Research Progress on LDH Corrosion-Resistant Films on Magnesium Alloy: A Review
Abstract
1. Introduction
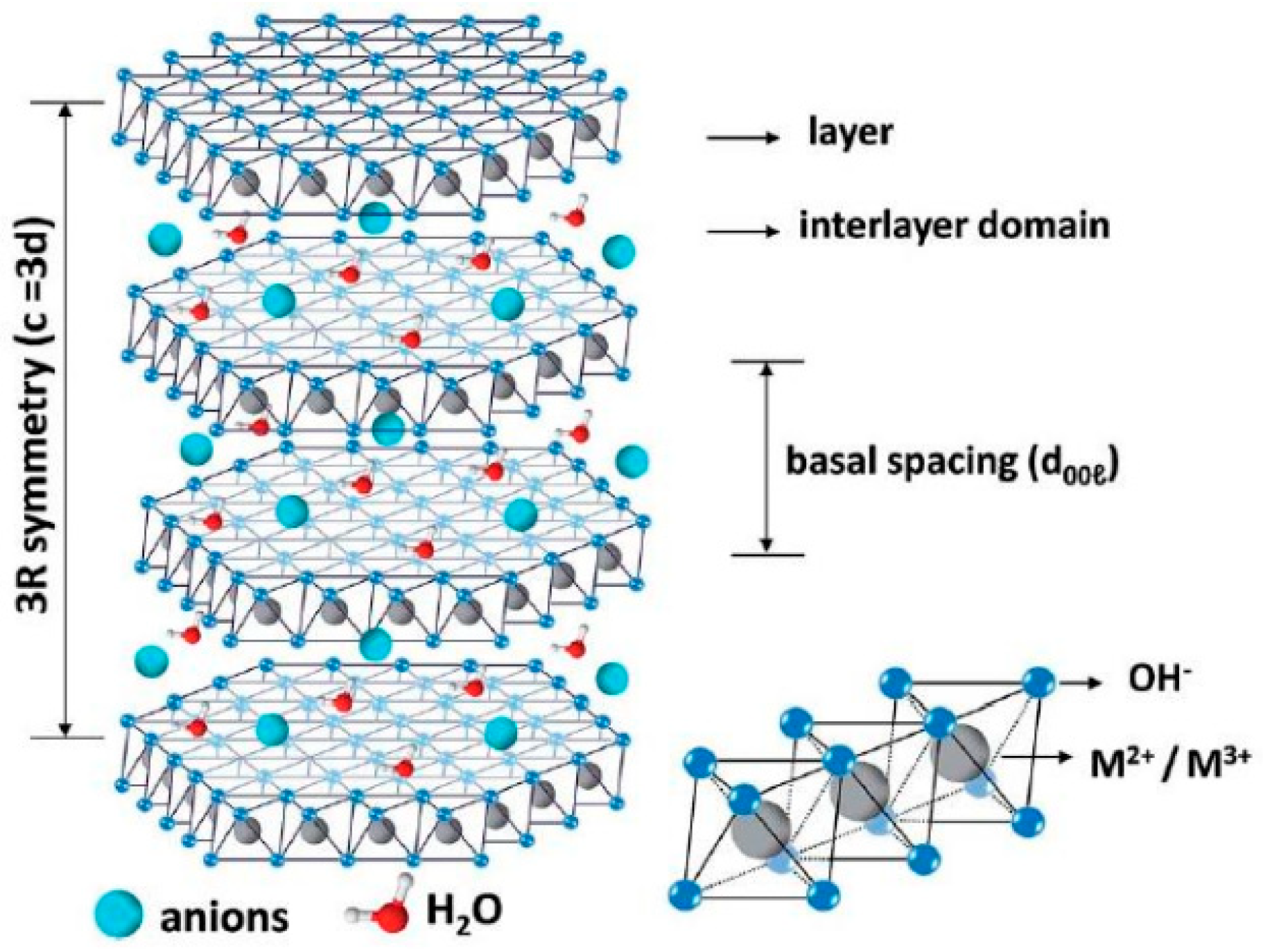
2. Structure and Performance Characteristics of LDH
3. Preparation Method for LDH Films on Mg Alloys
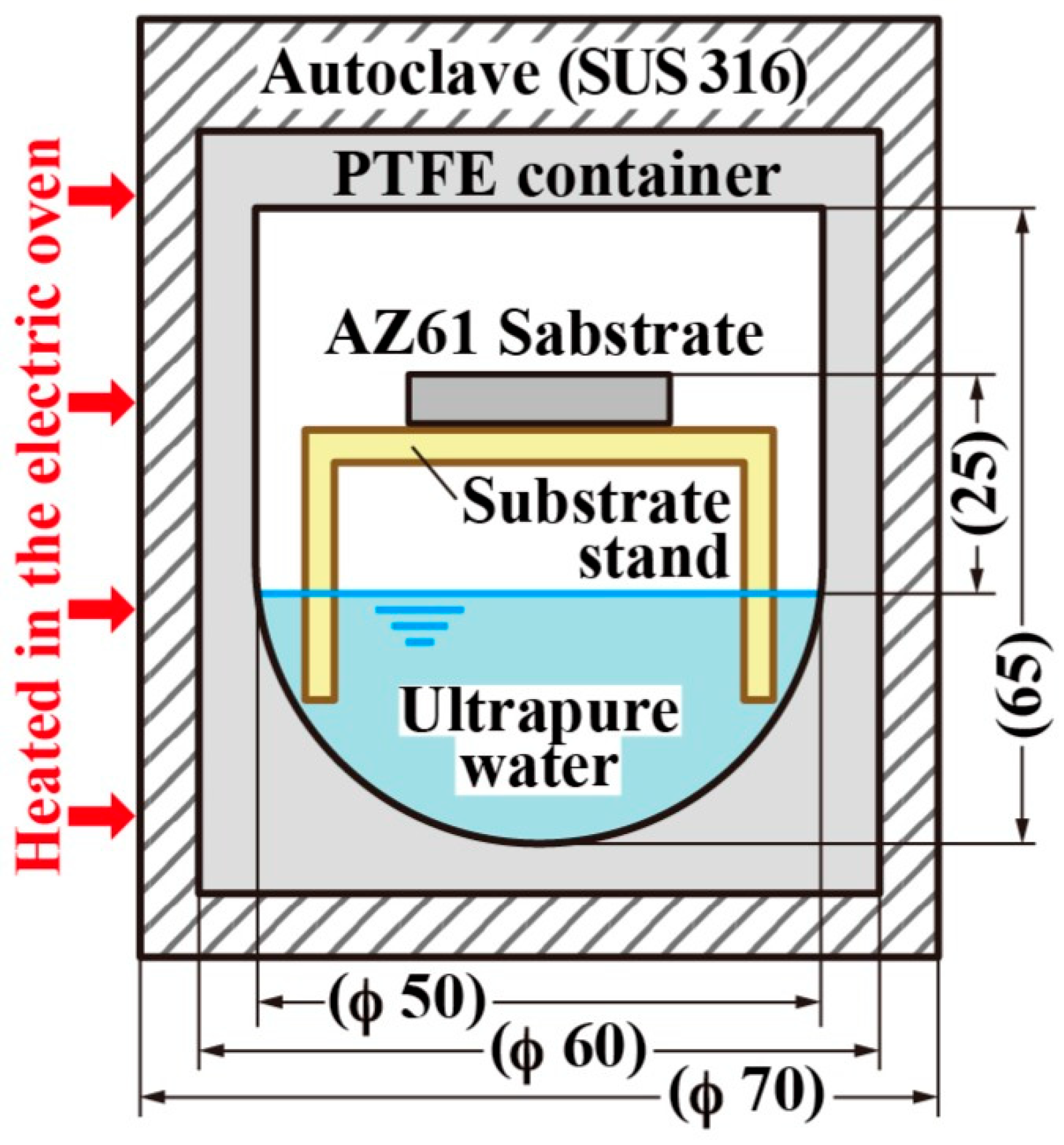
3.1. Hydrothermal Method
3.2. Steam Method
3.3. Impregnation Method
3.4. Electrochemical Deposition Method
3.5. Co-Precipitation Method
4. Functionalization of LDH Films on Mg Alloys
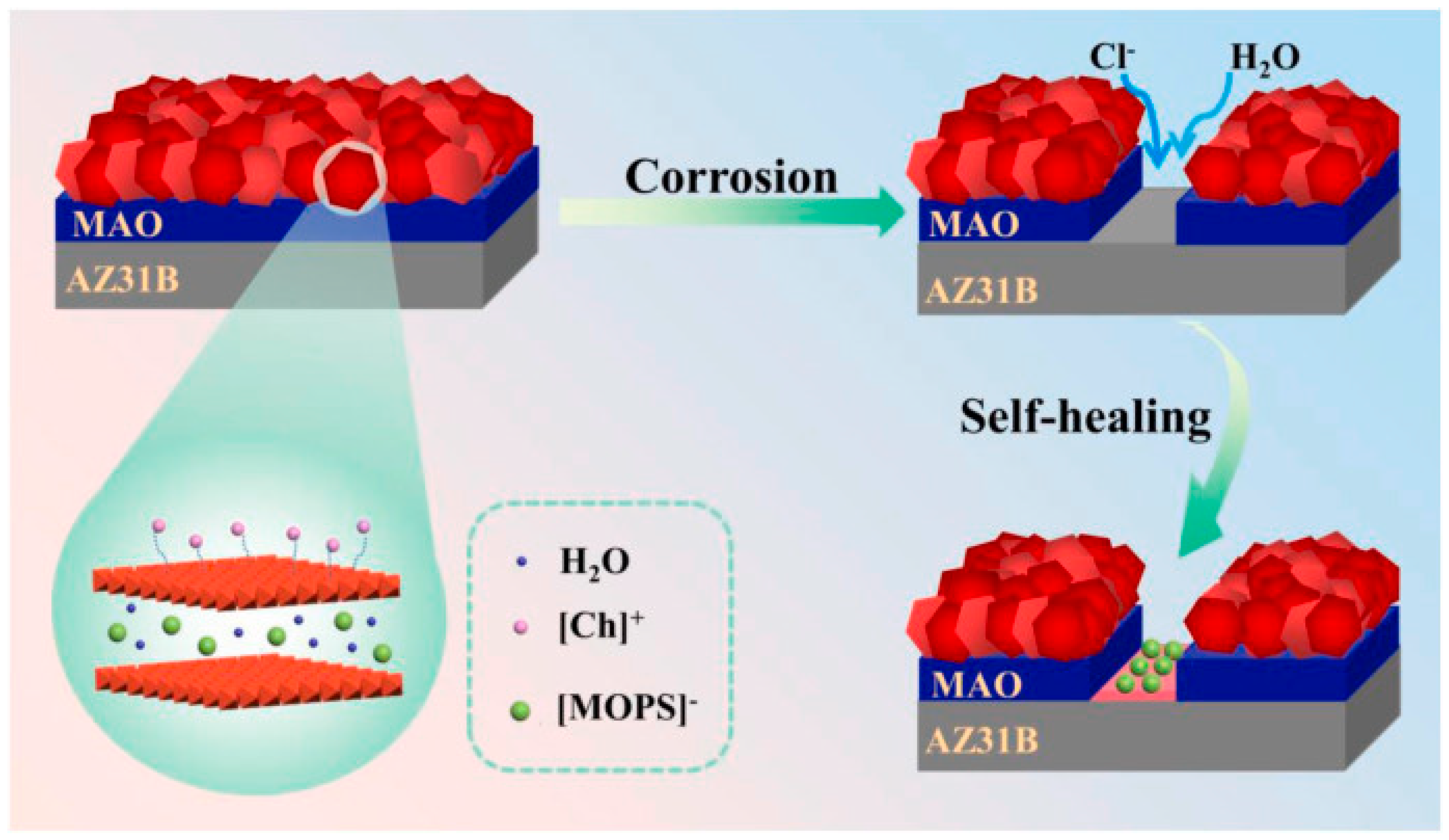
4.1. Self-Healing LDH Films
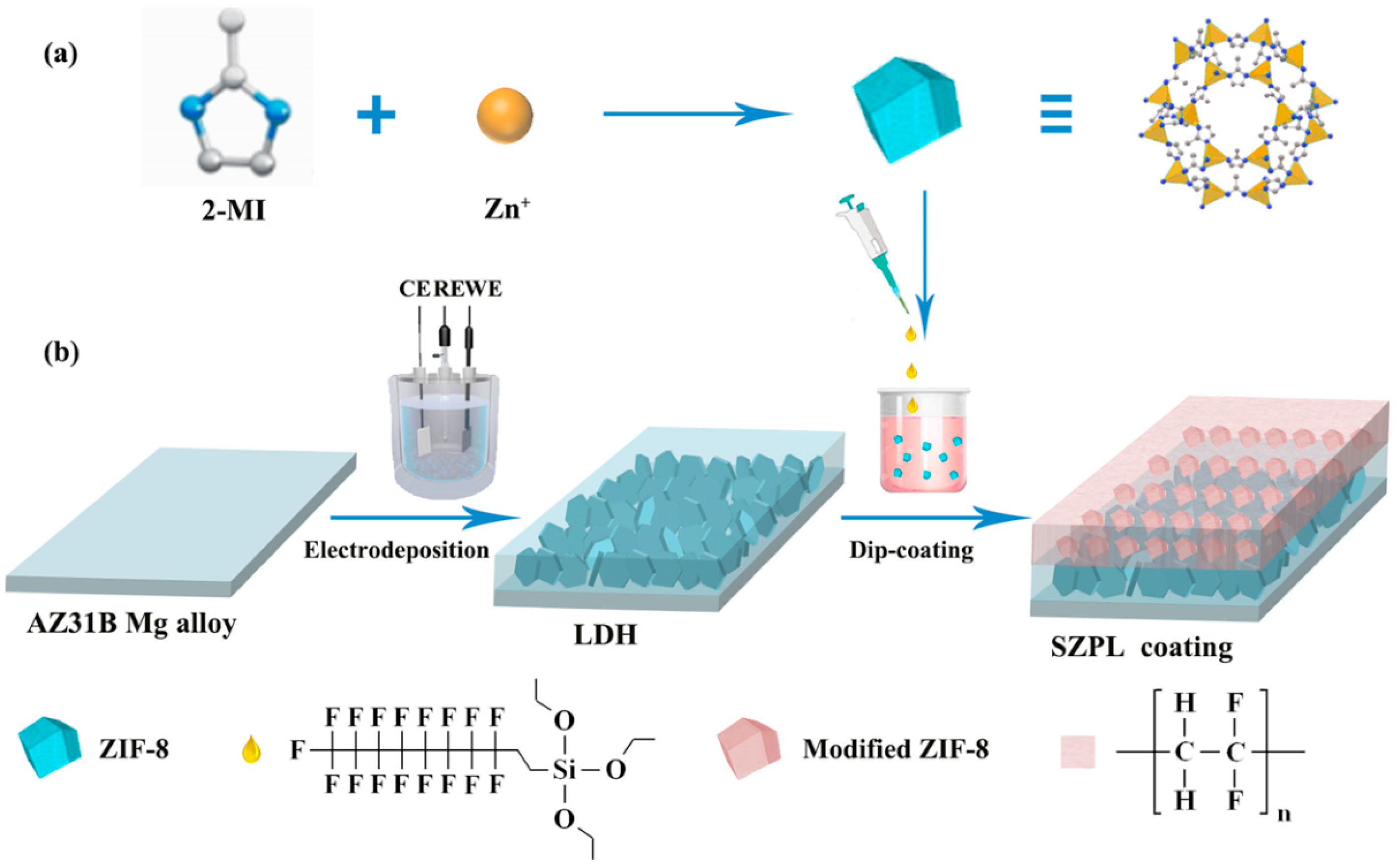
4.2. Superhydrophobic LDH Films
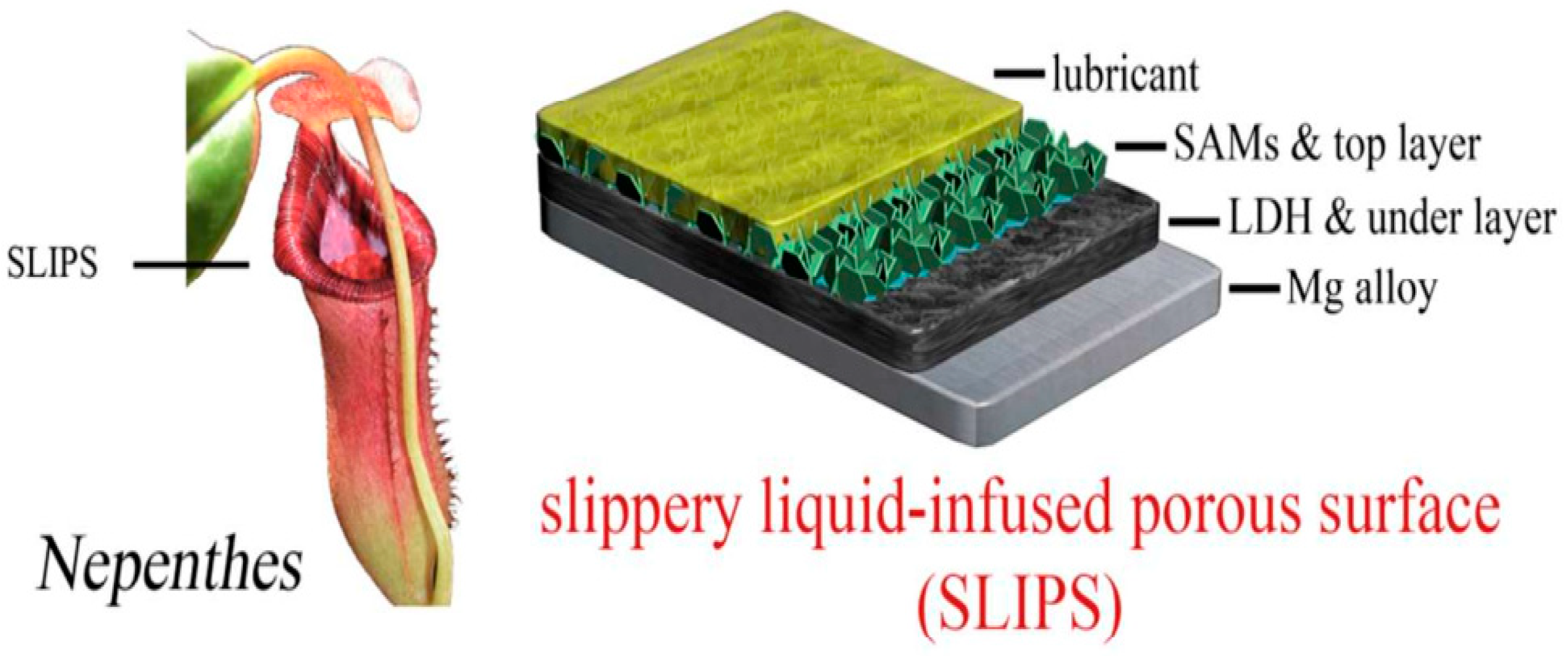
4.3. Slippery Liquid-Infused Porous Surface (SLIPS) LDH Films
4.4. Wear-Resistant LDH Films
5. Conclusions and Future Perspectives
- (1)
- Currently, the preparation and research of LDH films remain largely confined to laboratory-scale studies. To facilitate their transition to industrial applications, further optimization of both the synthesis methods and processing procedures is imperative.
- (2)
- Current research efforts are predominantly directed toward exploring the functional applications of LDHs, whereas systematic investigations into the intrinsic physicochemical properties of LDH films and their correlations with industrial application scenarios remain relatively limited.
- (3)
- Despite the excellent corrosion protection of the functionalized LDH coatings under laboratory conditions, they encounter significant challenges in complex industrial scenarios. To address this, LDH coatings as a pretreatment substrate paired with a topcoat can form a composite protection system—integrating their active protection and the topcoat’s efficient passive barrier performance—meeting industrial needs for corrosion-prone metals such as magnesium alloys better and delivering a more reliable protective solution.
- (4)
- Exploring the combination of LDH with surface treatment technologies such as plasma spraying, cold spraying and laser processing is expected to achieve the optimal performance coupling of green manufacturing, low cost, functionalization and high corrosion resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sahoo, S.K.; Sahoo, B.N.; Panigrahi, S.K. Investigation into machining performance of microstructurally engineered in-situ particle reinforced magnesium matrix composite. J. Magnes. Alloys 2023, 11, 916–935. [Google Scholar] [CrossRef]
- Wang, T.; Zha, M.; Du, C.F.; Jia, H.L.; Wang, C.; Guan, K.; Gao, Y.P.; Wang, H.Y. High strength and high ductility achieved in a heterogeneous lamella-structured magnesium alloy. Mater. Res. Lett. 2023, 11, 187–195. [Google Scholar] [CrossRef]
- Kim, J.; Pan, H.B. Effects of magnesium alloy corrosion on biological response–Perspectives of metal-cell interaction. Prog. Mater. Sci. 2023, 133, 101039. [Google Scholar] [CrossRef]
- Wang, G.G.; Weiler, J.P. Recent developments in high-pressure die-cast magnesium alloys for automotive and future applications. J. Magnes. Alloys 2023, 11, 78–87. [Google Scholar] [CrossRef]
- Kiani, F.; Lin, J.X.; Vahid, A.; Munir, K.; Wen, C.; Li, Y.C. Microstructures, mechanical properties, corrosion, and biocompatibility of extruded Mg-Zr-Sr-Ho alloys for biodegradable implant applications. J. Magnes. Alloys 2023, 11, 110–136. [Google Scholar] [CrossRef]
- Cai, L.; Mei, D.; Zhang, Z.Q.; Huang, Y.D.; Cui, L.Y.; Guan, S.K.; Chen, D.C.; Bobby-Kannan, M.; Zheng, Y.F.; Zeng, R.C. Advances in bioorganic molecules inspired degradation and surface modifications on Mg and its alloys. J. Magnes. Alloys 2022, 10, 670–688. [Google Scholar] [CrossRef]
- Zhang, A.M.; Lenin, P.; Zeng, R.C.; Bobby Kannan, M. Advances in hydroxyapatite coatings on biodegradable magnesium and its alloys. J. Magnes. Alloys 2022, 10, 1154–1170. [Google Scholar] [CrossRef]
- Huang, R.P.; Dong, Z.H.; Zhou, Y.Z.; Ye, T.L.; Liu, Z.G.; Tang, D.Y. Reinforced adhesion by in-situ growth converse chemical layers between substrates and electrosprayed organic coatings to improve anti-corrosion of magnesium alloy. Colloids Surf. A Physicochem. Eng. Asp. 2025, 723, 137419. [Google Scholar] [CrossRef]
- Pan, S.Q.; Zhang, F.; Wen, C.; Zeng, R.C. Advances in Mg–Al-layered double hydroxide steam coatings on Mg alloys: A review. J. Magnes. Alloys 2023, 11, 1505–1518. [Google Scholar] [CrossRef]
- Xia, X.C.; Pan, Y.; Yuan, X.; Nie, J.J.; Sun, J.L.; Yuan, Y.; Dong, Z.H. Research progress of surface corrosion protection technology for Mg alloys. Surf. Technol. 2023, 52, 37–50. [Google Scholar]
- Blawert, C.; Fechner, D.; Höche, D.; Heitmann, V.; Dietzel, W.; Kainer, K.U.; Živanović, P.; Scharf, C.; Ditze, A.; Gröbner, J.; et al. Magnesium secondary alloys: Alloy design for magnesium alloys with improved tolerance limits against impurities. Corros. Sci. 2010, 52, 2452–2468. [Google Scholar] [CrossRef]
- Song, G.L. Effect of tin modification on corrosion of AM70 magnesium alloy. Corros. Sci. 2009, 51, 2063–2070. [Google Scholar] [CrossRef]
- Ding, Y.; Wen, C.; Hodgson, P.; Li, Y. Effects of alloying elements on the corrosion behavior and biocompatibility of biodegradable magnesium alloys: A review. J. Mater. Chem. B 2014, 2, 1912–1933. [Google Scholar] [CrossRef]
- Huang, H.; Li, D.H.; Hou, L.F.; Du, H.Y.; Wei, H.; Liu, X.D.; Wang, Q.; Wei, Y.H. Advanced protective layer design on the surface of Mg-based metal and application in batteries: Challenges and progress. J. Power Sources 2022, 542, 231755. [Google Scholar] [CrossRef]
- Grubač, Z.; Rončević, I.Š.; Metikoš-Huković, M. Corrosion properties of the Mg alloy coated with polypyrrole films. Corros. Sci. 2016, 102, 310–316. [Google Scholar] [CrossRef]
- Chen, Y.N.; Wu, L.; Yao, W.H.; Wu, J.H.; Xiang, J.P.; Dai, X.W.; Wu, T.; Yuan, Y.; Wang, J.F.; Jiang, B.; et al. Development of metal-organic framework (MOF) decorated graphene oxide/MgAl-layered double hydroxide coating via microstructural optimization for anti-corrosion micro-arc oxidation coatings of magnesium alloy. J. Mater. Sci. Technol. 2022, 130, 12–26. [Google Scholar] [CrossRef]
- Lu, X.Y.; Feng, X.G.; Zuo, Y.; Zhang, P.; Zheng, C.B. Improvement of protection performance of Mg-rich epoxy coating on AZ91D magnesium alloy by DC anodic oxidation. Prog. Org. Coat. 2017, 104, 188–198. [Google Scholar] [CrossRef]
- Li, T.; Leng, Z.J.; Wang, X.T.; Wang, S.F.; Zhang, S.Q.; Yang, Y.S.; Zhou, J.X. Microstructure and electrical conductivity of electroless copper plating layer on magnesium alloy micro-arc oxidation coating. Trans. Nonferrous Met. Soc. China 2022, 32, 3950–3962. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Shen, X.L. Facile fabrication of robust superhydrophobic coating for enhanced corrosion protection on AZ91 magnesium alloy by electroless Ni-B/GO plating. Surf. Coat. Technol. 2023, 455, 129213. [Google Scholar] [CrossRef]
- Pereira, G.S.; Prada Ramirez, O.M.; Tavares Avila, P.R.; Avila, J.A.; Pinto, H.C.; Miyazaki, M.H.; de Melo, H.G.; Bose Filho, W.W. Cerium conversion coating and sol-gel coating for corrosion protection of the WE43 Mg alloy. Corros. Sci. 2022, 206, 110527. [Google Scholar] [CrossRef]
- Du, M.; Peng, M.K.; Mai, B.J.; Hu, F.Y.; Zhang, X.D.; Chen, Y.H.; Wang, C.H. A multifunctional hybrid inorganic-organic coating fabricated on magnesium alloy surface with antiplatelet adhesion and antibacterial activities. Surf. Coat. Technol. 2020, 384, 125336. [Google Scholar] [CrossRef]
- Bu, T.; Jia, R.J.; Ying, T.; Atrens, A.; Chen, P.B.; Zheng, D.J.; Cao, F.Y. A green conversion coating on a magnesium alloy for corrosion protection. Acta Metall. Sin. (Engl. Lett.) 2023, 36, 1630–1648. [Google Scholar] [CrossRef]
- Jayaraj, J.; Arun Kumar, S.A.; Srinivasan, A.; Raghu, K.G.; Arunchandran, C.; Rajinikanth, V. Corrosion and in vitro characteristics of cerium phosphate based chemical conversion coating on AZ31 magnesium alloy. Appl. Surf. Sci. 2024, 644, 158797. [Google Scholar] [CrossRef]
- Vaghefinazari, B.; Wierzbicka, E.; Visser, P.; Posner, R.; Arrabal, R.; Matykina, E.; Mohedano, M.; Blawert, C.; Zheludkevich, M.; Lamaka, S. Chromate-free corrosion protection strategies for magnesium alloys—A review: PART I—Pre-treatment and conversion coating. Materials 2022, 15, 8676. [Google Scholar] [CrossRef]
- Oni, B.A.; Tomomewo, O.S.; Evro, S.; Misiani, A.N.; Sanni, S.E. A review of anticorrosive, superhydrophobic and self-healing properties of coating-composites as corrosion barriers on magnesium alloys: Recent advances, challenges and future directions. J. Magnes. Alloys 2025, 13, 2435–2469. [Google Scholar] [CrossRef]
- Guo, L.; Wu, W.; Zhou, Y.F.; Zhang, F.; Zeng, R.C.; Zeng, J.M. Layered double hydroxide coatings on magnesium alloys: A review. J. Mater. Sci. Technol. 2018, 34, 1455–1466. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, K.M. Corrosion and protection of magnesium alloys: Recent advances and future perspectives. Coatings 2023, 13, 1533. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Tran, H.N.; Nguyen, T.V.; Vigneswaran, S.; Trinh, V.T.; Nguyen, T.D.; Ha Nguyen, T.H.; Mai, T.N.; Chao, H.P. Single-step removal of arsenite ions from water through oxidation-coupled adsorption using Mn/Mg/Fe layered double hydroxide as catalyst and adsorbent. Chemosphere 2022, 295, 133370. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.K.E.; Balan, P.; Birbilis, N. Advances in LDH coatings on Mg alloys for biomedical applications: A corrosion perspective. Appl. Clay Sci. 2021, 202, 105948. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.G.; Zeng, R.C.; Li, S.Q.; Cui, H.Z.; Song, L.; Han, E.H. Corrosion resistance of Mg–Al-LDH coating on magnesium alloy AZ31. Surf. Coat. Technol. 2014, 258, 1152–1158. [Google Scholar] [CrossRef]
- Taviot-Guého, C.; Prévot, V.; Forano, C.; Renaudin, G.; Mousty, C.; Leroux, F. Tailoring hybrid layered double hydroxides for the development of innovative applications. Adv. Funct. Mater. 2018, 28, 1703868. [Google Scholar] [CrossRef]
- Santos, R.M.M.; Tronto, J.; Briois, V.; Santilli, C.V. Thermal decomposition and recovery properties of ZnAl–CO3 layered double hydroxide for anionic dye adsorption: Insight into the aggregative nucleation and growth mechanism of the LDH memory effect. J. Mater. Chem. A 2017, 5, 9998–10009. [Google Scholar] [CrossRef]
- Li, C.Y.; Gao, L.; Fan, X.L.; Zeng, R.C.; Chen, D.C.; Zhi, K.Q. In vitro degradation and cytocompatibility of a low temperature in-situ grown self-healing Mg-Al LDH coating on MAO-coated magnesium alloy AZ31. Bioact. Mater. 2020, 5, 364–376. [Google Scholar] [CrossRef]
- Peng, F.; Wang, D.H.; Zhang, D.D.; Cao, H.L.; Liu, X.Y. The prospect of layered double hydroxide as bone implants: A study of mechanical properties, cytocompatibility and antibacterial activity. Appl. Clay Sci. 2018, 165, 179–187. [Google Scholar] [CrossRef]
- Pillado, B.; Mingo, B.; del Olmo, R.; Matykina, E.; Kooijman, A.M.; Gonzalez−Garcia, Y.; Arrabal, R.; Mohedano, M. LDH conversion films for active protection of AZ31 Mg alloy. J. Magnes. Alloys 2023, 11, 201–216. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.H.; Li, Y.D.; Yu, M.; Li, S.M.; Xue, B. Fabrication of inhibitor anion-intercalated layered double hydroxide host films on aluminum alloy 2024 and their anticorrosion properties. J. Coat. Technol. Res. 2015, 12, 293–302. [Google Scholar] [CrossRef]
- Miyata, S. Anion-exchange properties of hydrotalcite-like compounds. Clays Clay Miner. 1983, 31, 305–311. [Google Scholar] [CrossRef]
- Liang, S.Y.; Ren, W.W.; Lin, W.X.; Zou, L.C.; Cui, X.P.; Chen, J.F. In-situ preparation of MgCr-LDH nanolayer on the surface of micro-arc oxidation coating on magnesium alloy and its corrosion resistance mechanism. Rare Met. Mater. Eng. 2020, 49, 2830–2838. [Google Scholar]
- Wu, L.; Yang, D.N.; Zhang, G.; Zhang, Z.; Zhang, S.; Tang, A.T.; Pan, F.S. Fabrication and characterization of Mg-M layered double hydroxide films on anodized magnesium alloy AZ31. Appl. Surf. Sci. 2018, 431, 177–186. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, F.; Fang, L.; Guan, T.; Hu, J.; Zhang, S.F. A comparative study and optimization of corrosion resistance of ZnAl layered double hydroxides films intercalated with different anions on AZ31 Mg alloys. Surf. Coat. Technol. 2019, 358, 594–603. [Google Scholar] [CrossRef]
- Yarger, M.S.; Steinmiller, E.M.P.; Choi, K.S. Electrochemical synthesis of Zn−Al layered double hydroxide (LDH) films. Inorg. Chem. 2008, 47, 5859–5865. [Google Scholar] [CrossRef]
- Wu, L.; Ding, X.X.; Zheng, Z.C.; Tang, A.T.; Zhang, G.; Atrens, A.; Pan, F.S. Doublely-doped Mg-Al-Ce-V2O74− LDH composite film on magnesium alloy AZ31 for anticorrosion. J. Mater. Sci. Technol. 2021, 64, 66–72. [Google Scholar] [CrossRef]
- Wu, Y.L.; Wu, L.; Yao, W.H.; Jiang, B.; Wu, J.H.; Chen, Y.N.; Chen, X.B.; Zhan, Q.; Zhang, G.; Pan, F.S. Improved corrosion resistance of AZ31 Mg alloy coated with MXenes/MgAl-LDHs composite layer modified with yttrium. Electrochim. Acta 2021, 374, 137913. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, A.X.; Wang, W.; Chen, Y.P.; Li, W.; Liu, W.; Chen, M.F. The effects of reaction parameters on the corrosion resistance of an Mg-Al hydroxide coating via in situ growth on a biomedical magnesium alloy. Coatings 2022, 12, 1388. [Google Scholar] [CrossRef]
- Shulha, T.N.; Serdechnova, M.; Lamaka, S.V.; Wieland, D.C.F.; Lapko, K.N.; Zheludkevich, M.L. Chelating agent-assisted in situ LDH growth on the surface of magnesium alloy. Sci. Rep. 2018, 8, 16409. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Tsunakawa, M.; Shimada, Y.; Serizawa, A.; Ishizaki, T. Formation mechanism of Mg-Al layered double hydroxide-containing magnesium hydroxide films prepared on Ca-added flame-resistant magnesium alloy by steam coating. Surf. Coat. Technol. 2017, 328, 436–443. [Google Scholar] [CrossRef]
- Ishizaki, T.; Chiba, S.; Suzuki, H. In situ formation of anticorrosive Mg–Al layered double hydroxide-containing magnesium hydroxide film on magnesium alloy by steam coating. ECS Electrochem. Lett. 2013, 2, C15. [Google Scholar] [CrossRef]
- Zhang, J.M.; Lian, D.D.; Hou, A.R.; Wang, Z.H.; Zhang, M.C.; Wu, J.W.; Wang, C.C. Comparative Study on Microstructure, Corrosion Morphology, and Friction Wear Properties of Layered Double Hydroxide/Steam Coating Composite Coatings on Mg–Li Alloy. Adv. Eng. Mater. 2024, 26, 2400058. [Google Scholar] [CrossRef]
- Ishizaki, T.; Miyashita, T.; Inamura, M.; Nagashima, Y.; Serizawa, A. Effect of Al content in the Mg-based alloys on the composition and corrosion resistance of composite hydroxide films formed by steam coating. Materials 2019, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Shimada, Y.; Miyashita, T.; Serizawa, A.; Ishizaki, T. Effect of vapor pressure during the steam coating treatment on structure and corrosion resistance of the Mg(OH)2/Mg-Al LDH composite film formed on Mg alloy AZ61. Materials 2018, 11, 1659. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Pan, S.Q.; Zhang, F.; Cui, L.Y.; Li, S.Q.; Liu, C.B.; Zeng, R.C. Corrosion resistance of layered double hydroxide coating prepared by in-situ steam method on the surface of magnesium alloy: Effect of Al(NO3)3 concentration. Surf. Technol. 2024, 53, 43–55. [Google Scholar]
- Pan, S.Q.; Wang, J.M.; Zhang, F.; Xie, H.F.; Cui, L.Y.; Bobby Kannan, M.; Zou, Y.H.; Zeng, R.C. Corrosion resistance of rapidly formed in-situ steam Mg−Al LDH coating on AM50 Mg alloy pretreated with oxalic acid. Trans. Nonferrous Met. Soc. China 2024, 34, 1843–1863. [Google Scholar] [CrossRef]
- Lin, J.K.; Uan, J.Y. Formation of Mg, Al-hydrotalcite conversion coating on Mg alloy in aqueous HCO3−/CO32− and corresponding protection against corrosion by the coating. Corros. Sci. 2009, 51, 1181–1188. [Google Scholar] [CrossRef]
- Yu, B.L.; Lin, J.K.; Uan, J.Y. Applications of carbonic acid solution for developing conversion coatings on Mg alloy. Trans. Nonferrous Met. Soc. China 2010, 20, 1331–1339. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.W.; Shan, D.Y.; Han, E.H. In situ growth of Mg–Al hydrotalcite conversion film on AZ31 magnesium alloy. Corros. Sci. 2011, 53, 3281–3288. [Google Scholar] [CrossRef]
- Chen, J.; Song, Y.W.; Shan, D.Y.; Han, E.H. Study of the in situ growth mechanism of Mg–Al hydrotalcite conversion film on AZ31 magnesium alloy. Corros. Sci. 2012, 63, 148–158. [Google Scholar] [CrossRef]
- Syu, J.H.; Uan, J.Y.; Lin, M.C.; Lin, Z.Y. Optically transparent Li–Al–CO3 layered double hydroxide thin films on an AZ31 Mg alloy formed by electrochemical deposition and their corrosion resistance in a dilute chloride environment. Corros. Sci. 2013, 68, 238–248. [Google Scholar] [CrossRef]
- Wu, F.X.; Liang, J.; Peng, Z.J.; Liu, B.X. Electrochemical deposition and characterization of Zn-Al layered double hydroxides (LDHs) films on magnesium alloy. Appl. Surf. Sci. 2014, 313, 834–840. [Google Scholar] [CrossRef]
- Ouyang, Y.J.; Hu, T.; Wang, J.Y.; Xie, Z.H. Electrochemical Deposition and Characterization of Layered Double Hydroxide Film on Magnesium Alloys. J. Chin. Soc. Corros. Prot. 2019, 39, 453–457. [Google Scholar]
- Tai, Y.; Fu, J.J. Molybdenum intercalated hydrotalcite-superhydrophobic composite coating: Fabrication and application in corrosion protection of magnesium alloys. Corros. Prot. 2018, 39, 202–206. [Google Scholar]
- Wang, B.; Li, H.Z.; Dong, Z.H. Micro-arc oxidation-hydrotalcite composite coating: Fabrication and application in corrosion protection of AZ91 magnesium alloy. Mater. Prot. 2020, 53, 26–31. [Google Scholar]
- Cao, Y.H.; Zheng, D.J.; Zhang, F.; Pan, J.S.; Lin, C.J. Layered double hydroxide (LDH) for multi-functionalized corrosion protection of metals: A review. J. Mater. Sci. Technol. 2022, 102, 232–263. [Google Scholar] [CrossRef]
- Yang, J.Y.; Wang, Y.F.; Li, M.X.; Wang, T.S.; Wang, D.W.; Wang, C.; Zha, M.; Wang, H.Y. Recent progress in self-repairing coatings for corrosion protection on magnesium alloys and perspective of porous solids as novel carrier and barrier. J. Magnes. Alloys 2023, 11, 3585–3608. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Song, G.L.; Wu, P.P. Self-repairing functionality and corrosion resistance of in-situ Mg-Al LDH film on Al-alloyed AZ31 surface. J. Magnes. Alloys 2023, 11, 1567–1579. [Google Scholar] [CrossRef]
- Li, L.X.; Xie, Z.H.; Fernandez, C.; Wu, L.; Cheng, D.J.; Jiang, X.H.; Zhong, C.J. Development of a thiophene derivative modified LDH coating for Mg alloy corrosion protection. Electrochim. Acta 2020, 330, 135186. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Nomerovskii, A.D.; Filonina, V.S.; Ustinov, A.Y.; Gnedenkov, S.V. Design of self-healing PEO-based protective layers containing in-situ grown LDH loaded with inhibitor on the MA8 magnesium alloy. J. Magnes. Alloys 2023, 11, 3688–3709. [Google Scholar] [CrossRef]
- Song, Y.H.; Tang, Y.; Fang, L.; Wu, F.; Zeng, X.G.; Hu, J.; Zhang, S.F.; Jiang, B.; Luo, H.J. Enhancement of corrosion resistance of AZ31 Mg alloys by one-step in situ synthesis of ZnAl-LDH films intercalated with organic anions (ASP, La). J. Magnes. Alloys 2021, 9, 658–667. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Tang, A.T.; Ma, Y.L.; Song, G.L.; Zheng, D.J.; Jiang, B.; Atrens, A.; Pan, F.S. Active corrosion protection by a smart coating based on a MgAl-layered double hydroxide on a cerium-modified plasma electrolytic oxidation coating on Mg alloy AZ31. Corros. Sci. 2018, 139, 370–382. [Google Scholar] [CrossRef]
- Liu, Y.X.; Duan, J.Q.; Zhang, J.L.; Guo, X.G. Active corrosion protection of micro-arc oxidation-based composite coating on magnesium alloy: Multiple roles of ionic liquid modified layered double hydroxide. Ceram. Int. 2024, 50, 26160–26170. [Google Scholar] [CrossRef]
- Ma, G.J.; Liu, X.; Zhang, W.; Sun, G. Fabrication and application of superhydrophobic bionic surface. Rare Met. Mater. Eng. 2018, 47, 1866–1871. [Google Scholar]
- Wang, L.; Zhang, F.; Jiao, Z.J.; Cui, L.Y.; Huang, Y.D.; Li, S.Q.; Liu, C.B.; Zeng, R.C. Advances in LDHs for corrosion-resistant protection of Mg and Al alloys: A review. J. Magnes. Alloys 2025, 13, 923–947. [Google Scholar] [CrossRef]
- Wang, X.; Jing, C.; Chen, Y.X.; Wang, X.S.; Zhao, G.; Zhang, X.; Wu, L.; Liu, X.Y.; Dong, B.Q.; Zhang, Y.X. Active corrosion protection of super-hydrophobic corrosion inhibitor intercalated Mg–Al layered double hydroxide coating on AZ31 magnesium alloy. J. Magnes. Alloys 2020, 8, 291–300. [Google Scholar] [CrossRef]
- Yin, X.X.; Mu, P.; Wang, Q.T.; Li, J. Superhydrophobic ZIF-8-based dual-layer coating for enhanced corrosion protection of Mg alloy. ACS Appl. Mater. Interfaces 2020, 12, 35453–35463. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Song, G.L.; Wu, P.P.; Huang, J.F.; Zheng, D.J. A protective superhydrophobic Mg–Zn–Al LDH film on surface-alloyed magnesium. J. Alloys Compd. 2021, 855, 157550. [Google Scholar] [CrossRef]
- Zhang, J.L.; Gu, C.D.; Tu, J.P. Robust Slippery Coating with Superior Corrosion Resistance and Anti-Icing Performance for AZ31B Mg Alloy Protection. ACS Appl. Mater. Interfaces 2017, 798, 320–325. [Google Scholar] [CrossRef]
- Wong, T.S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef]
- Tian, G.Y.; Wang, J.S.; Su, H.; Xue, C.P.; Yang, X.H.; Li, Q.; Li, X.X.; Miao, Y.S.; Yang, Z.H. Bio-inspired self-healing slippery surfaces with smart multifunctionality on MgLi alloys. Prog. Org. Coat. 2024, 196, 108696. [Google Scholar] [CrossRef]
- Niu, C.C.; Lin, X.K.; Zhang, D.D.; Liu, J.P.; Xu, J.; He, Q. Slippery anti-biofouling coatings on Mg alloy with lubricant self-replenishing performance. Colloids Surf. A Physicochem. Eng. Asp. 2025, 714, 136587. [Google Scholar] [CrossRef]
- Yao, W.H.; Wu, L.; Jiang, B.; Pan, F.S. Slippery liquid-infused porous surface by ZnAl-layered double hydroxide on AZ31 Mg alloys. J. Taiwan Inst. Chem. Eng. 2023, 150, 105017. [Google Scholar] [CrossRef]
- Jiang, D.; Xia, X.C.; Hou, J.; Cai, G.Y.; Zhang, X.X.; Dong, Z.H. A novel coating system with self-reparable slippery surface and active corrosion inhibition for reliable protection of Mg alloy. Chem. Eng. J. 2019, 373, 285–297. [Google Scholar] [CrossRef]
- Yao, W.H.; Qin, J.; Chen, Y.H.; Wu, L.; Jiang, B.; Pan, F.S. SiO2 nanoparticles-containing slippery-liquid infused porous surface for corrosion and wear resistance of AZ31 Mg alloy. Mater. Des. 2023, 227, 111721. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Liu, Y.; Wang, H.; Luo, J. The relationship between surface microstructure and super-lubrication performance based on 2D LDHs. Mater. Today Nano 2023, 23, 100361. [Google Scholar] [CrossRef]

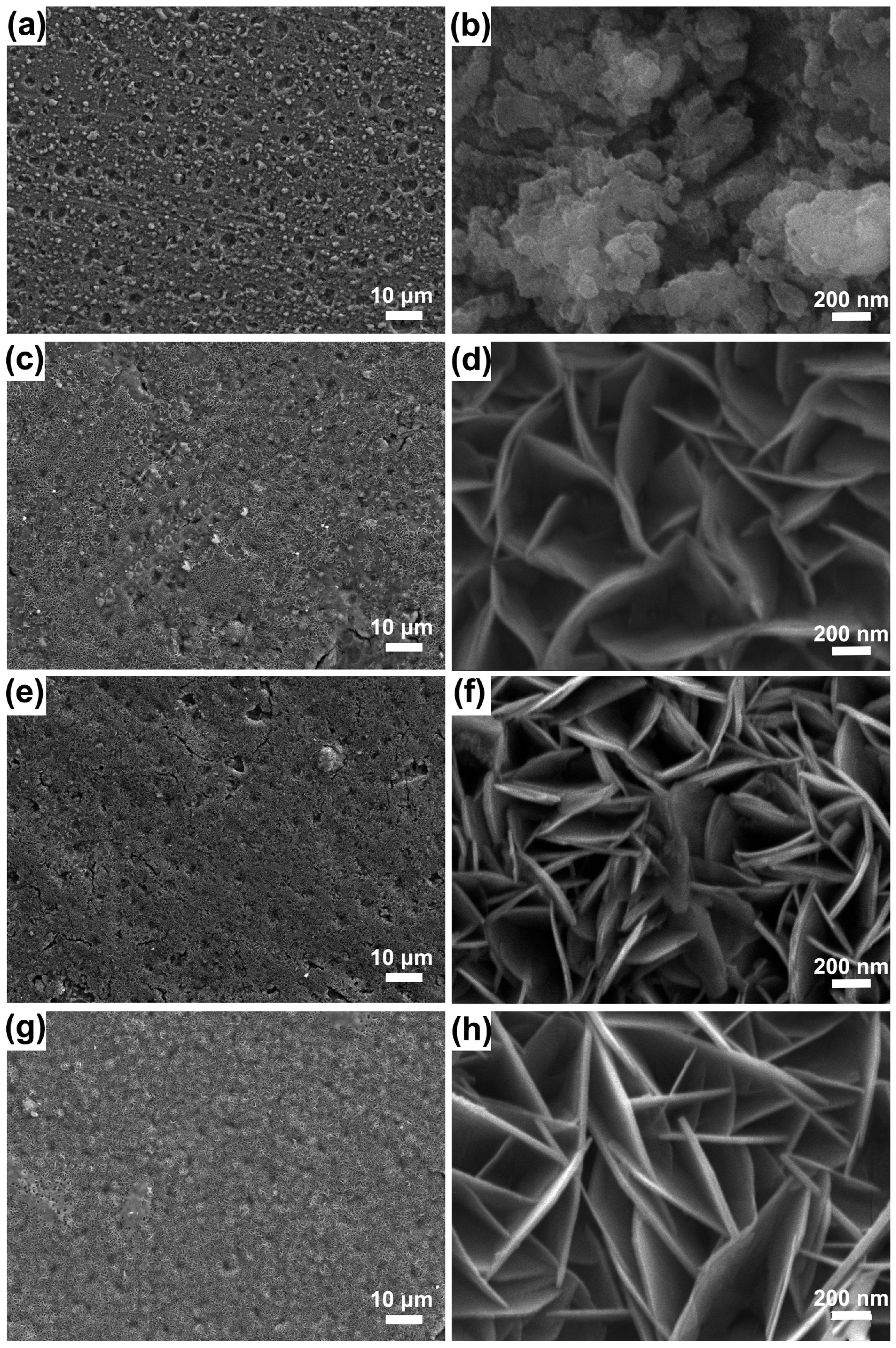

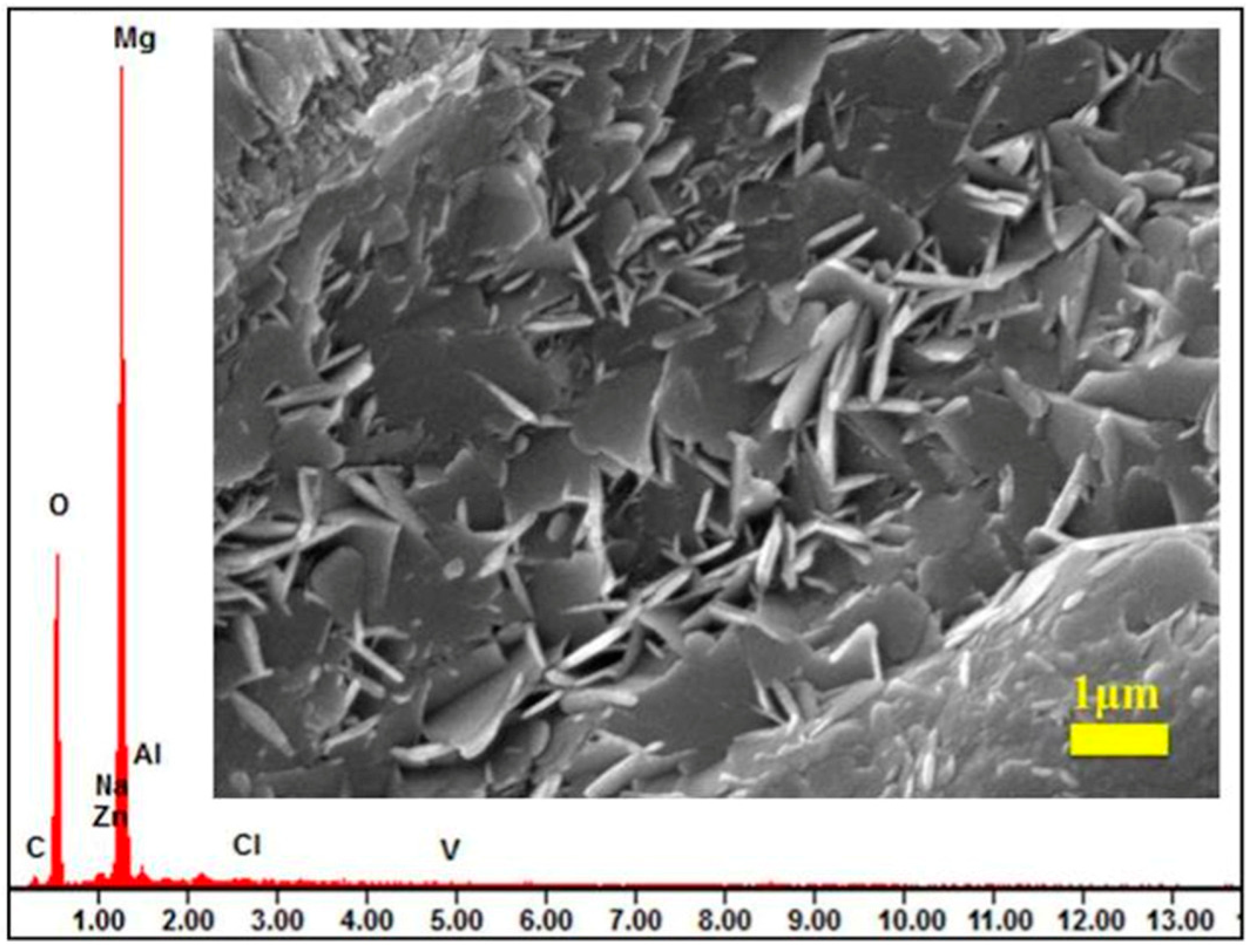

| Method | Conditions | Advantages | Disadvantages | Film Quality and Core Features | Corrosion Current Density (A/cm2) | Functional Expandability | Application Scenarios | References |
|---|---|---|---|---|---|---|---|---|
| Hydrothermal treatment | High temperature, high pressure | Stable performance, high process controllability, widely used | Long reaction time, high temperature, high equipment requirements, medium cost |
| 9.12 × 10−9–3.026 × 10−7 | Metal ion doping, anion intercalation | Automotive components, medical implants | [26,39,40,41,42,43,44,45] |
| Steam coating | High temperature, high pressure | High efficiency, environmentally friendly, low cost | High pressure, high safety requirements, limited types of Mg alloys, weak ion exchange capacity, presence of by-products |
| 8 × 10−9–2.4 × 10−8 | None | Mass-produced structural parts, green production scenarios | [46,47,48,49,50,51,52] |
| Impregnation method | Atmospheric pressure and room temperature | Simple method, easy operation, environmentally friendly, low cost | Weak functionalization, poor film durability, limited types of Mg alloys, with Al-rich grades (AZ31, AZ91) |
| 2.21 × 10−6– 10 × 10−6 | None | Small-batch samples, short-term storage | [26,53,54,55,56] |
| Electrodeposition | Atmospheric pressure and room temperature | Short reaction time, mild reaction conditions, simple equipment, coating not limited by substrate shape, suitable for large-sized components | High energy consumption, prone to by-products, high cost |
| 2.12 × 10−6–7.882 × 10−7 | Metal ion doping, anion intercalation | Components with complex shapes, large-scale production | [26,57,58,59] |
| Co-precipitation | High temperature, high pressure | Controllable chemical composition, wide scope of application | Complex operation, time-consuming operation, high equipment standards, high cost |
| 1.2 × 10−7–1.6 × 10−7 | Metal ion doping, anion intercalation | Customized functional coatings (marine/medical applications) | [60,61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Bai, X.; Chen, W. Research Progress on LDH Corrosion-Resistant Films on Magnesium Alloy: A Review. Materials 2025, 18, 5249. https://doi.org/10.3390/ma18225249
Li H, Bai X, Chen W. Research Progress on LDH Corrosion-Resistant Films on Magnesium Alloy: A Review. Materials. 2025; 18(22):5249. https://doi.org/10.3390/ma18225249
Chicago/Turabian StyleLi, Huan, Xue Bai, and Wenjin Chen. 2025. "Research Progress on LDH Corrosion-Resistant Films on Magnesium Alloy: A Review" Materials 18, no. 22: 5249. https://doi.org/10.3390/ma18225249
APA StyleLi, H., Bai, X., & Chen, W. (2025). Research Progress on LDH Corrosion-Resistant Films on Magnesium Alloy: A Review. Materials, 18(22), 5249. https://doi.org/10.3390/ma18225249






