.3. Relationship Between Mass Loss and Drying Shrinkage
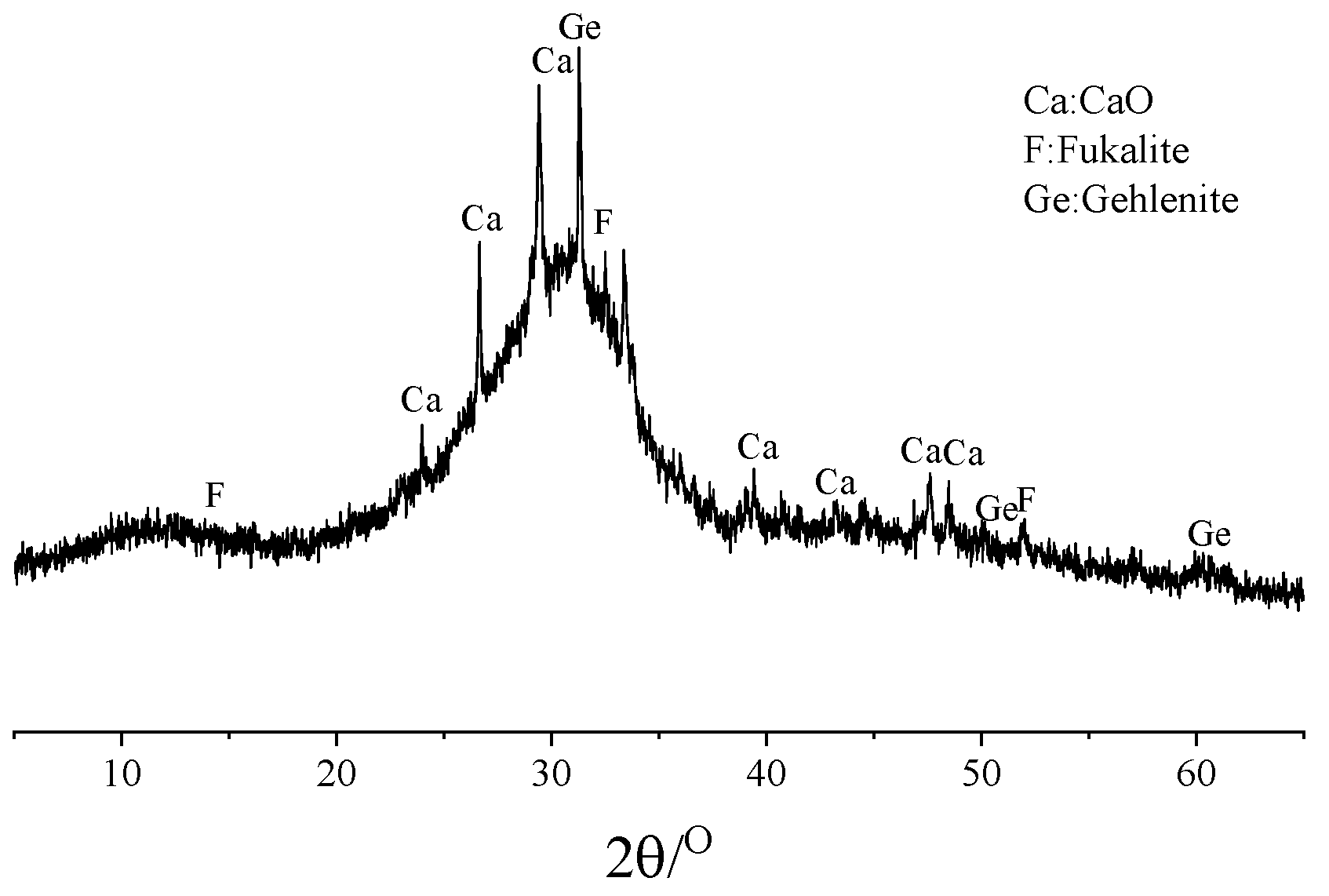
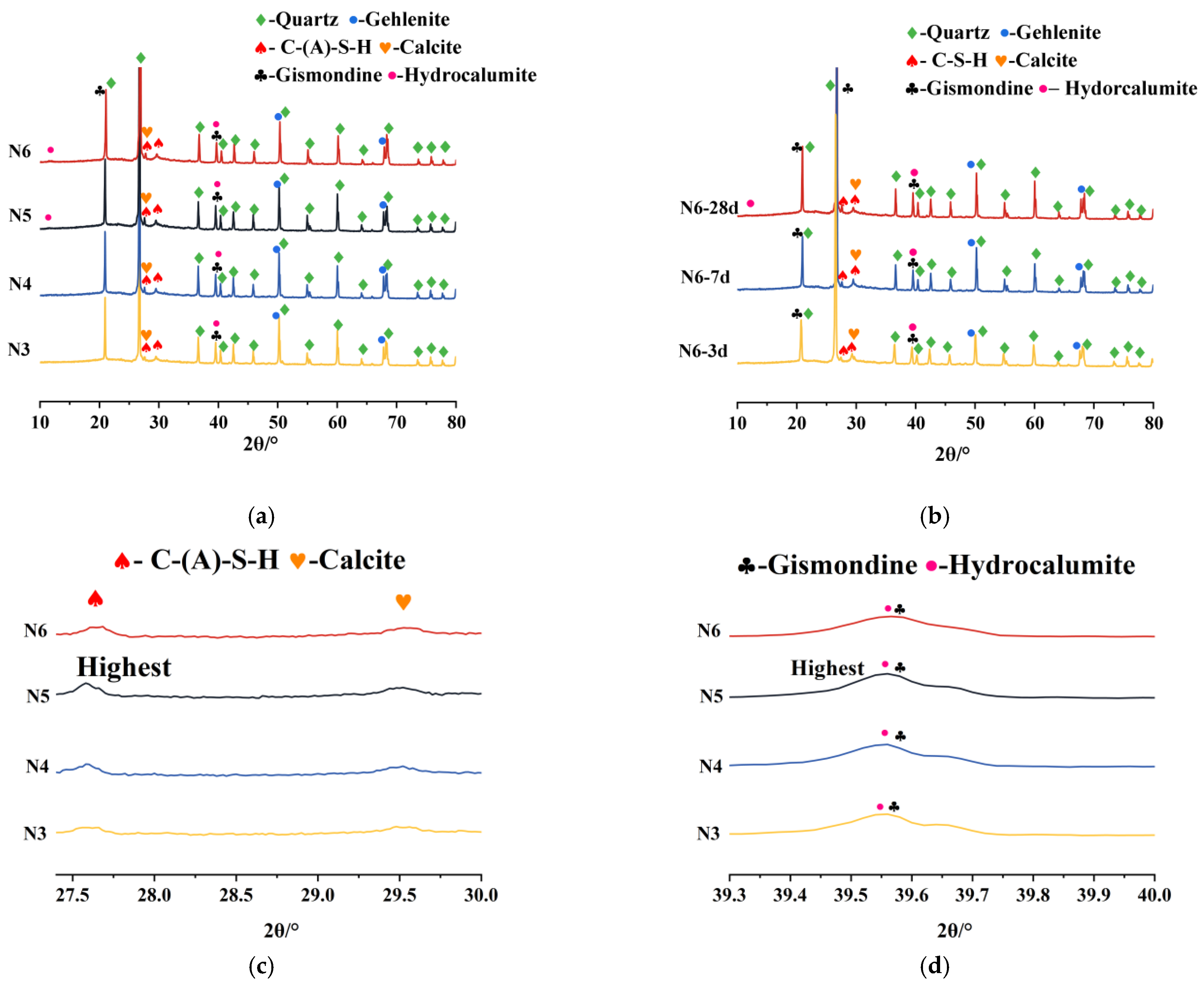
3.5. XRD
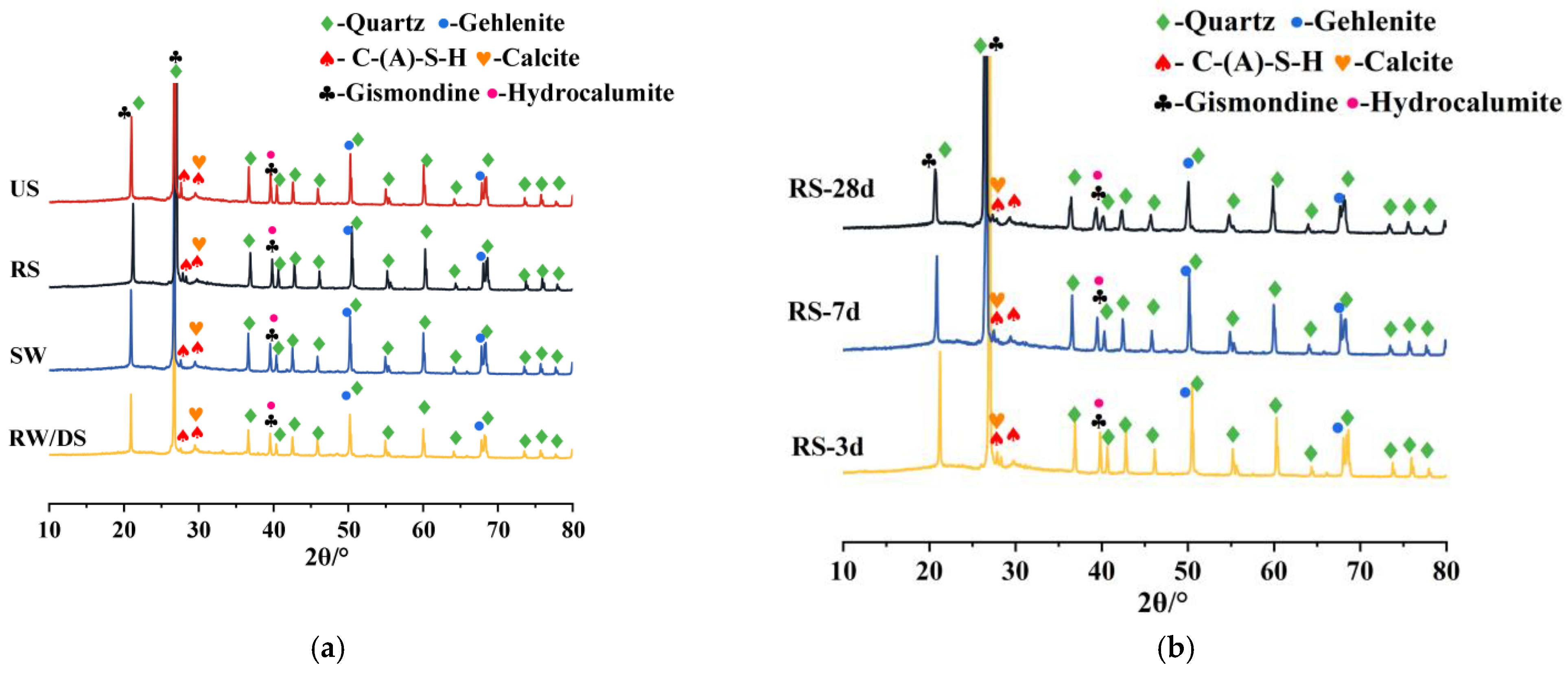
3.5.1. Impact of Seawater and Sea Sand
3.5.2. Impact of Alkali Equivalent
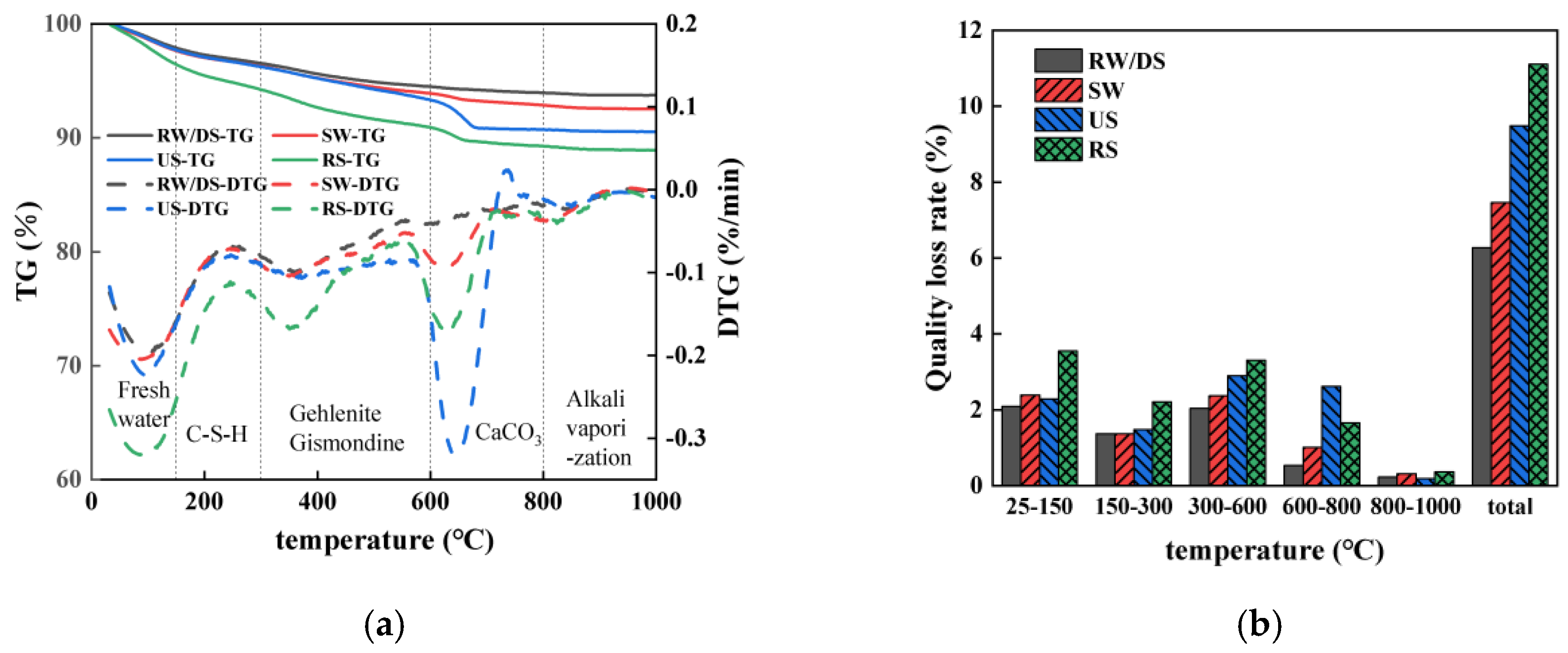
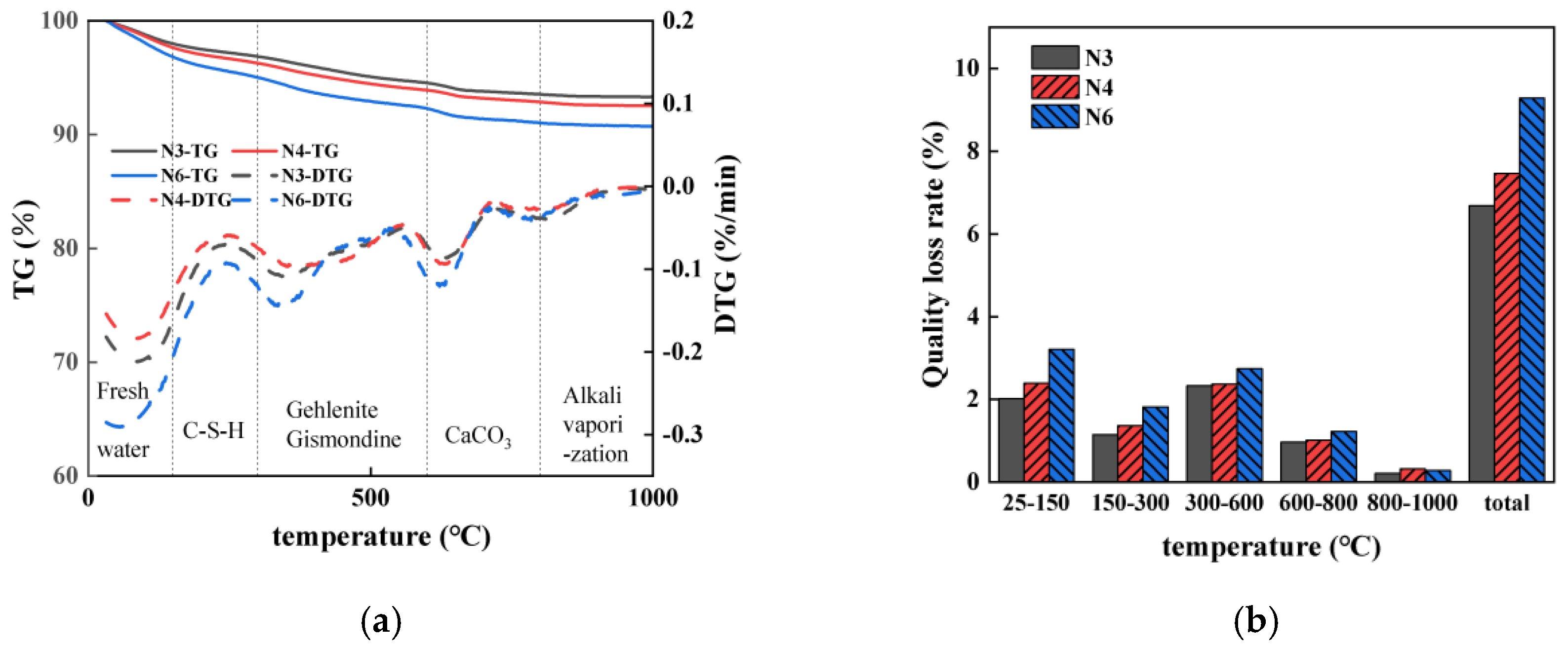
3.6. TG
3.6.1. Impact of Seawater and Sea Sand
3.6.2. Impact of Alkali Equivalent
3.6.3. Relationship Between Evaporable Water Content and Drying Shrinkage
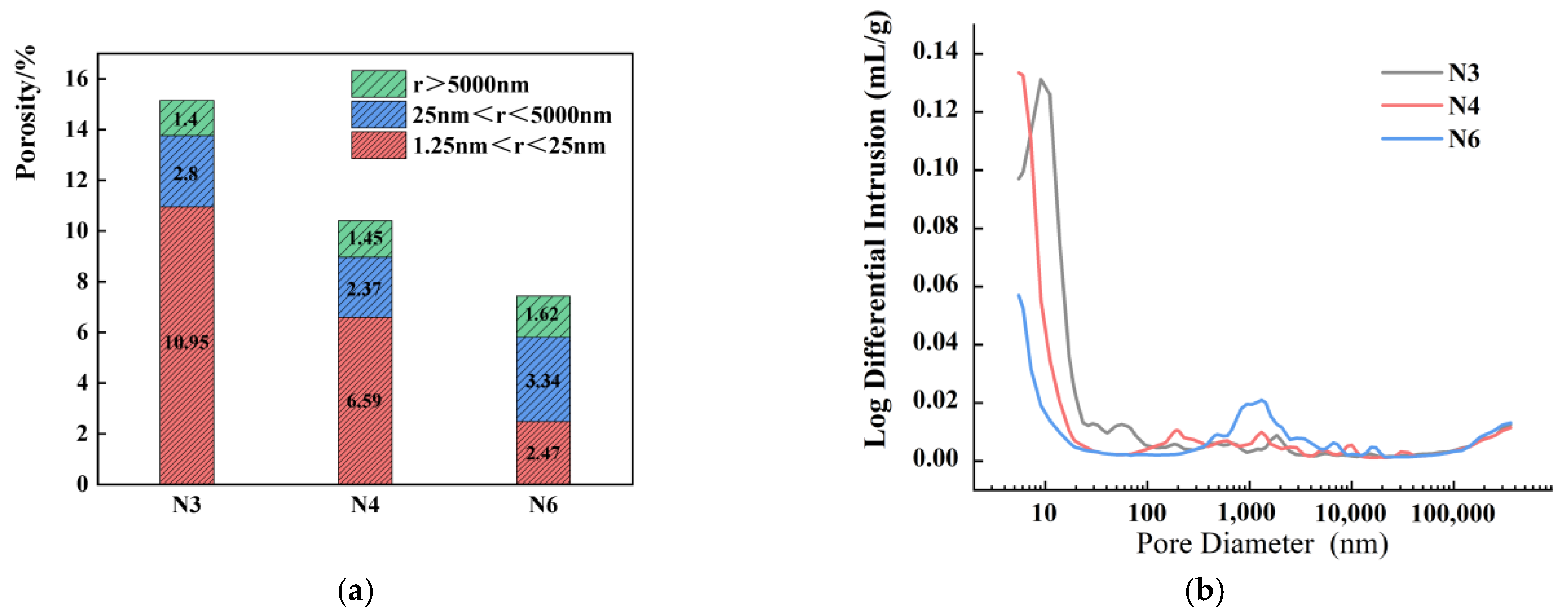
3.7. MIP
3.7.1. Impact of Seawater and Sea Sand
3.7.2. Impact of Alkali Equivalent
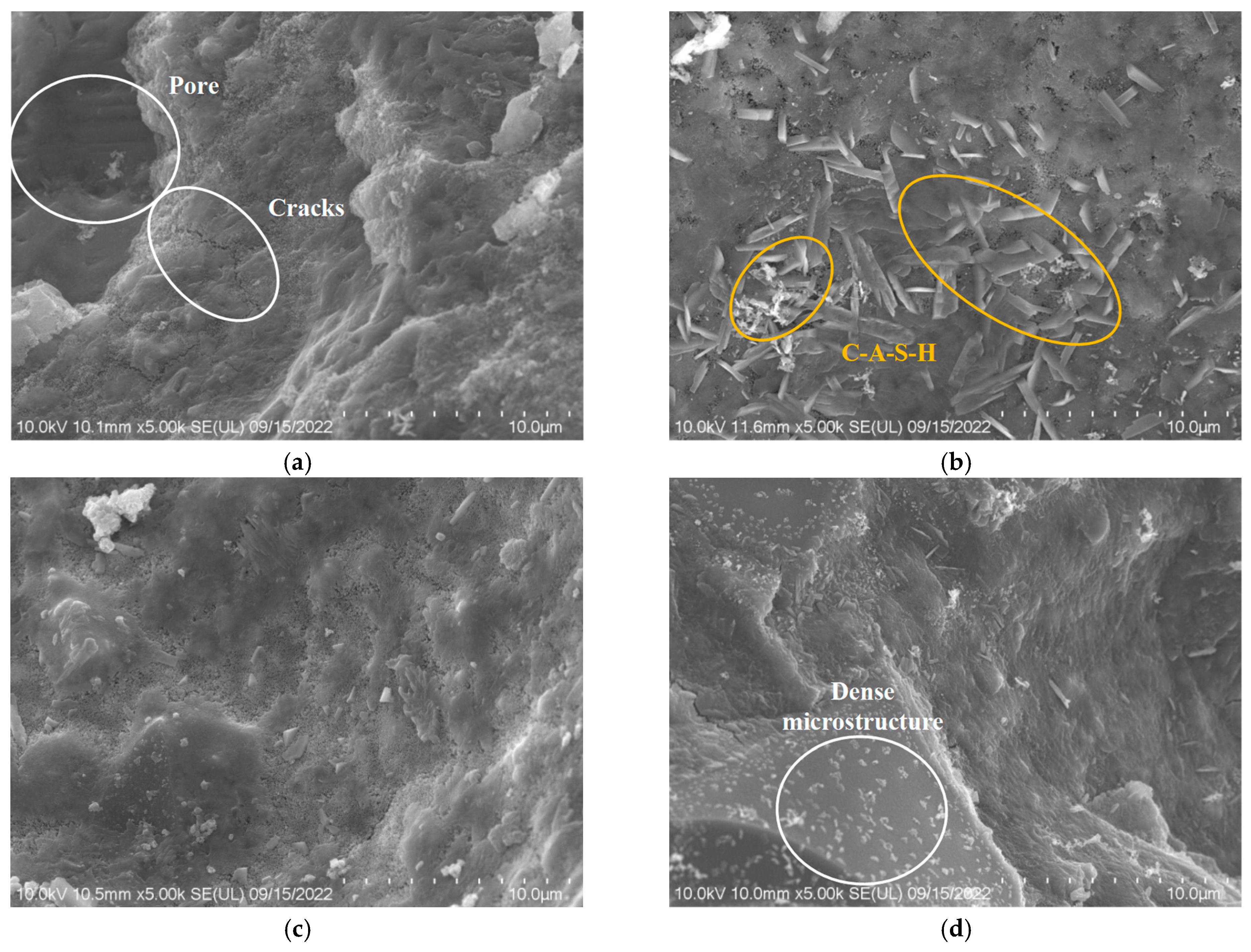
3.8. SEM
3.8.1. Impact of Seawater and Sea Sand
3.8.2. Impact of Alkali Equivalent
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Provis, J.L.; Berna, S.A. Geopolymers and related alkali-activated materials. Annu. Rev. Mater. Res. 2014, 44, 299–327. [Google Scholar] [CrossRef]
- Xi, F.; Davis, S.; Ciais, P.; Crawford-Brown, D.; Guan, D.; Pade, C.; Shi, T.; Syddall, M.; Lv, J.; Ji, L.; et al. Substantial global carbon uptake by cement carbonation. Nat. Geosci. 2016, 9, 880–883. [Google Scholar] [CrossRef]
- Turner, L.K.; Collins, F.G. Carbon dioxide equivalent (CO2-e) emissions: A comparison between geopolymer and OPC cement concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Assi, L.; Carter, K.; Deaver, E.; Anay, R.; Ziehl, P. Sustainable concrete: Building a greener future. J. Clean. Prod. 2018, 198, 1641–1651. [Google Scholar] [CrossRef]
- Roy, D.M.; Silsbee, M.R.; Wolfe-Confer, D. New rapid setting alkali activated cement compositions. MRS Proc. 1989, 179, 203. [Google Scholar] [CrossRef]
- Aiken, T.A.; Sha, W.; Kwasny, J.; Soutsos, M.N. Resistance of geopolymer and Portland cement based systems to silage effluent attack. Cem. Concr. Res. 2017, 92, 56–65. [Google Scholar] [CrossRef]
- Miller, S.A.; Horvath, A.; Monteiro, P.J.M. Readily implementable techniques can cut annual CO2 emissions from the production of concrete by over 20%. Environ. Res. Lett. 2016, 11, 074029. [Google Scholar] [CrossRef]
- Nishida, T.; Otsuki, N.; Ohara, H.; Garba-Say, Z.M.; Nagata, T. Some considerations for applicability of seawater as mixing water in concrete. J. Mater. Civ. Eng. 2015, 27, B4014004. [Google Scholar] [CrossRef]
- Xiao, J.Z.; Qiang, C.B.; Nanni, A.; Zhang, K. Use of sea-sand and seawater in concrete construction: Current status and future opportunities. Constr. Build. Mater. 2017, 155, 1101–1111. [Google Scholar] [CrossRef]
- Anthony, E.J.; Brunier, G.; Besset, M.; Goichot, M.; Dussouillez, P.; Nguyen, V.L. Linking rapid erosion of the Mekong river delta to human activities. Sci. Rep. 2015, 5, 14745. [Google Scholar] [CrossRef]
- Torres, A.; Brandt, J.; Lear, K.; Liu, J. A looming tragedy of the sand commons. Science 2017, 357, 970–971. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K. Viewpoints on using sea sand in concrete as fine aggregates in Japan. Concr. J. 1974, 12, 72–76. (In Japanese) [Google Scholar]
- Soeda, K.; Ichimura, T. Present state of corrosion inhibitors in Japan. Cem. Concr. Compos. 2003, 25, 117–122. [Google Scholar] [CrossRef]
- Hu, X.; Xiao, J.; Zhang, K.; Zhang, Q. The state-of-the-art study on durability of FRP reinforced concrete with seawater and sea sand. J. Build. Eng. 2022, 51, 104294. [Google Scholar] [CrossRef]
- Li, T.; Liu, X.; Zhang, Y.; Yang, H.; Zhi, Z.; Liu, L.; Ma, W.; Shah, S.P.; Li, W. Preparation of sea water sea sand high performance concrete (SHPC) and serving performance study in marine environment. Constr. Build. Mater. 2020, 254, 119114. [Google Scholar] [CrossRef]
- Ting, M.Z.Y.; Wong, K.S.; Rahman, M.E.; Joo, M.S. Mechanical and durability performance of marine sand and seawater concrete incorporating silicomanganeseslag as coarse aggregate. Constr. Build. Mater. 2020, 254, 119195. [Google Scholar] [CrossRef]
- He, X.; Zhou, J. Mechanical characteristics of sea-sand concrete in simulated marine environment. Constr. Build. Mater. 2021, 274, 122098. [Google Scholar] [CrossRef]
- Xu, Q.; Ji, T.; Yang, Z.; Ye, Y. Preliminary investigation of artificial reef concrete with sulphoaluminate cement, marine sand and sea water. Constr. Build. Mater. 2019, 211, 837–846. [Google Scholar] [CrossRef]
- Pan, D.; Yaseen, S.; Chen, K.; Niu, D.; Leung, C.; Li, Z. Study of the influence of seawater and sea sand on the mechanical and microstructural properties of concrete. J. Build. Eng. 2021, 42, 103006. [Google Scholar] [CrossRef]
- Limeira, J.; Agullo, L.; Etxeberria, M. Dredged marine sand as construction material. Eur. J. Environ. Civ. Eng. 2012, 16, 906–918. [Google Scholar] [CrossRef]
- Athira, S.S.; Neethu, S. Strength and durability of concrete using dredged sea sand as partial replacement of M-sand. Int. Res. J. Eng. Technol. 2016, 3, 1072–1075. [Google Scholar]
- Zhang, G.L.; Mo, L.W.; Chen, J.B.; Liu, J.Z.; He, Z.M. Research on influence of chloride ion in sea sand on the performance of concrete. Appl. Mech. Mater. 2012, 1801, 444–447. [Google Scholar] [CrossRef]
- Liu, W.; Chen, H.; Wang, D. Experimental research on durability of marine sand and seawater concrete. Constr. Build. Mater. 2020, 236, 117507. [Google Scholar]
- Mangat, P.S.; Ojedokun, O.O. Influence of curing on pore properties and strength of alkali activated mortars. Constr. Build. Mater. 2018, 188, 337–348. [Google Scholar] [CrossRef]
- Noushini, A.; Castel, A.; Aldred, J.; Rawal, A. Chloride diffusion resistance and chloride binding capacity of fly ash-based geopolymer concrete. Cem. Concr. Compos. 2020, 105, 103290. [Google Scholar] [CrossRef]
- Babaee, M.; Castel, A. Chloride diffusivity, chloride threshold, and corrosion initiation in reinforced alkali-activated mortars: Role of calcium, alkali, and silicate content. Cem. Concr. Res. 2018, 111, 56–71. [Google Scholar] [CrossRef]
- Lou, Y.; Kang, S.; Wu, W.; Wang, X.; Sun, H.; Chen, F.; Xu, M. Sulfate resistance of recycled powder-slag-based geopolymers under different erosive environments. Constr. Build. Mater. 2025, 462, 139950. [Google Scholar] [CrossRef]
- El-Didamony, H.; Amer, A.A.; Ela-ziz, H.A. Properties and durability of alkali-activated slag pastes immersed in sea water. Ceram. Int. 2012, 38, 3773–3780. [Google Scholar] [CrossRef]
- Reddy, D.V.; Edouard, J.-B.; Sobhan, K. Durability of fly ash-based geopolymer structural concrete in the marine environment. J. Mater. Civ. Eng. 2013, 25, 781–787. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Raman, R.K.S.; Al-Saadi, S. Thermal and mechanical properties of alkali-activated slag paste, mortar and concrete utilizing seawater and sea sand. Constr. Build. Mater. 2018, 159, 704–724. [Google Scholar] [CrossRef]
- Shi, J.J.; Deng, C.H.; Zhang, Y.M. Early corrosion behaviour of rebars embedded in the alkali-activated slag mortar. J. Build. Mater. 2016, 19, 969–975. (In Chinese) [Google Scholar]
- Zhang, B.; Zhu, H.; Cheng, Y.; Huseien, G.F.; Shah, K.W. Shrinkage mechanisms and shrinkage-mitigating strategies of alkali-activated slag composites: A critical review. Constr. Build. Mater. 2022, 318, 125993. [Google Scholar] [CrossRef]
- Rashad, A.M.; Morsi, W.M.; Khafaga, S.A. Effect of limestone powder on mechanical strength, durability and drying shrinkage of alkali-activated slag pastes. Innov. Infrastruct. Solut. 2021, 6, 127. [Google Scholar] [CrossRef]
- Mohammed, T.U.; Hamada, H.; Yamaji, T. Performance of seawater-mixed concrete in the tidal environment. Cem. Concr. Res. 2004, 34, 593–601. [Google Scholar] [CrossRef]
- Dewar, J.D. The Workability and Compressive Strength of Concrete Made with Sea Water; Cement and Concrete Association: London, UK, 1963. [Google Scholar]
- Yang, S.; Xu, J.; Zang, C.; Li, R.; Yang, Q.; Sun, S. Mechanical properties of alkali-activated slag concrete mixed by seawater and sea sand. Constr. Build. Mater. 2019, 196, 395–410. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, H.; Shah, K.W.; Dong, Z.; Wu, J. Performance evaluation and microstructure characterization of seawater and coral/sea sand alkali-activated mortars. Constr. Build. Mater. 2020, 259, 120403. [Google Scholar] [CrossRef]
- JGJ 52-2006; Standard for Quality and Testing Methods of Sand and Crushed Stone for Ordinary Concrete. Ministry of Construction of the People’s Republic of China: Beijing, China, 2006.
- ASTM D1141-98; Standard Practice for the Preparation of Substitute Ocean Water. ASTM International: West Conshohocken, PA, USA, 1998.
- GB/T 2419-2005; Test Method for Fluidity of Cement Mortar. Standardization Administration of China: Beijing, China, 2005.
- GB/T 17671-1999; Method of Testing Cements—Determination of Strength. Standardization Administration of China: Beijing, China, 1999.
- JGJ/T 70-2009; Standard for Test Method of Performance on Building Mortar. Ministry of Housing and Urban-Rural Development of the People’s Republic of China: Beijing, China, 2009.
- JC/T 603-2004; Standard Test Method for Drying Shrinkage of Mortar. Ministry of Housing and Urban-Rural Development of the People’s Republic of China: Beijing, China, 2004.
- IUPAC. Manual of symbols and terminology. J. Pure Appl. Chem. 1972, 31, 578. [Google Scholar]
- Nguyen, K.T.; Le, T.A.; Lee, K. Evaluation of the mechanical properties of sea sand-based geopolymer concrete and the corrosion of embedded steel bar. Constr. Build. Mater. 2018, 169, 462–472. [Google Scholar] [CrossRef]
- Lyu, X.; Robinson, N.I.; Elchalakani, M.; Johns, M.L.; Dong, M.; Nie, S. Sea sand seawater geopolymer concrete. J. Build. Eng. 2022, 50, 104141. [Google Scholar] [CrossRef]
- Newman, K. Sea-dredged aggregates for concrete. In Proceedings of the Symposium: Sea-Dredged Aggregates for Concrete, Sand and Gravel Association Great Britain, Buckinghamshire, UK, 9 December 1968. [Google Scholar]
- Sun, B.; Sun, Y.; Ye, G.; De Schutter, G. A mix design methodology of slag and fly ash-based alkali-activated paste. Cem. Concr. Compos. 2022, 126, 104368. [Google Scholar] [CrossRef]
- Shi, D.; Yao, Y.; Ye, J. Effects of seawater on mechanical properties, mineralogy and microstructure of calcium silicate slag-based alkali-activated materials. Constr. Build. Mater. 2019, 212, 569–577. [Google Scholar] [CrossRef]
- Rashad, A.M.; Khalil, M.H. An investigation of using seawater as mixing water for alkali-activated slag pastes. Innov. Infrastruct. Solut. 2022, 7, 152. [Google Scholar] [CrossRef]
- Li, Q.; Geng, H.; Shui, Z.; Huang, Y. Effect of metakaolin addition and seawater mixing on the properties and hydration of concrete. Appl. Clay Sci. 2015, 115, 51–60. [Google Scholar] [CrossRef]
- Bhaskar, S.; Smitha, M.; John, E. Relevance of sea water as mixing water in concrete. Eng. Technol. 2016, 5, 17084–17090. [Google Scholar]
- Rashad, A.M.; Bai, Y.; Basheer, P.A.M.; Milestone, N.B.; Collier, N.C. Hydration and properties of sodium sulfate activated slag. Cem. Concr. Compos. 2013, 37, 20–29. [Google Scholar] [CrossRef]
- Shinde, B.H.; Kadam, K.N. Strength properties of fly ash based geopolymer concrete with sea sand. Am. J. Eng. Res. 2016, 5, 129–132. [Google Scholar]
- Anbarasan, I.; Soundarapandian, N. Investigation of mechanical and microstructural properties of geopolymer concrete blended by dredged marine sand and manufactured sand under ambient curing conditions. Struct. Concr. 2019, 21, 992–1003. [Google Scholar] [CrossRef]
- Rashad, A.M.; Gharieb, M. An investigation on the effect of sea sand on the properties of fly ash geopolymer mortars. Innov. Infrastruct. Solut. 2021, 6, 53. [Google Scholar] [CrossRef]
- Puertas, F.; Martinez-Ramirez, S.; Alonso, S.; Vázquez, T. Alkali-activated fly ash/slag cements: Strength behaviour and hydration products. Cem. Concr. Res. 2000, 30, 1625–1632. [Google Scholar] [CrossRef]
- Shi, Z.; Shi, C.; Wan, S.; Ou, Z. Effect of alkali dosage on alkali-silica reaction in sodium hydroxide activated slag mortars. Constr. Build. Mater. 2017, 143, 16–23. [Google Scholar] [CrossRef]
- Fang, S.; Lam, E.S.S.; Li, B.; Wu, B. Effect of alkali contents, moduli and curing time on engineering properties of alkali activated slag. Constr. Build. Mater. 2020, 249, 118799. [Google Scholar] [CrossRef]
- Singh, A.; Bhadauria, S.S.; Mudgal, M.; Kushwah, S.S. Effect of alkali activator dosage on compressive and tensile strength of ground granulated blast furnace slag based geopolymer concrete. Can. J. Civ. Eng. 2022, 49, 73–82. [Google Scholar] [CrossRef]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. In situ ATR-FTIR study of the early stages of fly ash geopolymer gel formation. Langmuir 2007, 23, 9076–9082. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ou, G.; Qiu, Q.; Chen, X.; Hong, J.; Xing, F. Chloride transport and microstructure of concrete with/without fly ash under atmospheric chloride condition. Constr. Build. Mater. 2017, 146, 493–501. [Google Scholar] [CrossRef]
- Wei, T.F. Experimental Study on Early Shrinkage and Cracking of Sea Sand Mortar. Master’s Thesis, Harbin Engineering University, Harbin, China, 2018. [Google Scholar] [CrossRef]
- Meddah, M.S.; Lmbachiya, M.C.; Dhir, R.K. Potential use of binary and composite limestone cements in concrete production. Constr. Build. Mater. 2014, 58, 193–205. [Google Scholar] [CrossRef]
- Tongaroonsri, S.; Tangtermsirikul, S. Effect of mineral admixtures and curing periods on shrinkage and cracking age under restrained condition. Constr. Build. Mater. 2009, 23, 1050–1056. [Google Scholar] [CrossRef]
- Atis, C.D.; Bilim, C.; Celik, O.; Karahan, O. Influence of activator on the strength and drying shrinkage of alkali-activated slag mortar. Constr. Build. Mater. 2007, 23, 548–555. [Google Scholar] [CrossRef]
- Lee, N.K.; Jang, J.G.; Lee, H.K. Shrinkage characteristics of alkali-activated fly ash/slag paste and mortar at early ages. Cem. Concr. Compos. 2014, 53, 239–248. [Google Scholar] [CrossRef]
- Shi, D.; Ye, J.; Zhang, W. Effects of activator content on properties, mineralogy, hydration and microstructure of alkali-activated materials synthesized from calcium silicate slag and ground granulated blast furnace slag. J. Build. Eng. 2020, 32, 101791. [Google Scholar] [CrossRef]
- Liu, X.; Liu, E.; Fu, Y. Reduction in Drying Shrinkage and Efflorescence of Recycled Brick and Concrete Fine Powder-Slag-Based Geopolymer. Appl. Sci. 2023, 13, 2997. [Google Scholar] [CrossRef]
- Li, Z.; Lu, T.; Liang, X.; Dong, H.; Ye, G. Mechanisms of autogenous shrinkage of alkali-activated slag and fly ash pastes. Cem. Concr. Res. 2020, 135, 106107. [Google Scholar] [CrossRef]
- Fang, G.; Bahrami, H.; Zhang, M. Mechanisms of autogenous shrinkage of alkali-activated fly ash-slag pastes cured at ambient temperature within 24 h. Constr. Build. Mater. 2018, 171, 377–387. [Google Scholar] [CrossRef]
- Li, S. Shrinkage Properties of alkali-Excited Slag-Metakaolin Cementitious Materials. Master’s Thesis, Chongqing University, Chongqing, China, 2019. [Google Scholar] [CrossRef]
- Wu, Z. Study of the Effect of Expansion Agents on the Shrinkage of Alkali-Excited Materials. Master’s Thesis, Guangzhou University, Guangzhou, China, 2021. (In Chinese). [Google Scholar]
- Ruiz-Santaquiteria, C.; Skibsted, J.; Fernández-Jiménez, A.; Palomo, A. Alkaline solution/ binder ratio as a determining factor in the alkaline activation of aluminosilicates. Cem. Concr. Res. 2012, 42, 1242–1251. [Google Scholar] [CrossRef]
- Taghvayi, H.Q.; Behfarnia, K.; Khalili, M. The Effect of Alkali Concentration and Sodium Silicate Modulus on the Properties of Alkali-Activated Slag Concrete. J. Adv. Concr. Technol. 2018, 16, 293–305. [Google Scholar] [CrossRef]
- Jun, Y.; Kim, T.; Kim, J.H. Chloride-bearing characteristics of alkali-activated slag mixed with seawater: Effect of different salinity levels. Cem. Concr. Compos. 2020, 112, 103680. [Google Scholar] [CrossRef]
- Yu, J.; Lu, C.; Leung, C.K.Y.; Li, G. Mechanical properties of green structural concrete with ultrahigh-volume fly ash. Constr. Build. Mater. 2017, 147, 510–518. [Google Scholar] [CrossRef]
- Birnin-Yauri, U.A.; Glasser, F.P. Friedel’s salt, Ca2Al(OH)6(ClOH)·2H2O: Its solid solutions and their role in chloride binding. Cem. Concr. Res. 1998, 28, 1713–1723. [Google Scholar] [CrossRef]
- Rapin, J.P.; Renaudin, G.; Elkaim, E.; Francois, M. Structural transition of Friedel’s salt 3CaO·Al2O3·CaCl2·10H2O studied by synchrotron powder diffraction. Cem. Concr. Res. 2002, 32, 513–519. [Google Scholar] [CrossRef]
- Lv, W.; Sun, Z.; Su, Z. Study of seawater mixed one-part alkali activated GGBFS-fly ash. Cem. Concr. Compos. 2020, 106, 103484. [Google Scholar] [CrossRef]
- Ma, H.Q. Study on the Properties of Alkali-Excited Gangue-Slag Cementitious Materials and Durability of Concrete. Master’s Thesis, China University of Mining and Technology, Xuzhou, China, 2021. [Google Scholar]
- Ramachandran, V.S.; Paroli, R.M.; Beaudoin, J.J.; Delgado, A.H. Handbook of Thermal Analysis of Construction Materials. Thermochim. Acta 2003, 406, 249. [Google Scholar] [CrossRef]
- Hidalgo, A.; Domingo, C.; Garcia, C.; Petit, S.; Andrade, C.; Alonso, C. Microstructural changes induced in Portland cement-based materials due to natural and supercritical carbonation. J. Mater. Sci. 2008, 43, 3101–3111. [Google Scholar] [CrossRef]
- Yuan, Q.; Huang, Y.; Huang, T.; Yao, H.; Wu, Q.-H. Effect of activator on rheological properties of alkali-activated slag-fly ash pastes. J. Cent. South. Univ. 2022, 29, 282–295. [Google Scholar] [CrossRef]
- Zhang, G.; Lin, R.; Wang, Y.; Wang, X.-Y. Influence of K+ and CO32− in activator on high-temperature performance of alkali-activated slag-ceramic powder binary blends. Case Stud. Constr. Mater. 2022, 17, e01306. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Fu, L.; Wu, W.; Li, X. Preparation of one-part alkali-activated nickel slag binder using an optimal ball milling process. Constr. Build. Mater. 2022, 322, 125902. [Google Scholar] [CrossRef]
- Feng, S.; Zhu, J.; Wang, R.; Qu, Z.; Song, L.; Wang, H. The influence of CaO and MgO on the mechanical properties of alkali-activated blast furnace slag powder. Materials 2022, 15, 6128. [Google Scholar] [CrossRef]
- An, Q.; Pan, H.; Zhao, Q.; Wang, D. Strength development and microstructure of sustainable geopolymers made from alkali-activated ground granulated blast-furnace slag, calcium carbide residue, and red mud. Constr. Build. Mater. 2022, 356, 129279. [Google Scholar] [CrossRef]
- Ferone, C.; Colangelo, F.; Roviello, G. Application-oriented chemical optimization of a metakaolin based geopolymer. Materials 2013, 6, 1920–1939. [Google Scholar] [CrossRef]
- Xu, Z.; Yue, J.; Pang, G.; Li, R.; Zhang, P.; Xu, S. Influence of the Activator Concentration and Solid/Liquid Ratio on the Strength and Shrinkage Characteristics of Alkali-Activated Slag Geopolymer Pastes. Adv. Civ. Eng. 2021, 2021, 6631316. [Google Scholar] [CrossRef]
- Gao, X.; Liu, C.; Shui, Z.; Yu, R. Effects of Expansive Additives on the Shrinkage Behavior of Coal Gangue Based Alkali Activated Materials. Crystals 2021, 11, 816. [Google Scholar] [CrossRef]
- Gu, Y.; Fang, Y.; You, D.; Gong, Y.-F.; Zhu, C.-H. Properties and microstructure of alkali-activated slag cement cured at below- and about-normal temperature. Constr. Build. Mater. 2015, 79, 1–8. [Google Scholar] [CrossRef]
- Brough, A.R.; Atkinson, A. Sodium silicate-based, alkali-activated slag mortars: Part I. Strength, hydration and microstructure. Cem. Concr. Res. 2022, 32, 865–879. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Effect of pore size distribution on drying shrinking of alkali-activated slag concrete. Cem. Concr. Res. 2000, 30, 1401–1406. [Google Scholar] [CrossRef]
- Rashad, A.M.; Sadek, D.M.; Gharieb, M. Valorization of quartz powder for drying shrinkage and carbonation resistance of alkali-activated slag cement. Environ. Sci. Pollut. Res. 2022, 29, 45191–45203. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.M.E.; Basto, P.A.; Henrique, M.A.; Almeida, Y.M.B.; Neto, A.A.d.M. Optimizing the concentration of Na2O in alkaline activators to improve mechanical properties and reduce costs and CO2 emissions in alkali-activated mixtures. Constr. Build. Mater. 2022, 344, 128185. [Google Scholar] [CrossRef]







| Physical Property | DS | US | RS |
|---|---|---|---|
| moisture content/% | 0.28 | 0.54 | 4.80 |
| mud content/% | 1.20 | 1.52 | 2.46 |
| Chloride ion content/% | 0.050 | 0.163 | 0.003 |
| Shell content/% | 1.76 | 11.20 | / |
| Fineness modulus | 1.84 | 2.83 | 3.03 |
| Apparent density/kg/m3 | 2650 | 2640 | 2670 |
| Chemical Composition% | SiO2 | CaO | Al2O3 | K2O | Fe2O3 | MgO | Na2O |
|---|---|---|---|---|---|---|---|
| DS | 90.34 | 3.52 | 2.02 | 1.00 | 0.48 | 0.36 | 0.18 |
| US | 87.41 | 4.64 | 1.98 | 1.02 | 0.51 | 0.34 | 0.25 |
| RS | 93.70 | 2.16 | 2.22 | 0.93 | 0.44 | 0.53 | 0.12 |
| NaCl | MgCl2 | Na2SO4 | CaCl2 | KCl | NaHCO3 |
|---|---|---|---|---|---|
| 24.53 | 5.2 | 4.09 | 1.16 | 0.695 | 0.201 |
| Physical Quantities | Densities (g/cm3) | Specific Surface Area (m2/kg) | 7 Days Activity Index (%) | 28 Days Activity Index (%) | Heat Loss (%) | Chloride Ion Content (%) | Mobility Ratio (%) | Moisture Content (%) |
|---|---|---|---|---|---|---|---|---|
| Data | 2.92 | 425.00 | 78.00 | 96.00 | 1.02 | 0.03 | 103.00 | 0.36 |
| Oxide (%) | CaO | SiO2 | Al2O3 | MgO | SO3 | TiO2 | Na2O | Fe2O3 | MnO | K2O | Cl | SrO | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slag | 36.837 | 30.266 | 17.18 | 9.67 | 2.789 | 1.249 | 0.596 | 0.483 | 0.402 | 0.336 | 0.051 | 0.039 | 0.102 |
| Number | Group Name | Type of Water | Type of Sand | Water–Binder Ratio | Type of Alkali | Alkali Equivalent |
|---|---|---|---|---|---|---|
| 1 | RW | RW | DS | 0.43 | Na2SiO3 | 4% |
| 2 | SW | SW | DS | 0.43 | Na2SiO3 | 4% |
| 3 | DS | RW | DS | 0.43 | Na2SiO3 | 4% |
| 4 | US | RW | US | 0.43 | Na2SiO3 | 4% |
| 5 | RS | RW | RS | 0.43 | Na2SiO3 | 4% |
| 6 | N3 | SW | DS | 0.43 | Na2SiO3 | 3% |
| 7 | N4 | SW | DS | 0.43 | Na2SiO3 | 4% |
| 8 | N5 | SW | DS | 0.43 | Na2SiO3 | 5% |
| 9 | N6 | SW | DS | 0.43 | Na2SiO3 | 6% |
| Groups | RW/DS | SW | US | RS |
|---|---|---|---|---|
| Evaporable water content/% | 66.67 | 59.60 | 59.80 | 60.21 |
| Groups | N3 | N4 | N6 |
|---|---|---|---|
| Evaporable water content/% | 62.84 | 59.60 | 51.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Kang, S.; Shen, Y.; Song, C.; Lei, H.; Xie, W.; Chen, X.; Wang, J.; Wu, W.; Wang, X. Effects of Sand Type and Alkali Equivalent on Drying Shrinkage and Microstructure of Seawater–Sea Sand Alkali-Activated Slag Concrete. Materials 2025, 18, 1005. https://doi.org/10.3390/ma18051005
Zhang J, Kang S, Shen Y, Song C, Lei H, Xie W, Chen X, Wang J, Wu W, Wang X. Effects of Sand Type and Alkali Equivalent on Drying Shrinkage and Microstructure of Seawater–Sea Sand Alkali-Activated Slag Concrete. Materials. 2025; 18(5):1005. https://doi.org/10.3390/ma18051005
Chicago/Turabian StyleZhang, Jianbin, Sixiang Kang, Yanran Shen, Chenhao Song, Haoliang Lei, Wei Xie, Xianyun Chen, Jize Wang, Wenda Wu, and Xuefang Wang. 2025. "Effects of Sand Type and Alkali Equivalent on Drying Shrinkage and Microstructure of Seawater–Sea Sand Alkali-Activated Slag Concrete" Materials 18, no. 5: 1005. https://doi.org/10.3390/ma18051005
APA StyleZhang, J., Kang, S., Shen, Y., Song, C., Lei, H., Xie, W., Chen, X., Wang, J., Wu, W., & Wang, X. (2025). Effects of Sand Type and Alkali Equivalent on Drying Shrinkage and Microstructure of Seawater–Sea Sand Alkali-Activated Slag Concrete. Materials, 18(5), 1005. https://doi.org/10.3390/ma18051005






