A First-Principles Calculation Study of the Catalytic Properties of Two-Dimensional Bismuthene Materials for Carbon Dioxide Reduction
Abstract
1. Introduction
2. Computational Method and Materials
3. Results and Discussion
3.1. Surface Energy and Band Structures of Bismuthene
3.2. Free Energy Analysis of Intermediates in Bismuthene for RR
- *OCO → *OCHO;
- *OCO → *COOH;
- *H → H2.
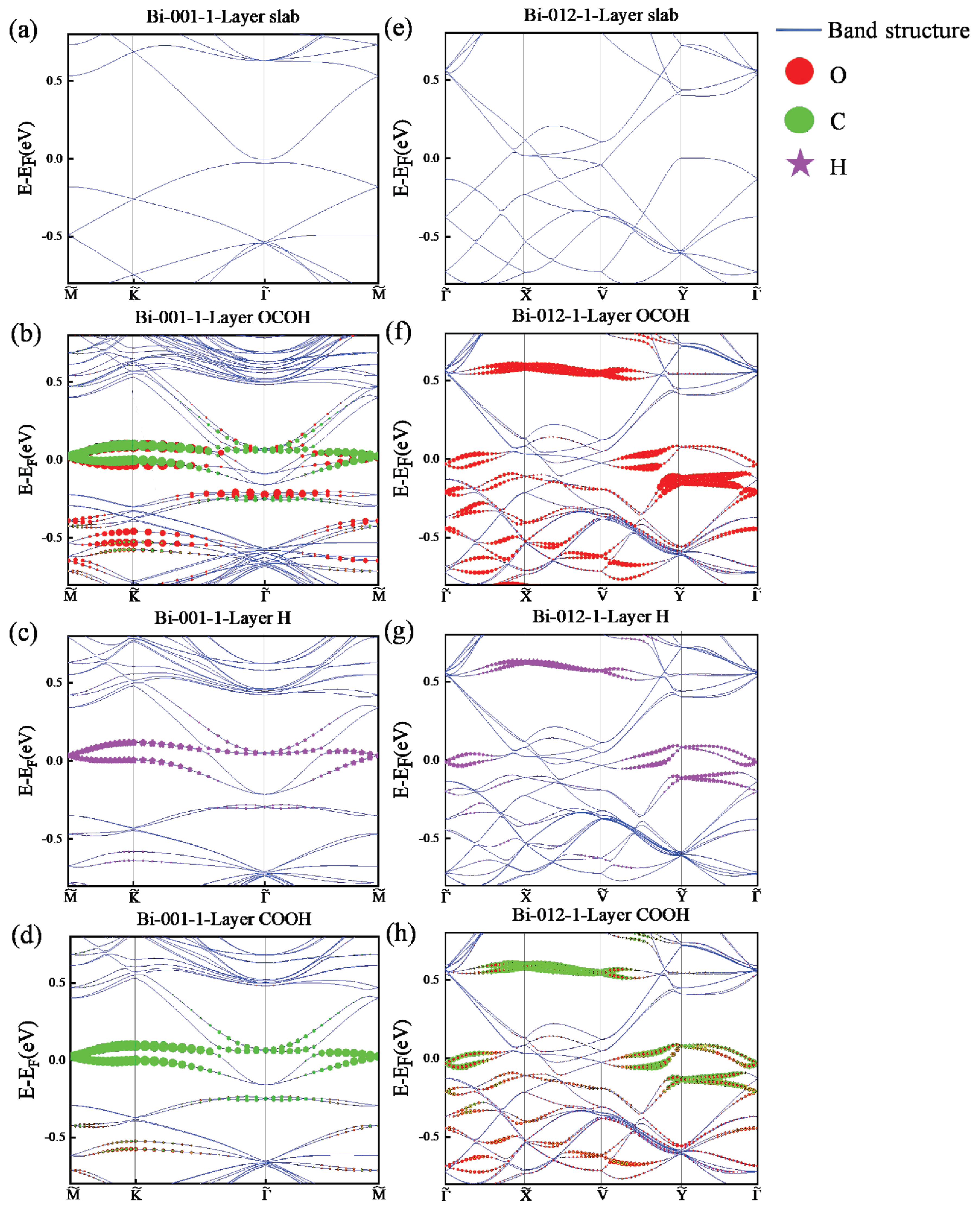
3.3. Calculation and Analysis of Energy Band of Intermediates in Bismuthene for RR
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Mayorov, A.S.; Gorbachev, R.V.; Morozov, S.V.; Britnell, L.; Jalil, R.; Ponomarenko, L.A.; Blake, P.; Novoselov, K.S.; Watanabe, K.; Taniguchi, T.; et al. Micrometer-Scale Ballistic Transport in Encapsulated Graphene at Room Temperature. Nano Lett. 2011, 11, 2396–2399. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.E. The birth of topological insulators. Nature 2010, 464, 194–198. [Google Scholar] [CrossRef]
- Schaibley, J.R.; Yu, H.Y.; Clark, G.; Rivera, P.; Ross, J.S.; Seyler, K.L.; Yao, W.; Xu, X.D. Valleytronics in 2D materials. Nat. Rev. Mater. 2016, 1, 16055. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Chia, X.Y.; Pumera, M. Characteristics and performance of two-dimensional materials for electrocatalysis. Nat. Catal. 2018, 1, 909–921. [Google Scholar] [CrossRef]
- Bellani, S.; Bartolotta, A.; Agresti, A.; Calogero, G.; Grancini, G.; Di Carlo, A.; Kymakis, E.; Bonaccorso, F. Solution-processed two-dimensional materials for next-generation photovoltaics. Chem. Soc. Rev. 2021, 50, 11870–11965. [Google Scholar] [CrossRef]
- Fu, X.B.; Pedersen, J.B.; Zhou, Y.Y.; Saccoccio, M.; Li, S.F.; Salinas, R.; Li, K.T.; Andersen, S.Z.; Xu, A.N.; Deissler, N.H.; et al. Continuous-flow electrosynthesis of ammonia by nitrogen reduction and hydrogen oxidation. Science 2023, 379, 707–712. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhao, J.X.; Cai, Q.H. Pyrrolic-nitrogen doped graphene: A metal-free electrocatalyst with high efficiency and selectivity for the reduction of carbon dioxide to formic acid: A computational study. Phys. Chem. Chem. Phys. 2016, 18, 5491–5498. [Google Scholar] [CrossRef]
- Schindler, F.; Cook, A.M.; Vergniory, M.G.; Wang, Z.J.; Parkin, S.S.P.; Bernevig, B.A.; Neupert, T. Higher-order topological insulators. Sci. Adv. 2018, 4, eaat0346. [Google Scholar] [CrossRef]
- Yeom, H.W.; Jin, K.H.; Jhi, S.H. Topological fate of edge states of single Bi bilayer on Bi(111). Phys. Rev. B 2016, 93, 075435. [Google Scholar] [CrossRef]
- Xue, Y.; Guo, Y.; Cui, H.; Zhou, Z. Catalyst Design for Electrochemical Reduction of CO2 to Multicarbon Products. Small Methods 2021, 5, 2100736. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Altaf, N.; Huang, L.; Gao, Y.; Wang, Q. Electrolytic cell design for electrochemical CO2 reduction. J. CO2 Util. 2020, 35, 90–105. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, X.; Zhou, Y.; Tan, Y.; Li, H.; Fu, J.; Liu, M. Electrocatalytic CO2 Reduction to C2+ Products in Flow Cells. Adv. Mater. 2024, 36, 2303902. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Weng, Z.; Wu, Z.; Zhong, Y.; Wu, Y.; Fang, J.; Wang, H. Coupled Metal/Oxide Catalysts with Tunable Product Selectivity for Electrocatalytic CO2 Reduction. ACS Appl. Mater. Interfaces 2017, 9, 28519–28526. [Google Scholar] [CrossRef]
- Guo, J.; Zhi, X.; Wang, D.; Qu, L.; Zavabeti, A.; Fan, Q.; Zhang, Y.; Butson, J.D.; Yang, J.; Wu, C.; et al. Surface-Enriched Room-Temperature Liquid Bismuth for Catalytic CO2 Reduction. Small 2024, 20, 2401777. [Google Scholar] [CrossRef]
- Cho, W.S.; Hong, D.M.; Dong, W.J.; Lee, T.H.; Yoo, C.J.; Lee, D.; Jang, H.W.; Lee, J.-L. Porously Reduced 2-Dimensional BiOCO Petals for Strain-Mediated Electrochemical CO Reduction to HCOOH. Energy Environ. Mater. 2024, 7, e12490. [Google Scholar] [CrossRef]
- Kaiyue, H.; Jiayu, T.; Zhifu, Z.; Daming, Z.; Xiangjiu, G. Direct Z-scheme photocatalytic systems based on vdW heterostructures for water splitting and CO2 reduction: Fundamentals and recent advances. Microstructures 2024, 4, 2024021. [Google Scholar] [CrossRef]
- Masoumi, Z.; Tayebi, M.; Tayebi, M.; Masoumi Lari, S.A.; Sewwandi, N.; Seo, B.; Lim, C.-S.; Kim, H.-G.; Kyung, D. Electrocatalytic Reactions for Converting CO2 to Value-Added Products: Recent Progress and Emerging Trends. Int. J. Mol. Sci. 2023, 24, 9952. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Cheah, M.H.; Lomoth, R.; Hammarström, L. Direct Detection of Key Intermediates during the Product Release in Rhenium Bipyridine-Catalyzed CO2 Reduction Reaction. ACS Catal. 2024, 14, 16324–16334. [Google Scholar] [CrossRef]
- Lee, J.; Lim, J.; Roh, C.W.; Whang, H.S.; Lee, H. Electrochemical CO2 reduction using alkaline membrane electrode assembly on various metal electrodes. J. CO2 Util. 2019, 31, 244–250. [Google Scholar] [CrossRef]
- Huang, Z.; Grim, R.G.; Schaidle, J.A.; Tao, L. The economic outlook for converting CO2 and electrons to molecules. Energy Environ. Sci. 2021, 14, 3664–3678. [Google Scholar] [CrossRef]
- Cao, B.; Li, F.-Z.; Gu, J. Designing Cu-Based Tandem Catalysts for CO2 Electroreduction Based on Mass Transport of CO Intermediate. ACS Catal. 2022, 12, 9735–9752. [Google Scholar] [CrossRef]
- Sliwa, M.; Zhang, H.; Gao, J.X.; Stephens, B.O.; Patera, A.J.; Raciti, D.; Hanrahan, P.D.; Warecki, Z.A.; Foley, D.L.; Livi, K.J.; et al. Selective CO2 Reduction Electrocatalysis Using AgCu Nanoalloys Prepared by a “Host-Guest” Method. Nano Lett. 2024, 24, 13911–13918. [Google Scholar] [CrossRef]
- Sun, H.H.; Wang, M.X.; Zhu, F.F.; Wang, G.Y.; Ma, H.Y.; Xu, Z.A.; Liao, Q.; Lu, Y.H.; Gao, C.L.; Li, Y.Y.; et al. Coexistence of Topological Edge State and Superconductivity in Bismuth Ultrathin Film. Nano Lett. 2017, 17, 3035–3039. [Google Scholar] [CrossRef]
- Han, N.; Wang, Y.; Yang, H.; Deng, J.; Wu, J.H.; Li, Y.F.; Li, Y.G. Ultrathin bismuth nanosheets from in situ topotactic transformation for selective electrocatalytic CO2 reduction to formate. Nat. Commun. 2018, 9, 1320. [Google Scholar] [CrossRef]
- Yang, H.; Han, N.; Deng, J.; Wu, J.H.; Wang, Y.; Hu, Y.P.; Ding, P.; Li, Y.F.; Li, Y.G.; Lu, J. Selective CO2 Reduction on 2D Mesoporous Bi Nanosheets. Adv. Energy Mater. 2018, 8, 1801536. [Google Scholar] [CrossRef]
- Cao, C.S.; Ma, D.D.; Gu, J.F.; Xie, X.Y.; Zeng, G.; Li, X.F.; Han, S.G.; Zhu, Q.L.; Wu, X.T.; Xu, Q. Metal-Organic Layers Leading to Atomically Thin Bismuthene for Efficient Carbon Dioxide Electroreduction to Liquid Fuel. Angew. Chem. Int. Ed. 2020, 59, 15014–15020. [Google Scholar] [CrossRef]
- Fan, J.; Zhao, X.; Mao, X.N.; Xu, J.; Han, N.; Yang, H.; Pan, B.B.; Li, Y.S.; Wang, L.; Li, Y.G. Large-Area Vertically Aligned Bismuthene Nanosheet Arrays from Galvanic Replacement Reaction for Efficient Electrochemical CO2 Conversion. Adv. Mater. 2021, 33, 2100910. [Google Scholar] [CrossRef]
- Li, Y.X.; Hui, D.P.; Sun, Y.Q.; Wang, Y.; Wu, Z.J.; Wang, C.Y.; Zhao, J.C. Boosting thermo-photocatalytic CO2 conversion activity by using photosynthesis-inspired electron-proton-transfer mediators. Nat. Commun. 2021, 12, 123. [Google Scholar] [CrossRef]
- Ma, W.X.; Bu, J.; Liu, Z.P.; Yan, C.; Yao, Y.; Chang, N.H.; Zhang, H.P.; Wang, T.; Zhang, J. Monoclinic Scheelite Bismuth Vanadate Derived Bismuthene Nanosheets with Rapid Kinetics for Electrochemically Reducing Carbon Dioxide to Formate. Adv. Funct. Mater. 2021, 31, 2006704. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.L.; Qu, Y.T.; Wang, X.; Huo, H.; Fan, Q.K.; Wang, J.; Yang, L.M.; Wu, Y.E. Bi-Based Metal-Organic Framework Derived Leafy Bismuth Nanosheets for Carbon Dioxide Electroreduction. Adv. Energy Mater. 2020, 10, 2001709. [Google Scholar] [CrossRef]
- Yang, F.; Elnabawy, A.O.; Schimmenti, R.; Song, P.; Wang, J.W.; Peng, Z.Q.; Yao, S.; Deng, R.P.; Song, S.Y.; Lin, Y.; et al. Bismuthene for highly efficient carbon dioxide electroreduction reaction. Nat. Commun. 2020, 11, 1088. [Google Scholar] [CrossRef]
- Siinor, L.; Lust, K.; Lust, E. Impedance study of adsorption of iodide ions at Bi(001) electrode from the aqueous solutions with constant ionic strength. J. Electroanal. Chem. 2007, 601, 39–46. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Lan, M.; Shao, T.-N.; Li, G.-Q.; Zhang, Y.; Huang, C.-Z.; Xiong, Z.-H.; Ma, X.-C.; Jia, J.-F.; Xue, Q.-K. STM study of a rubrene monolayer on Bi(001): Structural modulations. Phys. Rev. B 2011, 83, 235433. [Google Scholar] [CrossRef]
- Bychkov, Y.A.; Rashba, E.I. Oscillatory Effects and the Magnetic-Susceptibility of Carriers in Inversion-Layers. J. Phys. C Solid State Phys. 1984, 17, 6039–6045. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Wang, Y. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 1996, 54, 16533–16539. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Klimes, J.; Bowler, D.R.; Michaelides, A. Chemical accuracy for the van der Waals density functional. J. Phys. Condens. Matter 2010, 22, 022201. [Google Scholar] [CrossRef] [PubMed]
- Klimes, J.; Bowler, D.R.; Michaelides, A. Van der Waals density functionals applied to solids. Phys. Rev. B 2011, 83, 195131. [Google Scholar] [CrossRef]
- Reis, F.; Li, G.; Dudy, L.; Bauernfeind, M.; Glass, S.; Hanke, W.; Thomale, R.; Schäfer, J.; Claessen, R. Bismuthene on a SiC substrate: A candidate for a high-temperature quantum spin Hall material. Science 2017, 357, 287–290. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.L.; Ling, Y.Z.; Li, F.W.; Bond, A.M.; Zhang, J. Controllable Synthesis of Few-Layer Bismuth Subcarbonate by Electrochemical Exfoliation for Enhanced CO2 Reduction Performance. Angew. Chem. Int. Ed. 2018, 57, 13283–13287. [Google Scholar] [CrossRef]
- Available online: https://webbook.nist.gov/chemistry/ (accessed on 1 January 2023).
- Xu, A.; Wei, D.; Chen, X.; Yang, T.; Huang, Y.; He, H.; Xu, J. In situ transformation of bismuth-containing precursors into ultrathin bismuth nanosheets for enhanced electrochemical CO2 reduction. Chem. Eng. J. 2023, 452, 139227. [Google Scholar] [CrossRef]


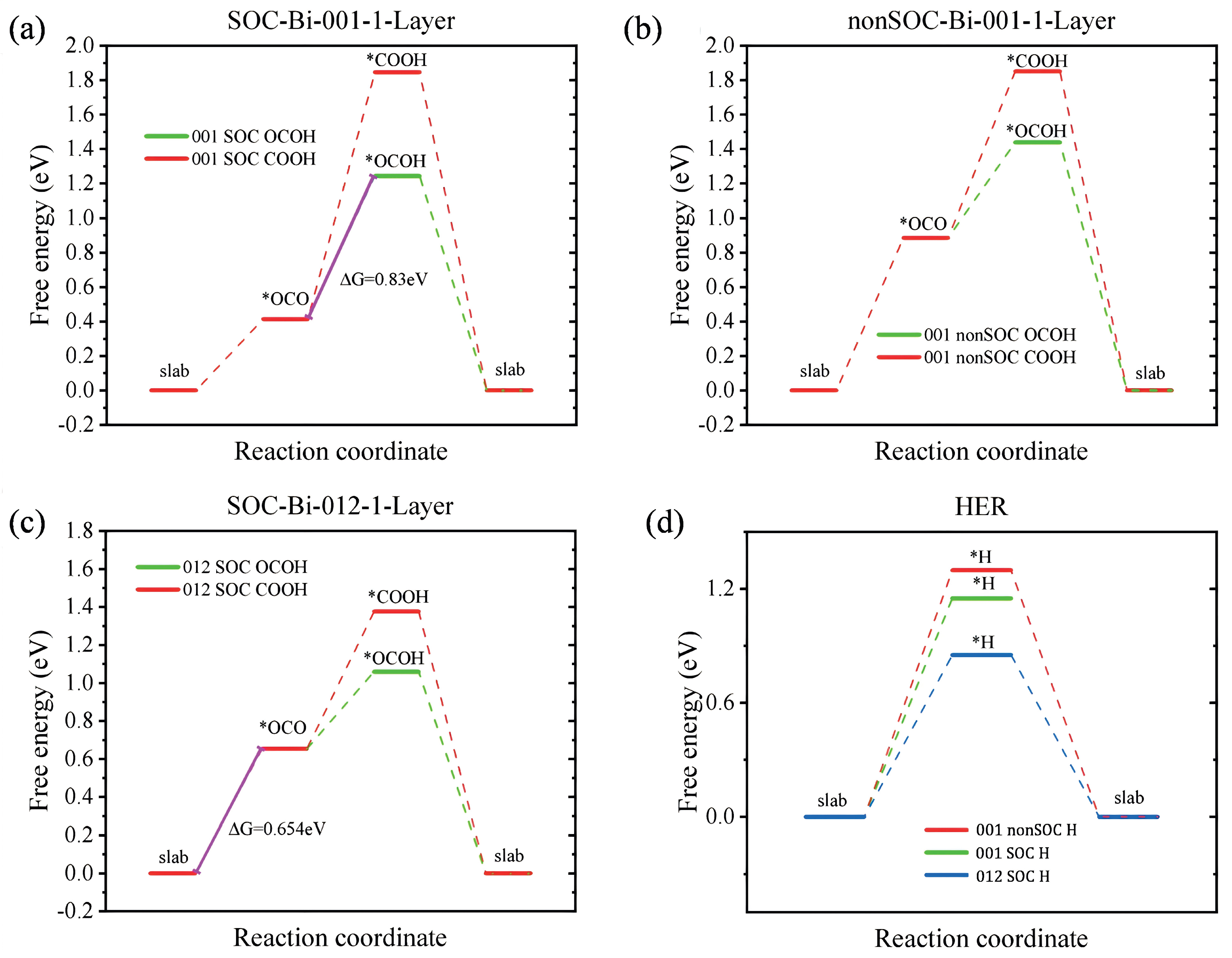
| Absorb Structure | G/eV | G/eV (U = 0 V) | G/eV (U = 0.1655 V) |
|---|---|---|---|
| Bi-SOC-001-1-Layer | |||
| slab | −167.247 | 0.000 | 0.000 |
| *OCO | −166.833 | 0.414 | 0.414 |
| *OCOH | −165.837 | 1.410 | 1.244 |
| *slab | −166.916 | 0.331 | 0.000 |
| *COOH | −165.235 | 2.012 | 1.846 |
| Bi-nonSOC-001-1-Layer | |||
| slab | −148.332 | 0.000 | 0.000 |
| *OCO | −147.447 | 0.885 | 0.885 |
| *OCOH | −146.727 | 1.605 | 1.439 |
| *slab | −148.001 | 0.331 | 0.000 |
| *COOH | −146.359 | 1.973 | 1.851 |
| Bi-SOC-012-1-Layer | |||
| slab | −102.207 | 0.000 | 0.000 |
| *OCO | −101.553 | 0.654 | 0.654 |
| *OCOH | −100.983 | 1.224 | 1.059 |
| *slab | −101.876 | 0.331 | 0.000 |
| *COOH | −100.666 | 1.541 | 1.376 |
| Absorb Structure | G/eV | G/eV |
|---|---|---|
| Bi-SOC-001-1-Layer | ||
| slab | −144.041 | 0.000 |
| *H | −142.770 | 1.272 |
| *slab | −144.041 | 0.000 |
| Bi-nonSOC-001-1-Layer | ||
| slab | −125.126 | 0.000 |
| *H | −123.828 | 1.298 |
| *slab | −125.126 | 0.000 |
| Bi-SOC-012-1-Layer | ||
| slab | −79.001 | 0.000 |
| *H | −78.149 | 0.852 |
| *slab | −79.001 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-T.; Yue, Q.; Wang, C.; Xu, Y.; Zhou, C. A First-Principles Calculation Study of the Catalytic Properties of Two-Dimensional Bismuthene Materials for Carbon Dioxide Reduction. Materials 2025, 18, 594. https://doi.org/10.3390/ma18030594
Wang C-T, Yue Q, Wang C, Xu Y, Zhou C. A First-Principles Calculation Study of the Catalytic Properties of Two-Dimensional Bismuthene Materials for Carbon Dioxide Reduction. Materials. 2025; 18(3):594. https://doi.org/10.3390/ma18030594
Chicago/Turabian StyleWang, Chang-Tian, Qinchi Yue, Changhao Wang, Yuanji Xu, and Chang Zhou. 2025. "A First-Principles Calculation Study of the Catalytic Properties of Two-Dimensional Bismuthene Materials for Carbon Dioxide Reduction" Materials 18, no. 3: 594. https://doi.org/10.3390/ma18030594
APA StyleWang, C.-T., Yue, Q., Wang, C., Xu, Y., & Zhou, C. (2025). A First-Principles Calculation Study of the Catalytic Properties of Two-Dimensional Bismuthene Materials for Carbon Dioxide Reduction. Materials, 18(3), 594. https://doi.org/10.3390/ma18030594









