Mechanical Properties of Composite Made from Bottom Ash Fractions of Municipal Waste Incineration Plant Products
Highlights
- Bottom ash (BA) demonstrates strong potential for use as a secondary binder in sustainable construction materials.
- Smaller particle size increases compressive strength.
- Thermal treatment at 1000 °C improves binding activity and raises water permeability values.
- The mechanical performance of BA-based composites is primarily controlled by chemical activation.
- Bottom ash can be reused in sustainable construction.
- Proper pretreatment enhances the reactivity of BA-based composites.
- The results demonstrate that proper thermal treatment can transform BA into a reactive component suitable for cementitious systems.
Abstract
1. Introduction
- The grain-size fraction used in the composite, which reflects the structural characteristics of the material;
- Exposure of the material to high temperatures, which represents an attempt to identify a method for improving its properties in the context of potential applications.
2. Materials and Methods
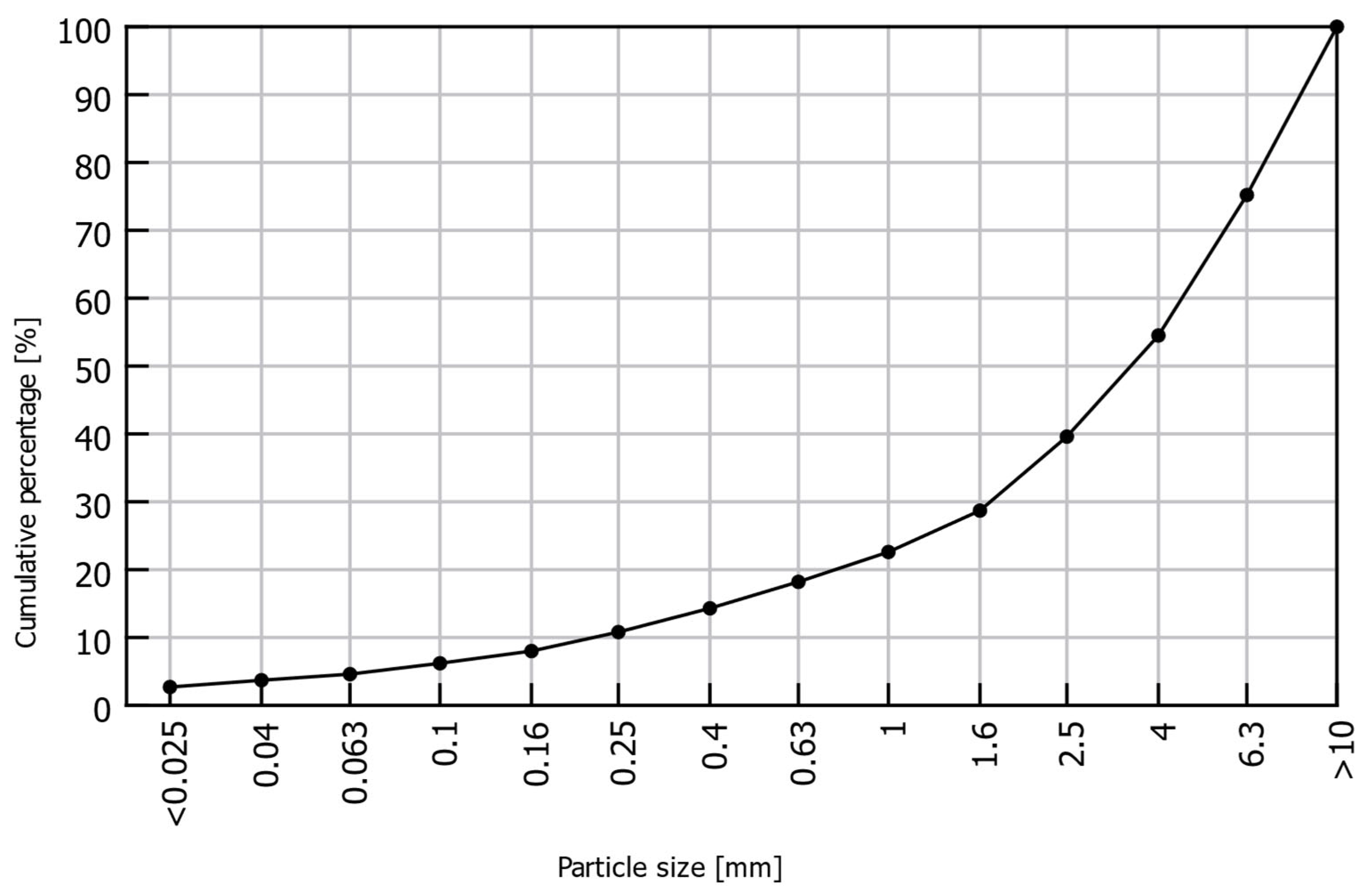
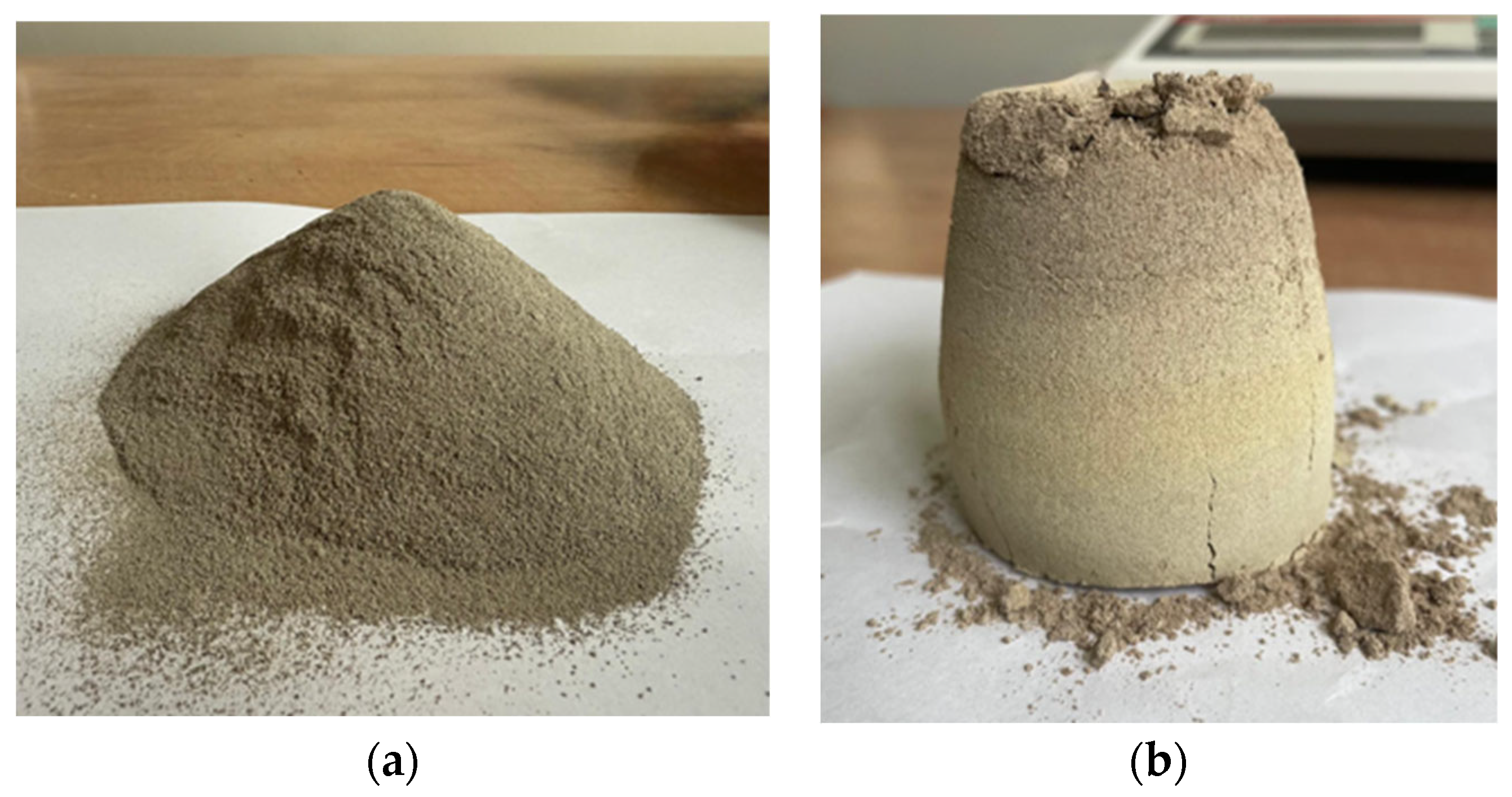
2.1. Material Origin and Preliminary Identification
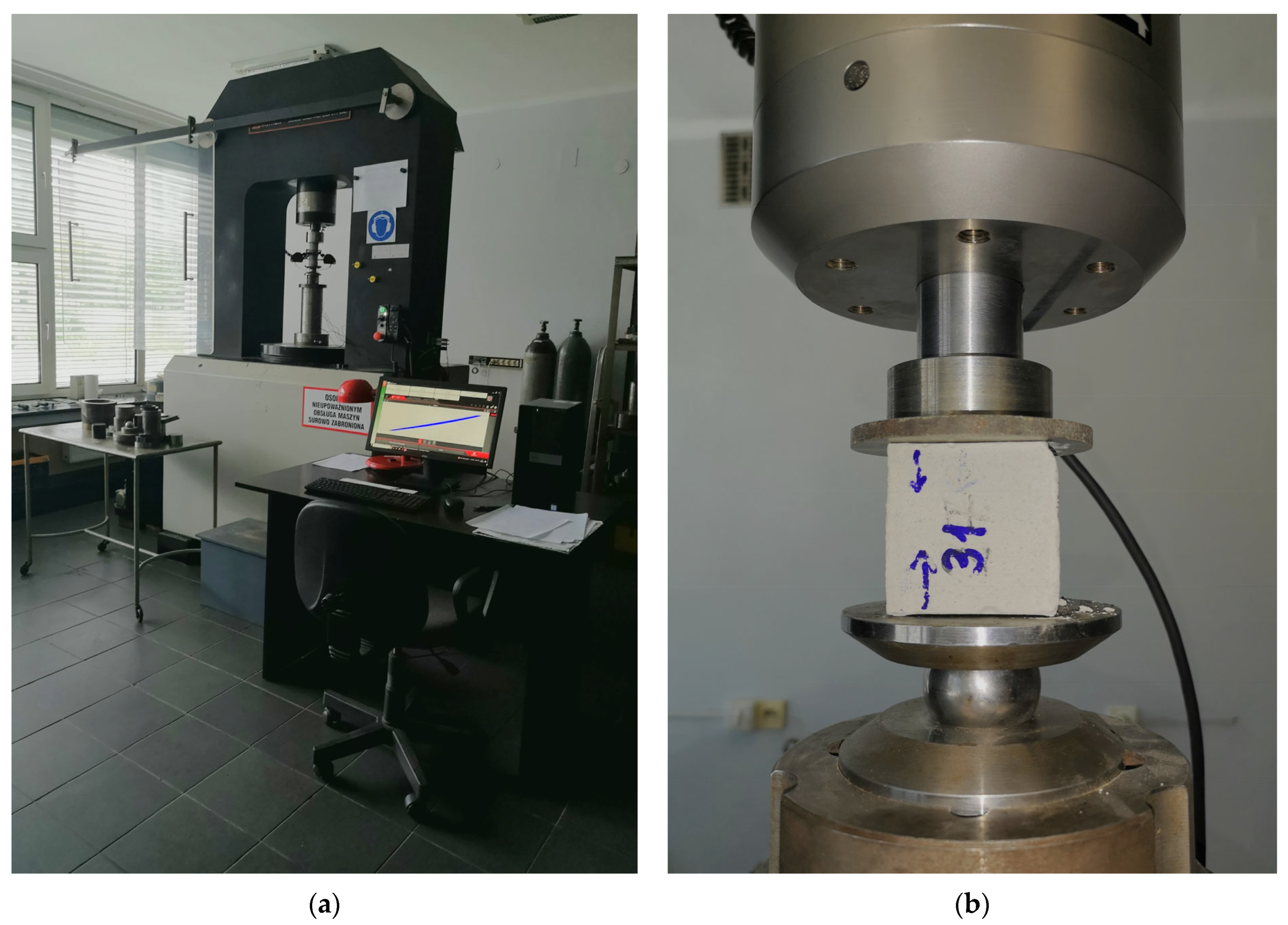
2.2. Compressive Strength Tests and Structural Properties
2.3. Water Permeability Coefficient
- l—sample height [m];
- Q—filtrated volume [m3];
- Δh—hydraulic head difference [m];
- A—cross-sectional area of the sample [m2];
- t—measurement duration [s].

3. Results
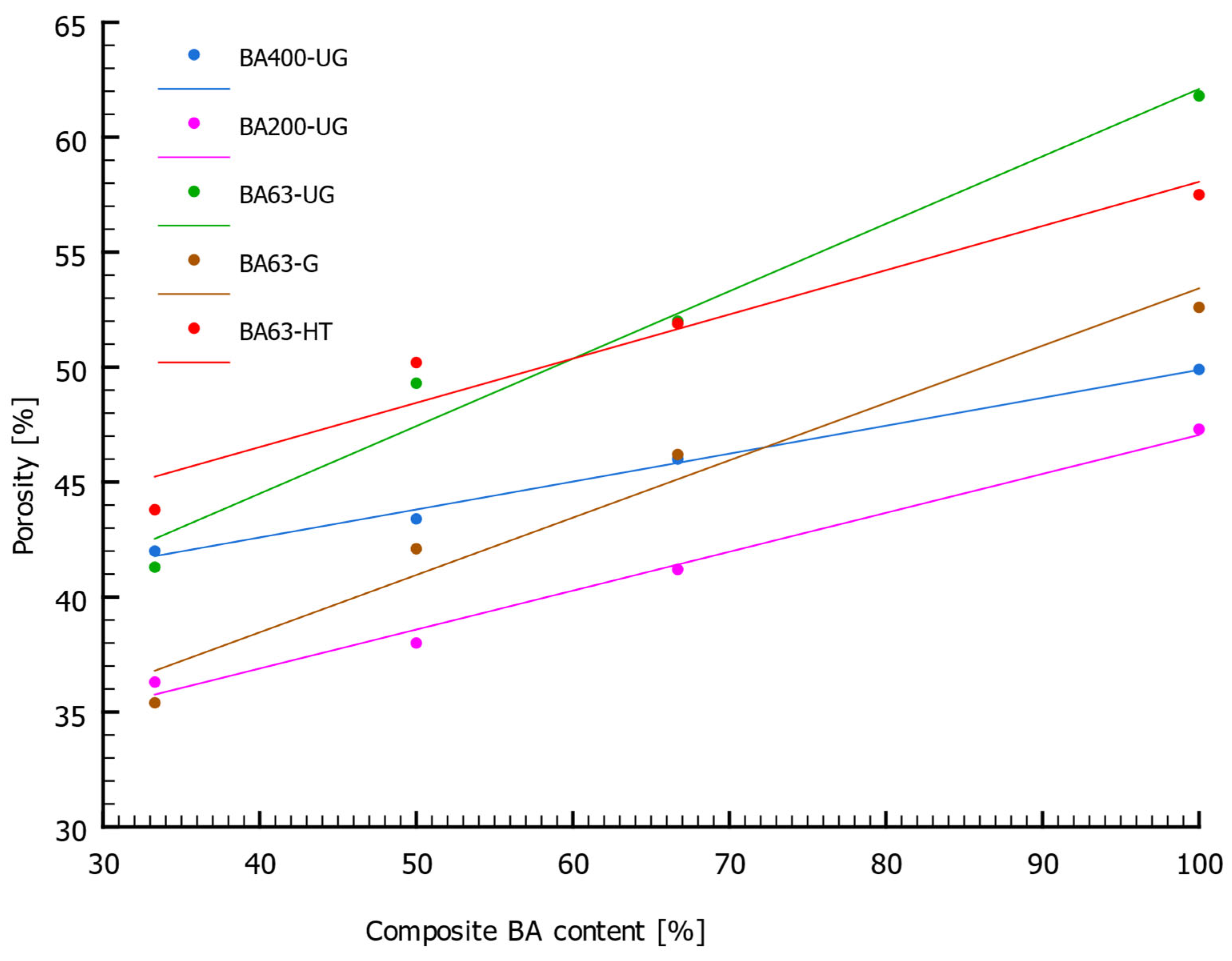
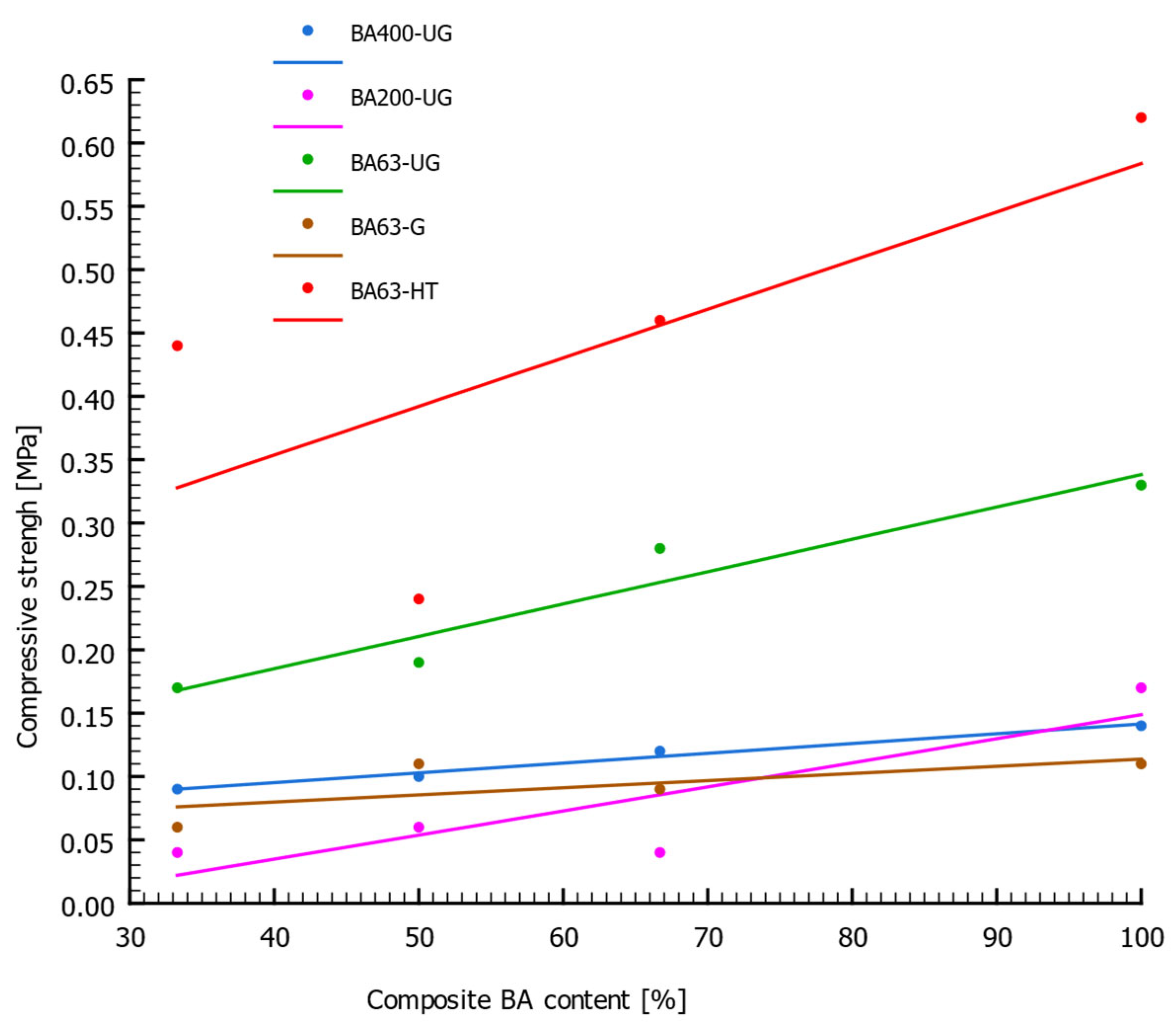
3.1. Structural and Compressive Strength Properties
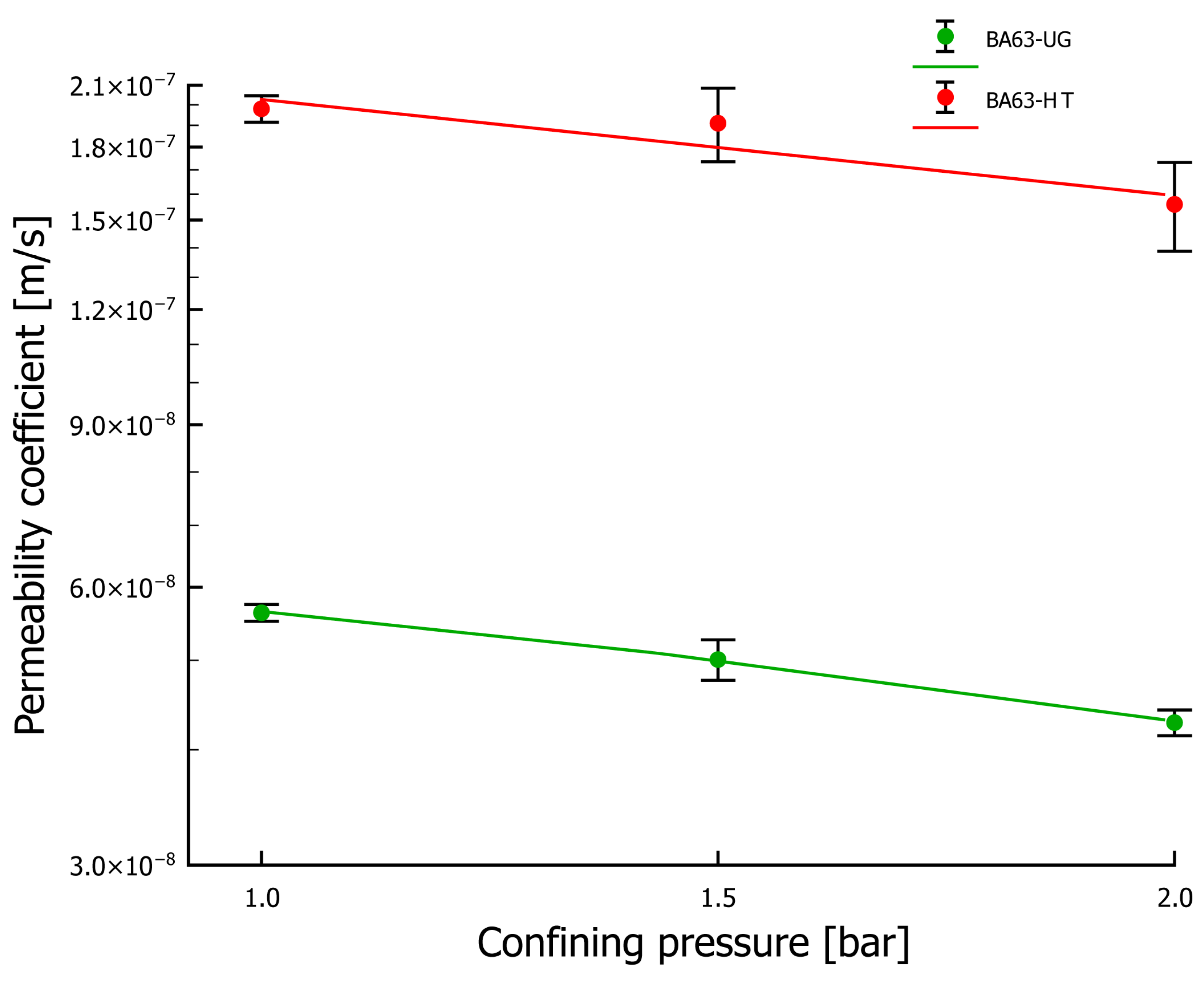
3.2. Water Permeability Coefficient Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Składowanie Odpadów. Available online: https://eur-lex.europa.eu/legal-content/PL/TXT/HTML/?uri=LEGISSUM:l21208 (accessed on 1 October 2025).
- EUR-Lex—L:2008:312:TOC—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:L:2008:312:TOC (accessed on 1 October 2025).
- O Zakładzie|Krakowski Holding Komunalny S.A. w Krakowie. Available online: https://khk.krakow.pl/en/eco-incinerator/about-the-plant/ (accessed on 7 October 2025).
- Ashraf, M.S.; Ghouleh, Z.; Shao, Y. Production of Eco-Cement Exclusively from Municipal Solid Waste Incineration Residues. Resour. Conserv. Recycl. 2019, 149, 332–342. [Google Scholar] [CrossRef]
- Clavier, K.A.; Paris, J.M.; Ferraro, C.C.; Townsend, T.G. Opportunities and Challenges Associated with Using Municipal Waste Incineration Ash as a Raw Ingredient in Cement Production—A Review. Resour. Conserv. Recycl. 2020, 160, 104888. [Google Scholar] [CrossRef]
- Dutka, B.; Rada, S.; Godyń, K.; Moldovan, D.; Chelcea, R.I.; Tram, M. Structural and Textural Characteristics of Municipal Solid Waste Incineration Bottom Ash Subjected to Periodic Seasoning. Sustainability 2024, 16, 9597. [Google Scholar] [CrossRef]
- del Valle-Zermeño, R.; Formosa, J.; Chimenos, J.M.; Martínez, M.; Fernández, A.I. Aggregate Material Formulated with MSWI Bottom Ash and APC Fly Ash for Use as Secondary Building Material. Waste Manag. 2013, 33, 621–627. [Google Scholar] [CrossRef]
- Chimenos, J.M.; Fernández, A.I.; Cervantes, A.; Miralles, L.; Fernández, M.A.; Espiell, F. Optimizing the APC Residue Washing Process to Minimize the Release of Chloride and Heavy Metals. Waste Manag. 2005, 25, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Polettini, A.; Pomi, R.; Sirini, P.; Testa, F. Properties of Portland Cement—Stabilised MSWI Fly Ashes. J. Hazard. Mater. 2001, 88, 123–138. [Google Scholar] [CrossRef]
- EUR-Lex—02000D0532-20150601—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A02000D0532-20150601 (accessed on 1 October 2025).
- Cornelis, G.; Van Gerven, T.; Vandecasteele, C. Antimony Leaching from MSWI Bottom Ash: Modelling of the Effect of PH and Carbonation. Waste Manag. 2012, 32, 278–286. [Google Scholar] [CrossRef]
- Kan, L.; Shi, R.; Zhao, Y.; Duan, X.; Wu, M. Feasibility Study on Using Incineration Fly Ash from Municipal Solid Waste to Develop High Ductile Alkali-Activated Composites. J. Clean. Prod. 2020, 254, 120168. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Carcelen-Taboada, V.; Fernández-Jiménez, A.; Palomo, A. Manufacture of Hybrid Cements with Fly Ash and Bottom Ash from a Municipal Solid Waste Incinerator. Constr. Build. Mater. 2016, 105, 218–226. [Google Scholar] [CrossRef]
- Zhang, S.; Ghouleh, Z.; Shao, Y. Use of Eco-Admixture Made from Municipal Solid Waste Incineration Residues in Concrete. Cem. Concr. Compos. 2020, 113, 103725. [Google Scholar] [CrossRef]
- Del Valle-Zermeño, R.; Formosa, J.; Prieto, M.; Nadal, R.; Niubó, M.; Chimenos, J.M. Pilot-Scale Road Subbase Made with Granular Material Formulated with MSWI Bottom Ash and Stabilized APC Fly Ash: Environmental Impact Assessment. J. Hazard. Mater. 2014, 266, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Ghouleh, Z.; Shao, Y. Turning Municipal Solid Waste Incineration into a Cleaner Cement Production. J. Clean. Prod. 2018, 195, 268–279. [Google Scholar] [CrossRef]
- Skrzypkowski, K.; Korzeniowski, W.; Poborska-Mlynarska, K. Binding Capability of Ashes and Dusts from Municipal Solid Waste Incineration with Salt Brine and Geotechnical Parameters of the Cemented Samples. Arch. Min. Sci. 2018, 63, 903–918. [Google Scholar] [CrossRef]
- Rehman, A.U.; Lee, S.M.; Kim, J.H. Use of Municipal Solid Waste Incineration Ash in 3D Printable Concrete. Process Saf. Environ. Prot. 2020, 142, 219–228. [Google Scholar] [CrossRef]
- Cho, B.H.; Nam, B.H.; An, J.; Youn, H. Municipal Solid Waste Incineration (MSWI) Ashes as Construction Materials-a Review. Materials 2020, 13, 3143. [Google Scholar] [CrossRef]
- Chen, B.; Perumal, P.; Aghabeyk, F.; Adediran, A.; Illikainen, M.; Ye, G. Advances in Using Municipal Solid Waste Incineration (MSWI) Bottom Ash as Precursor for Alkali-Activated Materials: A Critical Review. Resour. Conserv. Recycl. 2024, 204, 107516. [Google Scholar] [CrossRef]
- Sirico, A.; Bernardi, P.; Belletti, B.; Sciancalepore, C.; Milanese, D.; Paini, A.; Vignali, G. Environmental and Mechanical Analysis of Low-Carbon Concrete with Vitrified MSW Incineration Bottom Ash as Cement Replacement. Struct. Concr. 2024, 25, 2968–2990. [Google Scholar] [CrossRef]
- Vaitkus, A.; Škulteckė, J.; Šernas, O. A Test Road with Unbound Base and Sub-Base Course from MSWI Bottom Ash Mixtures. Buildings 2023, 13, 1311. [Google Scholar] [CrossRef]
- Chen, B.; Chen, J.; de Mendonça Filho, F.F.; Sun, Y.; van Zijl, M.B.; Copuroglu, O.; Ye, G. Characterization and Mechanical Removal of Metallic Aluminum (Al) Embedded in Weathered Municipal Solid Waste Incineration (MSWI) Bottom Ash for Application as Supplementary Cementitious Material. Waste Manag. 2024, 176, 128–139. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, X.; Ma, H.; Hou, D. Advanced Concrete Technology; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Zhang, B.; Ma, Y.; Yang, Y.; Zheng, D.; Wang, Y.; Ji, T. Improving the High Temperature Resistance of Alkali-Activated Slag Paste Using Municipal Solid Waste Incineration Bottom Ash. J. Build. Eng. 2023, 72, 106664. [Google Scholar] [CrossRef]
- Maldonado-Alameda, A.; Mañosa, J.; Miro-Escola, J.; Quintero-Payan, A.C.; Chimenos, J.M. Fluidised-Bed Incineration Bottom Ash as the Sole Precursor of Alkali-Activated Binders: A Comparison with Bottom Ash from Grate Incinerators. Constr. Build. Mater. 2023, 364, 130001. [Google Scholar] [CrossRef]
- Lancellotti, I.; Cannio, M.; Bollino, F.; Catauro, M.; Barbieri, L.; Leonelli, C. Geopolymers: An Option for the Valorization of Incinerator Bottom Ash Derived “End of Waste”. Ceram. Int. 2015, 41, 2116–2123. [Google Scholar] [CrossRef]
- Chen, B.; Zuo, Y.; Zhang, S.; de Lima Junior, L.M.; Liang, X.; Chen, Y.; van Zijl, M.B.; Ye, G. Reactivity and Leaching Potential of Municipal Solid Waste Incineration (MSWI) Bottom Ash as Supplementary Cementitious Material and Precursor for Alkali-Activated Materials. Constr. Build. Mater. 2023, 409, 133890. [Google Scholar] [CrossRef]
- Maldonado-Alameda, A.; Giro-Paloma, J.; Mañosa, J.; Formosa, J.; Chimenos, J.M. Alkali-Activated Binders Based on the Coarse Fraction of Municipal Solid Waste Incineration Bottom Ash. Bol. Soc. Esp. Cerám. Vidr. 2022, 61, 313–324. [Google Scholar] [CrossRef]
- Maldonado-Alameda, À.; Giro-Paloma, J.; Alfocea-Roig, A.; Formosa, J.; Chimenos, J.M.; Maldonado-Alameda, À.; Giro-Paloma, J.; Alfocea-Roig, A.; Formosa, J.; Chimenos, J.M. Municipal Solid Waste Incineration Bottom Ash as Sole Precursor in the Alkali-Activated Binder Formulation. Appl. Sci. 2020, 10, 4129. [Google Scholar] [CrossRef]
- Jin, L.; Huang, G.; Li, Y.; Zhang, X.; Ji, Y.; Xu, Z.; Jin, L.; Huang, G.; Li, Y.; Zhang, X.; et al. Positive Influence of Liquid Sodium Silicate on the Setting Time, Polymerization, and Strength Development Mechanism of MSWI Bottom Ash Alkali-Activated Mortars. Materials 2021, 14, 1927. [Google Scholar] [CrossRef]
- Huang, G.; Yang, K.; Chen, L.; Lu, Z.; Sun, Y.; Zhang, X.; Feng, Y.; Ji, Y.; Xu, Z. Use of Pretreatment to Prevent Expansion and Foaming in High-Performance MSWI Bottom Ash Alkali-Activated Mortars. Constr. Build. Mater. 2020, 245, 118471. [Google Scholar] [CrossRef]
- Huang, G.; Ji, Y.; Li, J.; Zhang, L.; Liu, X.; Liu, B. Effect of Activated Silica on Polymerization Mechanism and Strength Development of MSWI Bottom Ash Alkali-Activated Mortars. Constr. Build. Mater. 2019, 201, 90–99. [Google Scholar] [CrossRef]
- Li, Z.; Kondo, R.; Ikeda, K. Recycling of Waste Incineration Bottom Ash and Heavy Metal Immobilization by Geopolymer Production. J. Adv. Concr. Technol. 2021, 19, 259–279. [Google Scholar] [CrossRef]
- Godyń, K.; Dutka, B.; Rosik-Dulewska, C.; Ciesielczuk, T.; Głowacki, M. Assessment of the Possibility of Heavy Metals Elution from Municipal Solid Waste Incineration Bottom Ash into the Soil and Water. Desalination Water Treat. 2025, 323, 101282. [Google Scholar] [CrossRef]
- dos Santos, M.N.G.; dos Santos, C.M.; de Souza, M.T.G.; de Vasconcelos, E.A.; da Nóbrega, A.C.V.; Marinho, É.P. Use of Sodium Metasilicate as Silica Source and Stabilizing Agent in Two-Part Metakaolin–H2O2 Geopolymer Foams. Constr. Build. Mater. 2023, 391, 131907. [Google Scholar] [CrossRef]
- Castillo-Villar, K.K.; Eksioglu, S.; Taherkhorsandi, M. Integrating Biomass Quality Variability in Stochastic Supply Chain Modeling and Optimization for Large-Scale Biofuel Production. J. Clean. Prod. 2017, 149, 904–918. [Google Scholar] [CrossRef]
- Proces Termicznego Przekształcania Odpadów|Krakowski Holding Komunalny S.A. w Krakowie. Available online: https://khk.krakow.pl/en/eco-incinerator/green-energy-factory (accessed on 13 October 2025).
- Weryński, B. Mineralne Spoiwa Budowlane. In Budownictwo Ogólne; Materiały i Wyroby Budowlane; Stefańczyk, B., Ed.; Arkady: Warszawa, Poland, 2007; Volume 1, pp. 241–291. [Google Scholar]
- Um, N. Effect of Cl Removal in MSWI Bottom Ash via Carbonation with CO2 and Decomposition Kinetics of Friedel’s Salt. Mater. Trans. 2019, 60, 837–844. [Google Scholar] [CrossRef]
- Inkaew, K.; Saffarzadeh, A.; Shimaoka, T. Modeling the Formation of the Quench Product in Municipal Solid Waste Incineration (MSWI) Bottom Ash. Waste Manag. 2016, 52, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Godyń, K.; Dutka, B. Preliminary Studies of Slag and Ash from Incinerated Municipal Waste for Prospective Applications. Energies 2023, 16, 117. [Google Scholar] [CrossRef]
- Sun, C.; Sun, M.; Tao, T.; Qu, F.; Wang, G.; Zhang, P.; Li, Y.; Duan, J.; Sun, C.; Sun, M.; et al. Chloride Binding Capacity and Its Effect on the Microstructure of Mortar Made with Marine Sand. Sustainability 2021, 13, 4169. [Google Scholar] [CrossRef]
- Menéndez, E.; Vega, L.; Andrade, C. Use of Decomposition of Portlandite in Concrete Fire as Indicator of Temperature Progression into the Material. J. Therm. Anal. Calorim. 2012, 110, 203–209. [Google Scholar] [CrossRef]
- Lavagna, L.; Nisticò, R. An Insight into the Chemistry of Cement—A Review. Appl. Sci. 2022, 13, 203. [Google Scholar] [CrossRef]
- Tkaczewska, E.; Malata, G. Development of Properties of Portland Cement-Slag-Siliceous Fly Ash Mixtures in Relation to Their Composition. Cem.-Wapno-Beton Cem. Lime Concr. 2025, 29, 431–449. [Google Scholar] [CrossRef]
- PN-B-06265;2022-08; Concrete—Specification, Performance, Production and Conformity—National Annex to PN-EN 206+A2:2021-08. Polish Committee of Standardization: Warszawa, Poland, 2025. (In Polish)
- Zhu, W.; Chen, X.; Zhao, A.; Struble, L.J.; Yang, E.H. Synthesis of High Strength Binders from Alkali Activation of Glass Materials from Municipal Solid Waste Incineration Bottom Ash. J. Clean. Prod. 2019, 212, 261–269. [Google Scholar] [CrossRef]
- Zhu, W.; Teoh, P.J.; Liu, Y.; Chen, Z.; Yang, E.-H. Strategic Utilization of Municipal Solid Waste Incineration Bottom Ash for the Synthesis of Lightweight Aerated Alkali-Activated Materials. J. Clean. Prod. 2019, 235, 603–612. [Google Scholar] [CrossRef]
- ISO 17892-11:2019; Geotechnical Investigations and Testing. Laboratory Testing of Soils. Part 11. Permeability Tests. International Standard: Geneva, Switzerland, 2019.
- Lukschová, Š.; Přikryl, R.; Pertold, Z. Petrographic Identification of Alkali–Silica Reactive Aggregates in Concrete from 20th Century Bridges. Constr. Build. Mater. 2009, 23, 734–741. [Google Scholar] [CrossRef]
- Malaiškienė, J.; Spudulis, E.; Stonys, R. The Effect of Milled Municipal Solid Waste Incineration Bottom Ash on Cement Hydration and Mortar Properties. Materials 2023, 16, 2528. [Google Scholar] [CrossRef] [PubMed]
- Godyń, K.; Dutka, B.; Tram, M. Application of Petrographic and Stereological Analyses to Describe the Pore Space of Rocks as a Standard for the Characterization of Pores in Slags and Ashes Generated after the Combustion of Municipal Waste. Materials 2023, 16, 7706. [Google Scholar] [CrossRef]
- Abousnina, R.; Aljuaydi, F.; Benabed, B.; Almabrok, M.H.; Vimonsatit, V. A State-of-the-Art Review on the Influence of Porosity on the Compressive Strength of Porous Concrete for Infrastructure Applications. Buildings 2025, 15, 2311. [Google Scholar] [CrossRef]
- Bouzar, B.; Benzerzour, M.; Abriak, N.E. Evaluation of Mineral Eco-Binders from Secondary Raw Materials: Environmental Assessments. J. Mater. Cycles Waste Manag. 2024, 26, 2546–2566. [Google Scholar] [CrossRef]
- Chai, M.; Yao, J.; Song, H.; Li, Y.; Ying, Y.; Feng, J.; Yang, Z. Effects of Thermal Treatment on Mechanical Performance of Mortar with Bottom Ash Replacing Cement. J. Build. Eng. 2025, 100, 111742. [Google Scholar] [CrossRef]
- PN-EN 197-1:2012; Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements. Polish Committee of Standardization: Warszawa, Poland, 2013. (In Polish)
- Qiao, X.C.; Tyrer, M.; Poon, C.S.; Cheeseman, C.R. Novel Cementitious Materials Produced from Incinerator Bottom Ash. Resour. Conserv. Recycl. 2008, 52, 496–510. [Google Scholar] [CrossRef]
- Batu, V. Aquifer Hydraulics: A Comprehensive Guide to Hydrogeologic Data Analysis; John Wiley & Sons: Hoboken, NJ, USA, 1998; ISBN 978-0-471-18502-4. [Google Scholar]
- Dutka, B.; Godyń, K.; Skotniczny, P.; Tokarczyk, K.; Tram, M. Measurements of the Permeability Coefficient of Waste Coal Ash under Hydrostatic Pressure to Identify the Feasibility of Its Use in Construction. Recycling 2024, 9, 22. [Google Scholar] [CrossRef]
- Uler-Zefikj, M.; Godyń, K.; Tokarczyk, K.; Filkoski, R.V. Characterization of Municipal Solid Waste as Potential Fuel for Energy Needs. Materials 2025, 18, 2103. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, H.K. Coal Bottom Ash in Field of Civil Engineering: A Review of Advanced Applications and Environmental Considerations. KSCE J. Civ. Eng. 2015, 19, 1802–1818. [Google Scholar] [CrossRef]
- Ogunro, V.O.; Inyang, H.I.; Hooper, F.; Young, D.; Oturkar, A. Gradation Control of Bottom Ash Aggregate in Superpave Bituminous Mixes. J. Mater. Civ. Eng. 2004, 16, 604–613. [Google Scholar] [CrossRef]


| Sample Series | Fraction Size | Treatment |
|---|---|---|
| BA400-UG | <400 µm | unground |
| BA200-UG | <200 µm | unground |
| BA63-UG | <63 µm | unground |
| BA63-G | <63 µm | ground |
| BA63-HT | <63 µm | unground, thermally treated at 1000 °C |
| Specimen Name | SiO2 | CaO | Al2O3 | Fe2O3 | SO3 | MgO | K2O | Cl |
|---|---|---|---|---|---|---|---|---|
| wt% | ||||||||
| Portland cement | 21.62 | 62.12 | 2.37 | 1.51 | 2.03 | 1.57 | 0.84 | 0.097 |
| BA400-UG | 17.30 | 38.45 | 4.75 | 2.47 | 10.56 | 1.88 | 1.08 | 2.24 |
| BA200-UG | 15.67 | 41.52 | 4.51 | 2.40 | 10.81 | 1.90 | 1.07 | 2.27 |
| BA63-UG | 19.72 | 46.01 | 11.57 | 3.72 | 9.02 | 3.05 | 1.14 | 2.75 |
| BA63-G | 29.93 | 28.13 | 5.60 | 2.70 | 7.36 | 1.83 | 1.14 | 1.51 |
| BA63-HT | 22.43 | 49.16 | 8.29 | 3.29 | 8.73 | 2.01 | 0.90 | 1.90 |
| Sample Series | Sample Number | BA Content [%] | Water Content [%] |
|---|---|---|---|
| BA400-UG | 1 | 33.3 | 21.6 |
| 2 | 50.0 | 23.4 | |
| 3 | 66.7 | 29.4 | |
| 4 | 100 | 37.0 | |
| BA200-UG | 1 | 33.3 | 16.8 |
| 2 | 50.0 | 14.5 | |
| 3 | 66.7 | 18.3 | |
| 4 | 100 | 24.2 | |
| BA63-UG | 1 | 33.3 | 31.4 |
| 2 | 50.0 | 42.6 | |
| 3 | 66.7 | 52.4 | |
| 4 | 100 | 80.8 | |
| BA63-G | 1 | 33.3 | 15.4 |
| 2 | 50.0 | 17.9 | |
| 3 | 66.7 | 21.9 | |
| 4 | 100 | 33.9 | |
| BA63-HT | 1 | 33.3 | 32.0 |
| 2 | 50.0 | 45.3 | |
| 3 | 66.7 | 50.4 | |
| 4 | 100 | 92.0 |
| Serial Name | Sample Number | Rc [MPa] | ρ [g/cm3] | ρs [g/cm3] | n [%] |
|---|---|---|---|---|---|
| BA400-UG | 1 | 0.09 | 1.52 | 2.61 | 42.0 |
| 2 | 0.10 | 1.46 | 2.58 | 43.4 | |
| 3 | 0.12 | 1.38 | 2.55 | 46.0 | |
| 4 | 0.14 | 1.24 | 2.48 | 49.9 | |
| BA200-UG | 1 | 0.04 | 1.67 | 2.63 | 36.3 |
| 2 | 0.06 | 1.62 | 2.62 | 38.0 | |
| 3 | 0.04 | 1.53 | 2.60 | 41.2 | |
| 4 | 0.17 | 1.36 | 2.58 | 47.3 | |
| BA63-UG | 1 | 0.17 | 1.52 | 2.58 | 41.3 |
| 2 | 0.19 | 1.29 | 2.55 | 49.3 | |
| 3 | 0.28 | 1.21 | 2.52 | 52.0 | |
| 4 | 0.33 | 0.95 | 2.48 | 61.8 | |
| BA63-G | 1 | 0.06 | 1.70 | 2.64 | 35.4 |
| 2 | 0.11 | 1.52 | 2.63 | 42.1 | |
| 3 | 0.09 | 1.41 | 2.62 | 46.2 | |
| 4 | 0.11 | 1.23 | 2.59 | 52.6 | |
| BA63-HT | 1 | 0.44 | 1.51 | 2.69 | 43.8 |
| 2 | 0.24 | 1.35 | 2.71 | 50.2 | |
| 3 | 0.46 | 1.31 | 2.72 | 51.9 | |
| 4 | 0.62 | 1.17 | 2.76 | 57.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tram, M.; Sułkowska, K.; Jarosz, A.; Nowakowski, A. Mechanical Properties of Composite Made from Bottom Ash Fractions of Municipal Waste Incineration Plant Products. Materials 2025, 18, 5302. https://doi.org/10.3390/ma18235302
Tram M, Sułkowska K, Jarosz A, Nowakowski A. Mechanical Properties of Composite Made from Bottom Ash Fractions of Municipal Waste Incineration Plant Products. Materials. 2025; 18(23):5302. https://doi.org/10.3390/ma18235302
Chicago/Turabian StyleTram, Maciej, Katarzyna Sułkowska, Arkadiusz Jarosz, and Andrzej Nowakowski. 2025. "Mechanical Properties of Composite Made from Bottom Ash Fractions of Municipal Waste Incineration Plant Products" Materials 18, no. 23: 5302. https://doi.org/10.3390/ma18235302
APA StyleTram, M., Sułkowska, K., Jarosz, A., & Nowakowski, A. (2025). Mechanical Properties of Composite Made from Bottom Ash Fractions of Municipal Waste Incineration Plant Products. Materials, 18(23), 5302. https://doi.org/10.3390/ma18235302






