2. Materials and Methods
Powders of the raw materials, comprising Fe (<150 μm, 99%, Kojundo Chemical Lab. Co., Ltd., Saitama, Japan), Al (<150 μm, 99.99%, Kojundo Chemical Lab. Co., Ltd., Saitama, Japan), and grains of Si (2~5 mm, 99.999%, Kojundo Chemical Lab. Co., Ltd., Saitama, Japan) were weighed for prepared compositions of Al25.7Fe37.1Si37.1 (4%Al), Al26.8Fe36.6Si36.6 (10%Al), and Al27.5Fe36.2Si36.2 (14%Al), where 4, 10, and 14 at. % extra quantities of Al were added to the nominal composition of Al25Fe37.5Si37.5 to compensate for Al loss due to evaporation and oxidization during sintering at a later stage.
Pellet samples were prepared by two different routes; while samples with larger grains were prepared by a reactive sintering method [
16], those with smaller grains with nanometer-size scales were prepared by the consolidation of powder mechanically alloyed by high-energy ball milling.
For reactive sintering, first, Si was milled with ZrO
2 balls in a ZrO
2 vial at 700 rpm for 2 h using a planetary ball mill (Planet-min, Nagao System Inc., Kanagawa, Japan) to obtain a fine powder. The Si powder was mixed with Fe powder at 550 rpm for 1.75 h using the same machine, then Al powder was added and mixed at 500 rpm for 1.25 h. In these steps, the lower rates of rotation were employed to mix the powders of different elements well but not to alloy them [
16]. All operations were performed in an Ar atmosphere in a glove box equipped with a gas circulation purification system (UL-1000A, UNICO Ltd., Ibaraki, Japan). The mixed powder was loaded into a graphite die with a 12.4 mm inner diameter and sintered by hot pressing (HP) (SPD4000-I, Dai-ichi Kiden Co., Ltd., Tokyo, Japan) at 1223 K under a pressure of 84 MPa for 2 h under an argon atmosphere.
For preparing samples via high-energy ball milling, the powders of Al, Fe, and Si were milled with WC balls in a WC vial filled with argon gas in the glove box, where a viton O-ring was used to achieve better sealing for 70 h using a high-energy ball mill (Spex Mill 8000M, SPEX SamplePrep Corp., Metuchen, NJ, USA). For the No. 2 sample, while these operations were performed in air, the samples were repacked in the vial under Ar gas every 12 h in the glove box and the O-ring was replaced with a brand-new one every 30 h. For the No. 3 sample, the milling was performed in a WC vial sealed with Ar gas in a nylon bag for lower oxygen partial pressure than the No. 2 sample. Samples No. 4, 5, and 6 were milled in an Ar atmosphere in a glove box, where the oxygen level of the atmosphere was less than the detection limit of the zirconia-type oxygen analyzer of 0.001 ppm. The sample preparation conditions and bulk densities are summarized in
Table 1.
The milled powder was loaded into a graphite die with a 12.4 mm inner cylindrical diameter and sintered by spark plasma sintering (SPS) (SPD4000-I, Dai-ichi Kiden Co., Ltd., Tokyo, Japan) at 1223 K under a pressure of 84 MPa for 10 min in an argon atmosphere. After sintering, the thickness and weight of the sintered body were measured to calculate the bulk density.
The crystal structures were characterized via X-ray diffraction analysis (XRD) using Cu Kα radiation (UtimaIV, Rigaku Corp., Tokyo, Japan). The microstructure was observed using a scanning electron microscope combined with a focused ion beam (FIB-SEM) (AurigaLaser, Carl Zeiss Co., Ltd., Oberkochen, Germany), and the composition of samples was measured by energy-dispersive spectroscopy (EDS) (EMAX7693-H, HORIBA Corp., Kyoto, Japan). Oxygen concentrations were measured with an inert gas-fusion elemental analyzer (ONH836, LECO Corp., St. Joseph, MI, USA). The Seebeck coefficient and electrical conductivity were measured using a Seebeck coefficient/electric resistance measurement system (ZEM-3, Advance Riko, Inc., Kanagawa, Japan) simultaneously. The thermal diffusivity, α, and heat capacity, Cp, were measured by the laser flash method (LFA 447 Nanoflash, NETZSCH Japan K.K., Kanagawa, Japan). The thermal conductivity, κtot, was calculated by κtot = αρCp, where ρ is the bulk density of the sample and Cp is the heat capacity.
3. Results and Discussion
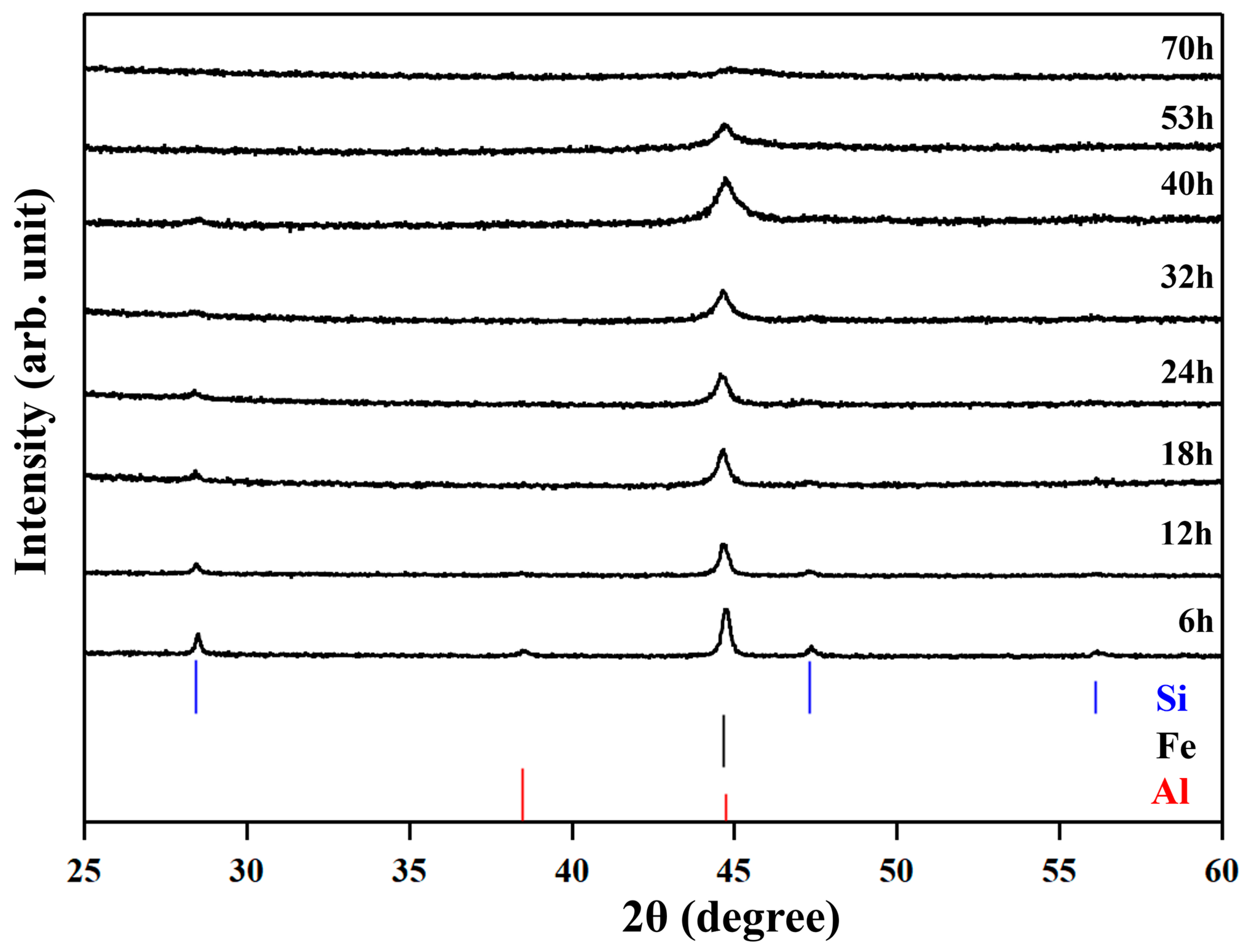
The XRD patterns of the No. 3 sample during the milling are shown in
Figure 1. The peaks are blunter for longer milling times. At 70 h, almost no peaks are recognized, suggesting that the crystallite diameters are less than 10 nm or samples are in an amorphous state.
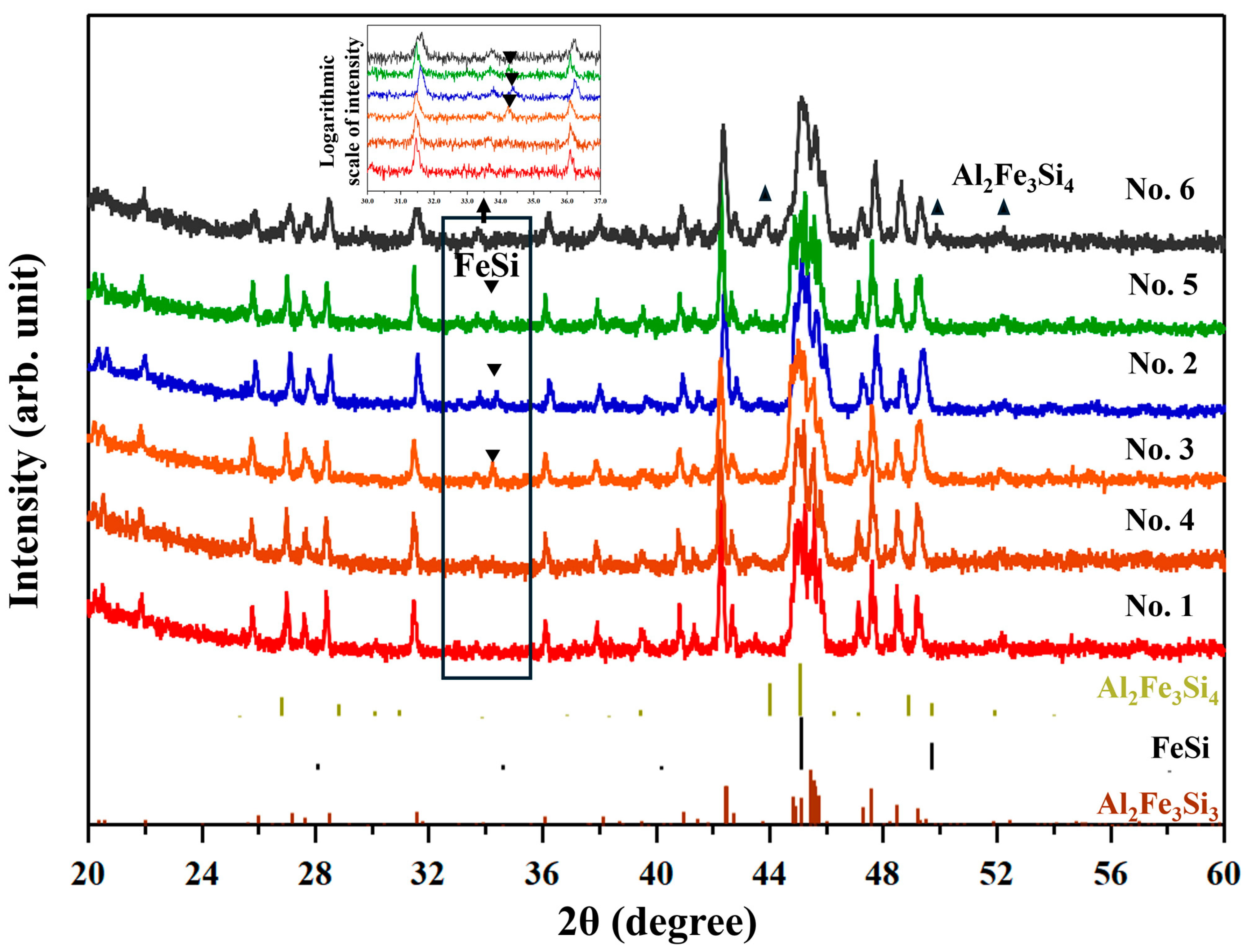
Figure 2 shows the XRD patterns of all samples after consolidation (SPS/HP). All samples have diffraction peaks which are identified as ones from the τ
1-Al
2Fe
3Si
3 phase. The No. 1–5 samples have an additional peak at around 35°, which is indexed as the ε-FeSi phase, similarly to previously reported samples [
17,
18,
19]. The No. 6 sample has a peak near 43°, which is identified as that from the τ
8-Al
2Fe
3Si
4 phase [
20]. According to the reference pattern of ε-FeSi of the ICDD database, there should be a second-strongest peak at around 49°. However, this peak is not clearly observed in the profiles of the current samples. The reason we deduced is that since the compositions of the ε-FeSi phase (containing Al in this study) and the t
1-Al
2Fe
3Si
3 phase (composition shifted from the stoichiometry) are different from the materials for the reference XRD patterns, the peak angles in the measured XRD profiles shift from the reference data. Actually, according to a previous work [
21], doping Al to ε-FeSi shifts the peak to a lower angle by 0.2–0.4°. This causes the overlapping with the peak from t
1-Al
2Fe
3Si
3. The presumption that the secondary phase is ε-FeSi will be confirmed later in this paper with the aid of SEM observations and EBSD analysis. Other peaks from the ε-FeSi phase or τ
8-Al
2Fe
3Si
4 phase overlap with peaks of the τ
1-Al
2Fe
3Si
3 phase (see
Figure S3 in Supplementary Materials for details). The diffraction peaks of oxides are not observed.
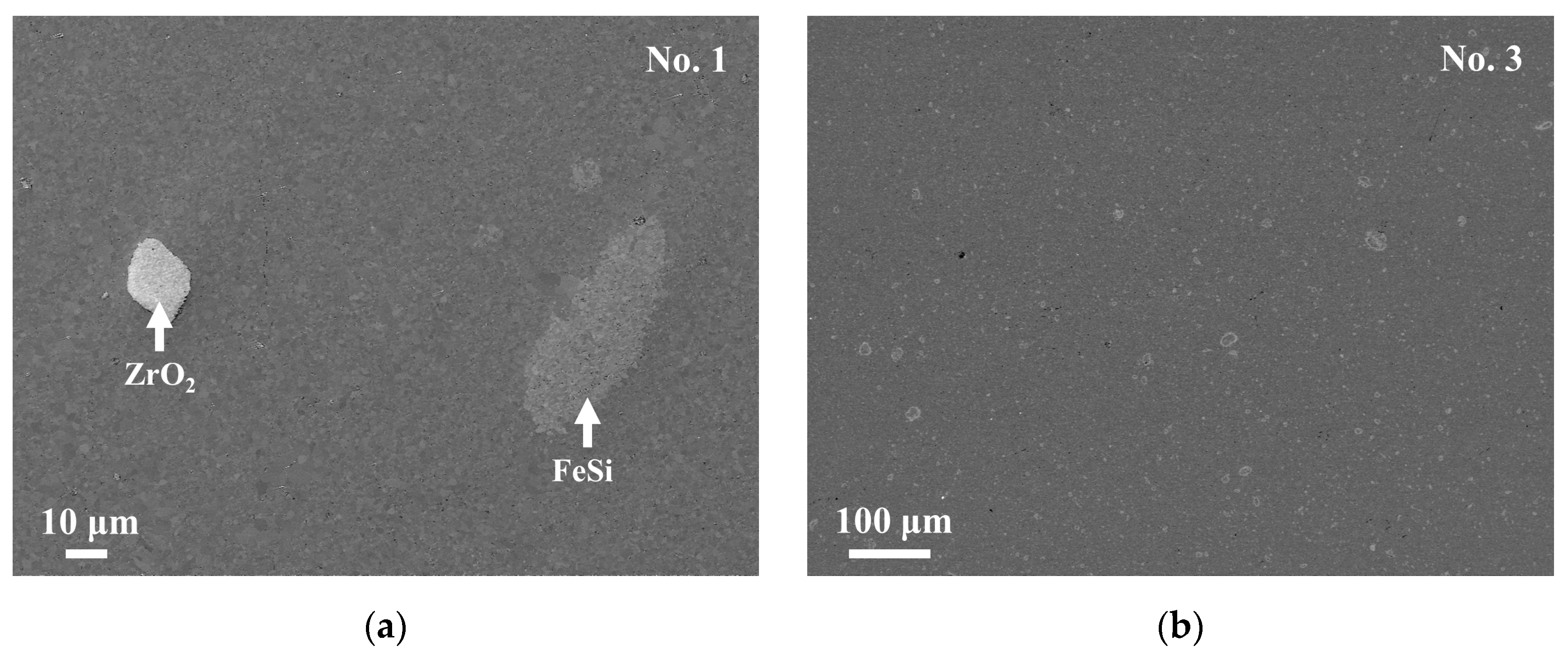
Figure 3a,b show the SEM images of the No. 1 and No. 3 samples. The chemical compositions of the respective phases obtained by EDS are shown in
Table 2. The results of XRD, SEM, and EDS suggest that the matrix phase of both the samples is the τ
1-Al
2Fe
3Si
3 phase. The bright phase is recognized in the No. 3 sample, as shown in
Figure 3b, but is too fine to be clearly analyzed for its chemical composition. The chemical composition will be discussed in the following section. In addition, the ZrO
2 phase, which is from the vial and balls during the milling process, is also observed, as shown in
Figure 3a.
The charging compositions of all the samples No. 1–6, which are schematically shown in
Figure 4a, were chosen so that the resulting compositions of the τ
1 phase would become Al
25Fe
37.5Si
37.5, supposing an Al loss of 4% for No. 1–4, 10% for No. 5, and 14% for No. 6, respectively, due to the evaporation or oxidation of Al during synthesis. These compositions are aligned on the line connecting Al and Al
25Fe
37.5Si
37.5 (hereafter called the “Al loss line”) in the composition triangle of the Al-Fe-Si system.
Recently, the tie lines of the Al-Fe-Si system have been experimentally studied by the sintered diffusion multiple method [
22]. According to that work, the tie lines between the τ
1 phase and the ε phase (shown as broken lines in
Figure 4b) are nearly parallel to the “Al loss line,” which is shown as the arrow connecting Al and Al
25Fe
37.5Si
37.5. Eventually, as long as the average compositions of the unoxidized regions go in the direction of the two-phase region between the τ
1 phase and the ε phase due to the loss of Al during synthesis, the samples will be composed of the τ
1 phase and the ε phase, and the resulting Al/Si ratios of the τ
1 phase in the samples will not vary much. The loss of Al can occur by the evaporation of Al, which causes the shift in the gross composition, or by the oxidization of Al, which causes the shift in the average composition of the unoxidized regions. Actually, samples No. 1–5 are composed of the τ
1 and ε phases, and their Al/Si ratios do not differ very much.
In addition to Al loss, oxidation of Si can occur, resulting in the average composition of unoxidized regions being shifted in the directions shown by the arrows going away from Si in the composition triangle, with the Al-richer compositions of the τ1 phase in samples of Al25Fe37.5Si37.5 being to some extent dependent on conditions.
The No. 6 sample, which has the highest Al content, is composed of the τ1 and τ8 phases of the Al-Fe-Si system. This suggests that the “Al loss line” did not reach the two-phase region between the τ1 phase and the ε phase because of the high Al content but did go in the direction of the two-phase region between the τ1 and τ8 phases.
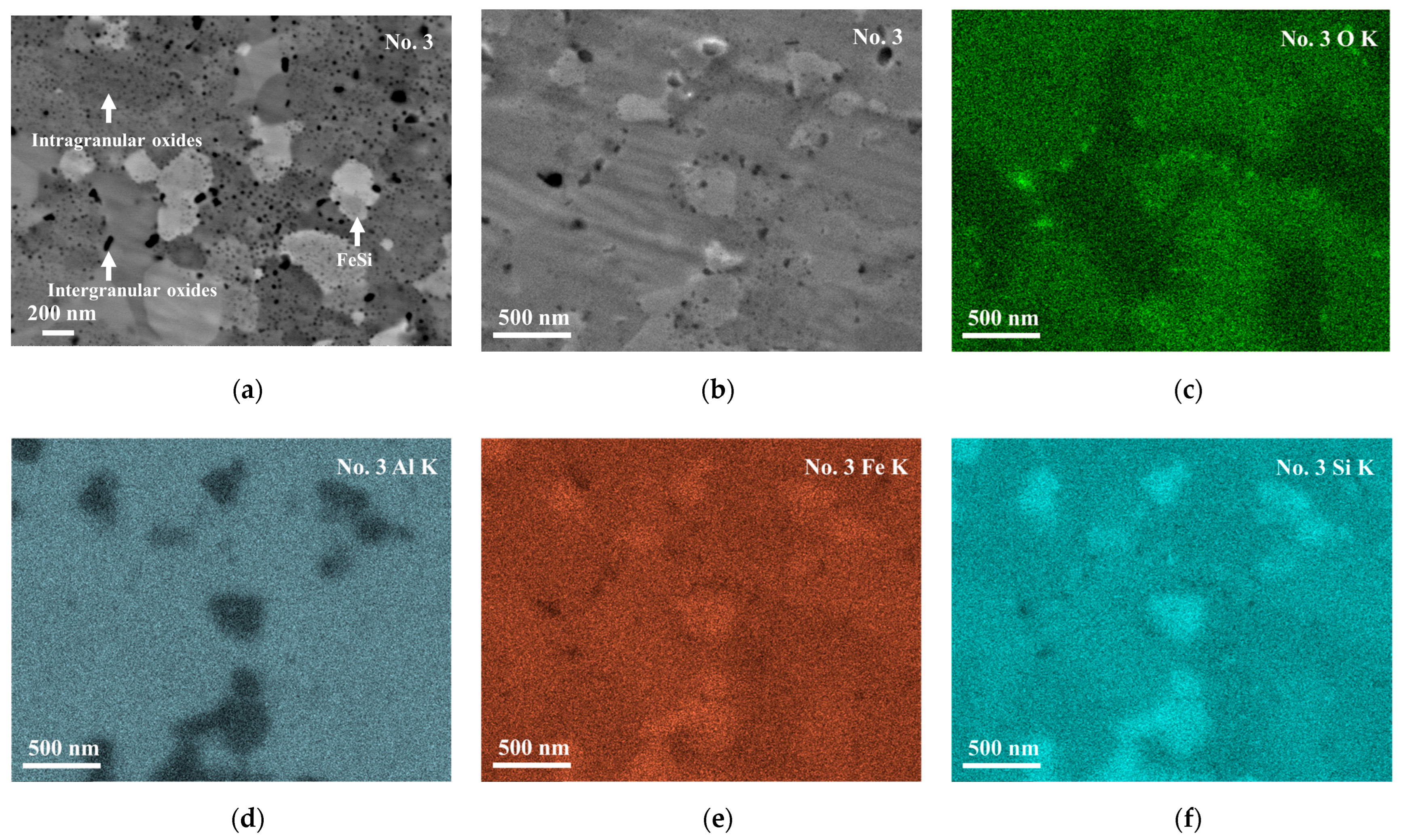
Figure 5a,b show a backscattered electron (BSE) image and secondary electron (SE) image of the No. 3 sample with a high magnification. For the bright phase in
Figure 5b, which corresponds to the bright phase in
Figure 3b, it is difficult to determine its exact chemical composition by EDS because its size-scale is less than 1 µm. Looking into the elemental mapping data, as shown
Figure 5c–f, it is found that the bright phase is rich in iron and silicon. From these data, together with the XRD profile (
Figure 2) and electron backscatter diffraction pattern (EBSD) (
Figure S4) image which show the existence of the ε-FeSi phase, and the reported phase diagram of the Al-Fe-Si phase [
20], the bright phase can be concluded to be ε-FeSi.
The sizes of ε-FeSi particles have been determined by analyzing SEM images using the ImageJ (version 1.54g) software for the No. 3 sample. The particle diameter distribution is shown in
Figure S5. The mean diameter is
= 0.23 μm, which is one order of magnitude smaller than those prepared by gas-atomization and SPS (
= 1.7 μm) [
23]. Thus, as suggested previously [
24], MA is more advantageous for realizing fine microstructures with nanometer-size scales.
The intragranular and intergranular black spots are observed in the No. 2–6 samples, similarly to those shown for No. 3 in
Figure 5a,b. They are found to have nanometer scales, while there are not so many similar black spots found in the No. 1 sample, as shown in
Figure S1.
Figure 5c–f show the oxygen, aluminum, iron, and silicon distributions, respectively, in the No. 3 sample of the same region as in
Figure 5b. The black spots in the SE image (
Figure 5b) show high concentrations of oxygen (
Figure 5c) and low concentrations of iron (
Figure 5e) and silicon (
Figure 5f). On the other hand, the aluminum (
Figure 5d) mapping does not show contrast at the same positions as the SE image. This suggests that the black spots are of aluminum oxide, while the oxides are too small to be analyzed for their exact chemical compositions. The aluminum concentration in the τ
1-Al
2Fe
3Si
3 phase is ~25 at. % while that in aluminum oxide is ~40 at. %, assuming that the oxides are of Al
2O
3. Since the difference in aluminum concentration is not very large and the size of the particles is smaller than the spatial resolution of EDS analysis, no clear contrast can be observed in the aluminum mapping (
Figure 5d).
The results of EDS in
Table 2 show that the oxygen concentrations of the No. 1–3 samples depend on the atmosphere in milling; they increase with the oxygen content of the atmosphere. In other words, the oxygen content, i.e., the volume fraction of the small dots of oxide, is controlled by the oxygen content of the atmosphere in milling. Since it is known that the concentrations of light elements determined by EDS are, in general, not necessarily accurate [
25], we double-checked the oxygen content of the No. 2 sample with inert gas-fusion elemental analyzers, and it is found to be 1.5 wt.% instead of the 5.3 wt.% found by EDS.
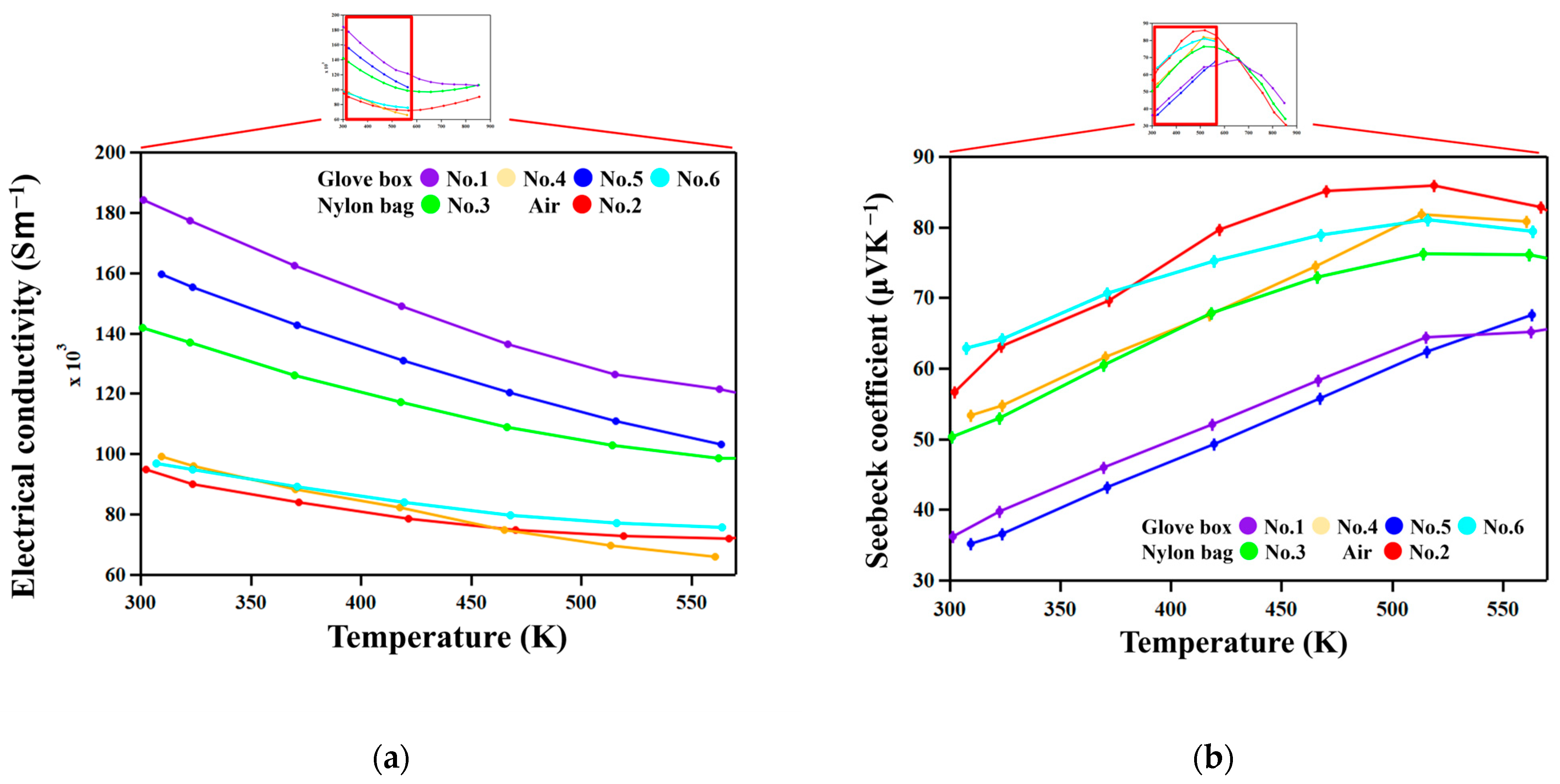
Figure 6 shows the temperature dependence of the electrical conductivity σ and Seebeck coefficient
S. All samples have positive Seebeck coefficients throughout the entire temperature range. σ decreased and
S increased with increasing temperature in all the samples, exhibiting degenerate semiconductor behavior.
Samples with higher σ have lower
S. This suggests that carrier concentration is the dominant factor affecting the difference in σ and
S among samples. The τ
1-Al
2Fe
3Si
3 phase has a composition range with variable Al/Si ratios from 21 at. % Al to 41.5 at. % Al [
20]. As reported in previous works [
19,
26], the Al/Si ratio determines the carrier concentration since excess Al and Si atoms generate holes and electrons, respectively. Actually, σ increases with increasing Al concentrations in the τ
1 phase, as shown in
Figure 7.
The error bars for the Al concentration in
Figure 7 might be too large, as evaluated by numerical values and shown in
Table 2, to discuss such a trend between the electrical conductivity and the Al concentration. Therefore, to check the validity of the aluminum concentration for all the samples, we determined the lattice parameters of each sample by the Rietveld analysis using the Rigaku-PDXL software (version 2.7.2.0) and show the results in
Figure S2. The lattice parameters
a, α, β, and γ are in good agreement with those reported in a previous work [
20]. The result of the lattice parameters of
b and
c does not show a clear dependence on Al concentration. This is possibly due to the overlap of peaks between the ε-FeSi phase and τ
1-Al
2Fe
3Si
3 phase. Thus, from another point of view, the ε-FeSi phase has high σ [
27] and might affect σ via the rule of mixtures. Therefore, we evaluated the volume fraction of the ε-FeSi phase by image analysis using the ImageJ software, and s is plotted as a function of it in
Figure 7. As a result, it is found that s depends on the Al concentration rather than the volume fraction of the ε-FeSi phase because the volume fraction of the ε-FeSi phase is less than 10% in all samples, which is too small to show a significant effect on σ. Focusing on the carrier transport across the interfaces between the ε-FeSi phase and τ
1-Al
2Fe
3Si
3 phase, it has been reported [
23] that they are of the ohmic-type, that is, they do not show an energy barrier and hence do not work as resistance for passing carriers.
The exceptionally high conductivity of the No. 1 sample, which was prepared by reactive sintering (
Table 1), can be attributed to the high mobility of carriers since nanometer-scale features are not observed in this sample only.
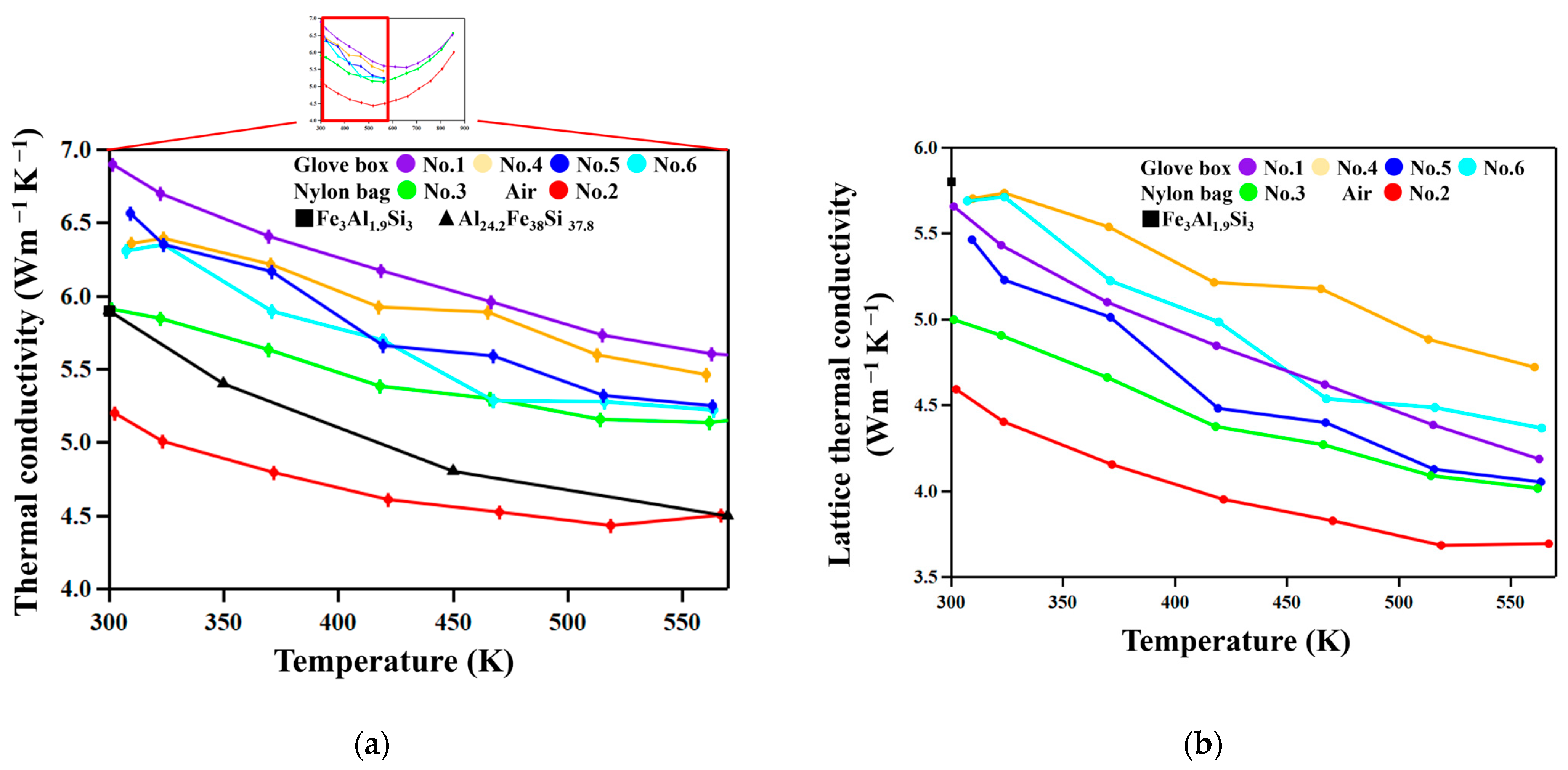
Figure 8 shows the temperature dependence of the total and lattice thermal conductivities, κ
tot and κ
lat, respectively. κ
lat was calculated using the Wiedemann–Franz law, κ
tot–
Lσ
T, where
L is the Lorenz number [
28]. We used an approximate expression for
L for degenerate semiconductors [
29],
L = 1.5 + exp[−|
S|/116] × 10
−8 WΩK
−2, where
S is in µVK
−1. No clear trend was recognized in the relation between chemical composition and κ
tot and κ
lat. On the other hand, samples prepared in nylon bags (No. 2 and 3) are lower than those prepared using the glove box.
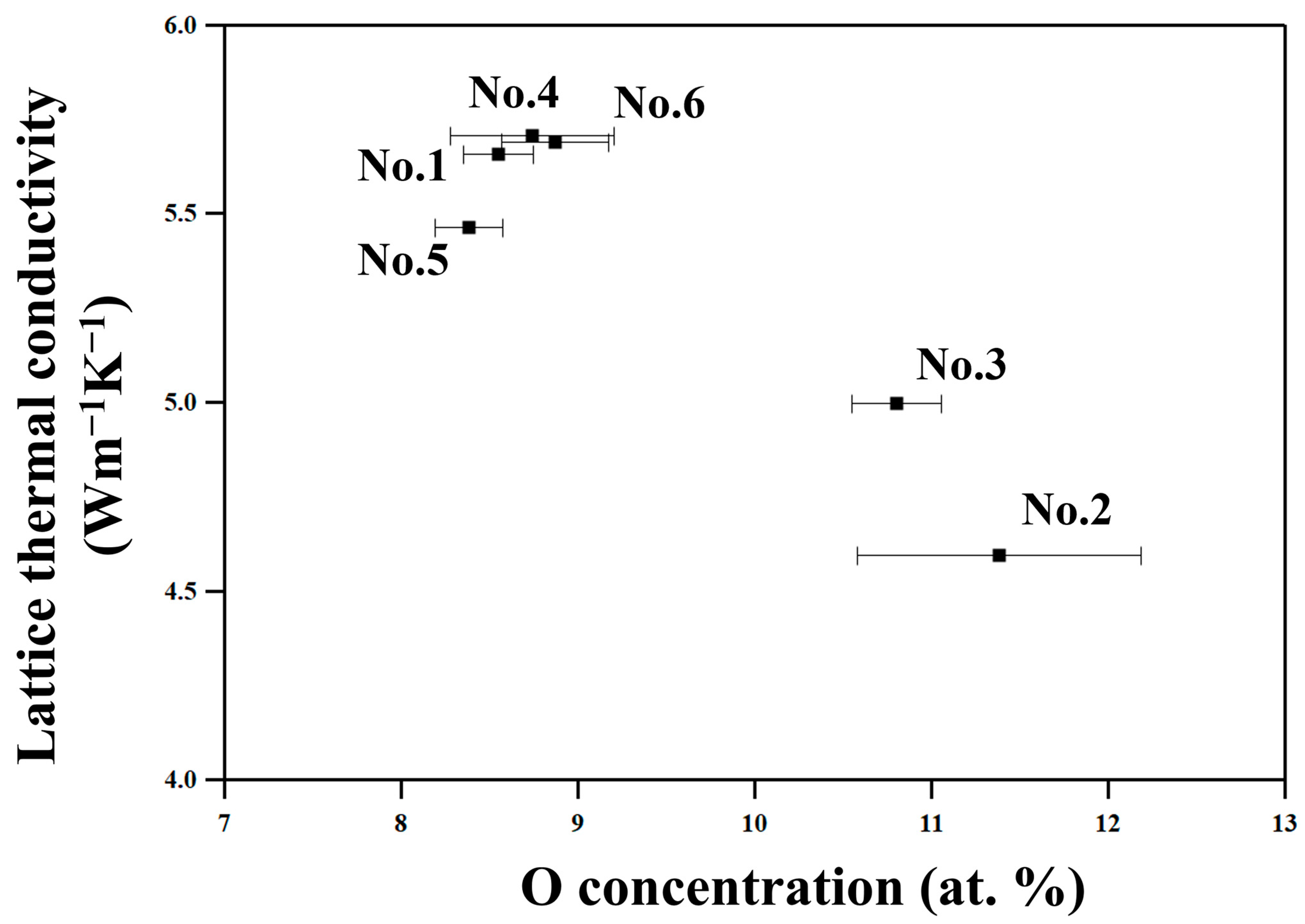
The No. 2 and 3 samples, which are milled in environments where samples are relatively easily oxidized and hence have higher oxygen concentrations (
Table 2), show lower κ
tot and κ
lat than the other samples, as
Figure 9 shows. This suggests that the oxide particles in nanometer scales effectively scatter phonons, resulting in such decreased thermal conductivities. On the other hand, the No. 1, 4, 5, and 6 samples show relatively high κ
tot and κ
lat since they were milled in an atmosphere with lower oxygen partial pressure in a glove box equipped with a gas circulation purification system, and thus have less oxide particles than samples No. 2 and 3.
We compare here the thermal conductivities of the FAST materials studied in this work with those of the FAST materials in the literature. As seen in
Figure 8b, the total thermal conductivity of sample No. 2 is lower than those reported previously for Fe
3Al
1.9Si
3 [
23] and Al
24.3Fe
38Si
37.8 [
26] compositions, but others are not. The reason is that the total thermal conductivity is contributed to by electrons and it should be significantly dependent on composition. Therefore, to unveil the effect of nanostructuring, it would be more suitable to look into the lattice aspect of thermal conductivity shown in
Figure 8b. As found in the figure, the lattice thermal conductivities of the No. 2 and 3 samples are significantly lower than that of Fe
3Al
1.9Si
3 [
23], which is not nanostructured, due to the scattering of phonons by oxide particles.
Thus, nanostructuring effectively reduces lattice thermal conductivity by particles of various sizes. A theoretical calculation for FAST materials based on first principles [
30] have revealed that the mean free path of phonons contributing to κ is below 500 nm and significant contribution is between 3 nm to 200 nm. Thus, particles size is important for reduced lattice thermal conductivity to be recognized. The SEM (
Figure 5) and EBSD (
Figure S4) images show that while the sizes of grains of the τ
1Al
2Fe
3Si
3 phase are of ~800 nm and 74% of the ε-FeSi particles are above 200 nm, intergranular and intragranular oxide phases are of tens of nanometers and of singlenanometer-size scales, respectively. According to the Debye–Callaway model [
31], the relaxation time for phonon scattering is expressed as the summation of relaxation time by various scattering modes,
τ−1 =
τD−1 +
τP−1 +
τB−1, where
τD−1,
τP−1, and
τB−1 are relaxation times for point-defect scattering, phonon–phonon scattering, and boundary scattering, respectively. Among these scattering modes, boundary scattering is dominant at low temperatures [
31].
The relaxation time of boundary scattering is expressed as τB−1 = υs/L, where υs is the velocity of sound and L is a characteristic length. Oxide particles effectively reduce the L of the matrix, resulting in a reduced relaxation time of boundary scattering, and effectively scatter phonons, resulting in a decreased thermal conductivity.
We checked the thermal stability of the nanostructures by annealing samples for one week in a vacuum at 573 K followed by microstructure observations and thermal diffusivity measurement. Regarding the results, the thermal diffusivity and oxide particle size are not changed by the annealing, as
Figure S6 shows.
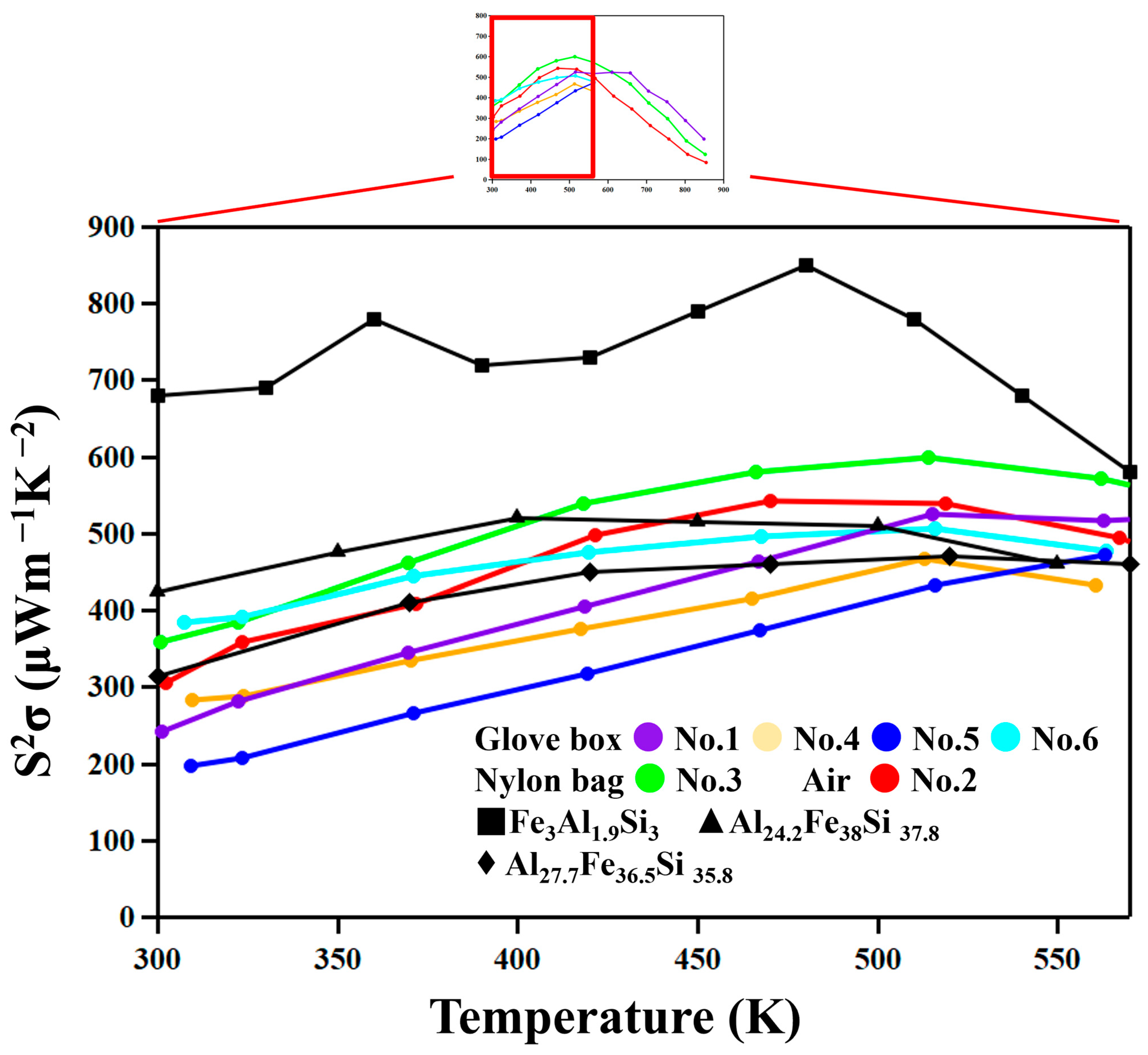
Figure 10 shows the temperature dependence of the power factor,
PF =
S2σ. The
PF of the No. 2 and 3 samples, which show low thermal conductivity due to the existence of nanoscale oxide particles, do not show significantly lower values compared with other samples. It is thus suggested that nanostructuring does not significantly reduce the mobility of carriers in these materials.
The temperature dependence of
zT is shown in
Figure S7. As seen in this figure,
zT values of the samples prepared by milling in a nylon bag (No. 3) and in air (No. 2), which have higher densities of oxide particles, are higher than others. This results from the relatively lower lattice thermal conductivities compared with other samples, as shown in
Figure 8. Thus, the effect of nanostructuring is positive.