Fungal-Derived Chitosan from In Vitro Mushroom Cultures as an Antimicrobial Matrix for Silver Nanoparticles in Advanced Bioactive Materials
Abstract
1. Introduction
2. Materials and Methods
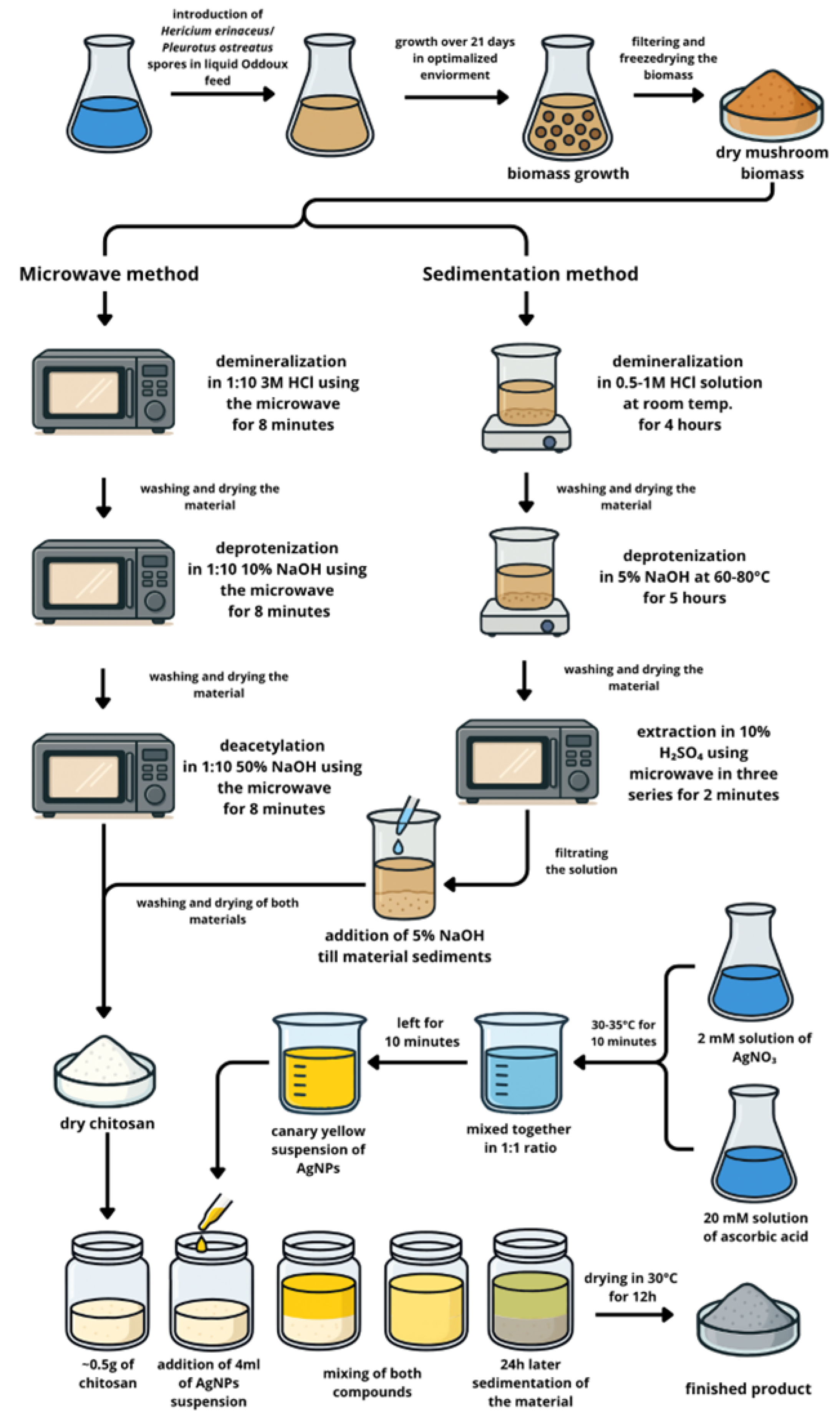
2.1. The Scheme of the Experiment
2.2. Materials Preparation
2.2.1. Biomass Material—In Vitro Cultures of H. erinaceus and P. ostreatus (Step 1)
2.2.2. Chitosan Extraction (Step 2)
Chitosan Extraction from In Vitro Cultures of H. erinaceus
Chitosan Extraction from In Vitro Cultures of P. ostreatus
2.2.3. Synthesis of AgNPs (Steps 3 and 4)
Stock Solution Preparation
Synthesis of Nanoparticles
2.3. Method Analysis
2.3.1. Microstructure Analysis
2.3.2. Analysis of the Surface and Grain Size of the Material
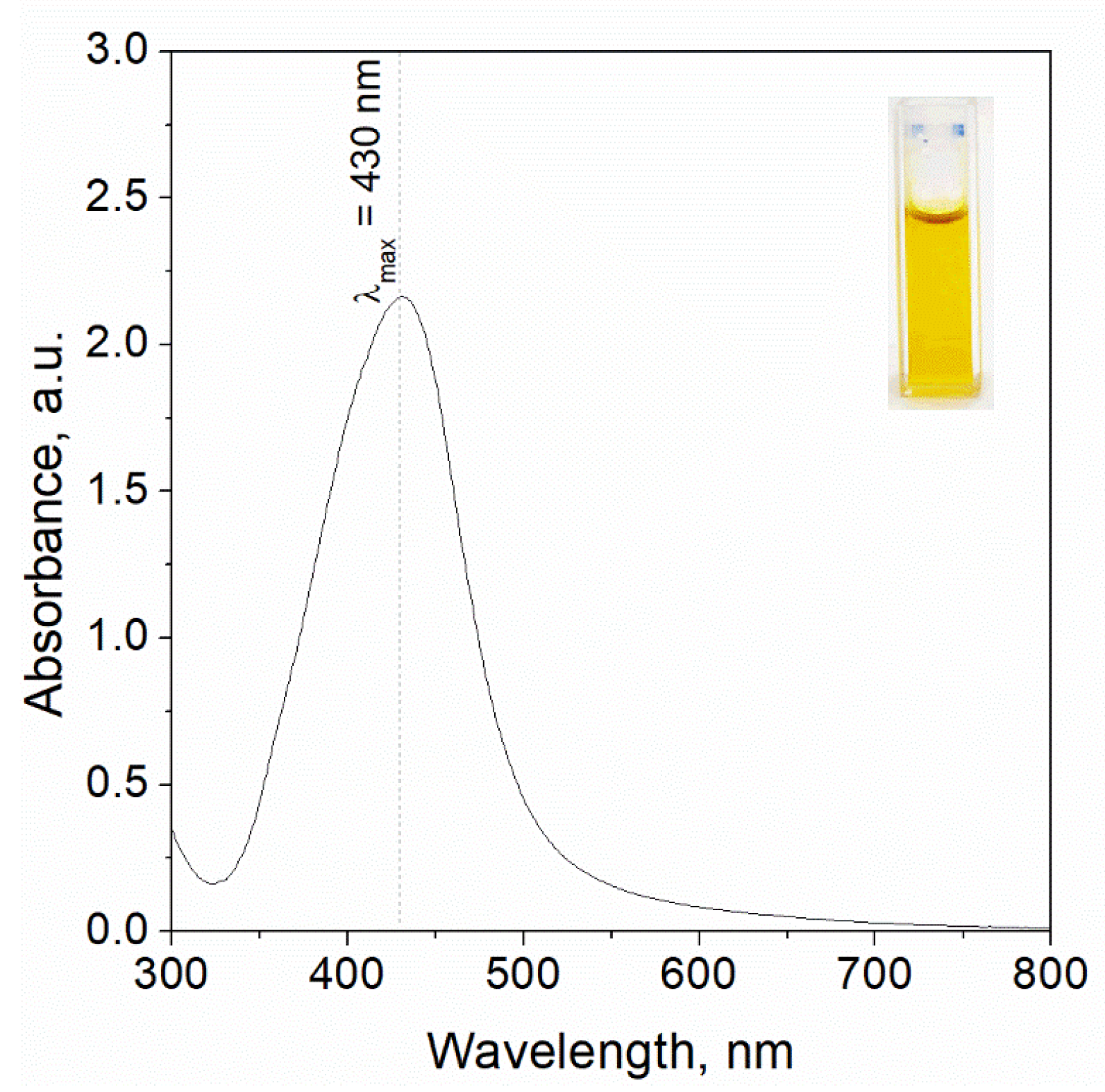
2.3.3. UV-Vis Analysis
2.3.4. FTIR-ATR Analysis
2.3.5. AAS Analysis
2.3.6. DSC Analysis
2.3.7. Microbiological Analysis
2.4. Reagents
2.4.1. Liquid Culture Media
2.4.2. Mineralization of Freeze-Dried Biomass
2.4.3. AAS Method Measurement
2.5. Statistical Analysis
3. Results
3.1. Characterization Materials
3.2. Structure Analysis (FTIR-ATR)
3.3. AAS Analysis
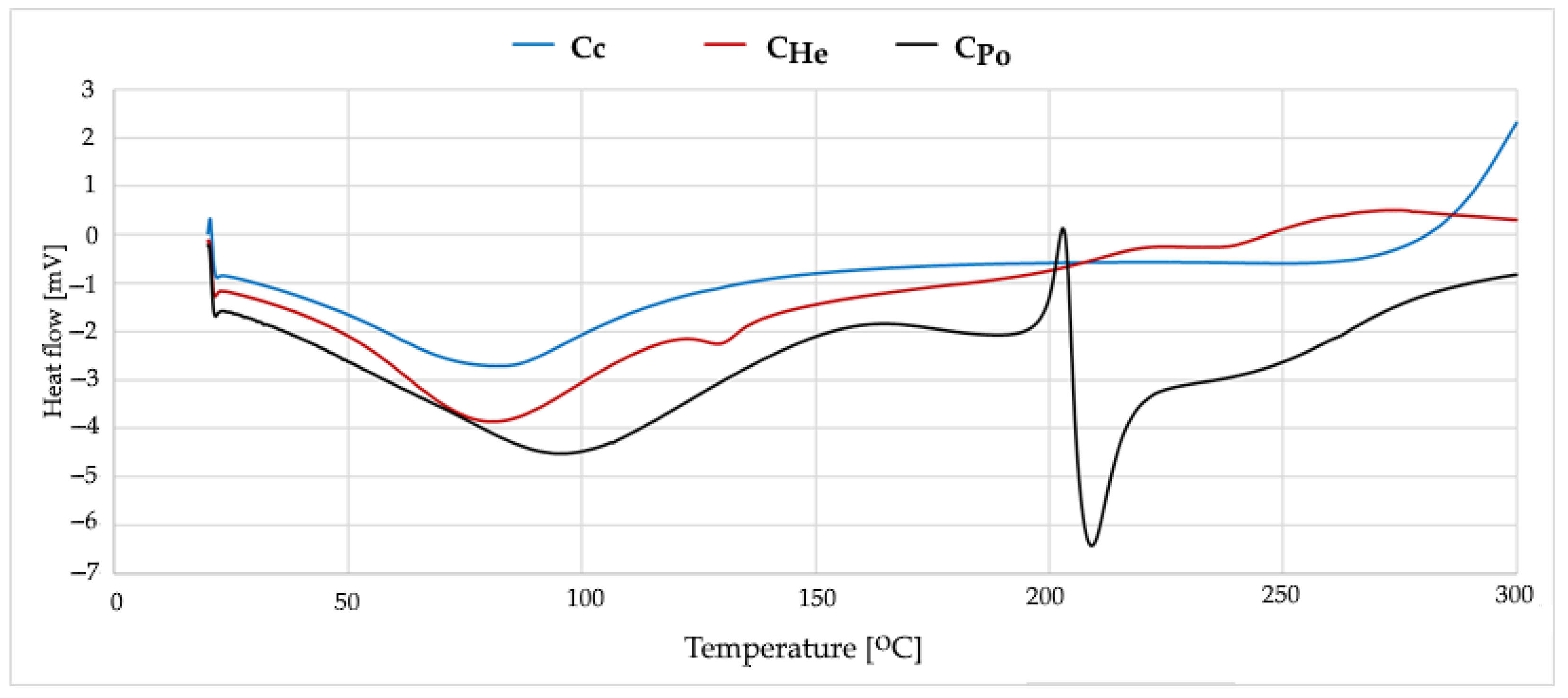
3.4. Thermal Stability Analysis of Chitosan
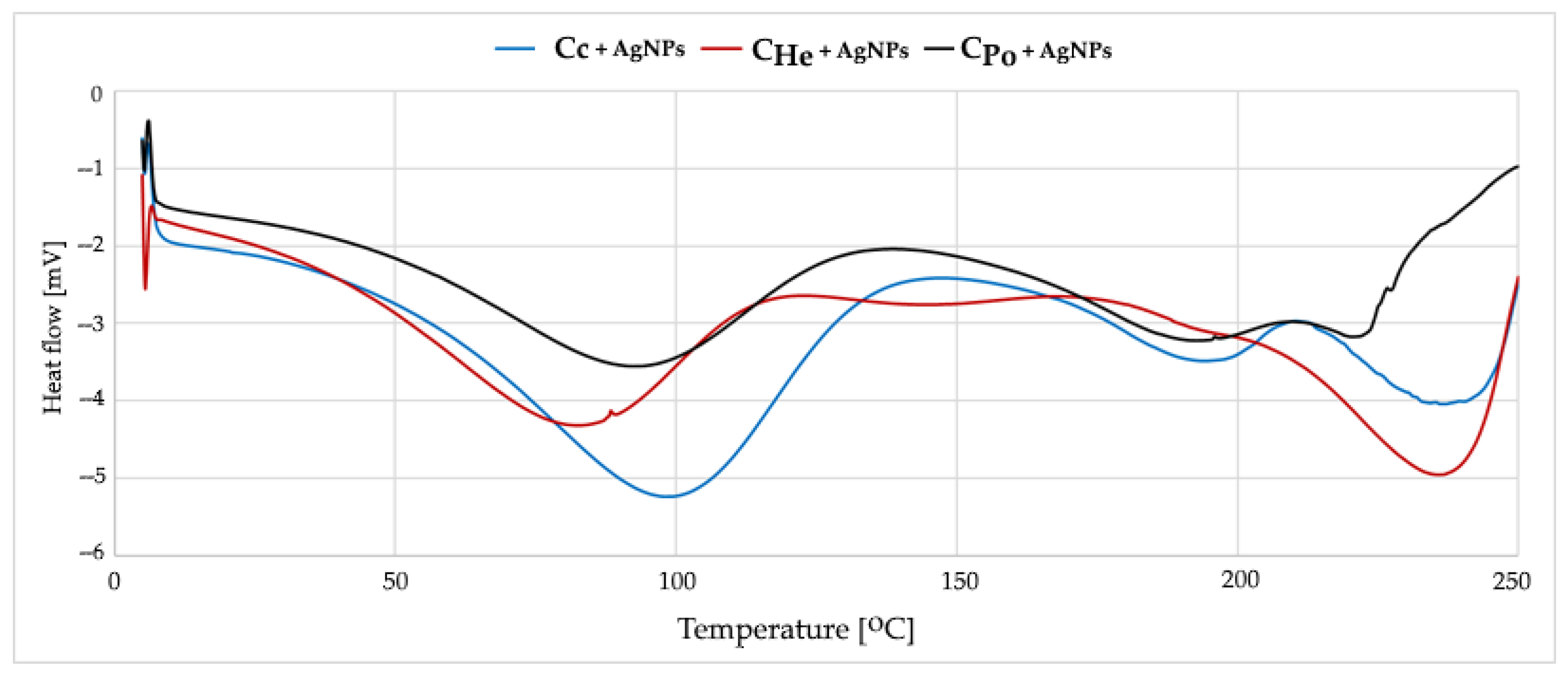
3.5. Effect of Silver Modification
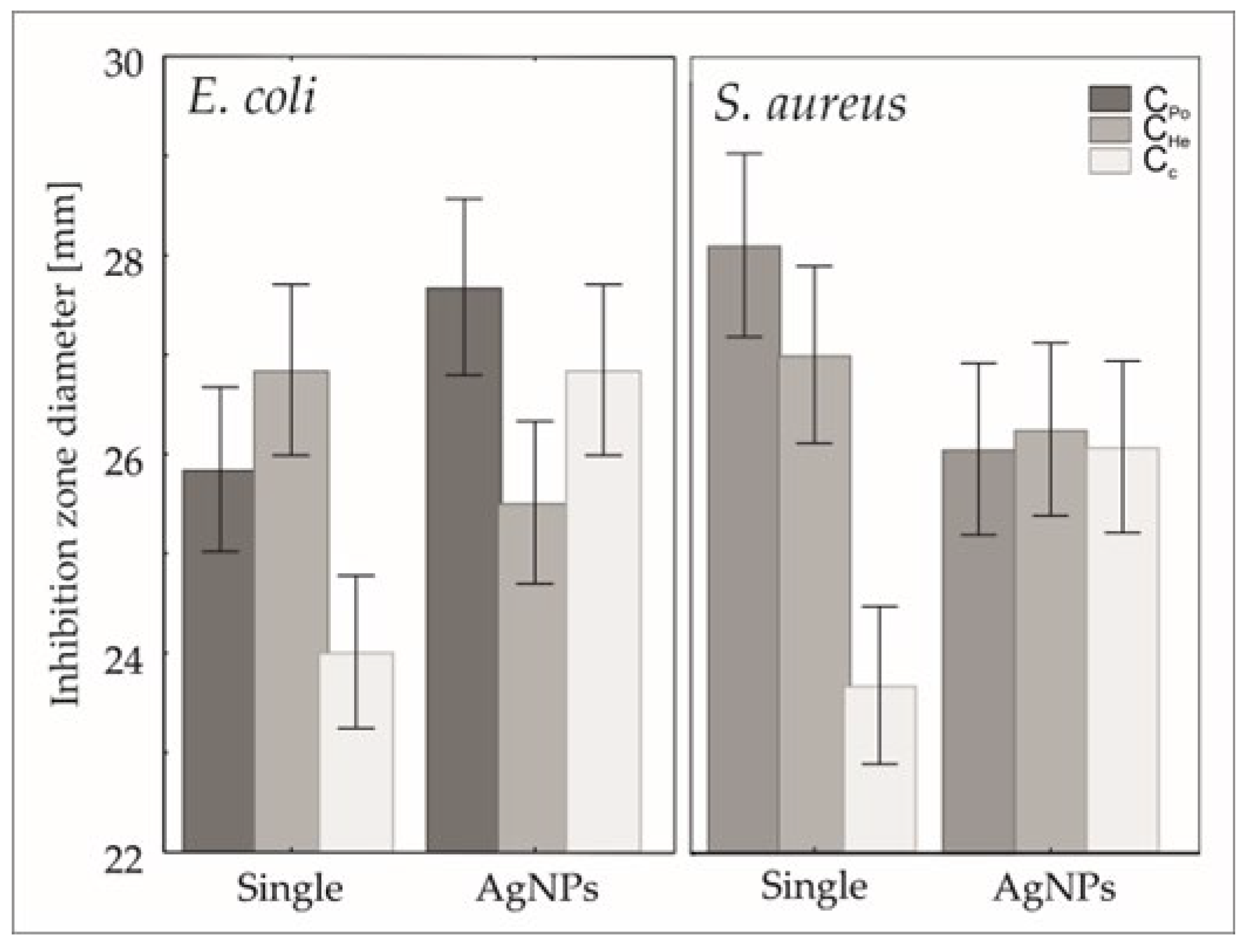
3.6. Microbiological Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, J.; Patel, D.; Rananavare, D.; Hudson, D.; Tran, M.; Schloss, R.; Langrana, N.; Berthiaume, F.; Kumar, S. Recent Advancements in Chitosan-Based Biomaterials for Wound Healing. J. Funct. Biomater. 2025, 16, 45. [Google Scholar] [CrossRef]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef] [PubMed]
- Jøraholmen, M.W.; Bhargava, A.; Julin, K.; Johannessen, M.; Škalko-Basnet, N. The Antimicrobial Properties of Chitosan Can Be Tailored by Formulation. Mar. Drugs 2020, 18, 96. [Google Scholar] [CrossRef]
- Andersen, T.; Mishchenko, E.; Flaten, G.; Sollid, J.; Mattsson, S.; Tho, I.; Škalko-Basnet, N. Chitosan-Based Nanomedicine to Fight Genital Candida Infections: Chitosomes. Mar. Drugs 2017, 15, 64. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial Applications of Crustacean By-Products (Chitin, Chitosan, and Chitooligosaccharides): A Review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Tapia, A.; Seña, R.; Zambrano, H.; Paredes, V. Extraction and Characterization of Chitosan Obtained from Shells of Crab (Callinectes bocourti and Callinectes sapidus). Int. J. Biol. Macromol. 2025, 320, 145963. [Google Scholar] [CrossRef]
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical Extraction and Modification of Chitin and Chitosan from Shrimp Shells. Eur. Polym. J. 2021, 159, 110709. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, Production and Commercial Applications of Fungal Chitosan: A Review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Peter, M.G. Chitin and Chitosan in Fungi. In Biopolymers Online; Steinbüchel, A., Ed.; Wiley: Hoboken, NJ, USA, 2002; ISBN 978-3-527-30290-1. [Google Scholar]
- Du, B.; Zhang, X.; Zhu, C.; Wu, Y.; Ji, H.; Zhang, Y.; Yue, X. Immunomodulatory and Antioxidant Effects of Polysaccharides from Pleurotus Ostreatus on Immunosuppressed Mice. Starch/Staerke 2022, 74, 2200009. [Google Scholar] [CrossRef]
- Khan, M.A.; Tania, M.; Liu, R.; Rahman, M.M. Hericium Erinaceus: An Edible Mushroom with Medicinal Values. J. Complement. Integr. Med. 2013, 10, 253–258. [Google Scholar] [CrossRef]
- Jedinak, A.; Sliva, D. Pleurotus Ostreatus Inhibits Proliferation of Human Breast and Colon Cancer Cells through P53-Dependent as Well as P53-Independent Pathway. Int. J. Oncol. 2008, 33, 1307–1313. [Google Scholar]
- Cui, W.; Song, X.; Li, X.; Jia, L.; Zhang, C. Structural Characterization of Hericium Erinaceus Polysaccharides and the Mechanism of Anti-T2DM by Modulating the Gut Microbiota and Metabolites. Int. J. Biol. Macromol. 2023, 242, 125165. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Huang, H. Extraction of a Novel Fungal Chitin from Hericium Erinaceus Residue Using Multistep Mild Procedures. Int. J. Biol. Macromol. 2020, 156, 1279–1286. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Ralte, V.; Zohmingliana, H.; Das, S.; Anal, J.M.H.; Lallianrawna, S.; Rokhum, S.L. A Review of Microbes Mediated Biosynthesis of Silver Nanoparticles and Their Enhanced Antimicrobial Activities. Heliyon 2024, 10, e32333. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Nie, P.; Zhao, Y.; Xu, H. Synthesis, Applications, Toxicity and Toxicity Mechanisms of Silver Nanoparticles: A Review. Ecotoxicol. Environ. Saf. 2023, 253, 114636. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.S.; Batista, J.G.S.; Rodrigues, M.Á.V.; Thipe, V.C.; Minarini, L.A.R.; Lopes, P.S.; Lugão, A.B. Advances in Silver Nanoparticles: A Comprehensive Review on Their Potential as Antimicrobial Agents and Their Mechanisms of Action Elucidated by Proteomics. Front. Microbiol. 2024, 15, 1440065. [Google Scholar] [CrossRef]
- Wu, J.; Niu, Y.; Jiao, Y.; Chen, Q. Fungal Chitosan from Agaricus bisporus (Lange) Sing. Chaidam Increased the Stability and Antioxidant Activity of Liposomes Modified with Biosurfactants and Loading Betulinic Acid. Int. J. Biol. Macromol. 2019, 123, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Chitosan-Based Scaffolds Incorporated with Silver Nanoparticles for the Treatment of Infected Wounds. Pharmaceutics 2024, 16, 327. [Google Scholar] [CrossRef]
- Krishnan, P.D.; Banas, D.; Durai, R.D.; Kabanov, D.; Hosnedlova, B.; Kepinska, M.; Fernandez, C.; Ruttkay-Nedecky, B.; Nguyen, H.V.; Farid, A.; et al. Silver Nanomaterials for Wound Dressing Applications. Pharmaceutics 2020, 12, 821. [Google Scholar] [CrossRef]
- Zhang, S.; Ali, M.; Nawaz, F.; Ali, N.; Khan, A.; Ali, F.; Khan, M.H.; Sidra; Ahmad, S.; Rahman, S.; et al. Scaffolds of Chitosan-Metallic Hybrids as Antimicrobial Wound Dressing. J. Mol. Liq 2025, 417, 126541. [Google Scholar] [CrossRef]
- Estévez-Martínez, Y.; Vázquez Mora, R.; Méndez Ramírez, Y.I.; Chavira-Martínez, E.; Huirache-Acuña, R.; Díaz-de-León-Hernández, J.N.; Villarreal-Gómez, L.J. Antibacterial Nanocomposite of Chitosan/Silver Nanocrystals/Graphene Oxide (ChAgG) Development for Its Potential Use in Bioactive Wound Dressings. Sci. Rep. 2023, 13, 10234. [Google Scholar] [CrossRef] [PubMed]
- Shehabeldine, A.M.; Salem, S.S.; Ali, O.M.; Abd-Elsalam, K.A.; Elkady, F.M.; Hashem, A.H. Multifunctional Silver Nanoparticles Based on Chitosan: Antibacterial, Antibiofilm, Antifungal, Antioxidant, and Wound-Healing Activities. J. Fungi 2022, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Aldakheel, F.M.; Wickramasinghe, R.; Thamaraiselvan, C.; Sayed, M.M.E.; Fagir, M.H.; Dein, D.K.E.; Mohsen, D. Green Silver Nanoparticle-Embedded Chitosan-Alginate Hydrogel: A Novel Antibacterial Approach for Potential Wound Healing. Polym. Polym. Compos. 2025, 33, 09673911251320463. [Google Scholar] [CrossRef]
- Vinanthi Rajalakshmi, K.S.; Balasubramanian, B.; Hinnakki, H.; Meyyazhagan, A.; Liu, W.-C.; Pappuswamy, M.; Kamyab, H.; Simancas-Racines, D.; Paari, K.A. Fungal Biopolymer-Based Nanoparticles for Wound Healing: Mechanisms, Applications, and Future Perspectives. Food Hydrocoll. Health 2025, 8, 100229. [Google Scholar] [CrossRef]
- Boureghda, Y.; Satha, H.; Bendebane, F. Chitin–Glucan Complex from Pleurotus Ostreatus Mushroom: Physicochemical Characterization and Comparison of Extraction Methods. Waste Biomass Valorization 2021, 12, 6139–6153. [Google Scholar] [CrossRef]
- Krakowska, A.; Zięba, P.; Włodarczyk, A.; Kała, K.; Sułkowska-Ziaja, K.; Bernaś, E.; Sękara, A.; Ostachowicz, B.; Muszyńska, B. Selected Edible Medicinal Mushrooms from Pleurotus Genus as an Answer for Human Civilization Diseases. Food Chem. 2020, 327, 127084. [Google Scholar] [CrossRef] [PubMed]
- Bahndral, A.; Shams, R.; Dash, K.K.; Chaudhary, P.; Mukarram Shaikh, A.; Béla, K. Microwave Assisted Extraction of Chitosan from Agaricus Bisporus: Techno-Functional and Microstructural Properties. Carbohydr. Polym. Technol. Appl. 2025, 9, 100730. [Google Scholar] [CrossRef]
- Kumar, T.; Senani, S.; Kumar, N.; Gautam, M.; Gupta, R.; Gupta, M. Extraction and characterization of chitin, chitosan and chitooligosaccharides from crab shell waste. Indian J. Anim. Res. 2017, 51, 1066–1072. [Google Scholar] [CrossRef]
- Kedar, K.; Nayak, S.; Bhaskar, V.H. Synthesis of Silver Nanoparticles by Chemical Reduction Method. Int. J. Pharm. Pharm. Res. 2022, 25, 364–376. [Google Scholar]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Binert-Kusztal, Ż.; Krakowska, A.; Skiba-Kurek, I.; Luty-Błocho, M.; Kula, A.; Olechowska-Jarząb, A.; Dorożyński, P.; Skalski, T. Potential Use of Cefiderocol and Nanosilver in Wound Dressings to Control Multidrug-Resistant Gram-Negative Bacteria. Molecules 2025, 30, 3072. [Google Scholar] [CrossRef]
- Purohit, G.; Rawat, D.S. Characterization Techniques for Chitosan and Its Based Nanocomposites. In Chitosan-Based Nanocomposite Materials; Gulati, S., Ed.; Springer Nature: Singapore, 2022; pp. 79–101. ISBN 978-981-19-5337-8. [Google Scholar]
- Chiu, F.-C. A Review on Conduction Mechanisms in Dielectric Films. Adv. Mater. Sci. Eng. 2014, 2014, 578168. [Google Scholar] [CrossRef]
- Indian Institute of Science (IISC)-INUP & VVIT; Srivani, A.; Vasanth, G.; East European University; Sharma, G.S.; Mallareddy Engineering College (MREC-A); Rao, S.; Annamalai University; Ramesh, P.; Mallareddy Engineering College; et al. SEM Imaging for Advanced Bio Materials. Radiol. Res. Diagn. Imaging 2023, 2, 1–3. [Google Scholar] [CrossRef]
- Huang, K.-S.; Wang, L.-S.; Wang, C.-Y.; Yang, C.-H.; Hsieh, C.-L.; Chen, S.-Y.; Shen, C.-Y.; Wang, J.-J. Synthesis and Anti-Fungal Effect of Silver Nanoparticles-Chitosan Composite Particles. Int. J. Nanomed. 2015, 10, 2685–2696. [Google Scholar] [CrossRef] [PubMed]
- Lercher, J.A. Chapter 13: Adsorption Methods for the Assessment of the Specific Surface Area, the Pore Size Distribution and the Active Sites of Heterogeneous Catalysts. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1999; Volume 123, pp. 543–566. ISBN 978-0-444-82963-4. [Google Scholar]
- Chuiko Institute of Surface Chemistry of National Academy of Sciences of Ukraine; Gun’ko, V.M. Features of BET Method Application to Various Adsorbents. Him. Fiz. Tehnol. Poverhni 2022, 13, 249–258. [Google Scholar] [CrossRef]
- Kuila, U.; Prasad, M. Application of Nitrogen Gas-Adsorption Technique for Characterization of Pore Structure of Mudrocks. Lead. Edge. 2013, 32, 1478–1485. [Google Scholar] [CrossRef]
- Beuselinck, L.; Govers, G.; Poesen, J.; Degraer, G.; Froyen, L. Grain-Size Analysis by Laser Diffractometry: Comparison with the Sieve-Pipette Method. Catena 1998, 32, 193–208. [Google Scholar] [CrossRef]
- Bartos, C.; Varga, P.; Szabó-Révész, P.; Ambrus, R. Physico-Chemical and In Vitro Characterization of Chitosan-Based Microspheres Intended for Nasal Administration. Pharmaceutics 2021, 13, 608. [Google Scholar] [CrossRef]
- Picker-Freyer, K.M.; Brink, D. Evaluation of Powder and Tableting Properties of Chitosan. AAPS PharmSciTech 2006, 7, E152–E161. [Google Scholar] [CrossRef]
- Rousta, M.H.; Ghasemi, N. Green Synthesis of Silver Nanoparticles Using a Mountain Plant Extract. Rev. Roum. Chim. 2019, 64, 143–152. [Google Scholar] [CrossRef]
- Pinjarkar, H.; Gaikwad, S.; Ingle, A.P.; Gade, A.; Rai, M. Phycofabrication of Silver Nanoparticles and Their Antibacterial Activity Against Human Pathogens. Adv. Mater. Lett. 2016, 7, 1010–1014. [Google Scholar] [CrossRef]
- Thangapandiyan, S.; Prema, P. Chemically fabricated silver nanoparticles enhance the activity of antibiotics against selected human bacterial pathogens. Int. J. Pharm. Sci. Res. 2012, 3, 1415–1422. [Google Scholar]
- Berasarte, I.; Bordagaray, A.; Garcia-Arrona, R.; Ostra, M.; Vidal, M. Silver Nanoparticles for the Colorimetric Determination of Electrolytes by UV–Vis Spectrophotometry and Digital Image Analysis. Sens. Bio-Sens. Res. 2025, 49, 100831. [Google Scholar] [CrossRef]
- Mousavi-Khattat, M.; Nourbakhshan, H.; Afrazeh, S.; Aminorroaya, S.H.; Shakeran, Z. Donkey Dung–Mediated Synthesis of Silver Nanoparticles and Evaluation of Their Antibacterial, Antifungal, Anticancer, and DNA Cleavage Activities. BioNanoScience 2022, 12, 877–889. [Google Scholar] [CrossRef]
- Barbes, L.; Radulescu, C.; Stihi, C. ATR-FTIR Spectrometry Characterisation of Polymeric Materials. Rom. Rep. Phys. 2014, 66, 765–777. [Google Scholar]
- Cubas Pereira, D.; Pupin, B.; De Simone Borma, L. Influence of Sample Preparation Methods on FTIR Spectra for Taxonomic Identification of Tropical Trees in the Atlantic Forest. Heliyon 2024, 10, e27232. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Chan, K.L.A. Applications of ATR-FTIR Spectroscopic Imaging to Biomedical Samples. Biochim. Biophys. Acta 2006, 1758, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, J.; Rouissi, T.; Brar, S.K.; Hegde, K.; Verma, M. Microwave-Assisted Extraction of Chitosan from Rhizopus Oryzae NRRL 1526 Biomass. Carbohydr. Polym. 2019, 219, 431–440. [Google Scholar] [CrossRef]
- Silva, M.B.d.O.; de Oliveira, S.A.; Rosa, D.d.S. Comparative Study on Microwave-Assisted and Conventional Chitosan Production from Shrimp Shell: Process Optimization, Characterization, and Environmental Impacts. J. Clean. Prod. 2024, 440, 140726. [Google Scholar] [CrossRef]
- Krakowska, A.; Reczyński, W.; Muszyńska, B. Optimization of the liquid culture medium composition to obtain the mycelium of Agaricus bisporus rich in essential minerals. Biol. Trace Elem. Res. 2016, 173, 231–240. [Google Scholar] [CrossRef]
- Muszyńska, B.; Krakowska, A.; Sułkowska-Ziaja, K.; Opoka, W.; Reczyński, W.; Baś, B. In vitro cultures and fruiting bodies of culinary-medicinal Agaricus bisporus (white button mushroom) as a source of selected biologically-active elements. J. Food Sci. Technol. 2015, 52, 7337–7344. [Google Scholar] [CrossRef]
- Chicea, D.; Nicolae-Maranciuc, A.; Chicea, L.-M. Silver Nanoparticles-Chitosan Nanocomposites: A Comparative Study Regarding Different Chemical Syntheses Procedures and Their Antibacterial Effect. Materials 2024, 17, 1113. [Google Scholar] [CrossRef]
- Santos, H.M.; Coutinho, J.P.; Amorim, F.A.C.; Lôbo, I.P.; Moreira, L.S.; Nascimento, M.M.; De Jesus, R.M. Microwave-Assisted Digestion Using Diluted HNO3 and H2O2 for Macro and Microelements Determination in Guarana Samples by ICP OES. Food Chem. 2019, 273, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Hiura, S.; Abe, H.; Koyama, K.; Koseki, S. Bayesian Generalized Linear Model for Simulating Bacterial Inactivation/Growth Considering Variability and Uncertainty. Front. Microbiol. 2021, 12, 674364. [Google Scholar] [CrossRef] [PubMed]
- Oberemko, A.; Salaberria, A.M.; Saule, R.; Saulis, G.; Kaya, M.; Labidi, J.; Baublys, V. Physicochemical and in Vitro Cytotoxic Properties of Chitosan from Mushroom Species (Boletus bovinus and Laccaria laccata). Carbohydr. Polym. 2019, 221, 1–9. [Google Scholar] [CrossRef]
- Poverenov, E.; Arnon-Rips, H.; Zaitsev, Y.; Bar, V.; Danay, O.; Horev, B.; Bilbao-Sainz, C.; McHugh, T.; Rodov, V. Potential of Chitosan from Mushroom Waste to Enhance Quality and Storability of Fresh-Cut Melons. Food Chem. 2018, 268, 233–241. [Google Scholar] [CrossRef]
- Westlake, J.R.; Tassie, A.; Kotoulas, K.T.; Chaloner, E.; Laabei, M.; Sarda, L.; Ghandi, R.; Burrows, A.D.; Xie, M. Mushroom-Derived Chitosan as an Alternative Feedstock for Active Packaging Films: Performance and Biodegradation. ACS Food Sci. Technol. 2025, 5, 2381–2394. [Google Scholar] [CrossRef]
- Erdoğmuş, S.F.; Altıntaş, Ö.E.; Çelïk, S. Production of Fungal Chitosan and Fabrication of Fungal Chitosan/Polycaprolactone Elec-trospun Nanofibers for Tissue Engineering. Microsc. Res. Tech. 2023, 86, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef]
- Pacheco, N.; Garnica-Gonzalez, M.; Gimeno, M.; Bárzana, E.; Trombotto, S.; David, L.; Shirai, K. Structural characterization of chitin and chitosan obtained by biological and chemical methods. Biomacromolecules 2011, 12, 285–290. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends. Food. Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef]
- Moussian, B. Chitin: Structure, Chemistry and Biology. In Targeting Chitin-Containing Organisms; Springer: Berlin/Heidelberg, Germany, 2019; Volume 1142, pp. 5–18. [Google Scholar] [CrossRef]
- Kabalak, M.S.; Aracagok, D.; Torun, M. Extraction, characterization and comparison of chitins from large bodied four Coleoptera and Orthoptera species. Int. J. Biol. Macromol. 2019, 145, 402–409. [Google Scholar] [CrossRef]
- Pan, J.; Li, Y.; Chen, K.; Zhang, Y.; Zhang, H. Enhanced physical and antimicrobial properties of alginate/chitosan composite aerogels based on electrostatic interactions and noncovalent crosslinking. Carbohyd. Poly. 2021, 266, 118102. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Synowiecki, J.; Al-Khateeb, N.A. Production, properties, and some new applications of chitin and its derivatives. Crit. Rev. Food Sci. Nutr. 2003, 43, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Kolmas, J.; Molenda, M. The Role of Zinc in Bone Tissue Health and Regeneration—A Review. Biol. Trace. Elem. Res. 2021, 201, 5640–5651. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Park, D.; Lee, L. Chitosan Combined with ZnO, TiO2 and Ag Nanoparticles for Antimicrobial Wound Healing Applications: A Mini Review of the Research Trends. Polymers 2017, 9, 21. [Google Scholar] [CrossRef]
- Mohd Affandy, M.A.; Rovina, K. Characterization of chitosan derived from mushroom sources: Physicochemical, morphological, thermal analysis. Sustain. Chem. Pharm. 2024, 40, 101624. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as Antimicrobial Agent: Applications and Mode of Action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Xu, Z.; Jiang, X.; Hu, C.; Zou, X. Preparation and Antibacterial Activity of Chitosan Nanoparticles. Carbohydr Res 2004, 339, 2693–2700. [Google Scholar] [CrossRef]
- Dutta, P.K.; Dutta, J.; Tripathi, V.S. Chitin and Chitosan: Chemistry, Properties and Applications. J. Sci. Ind. Res. 2004, 63, 20–31. [Google Scholar]
- Xu, Y.; Young, T.; Ge, L. Effect of chitosan characteristics on silver nanoparticle stability and antimicrobial efficacy. Int. J. Biol. Macromol. 2019, 132, 960–976. [Google Scholar]
- Bikram, S.; Chauhan, P.; Gupta, V.K. Interaction of nanoparticles with bacterial cell walls: Impact on antimicrobial activity. J. Nanobiotechnology 2018, 16, 54. [Google Scholar]
- Kumar, A.; Singh, V.; Rai, A.K. Influence of chitosan molecular weight and degree of deacetylation on antimicrobial activity. Carbohyd. Polym. 2020, 246, 116627. [Google Scholar]
- Suba, R.B.; Raj, R.D.S.; Keerthika, K.; Vinothini, V. Chitosan-Based Biomaterial in Wound Healing: A Review. Cureus 2024, 16, e55193. [Google Scholar] [CrossRef]
- Dev, A.S.; Mohan, N.; Mohan, R. Chitosan-Based Composite Scaffolds for Accelerated Epidermal-Dermal Wound Healing. Explor. BioMat-X 2025, 2, 101336. [Google Scholar] [CrossRef]
- Sousa, I.; Teixeira, S.; Souza, V.; Conde, M.; Bailon, G.; Cardoso, S.; Araújo, L.; Oliviera, E.; Ferreira, S.; Oliviera, T.; et al. Sustainable Extraction and Multimodal Characterization of Fungal Chitosan from Agaricus bisporus. Foods 2025, 14, 2785. [Google Scholar] [CrossRef]
- Pramanik, S.; Aggarwal, A.; Kadi, A.; Alhomrani, M.; Alamri, A.S.; Alsanie, W.F.; Koul, K.; Deepak, A.; Bellucci, S. Chitosan Alchemy: Transforming Tissue Engineering and Wound Healing. RSC Adv. 2024, 14, 19219–19256. [Google Scholar] [CrossRef] [PubMed]
- Naveedunissa, S.; Meenalotchani, R.; Manisha, M.; Ankul Singh, S.; Nirenjen, S.; Anitha, K.; Harikrishnan, N.; Prajapati, B.G. Advances in Chitosan Based Nanocarriers for Targetted Wound Healing Therapies: A Review. Carbohydr. Polym. Technol. Appl. 2025, 11, 100891. [Google Scholar] [CrossRef]


| Type of Chitosans/Parameters | Cc | CHe | CPo |
|---|---|---|---|
| Vmicro, cm3/g | 0.000014 | 0.000015 | 0.006631 |
| Smicro, m2/g | 1.0957 | 0.9842 | 0.3273 |
| Apore, nm | 1.5486 | 0.5617 | 81.0301 |
| Functional Group/Type of Vibration | Cc [cm−1] | CHe [cm−1] | CPo [cm−1] |
|---|---|---|---|
| C-H (bending) | 894 | 900 | 896 |
| C-O (stretching) | 1027 | 1026 | 1032 |
| C-O (stretching) | 1063 | - | 1064 |
| C-O-C (stretching asymmetric) | 1150 | 1155 | 1152 |
| C-O (stretching) | 1260 | 1265 | 1249 |
| C=N (stretching) | 1322 | 1316 | 1318 |
| CH3 (symmetrical deformation) | 1377 | 1374 | 1374 |
| CH2 (deformation) | 1422 | 1417 | 1417 |
| NH (bending) | 1589 | 1557 | 1515 |
| C=O (stretching) | 1651 | 1652 | 1615 |
| CH (stretching asymmetric) | 2871 | 2851 | 2882 |
| CH (stretching symmetric) | 2916 | 2918 | 2927 |
| NH (stretching) | 3291 | 3272 | 3248 |
| OH (stretching) | 3354 | 3359 | 3351 |
| Functional Group/Type of Vibration | Cc | CHe | CPo |
|---|---|---|---|
| Mg | 1.12 ± 0.10 | 1.64 ± 0.10 | 1.96 ± 0.10 |
| Zn | 2.96 ± 0.10 | 6.45 ± 0.50 | 8.36 ± 0.10 |
| Cu | 0.44 ± 0.04 | 0.85 ± 0.10 | 1.02 ± 0.10 |
| Fe | 5.11 ± 0.20 | 9.23 ± 0.20 | 8.60 ± 0.30 |
| Ca | 0.90 ± 0.10 | 3.24 ± 0.10 | 5.30 ± 0.20 |
| Na | 1.10 ± 0.10 | 0.79 ± 0.05 | 1.36 ± 0.20 |
| K | 3.11 ± 0.20 | 1.43 ± 0.20 | 1.78 ± 0.10 |
| Mn | 1.42 ± 0.10 | 0.77 ± 0.05 | 0.95 ± 0.10 |
| Effect | d.f. | Wald Stat. | p |
|---|---|---|---|
| E. coli | |||
| Intercept | 1 | 227,880.1 | 0.000 |
| Chitosan type | 2 | 27.1 | 0.000 |
| AgNPs | 1 | 7.2 | 0.007 |
| Chitosan type * AgNPs | 2 | 10.4 | 0.005 |
| S. aureus | |||
| Intercept | 1 | 244,102.3 | 0.000 |
| Chitosan type | 2 | 104.8 | 0.000 |
| AgNPs | 1 | 1.6 | 0.211 |
| Chitosan type * AgNPs | 2 | 70.9 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krakowska, A.; Műller, D.; Kula, A.; Skiba-Kurek, I.; Paczosa-Bator, B.; Muszyńska, B.; Skalski, T. Fungal-Derived Chitosan from In Vitro Mushroom Cultures as an Antimicrobial Matrix for Silver Nanoparticles in Advanced Bioactive Materials. Materials 2025, 18, 5342. https://doi.org/10.3390/ma18235342
Krakowska A, Műller D, Kula A, Skiba-Kurek I, Paczosa-Bator B, Muszyńska B, Skalski T. Fungal-Derived Chitosan from In Vitro Mushroom Cultures as an Antimicrobial Matrix for Silver Nanoparticles in Advanced Bioactive Materials. Materials. 2025; 18(23):5342. https://doi.org/10.3390/ma18235342
Chicago/Turabian StyleKrakowska, Agata, Dominik Műller, Anna Kula, Iwona Skiba-Kurek, Beata Paczosa-Bator, Bożena Muszyńska, and Tomasz Skalski. 2025. "Fungal-Derived Chitosan from In Vitro Mushroom Cultures as an Antimicrobial Matrix for Silver Nanoparticles in Advanced Bioactive Materials" Materials 18, no. 23: 5342. https://doi.org/10.3390/ma18235342
APA StyleKrakowska, A., Műller, D., Kula, A., Skiba-Kurek, I., Paczosa-Bator, B., Muszyńska, B., & Skalski, T. (2025). Fungal-Derived Chitosan from In Vitro Mushroom Cultures as an Antimicrobial Matrix for Silver Nanoparticles in Advanced Bioactive Materials. Materials, 18(23), 5342. https://doi.org/10.3390/ma18235342








