High-Level Lanthanide-Doped Upconversion Nanoparticles-Based Aptasensor to Increase Carcinoembryonic Antigen Detection Sensitivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Apparatus
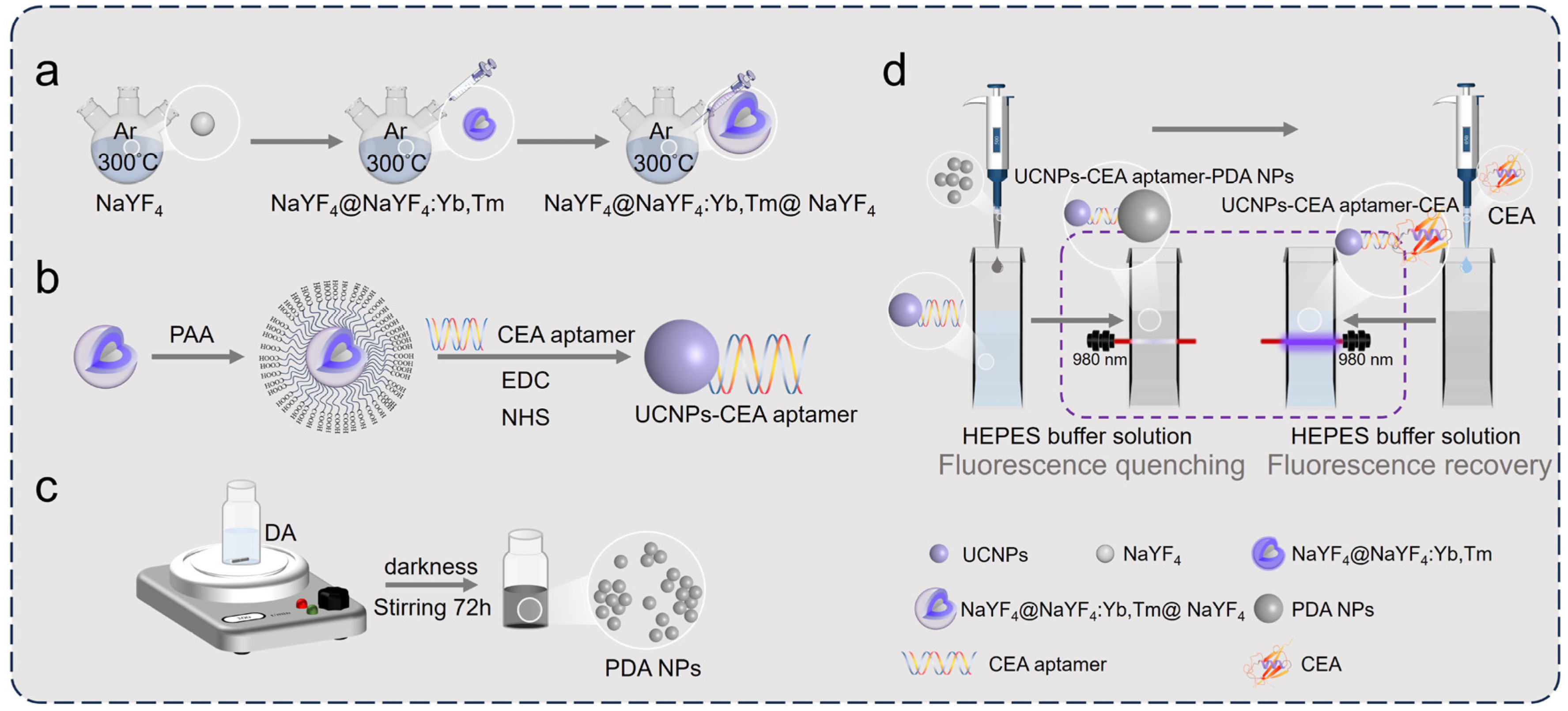
2.2. Synthesis Procedures
- (1)
- Preparation of bare core UCNPs
- (2)
- Preparation of core–shell-structured UCNPs
- (3)
- Preparation of PDA NPs
- (4)
- Coupling of UCNPs with CEA aptamer
- (5)
- Fluorescence quenching experiment
- (6)
- Determination of CEA in buffer solution/diluted fetal bovine serum
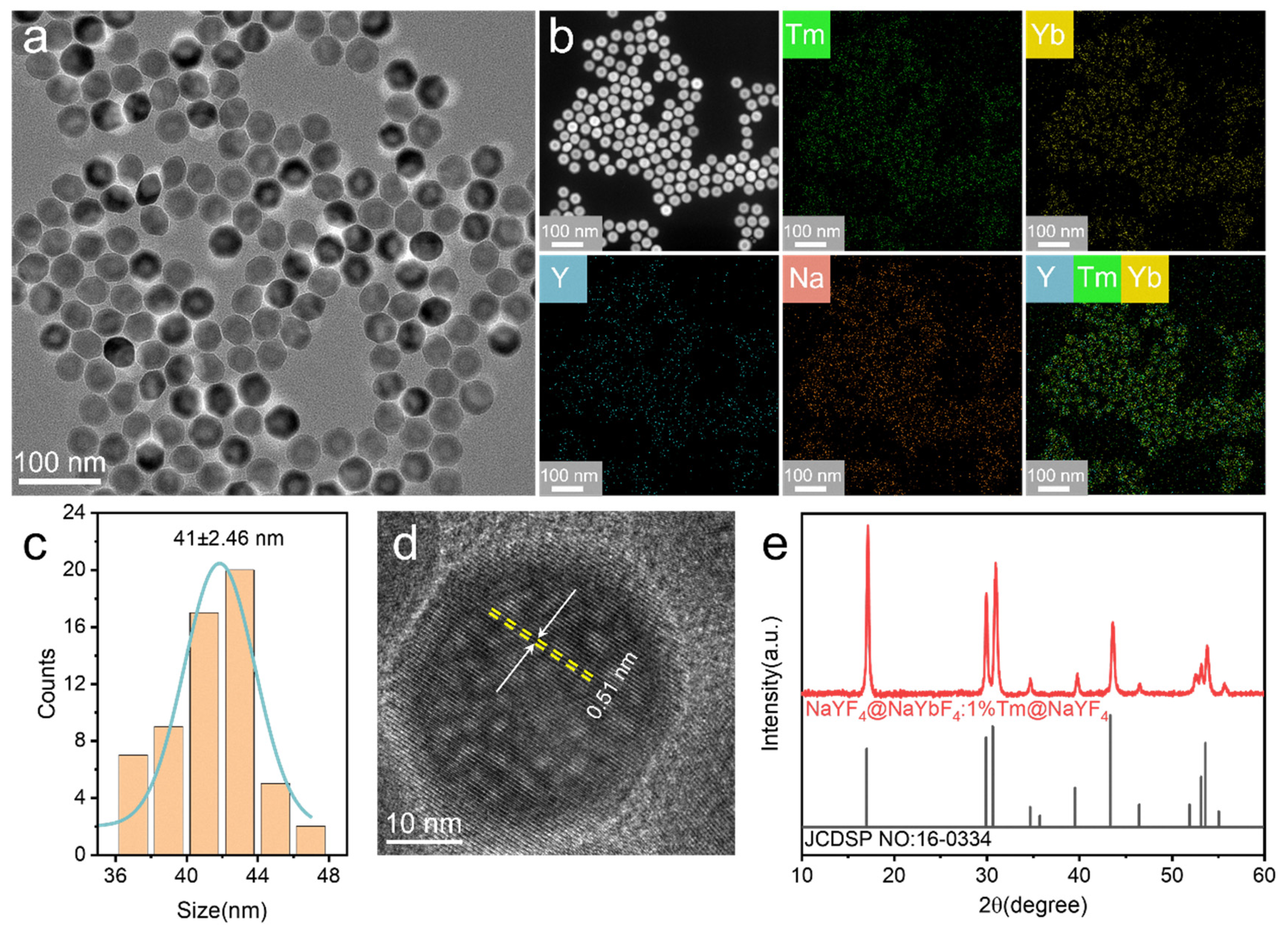
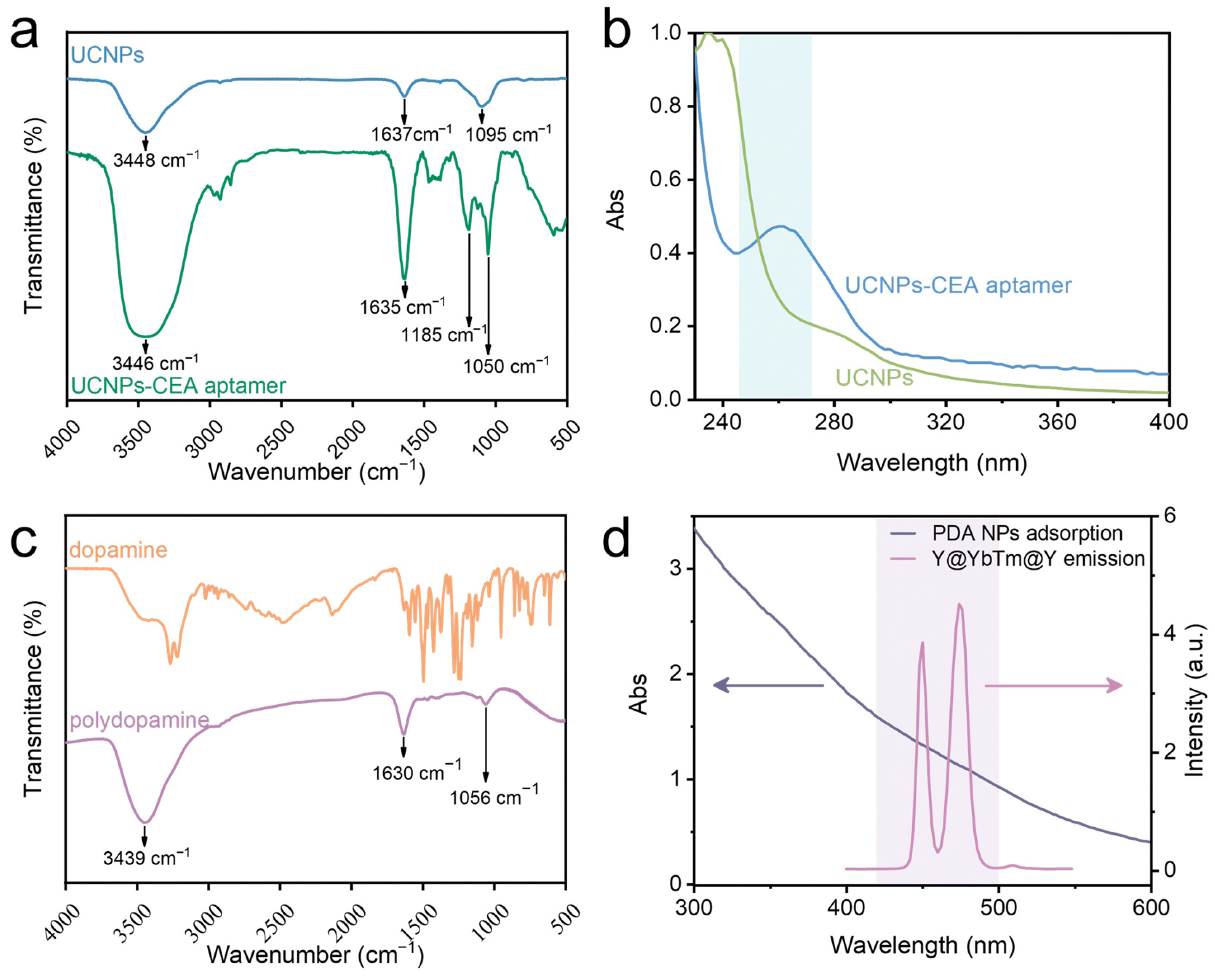
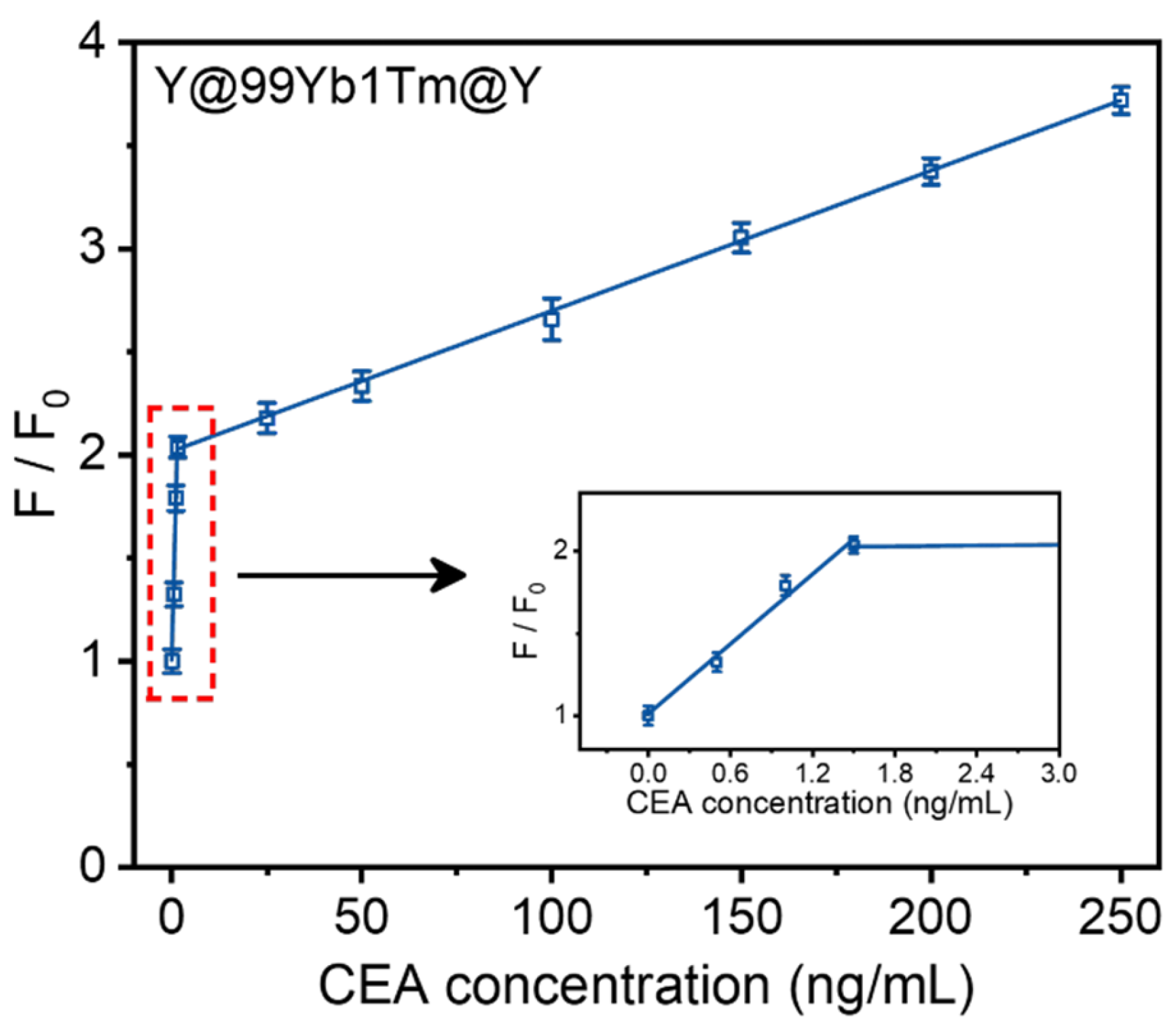
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jo, Y.; Lee, J.-H.; Cho, E.-S.; Lee, H.S.; Shin, S.-J.; Park, E.J.; Baik, S.H.; Lee, K.Y.; Kang, J. Clinical significance of early carcinoembryonic antigen change in patients with nonmetastatic colorectal cancer. Front. Oncol. 2022, 12, 739614. [Google Scholar] [CrossRef]
- Sorokin, J.J.; Kupchik, H.Z.; Zamcheck, N.; Dhar, P. A clinical comparison of two radioimmunoassays for carcinoembryonic antigen (CEA). Immunol. Commun. 1972, 1, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Tommasi, M.; Pieroni, C.; Cappelli, G. Radioimmunoassay of carcinoembryonic antigen: Observations and methodological variations. J. Nucl. Med. Allied. Sci. 1978, 22, 163–167. [Google Scholar]
- Schiedeck, T.H.K.; Wellm, C.; Roblick, U.J.; Broll, R.; Bruch, H.P. Diagnosis and monitoring of colorectal cancer by L6 blood serum polymerase chain reaction is superior to carcinoembryonic antigen-enzyme-linked immunosorbent assay. Dis. Colon. Rectum. 2003, 46, 818–825. [Google Scholar] [CrossRef]
- Huang, J.; Jiao, L.; Xu, W.; Fang, Q.; Wang, H.; Cai, X.; Yan, H.; Gu, W.; Zhu, C. Immobilizing enzymes on noble metal hydrogel nanozymes with synergistically enhanced peroxidase activity for ultrasensitive immunoassays by cascade signal amplification. ACS Appl. Mater. 2021, 13, 33383–33391. [Google Scholar] [CrossRef]
- Han, R.; Sun, Y.; Dai, Y.; Gao, D.; Wang, X.; Luo, C. A chemiluminescence aptasensor for sensitive detection of carcinoembryonic antigen based on dual aptamer-conjugates biorecognition. Sens. Actuators B Chem. 2021, 326, 128833. [Google Scholar] [CrossRef]
- Qin, X.; Zheng, C.; Li, Y.; Lu, X.; Mao, Q. Evaluation of the effect of hemolysis on quantitative chemiluminescent immunoassay results for 10 analytes. Clin. Lab. 2021, 67, 2423–2430. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, B.; Wang, Y.; Zhao, F.; Zeng, B. Electrochemiluminescence immunosensor for the detection of carcinoembryonic antigen based on oxygen vacancy-rich Co3O4 nanorods and luminol. ACS Appl. Nano Mater. 2021, 4, 7264–7271. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, J.; Zhou, P.; Liu, J.; Qiao, Z.; Yu, K.; Jiang, J.; Su, B. Electrochemiluminescence distance and reactivity of coreactants determine the sensitivity of bead-based immunoassays. Angew. Chem. Int. Ed. 2023, 62, 2216525. [Google Scholar]
- Xu, Z.; Liu, X.; Zong, C.; Zhang, Q.; Gai, H. Homogeneous immunoassay utilizing fluorescence resonance energy transfer from quantum dots to tyramide dyes deposited on full immunocomplexes. Analyst. 2023, 148, 4877–4884. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Li, G.; Zhou, J.; Zhang, Y.; Kang, K.; Ying, B.; Yi, Q.; Wu, Y. A light-up fluorescence resonance energy transfer magnetic aptamer-sensor for ultra-sensitive lung cancer exosome detection. J. Mater. Chem. B. 2021, 9, 2483–2493. [Google Scholar] [CrossRef]
- Song, J.; Chen, Z.; Huang, D.; Xu, B. Prognostic impact of pretreatment elevated and normalized carcinoembryonic antigen levels after neoadjuvant chemoradiotherapy in resected locally advanced rectal cancer patients. Cancer Manag. Res. 2021, 13, 3713–3721. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.D.; Shandilya, R.; Bhargava, A.; Kumar, R.; Tiwari, R.; Chaudhury, K.; Srivastava, R.K.; Goryacheva, I.Y.; Mishra, P.K. Quantum dot based nano-biosensors for detection of circulating cell free miRNAs in lung carcinogenesis: From biology to clinical translation. Front. Genet. 2018, 9, 616. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Liu, Z. An aptasensor for carcinoembryonic antigen based on upconversion fluorescence resonance energy transfer. Sens. Actuators B Chem. 2015, 206, 531–537. [Google Scholar] [CrossRef]
- Li, H.; Shi, L.; Sun, D.-e.; Li, P.; Liu, Z. Fluorescence resonance energy transfer biosensor between upconverting nanoparticles and palladium nanoparticles for ultrasensitive CEA detection. Biosens. Bioelectron. 2016, 86, 791–798. [Google Scholar] [CrossRef]
- Du, K.M.; Feng, J.; Gao, X.; Zhang, H.J. Nanocomposites based on lanthanide-doped upconversion nanoparticles: Diverse designs and applications. Light Sci. Appl. 2022, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Kotulska, A.M.; Pilch-Wrobel, A.; Lahtinen, S.; Soukka, T.; Bednarkiewicz, A. Upconversion FRET quantitation: The role of donor photoexcitation mode and compositional architecture on the decay and intensity based responses. Light Sci. Appl. 2022, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Sedlmeier, A.; Gorris, H.H. Surface modification and characterization of photon-upconverting nanoparticles for bioanalytical applications. Chem. Soc. Rev. 2015, 44, 1526–1560. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tu, L.P.; Zhang, Y.Q.; Huang, D.X.; Liu, X.X.; Zhang, X.R.; Du, J.R.; Fan, R.W.; Yang, C.H.; Kraemer, K.W.; et al. Size-dependent lanthanide energy transfer amplifies upconversion luminescence quantum yields. Nat. Photonics 2024, 18, 440–449. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta. Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.A.; Parchur, A.K.; Li, Y.; Jia, T.; Lv, R.; Wang, Y.; Chen, G. Cytotoxicity and genotoxicity evaluation of chemically synthesized and functionalized upconversion nanoparticles. Coord. Chem. Rev. 2024, 504, 215672. [Google Scholar] [CrossRef]
- Ansari, A.A.; Aldajani, K.M.; AlHazaa, A.N.; Albrithen, H.A. Recent progress of fluorescent materials for fingermarks detection in forensic science and anti-counterfeiting. Coord. Chem. Rev. 2022, 462, 214523. [Google Scholar] [CrossRef]
- Park, Y.I.; Lee, K.T.; Suh, Y.D.; Hyeon, T. Upconverting nanoparticles: A versatile platform for wide-field two-photon microscopy and multi-modal in vivo imaging. Chem. Soc. Rev. 2015, 44, 1302–1317. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Park, J.H.; Kim, M.; Kim, J.S.; Shim, T.S. Fabrication of a tunable photothermal actuator via in situ oxidative polymerization of polydopamine nanoparticles in hydrogel bilayers. Soft Matter 2022, 18, 4604–4612. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ren, X.; Liu, Y.; Wang, L.; Zhou, Y.; Zhang, Y.; Yan, Z.; Lan, X.; Guo, L. Multifunctional carboxymethyl chitosan/oxidized carboxymethyl cellulose hydrogel loaded with ginsenoside Rg1 and polydopamine nanoparticles for infected diabetic wound healing. Int. J. Biol. Macromol. 2024, 282, 136686. [Google Scholar] [CrossRef] [PubMed]
- Chahar, D.; Yadav, B.; Venkatesu, P. Interactions of polydopamine nanoparticles with serine and cysteine proteases: Implications for enhancing protein stabilization and enzyme activity. ACS Appl. Nano Mater. 2024, 7, 26202–26214. [Google Scholar] [CrossRef]
- Ji, H.; Zhang, H.; Liu, G.; Hao, X. Nanoparticles based on polydopamine and honokiol for combination therapy of colorectal cancer. ACS Appl. Nano Mater. 2024, 7, 16852–16863. [Google Scholar] [CrossRef]
- Han, Y.-P.; Shen, L.; Li, Z.; Hu, C.-G.; Liu, Z.-H. An aptasensor based on upconversion nanoparticles-polydopamine nanoparticles nanosystem for detection of carcinoembryonic antigen. Chin. J. Anal. Chem. 2018, 46, 1178–1185. [Google Scholar]
- Yu, D.; Zha, Z.; Tang, S.; Qiu, Y.; Liu, D. Modification-free fluorescent biosensor for CEA based on polydopamine-coated upconversion nanoparticles. J. Fluoresc. 2022, 32, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Krämer, K.W.; Biner, D.; Frei, G.; Güdel, H.U.; Hehlen, M.P.; Lüthi, S.R. Hexagonal sodium yttrium fluoride based green and blue emitting upconversion phosphors. Chem. Mater. 2004, 16, 1244–1251. [Google Scholar] [CrossRef]
- Johnson, N.J.J.; He, S.; Diao, S.; Chan, E.M.; Dai, H.J.; Almutairi, A. Direct evidence for coupled surface and concentration quenching dynamics in lanthanide-doped nanocrystals. J. Am. Chem. Soc 2017, 139, 3275–3282. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Li, Q.Q.; Xue, B.; Li, C.X.; Chang, Y.L.; Zhang, Y.L.; Liu, X.M.; Tu, L.P.; Zhang, H.; Kong, X.G. Employing shells to eliminate concentration quenching in photonic upconversion nanostructure. Nanoscale 2017, 9, 7941–7946. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Xu, X.; Wang, F.; Zhou, Z.; Liu, D.; Zhao, J.; Guan, M.; Lang, C.I.; Jin, D. Optimal sensitizer concentration in single upconversion nanocrystals. Nano Lett. 2017, 17, 2858–2864. [Google Scholar] [CrossRef]
- Gao, D.; Chen, B.J.; Sha, X.Z.; Zhang, Y.H.; Chen, X.; Wang, L.; Zhang, X.Z.; Zhang, J.S.; Cao, Y.Z.; Wang, Y.C.; et al. Near infrared emissions from both high efficient quantum cutting (173%) and nearly-pure-color upconversion in NaY(WO4)2:Er3+/Yb3+ with thermal management capability for silicon-based solar cells. Light Sci. Appl. 2024, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Gargas, D.J.; Chan, E.M.; Ostrowski, A.D.; Aloni, S.; Altoe, M.V.P.; Barnard, E.S.; Sanii, B.; Urban, J.J.; Milliron, D.J.; Cohen, B.E.; et al. Engineering bright sub-10-nm upconverting nanocrystals for single-molecule imaging. Nat. Nanotechnol. 2014, 9, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Chen, G.; Baev, A.; He, G.S.; Shao, W.; Damasco, J.; Prasad, P.N. Alleviating luminescence concentration quenching in upconversion nanoparticles through organic dye sensitization. J. Am. Chem. Soc. 2016, 138, 15130–15133. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, J.; Liu, X. Direct evidence of a surface quenching effect on size-dependent luminescence of upconversion nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 7456–7460. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Z.; Luo, X.; Wan, Q.; Qiu, R.; Wang, S. An ultrasensitive homogeneous aptasensor for carcinoembryonic antigen based on upconversion fluorescence resonance energy transfer. Talanta 2019, 195, 33–39. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Li, X. Solvothermal synthesis and modification of NaYF4: Yb/Er@NaLuF4: Yb for enhanced up-conversion luminescence for bioimaging. RSC Adv. 2019, 9, 42163–42171. [Google Scholar] [CrossRef]
- Ma, S.; Qi, Y.-X.; Jiang, X.-Q.; Chen, J.-Q.; Zhou, Q.-Y.; Shi, G.; Zhang, M. Selective and sensitive monitoring of cerebral antioxidants based on the dye-Labeled DNA/polydopamine conjugates. Anal. Chem. 2016, 88, 11647–11653. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.H.; Cheng, Y.; Xu, J.; Lin, H.; Wang, Y.S. Thermo-enhanced upconversion luminescence in inert-core/active-shell UCNPs: The inert core matters. Nanoscale 2021, 13, 6569–6576. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.Y.; Gao, H.P.; Mao, Y.L. Understanding the effect of Mn2+ on Yb3+/Er3+ upconversion and obtaining a maximum upconversion fluorescence enhancement in inert-core/active-shell/inert-shell structures. RSC Adv. 2016, 6, 83321–83327. [Google Scholar] [CrossRef]
- Zuo, J.; Tu, L.; Li, Q.; Feng, Y.; Que, I.; Zhang, Y.; Liu, X.; Xue, B.; Cruz, L.J.; Chang, Y.; et al. Near infrared light sensitive ultraviolet−blue nanophotoswitch for imaging-guided “Off−On” therapy. ACS Nano 2018, 12, 3217–3225. [Google Scholar] [CrossRef]
- Kamaç, M.B.; Altun, M.; Yilmaz, M.; Aktan, A.Y.; Aktan, S.; Sezgintürk, M.K. Point-of-care testing: A disposable label-free electrochemical CA125 and HE4 immunosensors for early detection of ovarian cancer. Biomed. Microdevices 2023, 25, 18. [Google Scholar]
- Kamaç, M.B.; Altun, M.; Yilmaz, M.; Sezgintürk, M.K. A label-free dual immunosensor for the simultaneous electrochemical determination of CA125 and HE4 biomarkers for the early diagnosis of ovarian cancer. Anal. Bioanal. Chem. 2023, 415, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Dong, B.; Zhou, D.L.; Yin, Z.; Cui, S.B.; Xu, W.; Chen, B.J.; Song, H.W. Paper-based upconversion fluorescence resonance energy transfer biosensor for sensitive detection of multiple cancer biomarkers. Sci. Rep. 2016, 6, 23406. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Feng, F.; Chen, Z.Z.; Bai, Y.F.; Guo, F.F.; Wu, F.Y.; Zhou, G. Sensitive detection of carcinoembryonic antigen using surface plasmon resonance biosensor with gold nanoparticles signal amplification. Talanta 2015, 140, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhang, G.; Yang, W.; Qiu, B.; Chen, G. CEA fluorescence biosensor based on the FRET between polymer dots and Au nanoparticles. Chem. Comm. 2012, 48, 9918–9920. [Google Scholar] [CrossRef]
- Li, X.H.; Sun, W.M.; Wu, J.; Gao, Y.; Chen, J.H.; Chen, M.; Ou, Q.S. An ultrasensitive fluorescence aptasensor for carcino-embryonic antigen detection based on fluorescence resonance energy transfer from upconversion phosphors to Au nanoparticles. Anal. Methods 2018, 10, 1552–1559. [Google Scholar] [CrossRef]
- Bhuckory, S.; Wegner, K.D.; Qiu, X.; Wu, Y.T.; Jennings, T.L.; Incamps, A.; Hildebrandt, N. Triplexed CEA-NSE-PSA immunoassay using time-gated terbium-to-quantum dot FRET. Molecules 2020, 25, 3679. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Wang, C.Q.; Pan, X.H.; Liu, S.Q. A high-throughput homogeneous immunoassay based on Förster resonance energy transfer between quantum dots and gold nanoparticles. Anal. Chim. Acta 2013, 763, 43–49. [Google Scholar] [CrossRef] [PubMed]


| Type of Sensor | Label | Limit of Detection (LOD) (ng/mL) | Linear Concentration Range | Reference |
|---|---|---|---|---|
| FRET | UCNPs@PDA/AuNPs-CEA aptamer | 0.031 | 0.1 ng/mL to 100 ng/mL | [29] |
| FRET | Magnetic NPs/UCNPs | 0.1 | 0.1 ng/mL to 40 ng/mL | [14] |
| FRET | UCNPs/FITC | 0.89 | 0.1 ng/mL to 100 ng/mL | [46] |
| FRET | UCNPs/carbon nanoparticle | 1.0 | 1 ng/mL to 60 ng/mL | [47] |
| FRET | Poly(9,9-dioctylfluorenyl-2,7-diyl) dots (PFO dots)/Au-NPs | 2.0 | 0.1 ng/mL to 10 ng/mL | [48] |
| FRET | UCNPs/Au-NPs | 0.02 | 0.05 ng/mL to 2.0ng/mL | [49] |
| FRET | Time-gated terbium/quantum dots | 3.6 | 0 ng/mL to 120 ng/mL | [50] |
| FRET | Quantum dots/gold nanoparticles | 0.3 | 1 ng/mL to 110 ng/mL | [51] |
| FRET | Quantum dots/tyramide Alexa 594 | 0.28 | 0.08 ng/mL to 20 ng/mL | [10] |
| FRET | UCNPs/PDA NPs | 0.0117 or 1.14 | 0 ng/mL to 1.5 ng/mL or 1.5 ng/mL to 250 ng/mL | This work |
| Sample No. | Added (ng/mL) | Found (ng/mL) | Recovery (%) | Relative Standard Deviation (%, n=3) |
|---|---|---|---|---|
| 1 | 0.5 | 0.482 | 96.4 | 2.34 |
| 2 | 1.5 | 1.467 | 97.76 | 4.13 |
| 3 | 10 | 10.872 | 108.7 | 3.5 |
| 4 | 50 | 46.1 | 92.2 | 5.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, L.; Sun, Q.; Wei, S.; Gong, D.; Wang, E.; Chen, Y.; Xia, L.; Liu, X.; Tu, L.; Shao, L.; et al. High-Level Lanthanide-Doped Upconversion Nanoparticles-Based Aptasensor to Increase Carcinoembryonic Antigen Detection Sensitivity. Materials 2025, 18, 796. https://doi.org/10.3390/ma18040796
Niu L, Sun Q, Wei S, Gong D, Wang E, Chen Y, Xia L, Liu X, Tu L, Shao L, et al. High-Level Lanthanide-Doped Upconversion Nanoparticles-Based Aptasensor to Increase Carcinoembryonic Antigen Detection Sensitivity. Materials. 2025; 18(4):796. https://doi.org/10.3390/ma18040796
Chicago/Turabian StyleNiu, Lujun, Qiren Sun, Shijia Wei, Dixiang Gong, Enhui Wang, Yan Chen, Lu Xia, Xingyu Liu, Langping Tu, Long Shao, and et al. 2025. "High-Level Lanthanide-Doped Upconversion Nanoparticles-Based Aptasensor to Increase Carcinoembryonic Antigen Detection Sensitivity" Materials 18, no. 4: 796. https://doi.org/10.3390/ma18040796
APA StyleNiu, L., Sun, Q., Wei, S., Gong, D., Wang, E., Chen, Y., Xia, L., Liu, X., Tu, L., Shao, L., Li, H., & Zuo, J. (2025). High-Level Lanthanide-Doped Upconversion Nanoparticles-Based Aptasensor to Increase Carcinoembryonic Antigen Detection Sensitivity. Materials, 18(4), 796. https://doi.org/10.3390/ma18040796






