Construction of Lunar Soil Simulants-Based Aluminum-Ion Battery Systems
Abstract
1. Introduction
2. Experimental Section
2.1. Sample Preparation
2.2. Materials Characterization
2.3. Electrochemical Measurements
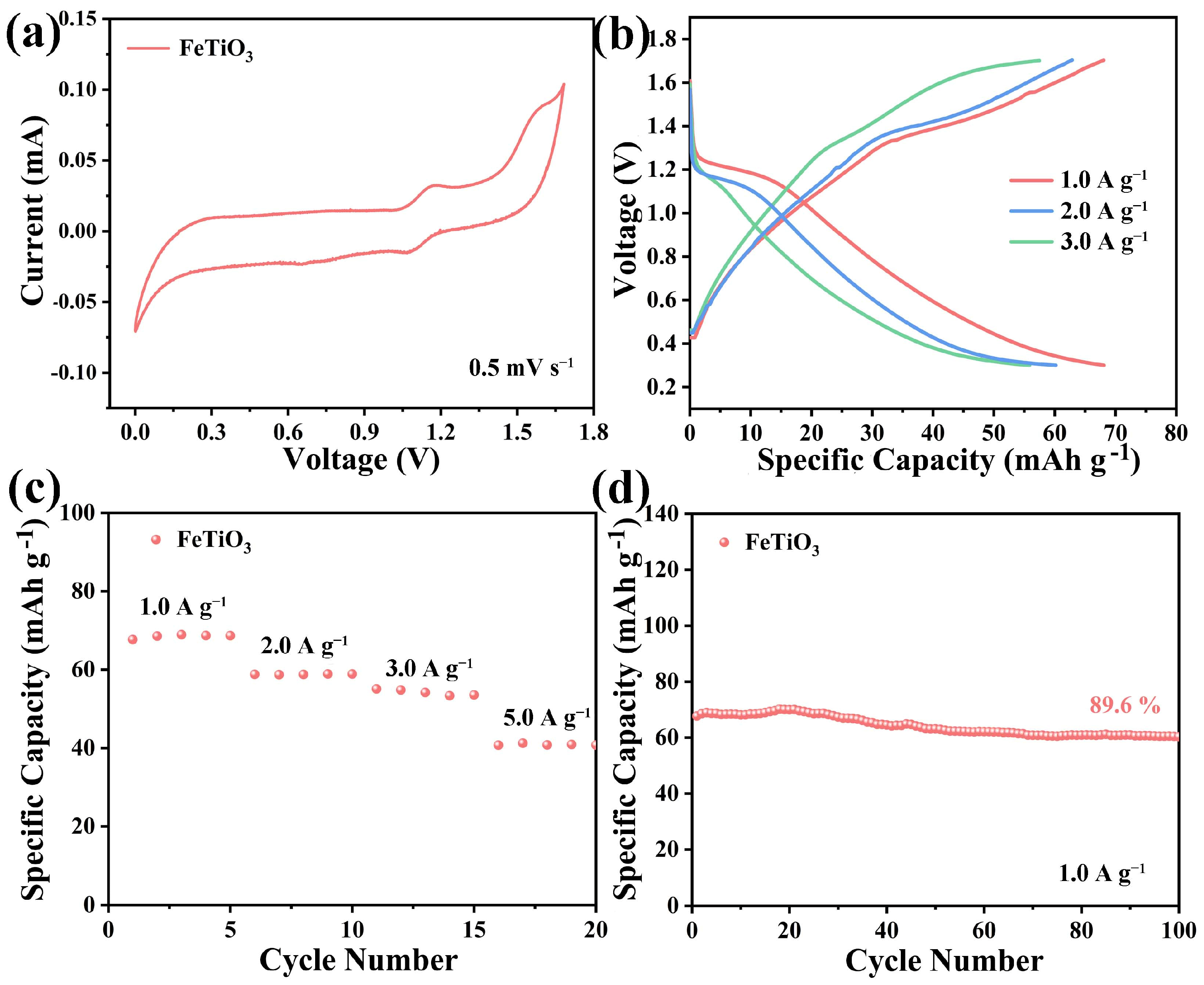
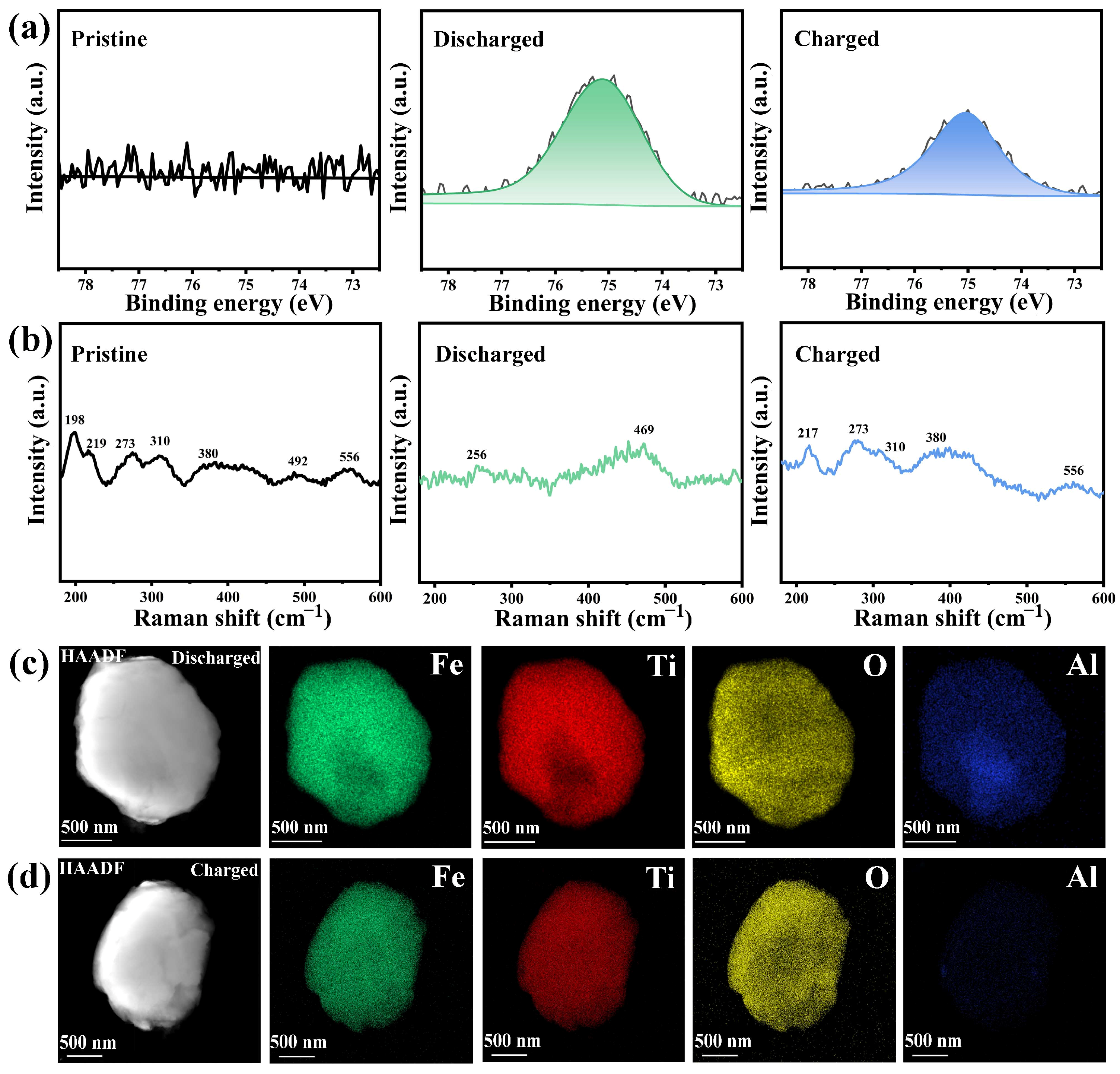
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, W.; Lin, Y. New lunar samples returned by Chang’e-5: Opportunities for new discoveries and international collaboration. Innovation 2021, 2, 100070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Su, F.; Avice, G.; Stuart, F.M.; Zheng, Y.; Liu, Z.; Guo, W.; Smith, T.; Liu, R.; Lu, C.; et al. Presence of non-solar derived krypton and xenon unveiled by Chang’e-5 lunar soils. Earth Planet. Sci. Lett. 2024, 637, 118725. [Google Scholar] [CrossRef]
- Yue, Z.; Di, K.; Michael, G.; Gou, S.; Lin, Y.; Liu, J. Martian surface dating model refinement based on Chang’E-5 updated lunar chronology function. Earth Planet. Sci. Lett. 2022, 595, 117765. [Google Scholar] [CrossRef]
- Zhang, W.; Liang, Q.; Li, X.; Ma, L.-P.; Li, X.; Zhao, Z.; Zhang, R.; Cao, H.; Wang, Z.; Li, W.; et al. Discovery of natural few-layer graphene on the Moon. Natl. Sci. Rev. 2024, 11, nwae211. [Google Scholar] [CrossRef] [PubMed]
- Kobaka, J.; Katzer, J.; Seweryn, K.; Srokosz, P.; Bujko, M.; Konečný, P. A study of lunar soil simulants from construction and building materials perspective. Case Stud. Constr. Mater. 2023, 18, e02082. [Google Scholar] [CrossRef]
- Guo, Z.; Xing, D.; Xi, X.; Yue, X.; Liang, C.; Hao, B.; Zheng, Q.; Gutnikov, S.I.; Lazoryak, B.I.; Ma, P. Production of fibres from lunar soil: Feasibility, applicability and future perspectives. Adv. Fiber Mater. 2022, 4, 923–937. [Google Scholar] [CrossRef]
- Zhou, C.; Gao, Y.; Zhou, Y.; She, W.; Shi, Y.; Ding, L.; Miao, C. Properties and characteristics of regolith-based materials for extraterrestrial construction. Engineering 2024, 37, 159–181. [Google Scholar] [CrossRef]
- Han, W.; Zhou, Y.; Dang, F.; Zhou, C.; Ding, L. Spark plasma sintering of HUST-1 lunar regolith simulant and its thermal shock resistance properties. Adv. Space Res. 2024, 73, 1992–2003. [Google Scholar] [CrossRef]
- Lin, H.; Xu, R.; Li, S.; Chang, R.; Hui, H.; Liu, Y.; Tian, H.; Fan, K.; He, Z.; He, H.; et al. Higher water content observed in smaller size fraction of Chang’e-5 lunar regolith samples. Sci. Bull. 2024, 69, 3723–3729. [Google Scholar] [CrossRef]
- Tian, H.C.; Hao, J.; Lin, Y.; Xu, Y.; Jin, Z.; Zhang, C.; Yang, W.; Hu, S.; Li, R.; Yue, Z.; et al. Distribution and abundance of solar wind-derived water in chang’E-5 core samples and its implications. Geophys. Res. Lett. 2024, 51, e2023GL107005. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, L.; Zhu, X.; Tu, W.; Zhou, Y.; Liu, R.; Sun, J.; Tao, B.; Wang, C.; Yu, X.; et al. Extraterrestrial photosynthesis by Chang’E-5 lunar soil. Joule 2022, 6, 1008–1014. [Google Scholar] [CrossRef]
- Zhou, S.; Lu, C.; Zhu, X.; Li, F. Preparation and characterization of high-strength geopolymer based on BH-1 lunar soil simulant with low alkali content. Engineering 2021, 7, 1631–1645. [Google Scholar] [CrossRef]
- Palos, M.F.; Serra, P.; Fereres, S.; Stephenson, K.; González-Cinca, R. Lunar ISRU energy storage and electricity generation. Acta Astronaut. 2020, 170, 412–420. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, T.; Lv, X.; Zhang, G.; Wang, C.; Gu, J.; Zhang, X.; Wang, Q.; Chen, X.; Quan, X.; et al. Investigation on a lunar energy storage and conversion system based on the in-situ resources utilization. Energy 2023, 268, 126681. [Google Scholar] [CrossRef]
- Zhong, Y.; Low, J.; Zhu, Q.; Jiang, Y.; Yu, X.; Wang, X.; Zhang, F.; Shang, W.; Long, R.; Yao, Y.; et al. In situ resource utilization of lunar soil for highly efficient extraterrestrial fuel and oxygen supply. Natl. Sci. Rev. 2023, 10, nwac200. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, W.; Yang, H.; Zhou, H. Electrolyte design principles for low-temperature lithium-ion batteries. eScience 2023, 3, 100170. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, S. Recent advances in cathode catalyst architecture for lithium–oxygen batteries. eScience 2023, 3, 100123. [Google Scholar] [CrossRef]
- Zeng, X.; Li, M.; Abd El-Hady, D.; Alshitari, W.; Al-Bogami, A.S.; Lu, J.; Amine, K. Commercialization of lithium battery technologies for electric vehicles. Adv. Energy Mater. 2019, 9, 1900161. [Google Scholar] [CrossRef]
- Masias, A.; Marcicki, J.; Paxton, W. Opportunities and challenges of lithium ion batteries in automotive applications. ACS Energy Lett. 2021, 6, 621–630. [Google Scholar] [CrossRef]
- Khan, M.M.R.; Chakraborty, N. Conducting polymer-based gel materials: Synthesis, morphology, thermal properties, and applications in supercapacitors. Gels 2024, 10, 553. [Google Scholar] [CrossRef]
- Khan, M.M.R.; Rumon, M.M.H. Recent Progress on the synthesis, morphological topography, and battery applications of polypyrrole-based nanocomposites. Polymers 2024, 16, 3277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Niu, J.; Zhao, Z.; Wang, Q. Research on the effect of thermal runaway gas components and explosion limits of lithium-ion batteries under different charge states. J. Energy Storage 2022, 45, 103759. [Google Scholar] [CrossRef]
- Zhang, Q.; Niu, J.; Yang, J.; Liu, T.; Bao, F.; Wang, Q. In-situ explosion limit analysis and hazards research of vent gas from lithium-ion battery thermal runaway. J. Energy Storage 2022, 56, 106146. [Google Scholar] [CrossRef]
- Xie, K.; Shi, Z.; Xu, J.; Hu, X.; Gao, B.; Wang, Z. Aluminothermic reduction-molten salt electrolysis using inert anode for oxygen and Al-base alloy extraction from lunar soil simulant. JOM 2017, 69, 1963–1969. [Google Scholar] [CrossRef]
- Du, K.; Liu, Y.; Zhao, Y.; Li, H.; Liu, H.; Sun, C.; Han, M.; Ma, T.; Hu, Y. High-entropy prussian blue analogues enable lattice respiration for ultrastable aqueous aluminum-ion batteries. Adv. Mater. 2024, 36, 2404172. [Google Scholar] [CrossRef]
- Su, J.; Zhang, M.; Tian, H.; Han, M.; Sun, Z.; Du, K.; Cui, F.; Li, J.; Huang, W.; Hu, Y. Synergistic π-conjugation organic cathode for ultra-stable aqueous aluminum batteries. Small 2024, 20, 2312086. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, R.; Zhi, X.; Liu, D.; Wang, Y.; Feng, Z.; Sun, T. Aluminum vacancy-rich MOF-derived carbon nanosheets for high-capacity and long-life aqueous aluminum-ion battery. EcoEnergy 2024, 2, 466–477. [Google Scholar] [CrossRef]
- Du, K.; Sun, C.-H.; Su, J.-W.; Zhao, Y.-Q.; Hou, J.-W.; Hu, Y.-X. Paving pathway for reliable cathodes development in aqueous aluminum-ion batteries: A mini review. Rare Met. 2024, 43, 6164–6182. [Google Scholar] [CrossRef]
- Yu, L.; Liu, J.; Xu, X.; Zhang, L.; Hu, R.; Liu, J.; Ouyang, L.; Yang, L.; Zhu, M. Ilmenite nanotubes for high stability and high rate sodium-ion battery anodes. ACS Nano 2017, 11, 5120–5129. [Google Scholar] [CrossRef]
- Dong, J.; Dong, Y.; Ren, N.; Zhang, L.; Li, Y.; He, H.; Chen, C. Realizing high-performance lithium storage by fabricating FeTiO3 nanoparticle-impregnated multichannel carbon nanofibers with promoted reaction kinetics. ACS Appl. Mater. Interfaces 2022, 14, 46513–46522. [Google Scholar] [CrossRef]
- Tao, T.; Glushenkov, A.M.; Rahman, M.M.; Chen, Y. Electrochemical reactivity of ilmenite FeTiO3, its nanostructures and oxide-carbon nanocomposites with lithium. Electrochim. Acta 2013, 108, 127–134. [Google Scholar] [CrossRef]
- Brugnetti, G.; Fiore, M.; Lorenzi, R.; Paleari, A.; Ferrara, C.; Ruffo, R. FeTiO3 as anode material for sodium-ion batteries: From morphology control to decomposition. ChemElectroChem 2020, 7, 1713–1722. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Liao, S.; Yin, Z.; Hsu, W. Mineral chemistry and 3D tomography of a Chang’E 5 high-Ti basalt: Implication for the lunar thermal evolution history. Sci. Bull. 2022, 67, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Zhang, G.; Dong, K.; Deng, X.; Gao, X.; Yang, Y.; Xiao, Y.; Bai, X.; Liang, K.; et al. Size, morphology, and composition of lunar samples returned by Chang’E-5 mission. Sci. China Phys. Mech. Astron. 2021, 65, 229511. [Google Scholar] [CrossRef]
- Li, Q.-L.; Zhou, Q.; Liu, Y.; Xiao, Z.; Lin, Y.; Li, J.-H.; Ma, H.-X.; Tang, G.-Q.; Guo, S.; Tang, X.; et al. Two-billion-year-old volcanism on the Moon from Chang’e-5 basalts. Nature 2021, 600, 54–58. [Google Scholar] [CrossRef]
- Pieters, C.M.; Taylor, L.A.; Noble, S.K.; Keller, L.P.; Hapke, B.; Morris, R.V.; Allen, C.C.; McKay, D.S.; Wentworth, S. Space weathering on airless bodies: Resolving a mystery with lunar samples. Meteorit. Planet. Sci. 2010, 35, 1101–1107. [Google Scholar] [CrossRef]
- Keller, L.; Mckay, D. The nature and origin of rims on lunar soil grains. Geochim. Cosmochim. Acta 1997, 61, 2331–2341. [Google Scholar] [CrossRef]
- Meng, Q.; Yuan, Z.; Yu, L.; Xu, Y.; Du, Y. Study on the activation mechanism of lead ions in the flotation of ilmenite using benzyl hydroxamic acid as collector. J. Ind. Eng. Chem. 2018, 62, 209–216. [Google Scholar] [CrossRef]
- Silveira, J.E.; Paz, W.S.; Garcia-Muñoz, P.; Zazo, J.A.; Casas, J.A. UV-LED/ilmenite/persulfate for azo dye mineralization: The role of sulfate in the catalyst deactivation. Appl. Catal. B Environ. 2017, 219, 314–321. [Google Scholar] [CrossRef]
- Dai, C.; Chen, P.; Yang, Y.; Sun, W. Selective flotation of ilmenite from titanaugite using 2-amino-1,3-propanediol as a novel depressant. Colloid Surf. A 2024, 681, 132752. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, S.; Li, J.; Sun, C.; Du, K.; Wang, C.; Han, M.; Geng, J.; Long, Y.; Hu, Y. Construction of Lunar Soil Simulants-Based Aluminum-Ion Battery Systems. Materials 2025, 18, 471. https://doi.org/10.3390/ma18030471
Su S, Li J, Sun C, Du K, Wang C, Han M, Geng J, Long Y, Hu Y. Construction of Lunar Soil Simulants-Based Aluminum-Ion Battery Systems. Materials. 2025; 18(3):471. https://doi.org/10.3390/ma18030471
Chicago/Turabian StyleSu, Shaokang, Jingzhen Li, Chunhao Sun, Kai Du, Chengjie Wang, Mingshan Han, Jing Geng, Yongde Long, and Yuxiang Hu. 2025. "Construction of Lunar Soil Simulants-Based Aluminum-Ion Battery Systems" Materials 18, no. 3: 471. https://doi.org/10.3390/ma18030471
APA StyleSu, S., Li, J., Sun, C., Du, K., Wang, C., Han, M., Geng, J., Long, Y., & Hu, Y. (2025). Construction of Lunar Soil Simulants-Based Aluminum-Ion Battery Systems. Materials, 18(3), 471. https://doi.org/10.3390/ma18030471




