Different Methods for Calculation of Activation Energies During Non-Isothermal Annealing of Mg72Zn27Pt1 and Mg72Zn27Cu1 Metallic Glasses
Abstract
1. Introduction
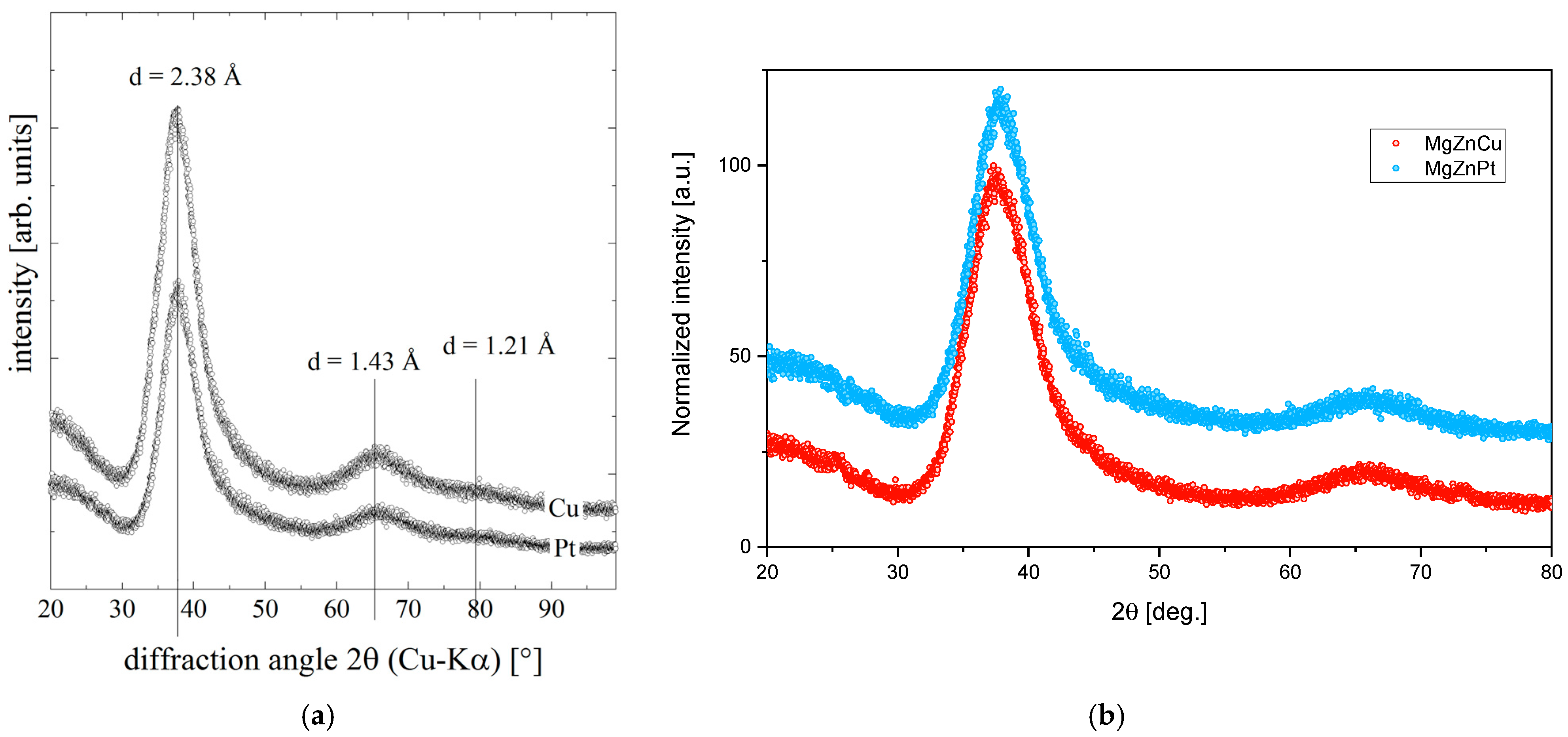
2. Materials and Methods
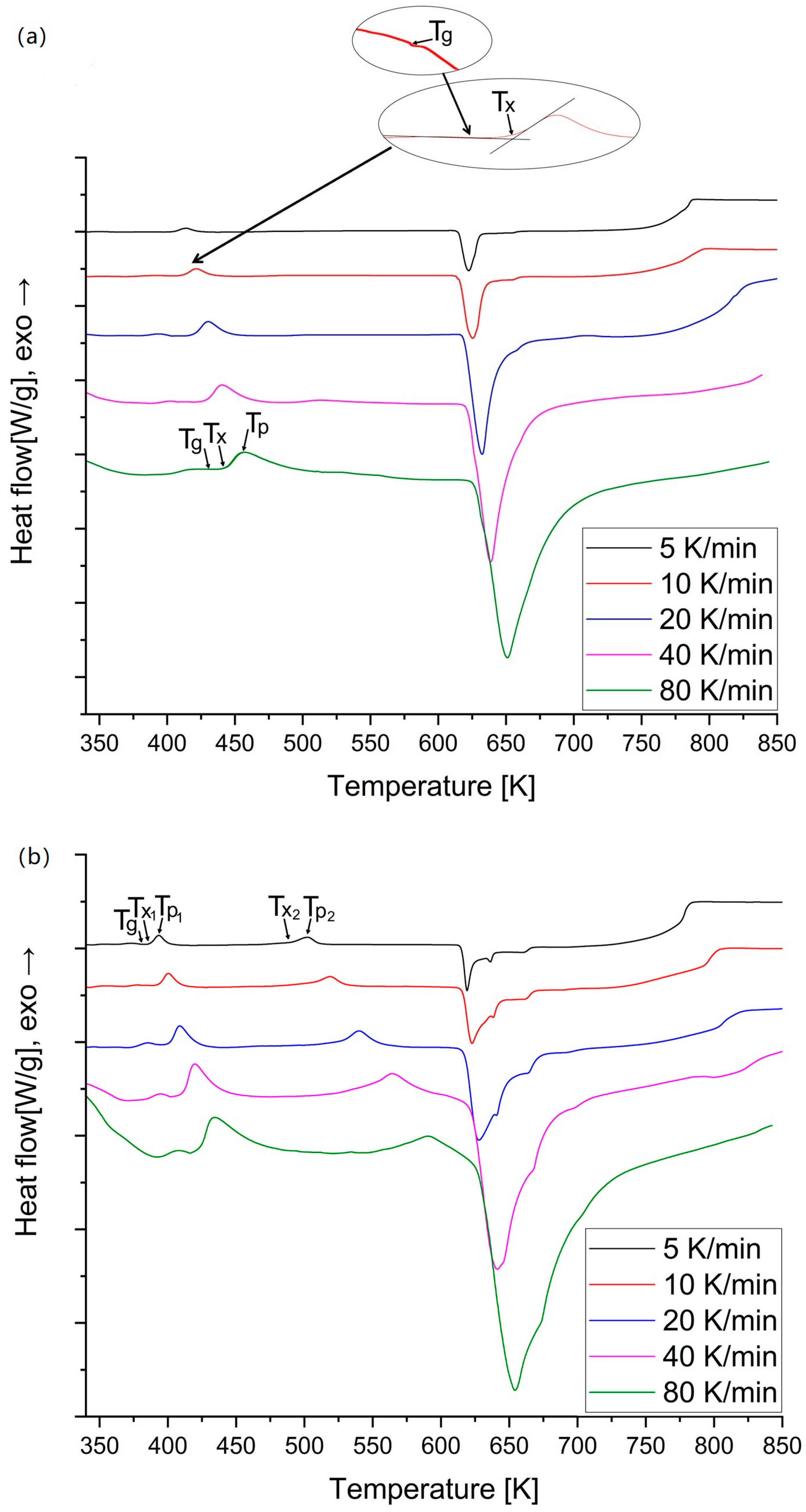
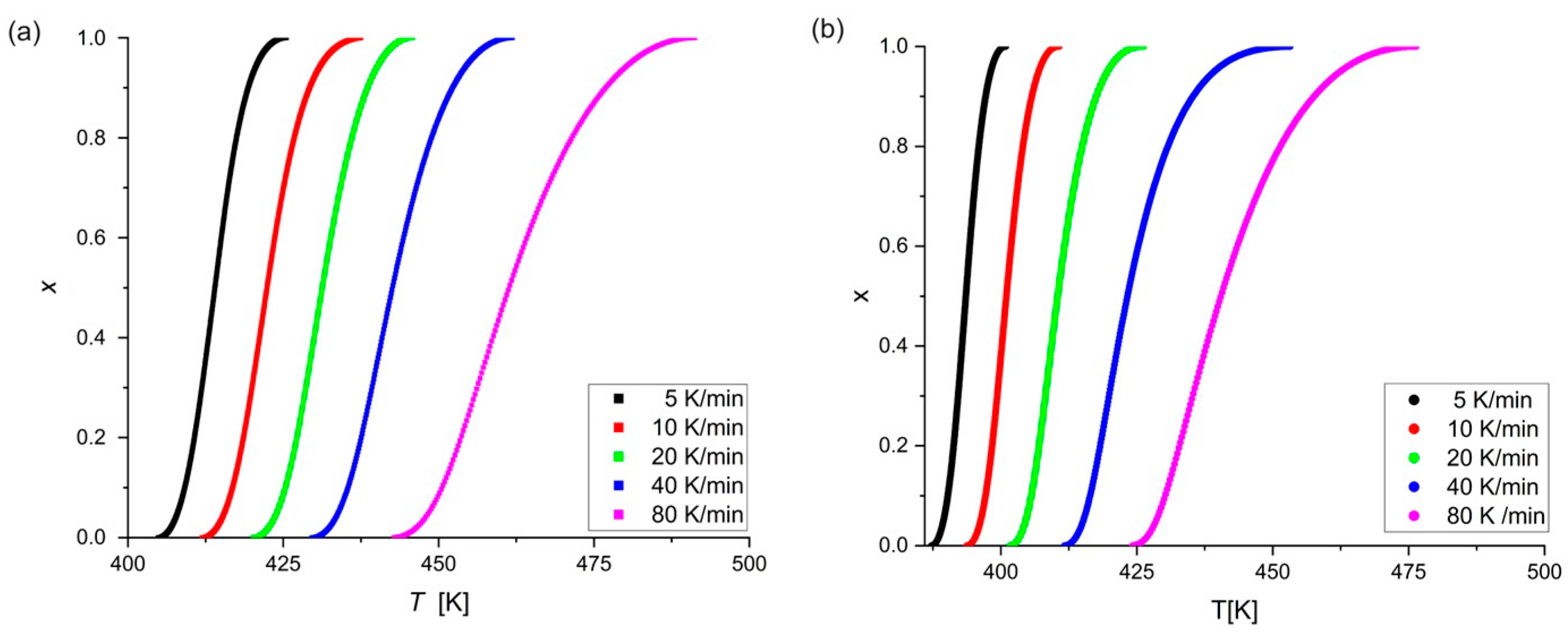
3. Results
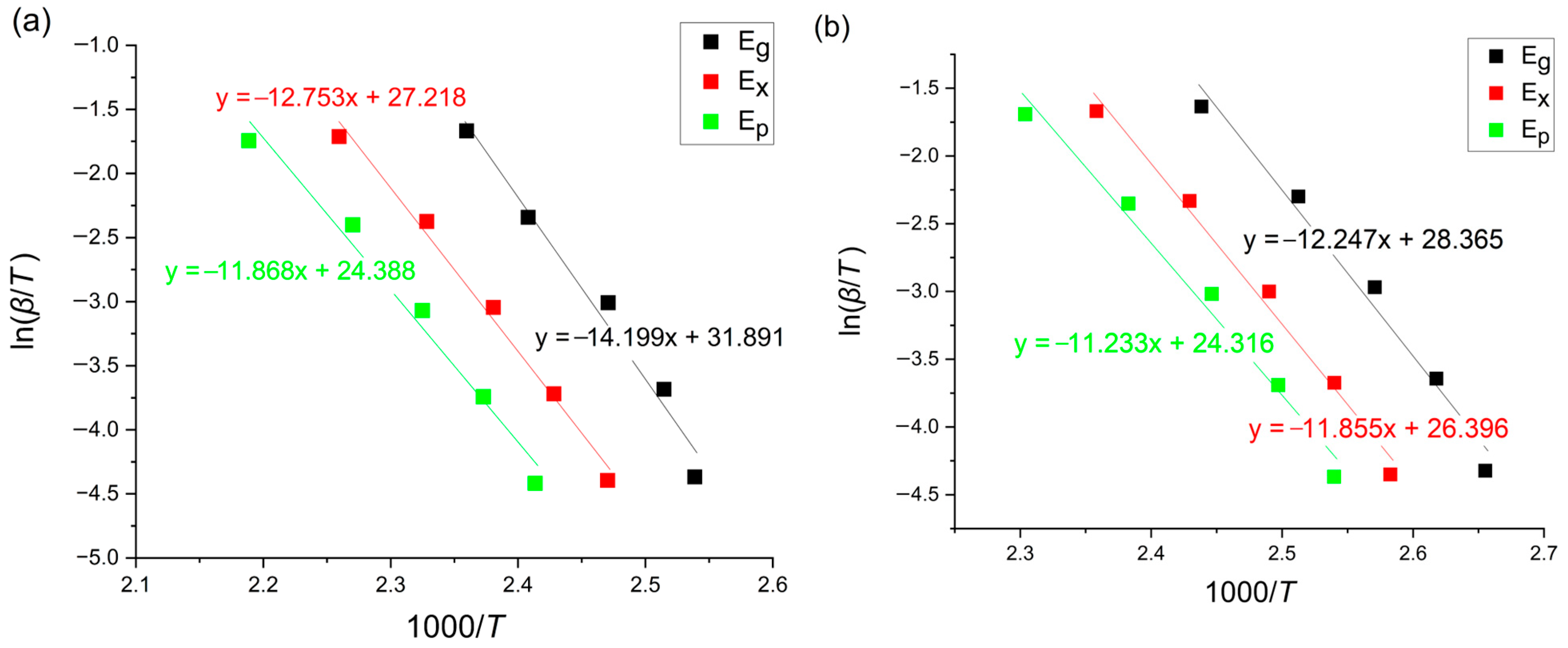
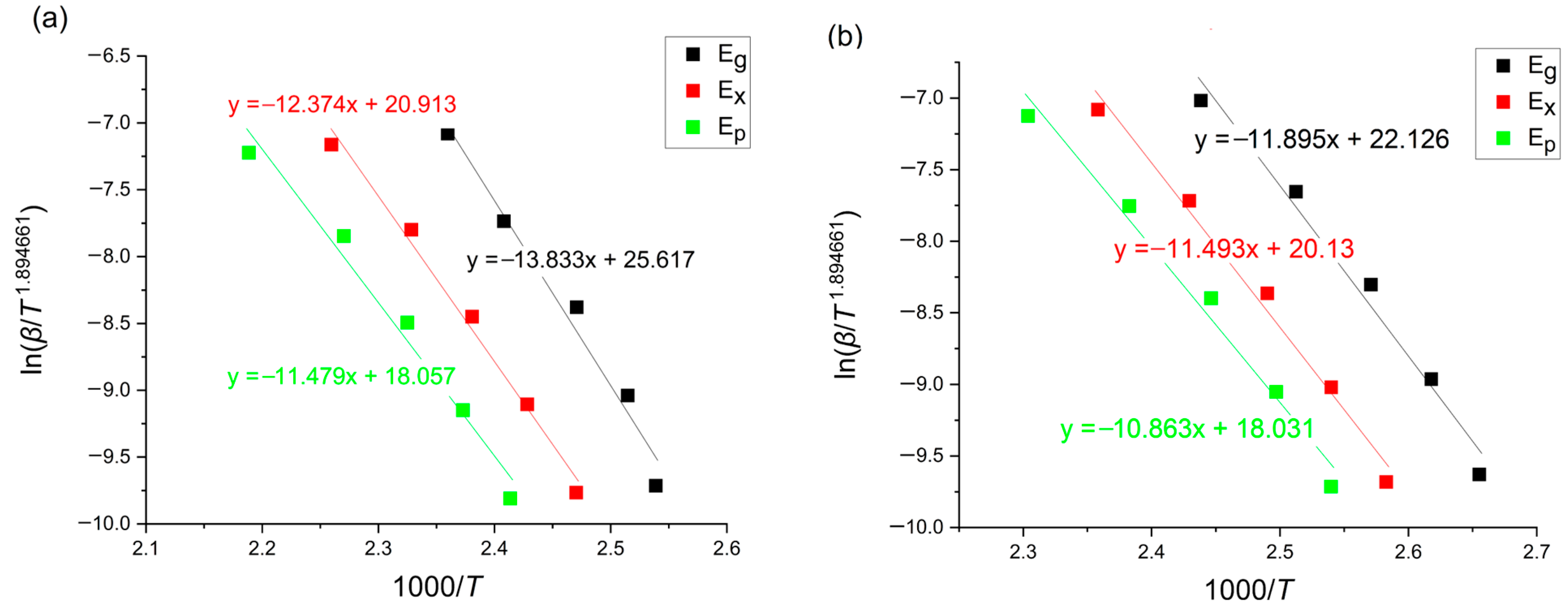
3.1. Kissinger Method
3.2. Ozawa–Flynn–Wall Method (OFW)
3.3. Boswell Method
3.4. Tang Method
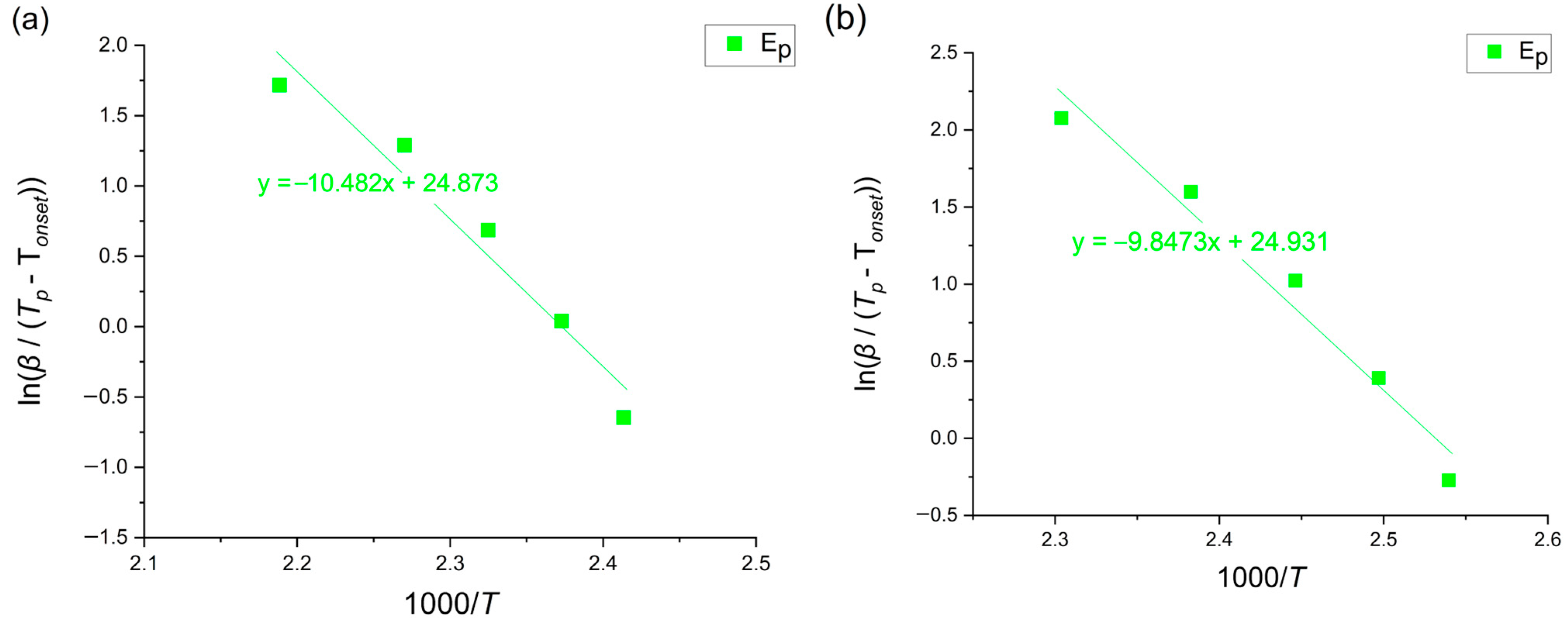
3.5. Augis–Bennett Method
3.6. Gao–Wang Method
4. Conclusions
- Crystallization proceeds with one exothermic peak for amorphous Mg72Zn27Pt1 alloy and with two exothermic peaks for amorphous Mg72Zn27Cu1 alloy, respectively.
- The characteristic temperatures , and are strongly dependent on the heating rate during non-isothermal annealing. The addition of Pt and Cu increases the characteristic temperatures toward higher values and, consequently, the stability of metallic glass compared to two-component Mg72Zn28 glass [17], especially that with Pt, due to its higher melting point and different atom size to those of Mg and Zn.
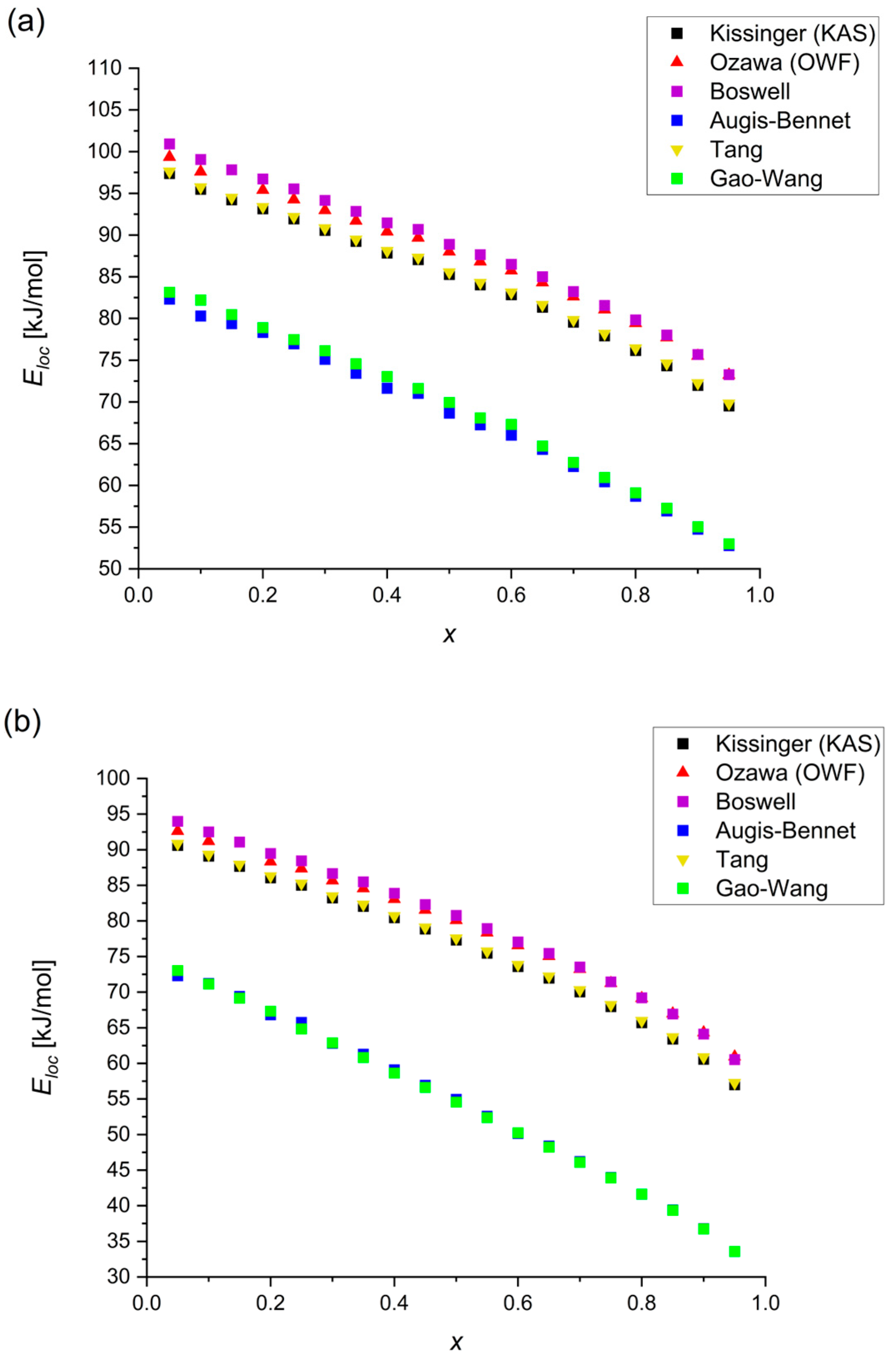
- The activation energies for the amorphous Mg72Zn27Pt1 alloy fluctuate in the range of 114.60–117.99 kJ/mol, 102.46–105.98 kJ/mol and 71.16–98.62 kJ/mol for , and , respectively, whereas, for Mg72Zn27Cu1, the calculated values are in the range of 98.51–101.77 kJ/mol, 95.15–98.51 kJ/mol and 55.15–93.34 kJ/mol for , and , respectively.
- Based on the , and local energy values, it can be confirmed that both alloys are meta-stable in the amorphous state and crystallization occurs spontaneously.
- The most similar values of the activation energy for three characteristic regions are given by the Kissinger, Ozawa, Tang and Boswell methods, because the structures of their equations are similar. The Boswell method gives the highest results, and the Augis–Bennett and Gao–Wang methods give significantly lower results.
- The Augis–Bennett and Gao–Wang methods allow for the calculation of the activation energy at the crystallization peak and they are the only ones that consider or .
- The use of the Gao–Wang method to analyze an alloy with two crystallization peaks may be difficult due to the inclusion of .
- The Augis–Bennett method, despite only slightly lower values for the peak activation energies, shows significantly lower values for the local activation energy.
- Taking into account the ease of their formulas, their best convergence, and their widespread use in the literature, the KAS and OFW methods will work very well for all comparisons.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, A.T.; Pratap, A. Kinetics of crystallization of Zr52Cu18Ni14Al10Ti6 metallic glass. J. Therm. Anal. Calorim. 2012, 10, 159–165. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Inoue, A. Bulk Metallic Glasses; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Lu, S.; Sun, S.; Li, K.; Li, H.; Huang, X.; Tu, G. The effect of Y addition on the crystallization behaviors of Zr-Cu-Ni-Al bulk metallic glasses. J. Alloys Compd. 2019, 799, 501–512. [Google Scholar] [CrossRef]
- Thomas, L.C. An Introduction To The Techniques of Differential Scanning Calorimetry (DSC) and Modulated Dsc®. 2005. Available online: https://api.core.ac.uk/oai/oai:ruc.udc.es:2183/11493 (accessed on 11 February 2021).
- Avrami, M. Granulation, phase change, and microstructure kinetics of phase change. III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Johnson, W.A.; Mehl, R.F. Reaction Kinetics in Processes of Nucleation and Growth. Am. Inst. Min. Metall. Eng. 1939, 135, 416–458. [Google Scholar]
- Matusita, K.; Sakka, S.; Matsui, Y. Determination of the activation energy for crystal growth by differential thermal analysis. J. Mater. Sci. 1975, 10, 961–966. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Ozawa, J.E. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. General treatment of the thermogravimetry of polymers. J. Res. Natl. Inst. Stand. Technol. 1966, 70, 487–523. [Google Scholar] [CrossRef]
- Boswell, P.G. On the calculation of activation energies using a modified Kissinger method. J. Therm. Anal. 1980, 18, 353–358. [Google Scholar] [CrossRef]
- Tang, W.; Liu, Y.; Zhang, H.; Wang, C. New approximate formula for Arrhenius temperature integral. Thermochim. Acta 2003, 408, 39–43. [Google Scholar] [CrossRef]
- Augis, J.; Bennett, A. Calculation of the Avrami parameters for heterogeneous solid state reactions using a modification of the Kissinger method. J. Therm. Anal. 1978, 13, 283–292. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Wang, W. On the activation energy of crystallization in metallic glasses. J. Non-Cryst. Solids 1986, 81, 129–134. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 2007, 6, 183–195. [Google Scholar] [CrossRef]
- Pierwoła, A.; Lelito, J.; Krawiec, H.; Szucki, M.; Gondek, Ł.; Kozieł, T.; Babilas, R. Non-Isothermal Analysis of the Crystallization Kinetics of Amorphous Mg72Zn27Pt1 and Mg72Zn27Ag1 Alloys. Materials 2024, 17, 408. [Google Scholar] [CrossRef] [PubMed]
- Calka, A.; Madhava, M.; Polk, D.E.; Giessen, B.C. A Transition-Metal-Free Amorphous Alloy: Mg.70Zn.30. Scr. Metall. 1977, II, 65–70. [Google Scholar] [CrossRef]
- Opitek, B.; Gracz, B.; Lelito, J.; Krajewski, W.K.; Łucarz, M.; Bała, P.; Kozieł, T.; Gondek, Ł.; Szucki, M. Crystallization Kinetics Analysis of the Binary Amorphous Mg72Zn28 Alloy. Materials 2023, 16, 2727. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Atomic Radius in the Periodic Table of Elements. 2023. Available online: https://pubchem.ncbi.nlm.nih.gov/periodic-table/atomic-radius (accessed on 18 August 2024).
- Opitek, B.; Lelito, J.; Szucki, M.; Piwowarski, G.; Gondek, Ł.; Rogal, Ł. Analysis of the Crystallization Kinetics and Thermal Stability of the Amorphous Mg72Zn24Ca4 Alloy. Materials 2021, 14, 3583. [Google Scholar] [CrossRef]
- Wang, J.; Meng, L.; Xie, W.; Ji, C.; Wang, R.; Zhang, P.; Jin, L.; Sheng, L.; Zheng, Y. Corrosion and in vitro cytocompatibility investigation on the designed Mg-Zn-Ag metallic glasses for biomedical application. J. Magnes. Alloys 2022, 15, 2172. [Google Scholar] [CrossRef]
- Rashidi, R.; Malekana, M.; Gholamipour, R. Crystallization kinetics of Cu47Zr47Al6 and (Cu47Zr47Al6)99Sn1 bulk metallic glasses. J. Non-Cryst. Solids 2018, 498, 272–280. [Google Scholar] [CrossRef]
- Mohammadi Rahvard, M.; Tamizifar, M.; Boutorabi, S.M.A. The effect of Ag addition on the non-isothermal crystallization kinetics and fragility of Zr56Co28Al16 bulk metallic glass. J. Non-Cryst. Solids 2018, 481, 74–84. [Google Scholar] [CrossRef]
- Rezaei-Shahreza, P.; Seifoddini, A.; Hasani, S.; Jaafari, Z.; Śliwa, A.; Nabiałek, M. Isokinetic Analysis of Fe41Co7Cr15Mo14Y2C15B6 Bulk Metallic Glass: Effect of Minor Copper Addition. Materials 2020, 13, 3704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X. Applications of Kinetic Methods in Thermal Analysis: A Review. Eng. Sci. 2021, 14, 1–13. [Google Scholar] [CrossRef]
- Amri, A.; Hendri, Y.B.; Saputra, E.; Heltina, D.; Yin, C.-Y.; Rahman, M.M.; Minakshi, M.; Mondinos, N.; Jiang, Z.-T. Formation kinetics of sol-gel derived LiFePO4 olivine analyzed by reliable non-isothermal approach. Ceram. Int. 2022, 48, 17729–17737. [Google Scholar] [CrossRef]




| Alloy | β [K/min] | [K] | [K] | [K] | [K] | [K] | [K] | [K] |
|---|---|---|---|---|---|---|---|---|
| Mg72Zn27Pt1 | 5 | 393.90 | 404.82 | 414.35 | 426.88 | — | — | — |
| 10 | 397.69 | 411.85 | 421.45 | 437.47 | — | — | — | |
| 20 | 404.75 | 420.07 | 430.14 | 448.85 | — | — | — | |
| 40 | 415.29 | 429.50 | 440.50 | 461.86 | — | — | — | |
| 80 | 423.80 | 442.57 | 456.93 | 491.17 | — | — | — | |
| Mg72Zn27Cu1 | 5 | 376.64 | 387.20 | 393.76 | 400.90 | 487.92 | 502.72 | 510.39 |
| 10 | 382.00 | 393.72 | 400.48 | 410.69 | 505.90 | 519.56 | 529.39 | |
| 20 | 389.00 | 401.60 | 408.79 | 426.25 | 528.08 | 540.29 | 557.17 | |
| 40 | 398.00 | 411.63 | 419.71 | 453.18 | 551.02 | 565.14 | 588.27 | |
| 80 | 410.11 | 424.06 | 434.08 | 476.35 | 579.91 | 591.59 | 617.22 |
| Method | Glass Transition | Onset of Crystallization | Crystallization Peak | Parameter for Slope Multiplying |
|---|---|---|---|---|
| Kissinger | ||||
| Ozawa–Flynn–Wall | ||||
| Boswell | ||||
| Tang | ||||
| Augis–Bennett | ||||
| Gao–Wang |
| Alloy | Method | [kJ/mol] | [kJ/mol] | [kJ/mol] |
|---|---|---|---|---|
| Mg72Zn27Pt1 | Kissinger | 114.60 | 102.46 | 95.01 |
| Ozawa–Flynn–Wall | 115.43 | 104.12 | 97.22 | |
| Boswell | 117.99 | 105.98 | 98.62 | |
| Tang | 114.79 | 102.68 | 95.25 | |
| Augis–Bennett | 87.11 | |||
| Gao–Wang | 71.16 | |||
| Mg72Zn27Cu1 | Kissinger | 98.51 | 95.15 | 89.91 |
| Ozawa–Flynn–Wall | 99.88 | 96.88 | 92.03 | |
| Boswell | 101.77 | 98.51 | 93.34 | |
| Tang | 98.71 | 95.37 | 90.14 | |
| Augis–Bennett | 81.83 | |||
| Gao–Wang | 55.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierwoła, A.; Lelito, J.; Szucki, M.; Krawiec, H. Different Methods for Calculation of Activation Energies During Non-Isothermal Annealing of Mg72Zn27Pt1 and Mg72Zn27Cu1 Metallic Glasses. Materials 2025, 18, 694. https://doi.org/10.3390/ma18030694
Pierwoła A, Lelito J, Szucki M, Krawiec H. Different Methods for Calculation of Activation Energies During Non-Isothermal Annealing of Mg72Zn27Pt1 and Mg72Zn27Cu1 Metallic Glasses. Materials. 2025; 18(3):694. https://doi.org/10.3390/ma18030694
Chicago/Turabian StylePierwoła, Aleksandra, Janusz Lelito, Michał Szucki, and Halina Krawiec. 2025. "Different Methods for Calculation of Activation Energies During Non-Isothermal Annealing of Mg72Zn27Pt1 and Mg72Zn27Cu1 Metallic Glasses" Materials 18, no. 3: 694. https://doi.org/10.3390/ma18030694
APA StylePierwoła, A., Lelito, J., Szucki, M., & Krawiec, H. (2025). Different Methods for Calculation of Activation Energies During Non-Isothermal Annealing of Mg72Zn27Pt1 and Mg72Zn27Cu1 Metallic Glasses. Materials, 18(3), 694. https://doi.org/10.3390/ma18030694






