Controlling the All-Solid Surface Reaction Between an Li1.3Al0.3Ti1.7(PO4)3 Electrolyte and Anode Through the Insertion of Ag and Al2O3 Nano-Interfacial Layers
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Sintering of Li1.3Al0.3Ti1.7(PO4)3 (LATP)
2.2. Nano-Al2O3 and Ag Layer Coating Methods
2.3. Battery Performance Evaluation of Thin-Film Coated LATP Solid-State Electrolyte
3. Results and Discussion
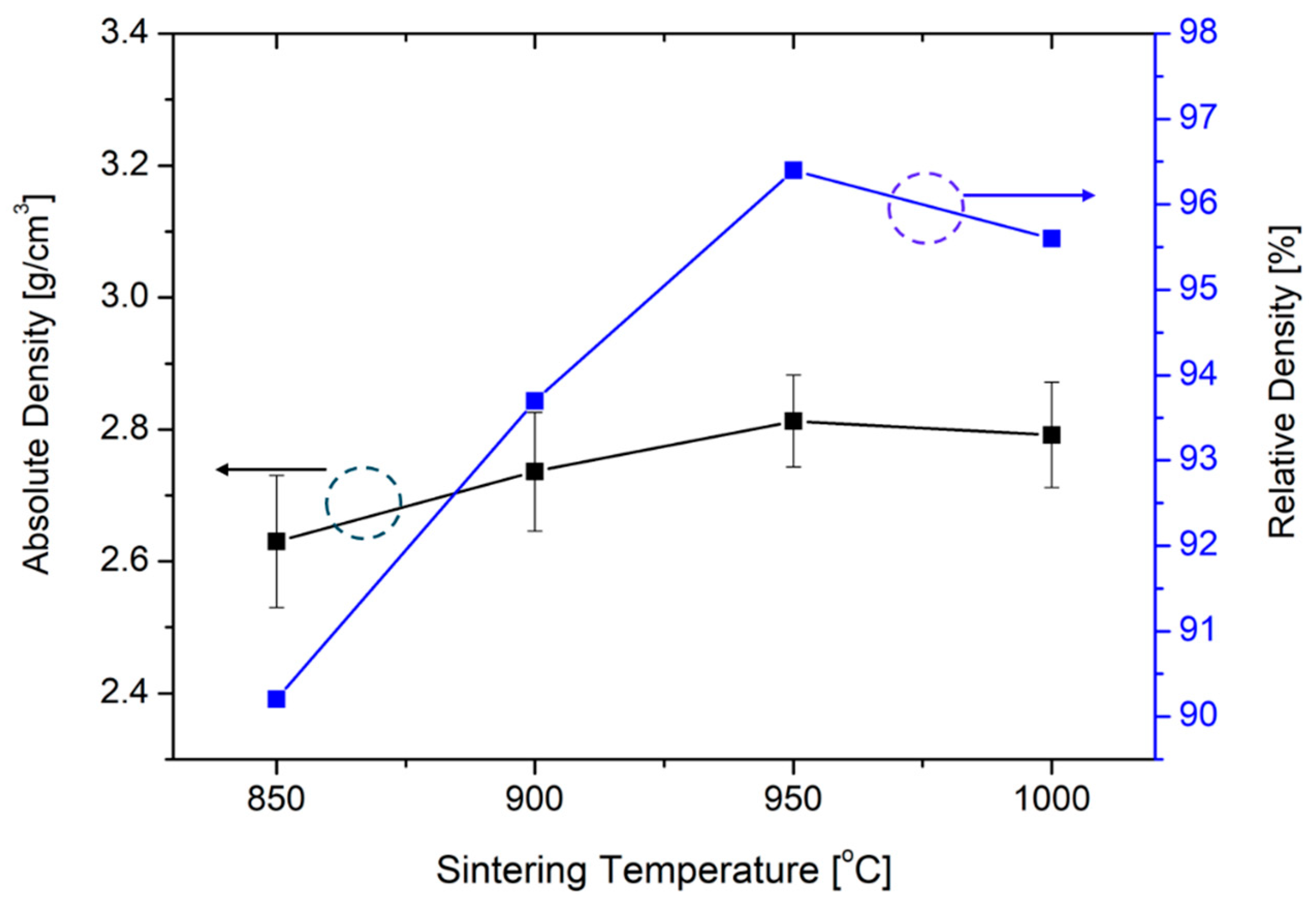
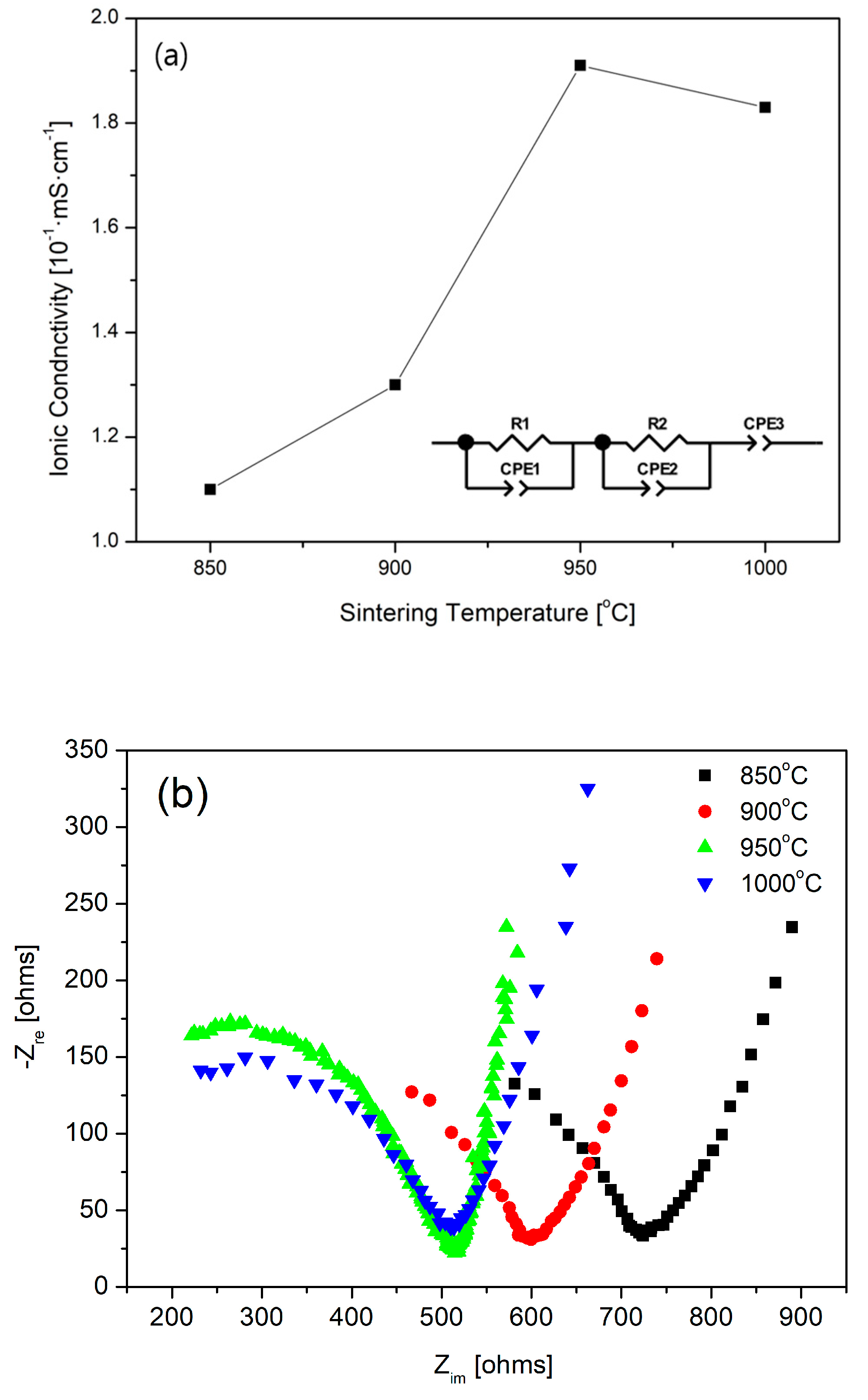
3.1. Synthesis of LATP Solid Electrolyte and Structural Characterization
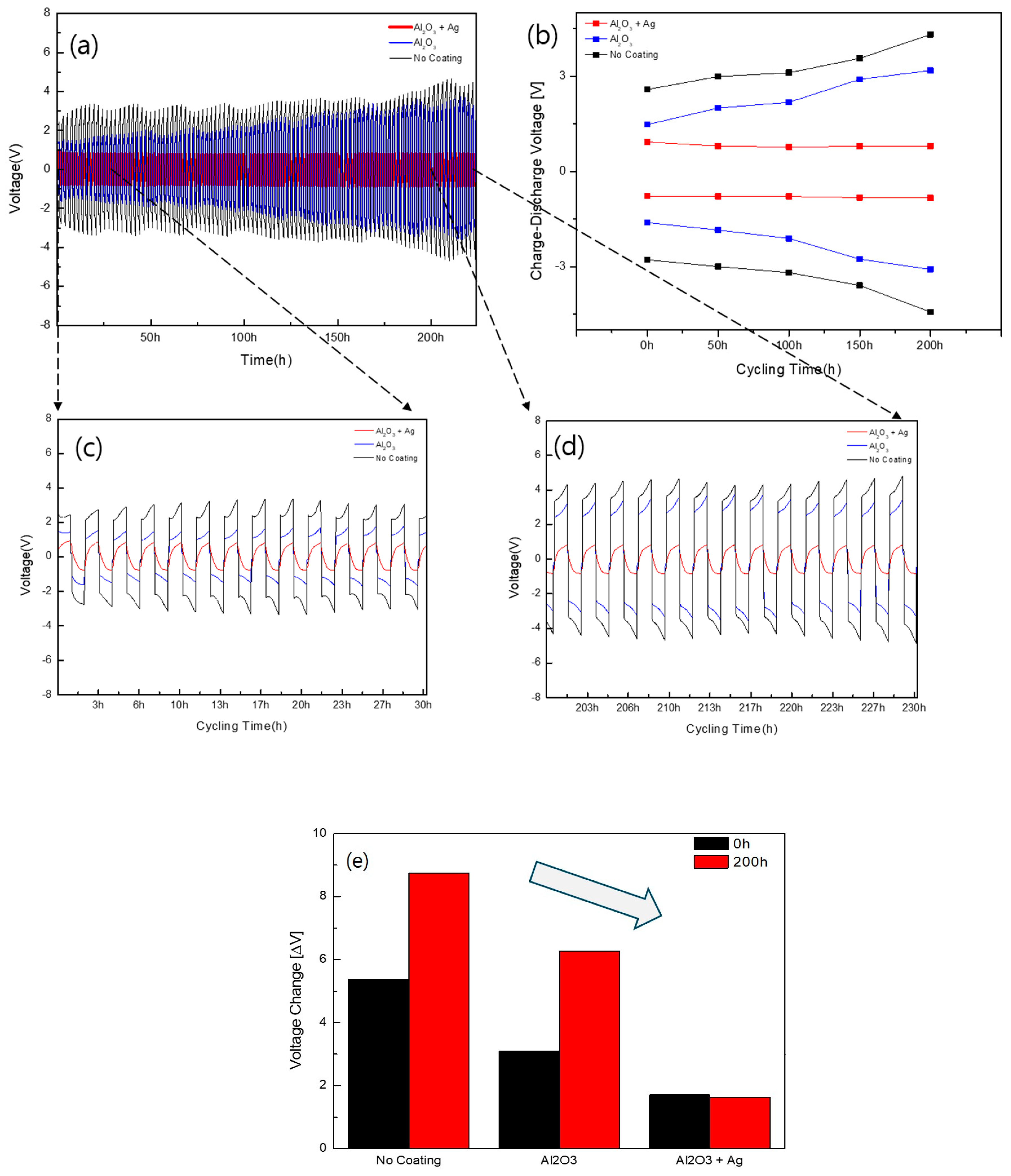
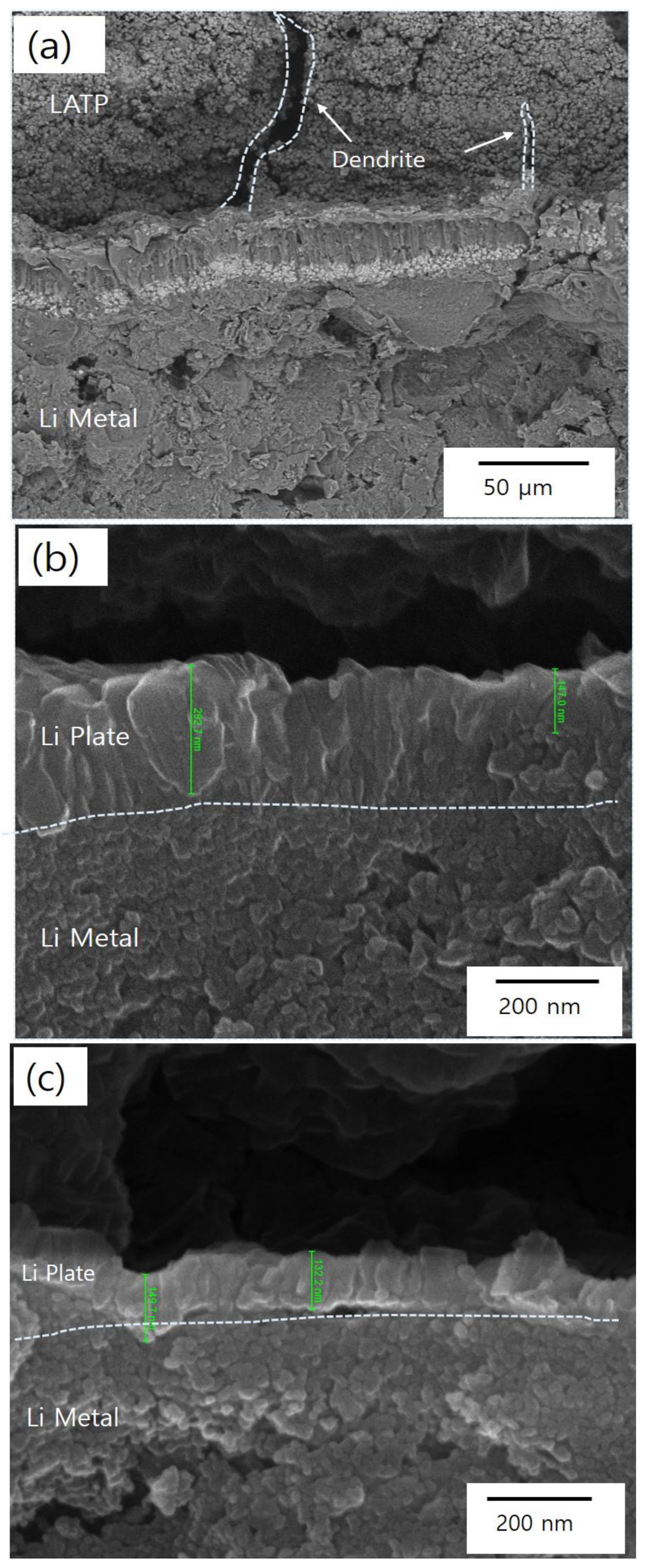
3.2. Evaluation of Electrochemical Stability of Solid Electrolytes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Turcheniuk, K.; Bondarev, D.; Amatucci, G.G.; Yushin, G. Battery materials for low-cost electric transportation. Mater. Today 2021, 42, 57–72. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Hubble, D.; Brown, D.E.; Zhao, Y.; Fang, C.; Lau, J.; McCloskey, B.D.; Liu, G. Liquid electrolyte development for low-temperature lithium-ion batteries. Energy Environ. Sci. 2022, 15, 550–578. [Google Scholar] [CrossRef]
- Monroe, C.; Newman, J. The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J. Electrochem. Soc. 2005, 152, A396. [Google Scholar] [CrossRef]
- Henriksen, M.; Vaagsaether, K.; Lundberg, J.; Forseth, S.; Bjerketvedt, D. Laminar burning velocity of gases vented from failed Li-ion batteries. J. Power Sources 2021, 506, 230141. [Google Scholar] [CrossRef]
- Roth, E.P.; Orendorff, C.J. How electrolytes influence battery safety. Electrochem. Soc. Interface 2012, 21, 45–49. [Google Scholar] [CrossRef]
- Duffner, F.; Kronemeyer, N.; Tübke, J.; Leker, J.; Winter, M.; Schmuch, R. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nat. Energy 2021, 6, 123–134. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A.; Zaghib, K.; Groult, H. Comparative issues of cathode materials for Li-ion batteries. Inorganics 2014, 2, 132–154. [Google Scholar] [CrossRef]
- Hausbrand, R.; Cherkashinin, G.; Ehrenberg, H.; Gröting, M.; Albe, K.; Hess, C.; Jaegermann, W. Fundamental degradation mechanisms of layered oxide Li-ion battery cathode materials: Methodology, insights and novel approaches. Mater. Sci. Eng. B 2015, 192, 3–25. [Google Scholar] [CrossRef]
- Myung, S.-T.; Maglia, F.; Park, K.-J.; Yoon, C.S.; Lamp, P.; Kim, S.-J.; Sun, Y.-K. Nickel-rich layered cathode materials for automotive lithium-ion batteries: Achievements and perspectives. ACS Energy Lett. 2017, 2, 196–223. [Google Scholar] [CrossRef]
- Kim, U.-H.; Kuo, L.-Y.; Kaghazchi, P.; Yoon, C.S.; Sun, Y.-K. Quaternary layered Ni-Rich NCMA cathode for lithium-ion batteries. ACS Energy Lett. 2019, 4, 576–582. [Google Scholar] [CrossRef]
- Zhou, P.; Meng, H.; Zhang, Z.; Chen, C.; Lu, Y.; Cao, J.; Cheng, F.; Chen, J. Stable layered Ni-Rich LiNi0.9Co0.07Al0.03O2 microspheres assembled with nanoparticles as high-performance cathode materials for lithium-ion batteries. J. Mater. Chem. A 2017, 5, 2724–2731. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, A.; Lee, J.Y. Synthesis and characterization of LiNi1−x−yCoxMnyO2 as the cathode materials of secondary lithium batteries. J. Power Sources 1999, 81–82, 416–419. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, B.; Lu, Z.; Liu, N.; Zhu, J. Challenges and recent progress in the development of si anodes for lithium-ion battery. Adv. Energy Mater. 2017, 7, 1700715. [Google Scholar] [CrossRef]
- Kim, J.-S.; Yoon, G.; Kim, S.; Sugata, S.; Yashiro, N.; Suzuki, S.; Lee, M.-J.; Kim, R.; Badding, M.; Song, Z.; et al. Surface engineering of inorganic solid-state electrolytes via interlayers strategy for developing long-cycling quasi-all-solid-state lithium batteries. Nat. Commun. 2023, 14, 782. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Fujiki, S.; Jung, C.; Suzuki, N.; Yashiro, N.; Omoda, R.; Ko, D.-S.; Shiratsuchi, T.; Sugimoto, T.; Ryu, S.; et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver-carbon composite anodes. Nat. Energy 2020, 5, 299–308. [Google Scholar] [CrossRef]
- Franco Gonzalez, A.; Yang, N.-H.; Liu, R.-S. Silicon anode design for lithium-ion batteries: Progress and perspectives. J. Phys. Chem. C 2017, 121, 27775–27787. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, W.; Kang, W.; Ye, Y.; Pan, Q.; Zhang, X.; Ke, Y.; Wang, C.; Qiu, Z.; Tang, Y. A review on silicon nanowire-based anodes for next-generation high-performance lithium-ion batteries from a material-based perspective. Sustain. Energy Fuels 2020, 4, 1577–1594. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Chen, Y.-T.; Yang, H.; Bao, W.; Sreenarayanan, B.; Doux, J.-M.; Li, W.; Lu, B.; Ham, S.-Y.; Sayahpour, B.; et al. Carbon-free high-loading silicon anodes enabled by sulfide solid electrolytes. Science 2021, 373, 1494–1499. [Google Scholar] [CrossRef] [PubMed]
- Bachman, J.C.; Muy, S.; Grimaud, A.; Chang, H.-H.; Pour, N.; Lux, S.F.; Paschos, O.; Maglia, F.; Lupart, S.; Lamp, P.; et al. Inorganic solid-state electrolytes for lithium batteries: Mechanisms and properties governing ion conduction. Chem. Rev. 2016, 116, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Kalhoff, J.; Eshetu, G.G.; Bresser, D.; Passerini, S. Safer electrolytes for lithium-ion batteries: State of the art and perspectives. ChemSusChem 2015, 8, 2154–2175. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, K.; Chen, T.; Zhang, X.; Armand, M.; Chen, S. Recent progress in all-solid-state lithium batteries: The emerging strategies for advanced electrolytes and their interfaces. Energy Storage Mater. 2020, 31, 401–433. [Google Scholar] [CrossRef]
- Zhao, Y.; Ding, Y.; Li, Y.; Peng, L.; Byon, H.R.; Goodenough, J.B.; Yu, G. A chemistry and material perspective on lithium redox flow batteries towards high-density electrical energy storage. Chem. Soc. Rev. 2015, 44, 7968–7996. [Google Scholar] [CrossRef]
- Sakuda, A.; Hayashi, A.; Takigawa, Y.; Higashi, K.; Tatsumisago, M. Evaluation of elastic modulus of Li2S−P2S5 glassy solid electrolyte by ultrasonic sound velocity measurement and compression test. J. Ceram. Soc. Jpn. 2013, 121, 946–949. [Google Scholar] [CrossRef]
- Tatsumisago, M.; Takano, R.; Tadanaga, K.; Hayashi, A. Preparation of Li3BO3–Li2SO4 glass–ceramic electrolytes for all-oxide lithium batteries. J. Power Sources 2014, 270, 603–607. [Google Scholar] [CrossRef]
- Sakuda, A.; Hayashi, A.; Tatsumisago, M. Sulfide solid electrolyte with favorable mechanical property for all-solid-state lithium battery. Sci. Rep. 2013, 3, 2261. [Google Scholar] [CrossRef]
- Iriyama, Y.; Wadaguchi, M.; Yoshida, K.; Yamamoto, Y.; Motoyama, M.; Yamamoto, T. 5V-class bulk-type all-solid-state rechargeable lithium batteries with electrode-solid electrolyte composite electrodes prepared by aerosol deposition. J. Power Sources 2018, 385, 55–61. [Google Scholar] [CrossRef]
- Zhu, J.; Li, X.; Wu, C.; Gao, J.; Xu, H.; Li, Y.; Guo, X.; Li, H.; Zhou, W. A multilayer ceramic electrolyte for all-solid-state Li batteries. Angew. Chem. Int. Ed. Engl. 2021, 60, 3781–3790. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guan, Z.; Chu, F.; Xue, Z.; Wu, F.; Yu, Y. Air-stable inorganic solid-state electrolytes for high energy density lithium batteries: Challenges, strategies, and prospects. InfoMat 2022, 4, e12248. [Google Scholar] [CrossRef]
- Wang, Y.; Richards, W.D.; Ong, S.P.; Miara, L.J.; Kim, J.C.; Mo, Y.; Ceder, G. Design principles for solid-state lithium superionic conductors. Nat. Mater. 2015, 14, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, M.; Mitsuishi, K.; Okumura, T.; Ishigaki, N.; Iriyama, Y. Fabrication of oxide-based all-solid-state batteries by a sintering process based on function sharing of solid electrolytes. ACS Appl. Mater. Interfaces 2022, 14, 48547–48557. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Dolocan, A.; Cui, Z.; Xin, S.; Xue, L.; Xu, H.; Park, K.; Goodenough, J.B. Garnet electrolyte with an ultralow interfacial resistance for Li-metal batteries. J. Am. Chem. Soc. 2018, 140, 6448–6455. [Google Scholar] [CrossRef]
- Han, F.; Westover, A.S.; Yue, J.; Fan, X.; Wang, F.; Chi, M.; Leonard, D.N.; Dudney, N.J.; Wang, H.; Wang, C. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat. Energy 2019, 4, 187–196. [Google Scholar] [CrossRef]
- Lu, W.; Xue, M.; Zhang, C. Modified Li7La3Zr2O12 (LLZO) and LLZO-polymer composites for solid-state lithium batteries. Energy Storage Mater. 2021, 39, 108–129. [Google Scholar] [CrossRef]
- Sun, H.; Kang, S.; Cui, L. Prospects of LLZO type solid electrolyte: From material design to battery application. Chem. Eng. J. 2023, 454, 140375. [Google Scholar] [CrossRef]
- Han, X.; Gong, Y.; Fu, K.; He, X.; Hitz, G.T.; Dai, J.; Pearse, A.; Liu, B.; Wang, H.; Rubloff, G.; et al. Negating Interfacial Impedance in Garnet-Based Solid-State Li Metal Batteries. Nat. Mater. 2017, 16, 572–579. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.-S.; Miara, L.; Wang, Y.; Jung, S.-K.; Park, S.Y.; Song, Z.; Kim, H.; Badding, M.; Chang, J.; et al. High energy and durable lithium metal batteries using garnet-type solid electrolytes with tailored lithium-metal compatibility. Nat. Commun. 2022, 13, 1883. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, K.; Sun, X. Addressing interfacial issues in liquid-based and solid-state batteries by atomic and molecular layer deposition. Joule 2018, 2, 2583–2604. [Google Scholar] [CrossRef]
- López-Aranguren, P.; Reynaud, M.; Głuchowski, P.; Bustinza, A.; Galceran, M.; López del Amo, J.M.; Armand, M.; Casas-Cabanas, M. Crystalline LiPON as a bulk-type solid electrolyte. ACS Energy Lett. 2021, 6, 445–450. [Google Scholar] [CrossRef]
- Hartmann, P.; Leichtweiss, T.; Busche, M.R.; Schneider, M.; Reich, M.; Sann, J.; Adelhelm, P.; Janek, J. Degradation of NASICON-type materials in contact with lithium metal: Formation of mixed conducting interphases (MCI) on solid electrolytes. J. Phys. Chem. C 2013, 117, 21064–21074. [Google Scholar] [CrossRef]
- Wang, S.; Ding, Y.; Zhou, G.; Yu, G.; Manthiram, A. Durability of the Li1+xTi2–xAlx(PO4)3 solid electrolyte in lithium–sulfur batteries. ACS Energy Lett. 2016, 1, 1080–1085. [Google Scholar] [CrossRef]
- Lee, R.-H.; Lee, D.-W.; Lee, J.-K.; Kim, K.-N.; Yoon, J.-R.; Lee, S.-H. Electrical and ionic conductivity of Li2O-B2O3-Al2O3 glass electrolyte for solid-state batteries. J. Energy Storage 2024, 77, 110018. [Google Scholar] [CrossRef]
- Okumura, T.; Taminato, S.; Miyazaki, Y.; Kitamura, M.; Saito, T.; Takeuchi, T.; Kobayashi, H. LISICON-based amorphous oxide for bulk-type all-solid-state lithium-ion battery. ACS Appl. Energy Mater. 2020, 3, 3220–3229. [Google Scholar] [CrossRef]
- Dantas, N.O.; Silva, V.A.; Neto, O.O.D.; Nascimento, M.L.F. Control of crystallization kinetics and study of the thermal, structural and morphological properties of an Li2O–B2O3–Al2O3 vitreous system. Braz. J. Phys. 2012, 42, 347–354. [Google Scholar] [CrossRef]
- Cheng, D.; Wynn, T.A.; Wang, X.; Wang, S.; Zhang, M.; Shimizu, R.; Bai, S.; Nguyen, H.; Fang, C.; Kim, M.; et al. Unveiling the stable nature of the solid electrolyte interphase between lithium metal and LiPON via cryogenic electron microscopy. Joule 2020, 4, 2484–2500. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, A.; Li, N.; Li, S.; Zangiabadi, A.; Li, T.-D.; Huang, W.; Li, A.C.; Jin, T.; Song, Q.; et al. Stabilizing solid electrolyte-anode interface in Li-metal batteries by boron nitride-based nanocomposite coating. Joule 2019, 3, 1510–1522. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Q.; Zhao, Y.; Wang, B.; Kaghazchi, P.; Adair, K.R.; Li, R.; Zhang, C.; Liu, J.; Kuo, L.-Y.; et al. Stabilizing the interface of NASICON solid electrolyte against Li metal with atomic layer deposition. ACS Appl. Mater. Interfaces 2018, 10, 31240–31248. [Google Scholar] [CrossRef]
- Bucharsky, E.C.; Schell, K.G.; Hupfer, T.; Hoffmann, M.J.; Rohde, M.; Seifert, H.J. Thermal properties and ionic conductivity of Li1,3Ti1,7Al0,3(PO4)3 solid electrolytes sintered by field-assisted sintering. Ionics 2016, 22, 1043–1049. [Google Scholar] [CrossRef]
- Moon, J.I.; Cho, H.C.; Song, J.H. Synthesis and Conductive Properties of Li1+xAlxTi2-x(PO4)3 (x = 0, 0.3, 0.5) by sol–gel Method. Korean J. Mater. Res. 2012, 22, 346–351. [Google Scholar] [CrossRef]
- Choi, S.K.; Choi, J.; Yang, M. A study on the microstructures and ionic conductivity of Li1.3Al0.3Ti1.7(PO4)3 with Different Synthesis Routes. J. Powder Mater. 2023, 30, 107–115. [Google Scholar] [CrossRef]
- Yamada, H.; Morimoto, N.; Mukohara, H.; Tojo, T.; Yano, S.; Magome, E.; Morimura, T.; Bekarevich, R.; Mitsuishi, K. Concerted influence of microstructure and adsorbed water on lithium-ion conduction of Li1.3Al0.3Ti1.7(PO4)3. J. Power Sources 2021, 511, 230422. [Google Scholar] [CrossRef]
- Kang, S.-G.; Kim, D.-H.; Kim, B.-J.; Yoon, C.-B. Sn-substituted argyrodite Li6PS5Cl solid electrolyte for improving interfacial and atmospheric stability. Materials 2023, 16, 2751. [Google Scholar] [CrossRef]
- Meng, X.; Yang, X.-Q.; Sun, X. Emerging applications of atomic layer deposition for lithium-ion battery studies. Adv. Mater. 2012, 24, 3589–3615. [Google Scholar] [CrossRef]
- Ahmed, B.; Xia, C.; Alshareef, H.N. Electrode surface engineering by atomic layer deposition: A promising pathway toward better energy storage. Nano Today 2016, 11, 250–271. [Google Scholar] [CrossRef]
- Sui, D.; Liu, J. Constriction-Susceptible Lithium Support for Fast Cycling of Solid-State Lithium Metal Battery. Chin. Chem. Lett. 2025, 36, 110417. [Google Scholar] [CrossRef]
- Li, J.; Zhou, M.; Wu, H.-H.; Wang, L.; Zhang, J.; Wu, N.; Pan, K.; Liu, G.; Zhang, Y.; Han, J.; et al. Machine Learning-Assisted Property Prediction of Solid-State Electrolyte. Adv. Energy Mater. 2024, 14, 2304480. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, G.; Kim, B.; Hwang, I.; Kim, J.; Kim, J.; Yoon, C.-B. Controlling the All-Solid Surface Reaction Between an Li1.3Al0.3Ti1.7(PO4)3 Electrolyte and Anode Through the Insertion of Ag and Al2O3 Nano-Interfacial Layers. Materials 2025, 18, 609. https://doi.org/10.3390/ma18030609
Song G, Kim B, Hwang I, Kim J, Kim J, Yoon C-B. Controlling the All-Solid Surface Reaction Between an Li1.3Al0.3Ti1.7(PO4)3 Electrolyte and Anode Through the Insertion of Ag and Al2O3 Nano-Interfacial Layers. Materials. 2025; 18(3):609. https://doi.org/10.3390/ma18030609
Chicago/Turabian StyleSong, Gwanhee, Bojoong Kim, Inkook Hwang, Jiwon Kim, Jinmo Kim, and Chang-Bun Yoon. 2025. "Controlling the All-Solid Surface Reaction Between an Li1.3Al0.3Ti1.7(PO4)3 Electrolyte and Anode Through the Insertion of Ag and Al2O3 Nano-Interfacial Layers" Materials 18, no. 3: 609. https://doi.org/10.3390/ma18030609
APA StyleSong, G., Kim, B., Hwang, I., Kim, J., Kim, J., & Yoon, C.-B. (2025). Controlling the All-Solid Surface Reaction Between an Li1.3Al0.3Ti1.7(PO4)3 Electrolyte and Anode Through the Insertion of Ag and Al2O3 Nano-Interfacial Layers. Materials, 18(3), 609. https://doi.org/10.3390/ma18030609






