Preparation and Anti-Corrosion Performance Investigation of Ni–SiC Composites Produced at Different Ultrasonic Powers
Abstract
1. Introduction
2. Materials and Methods
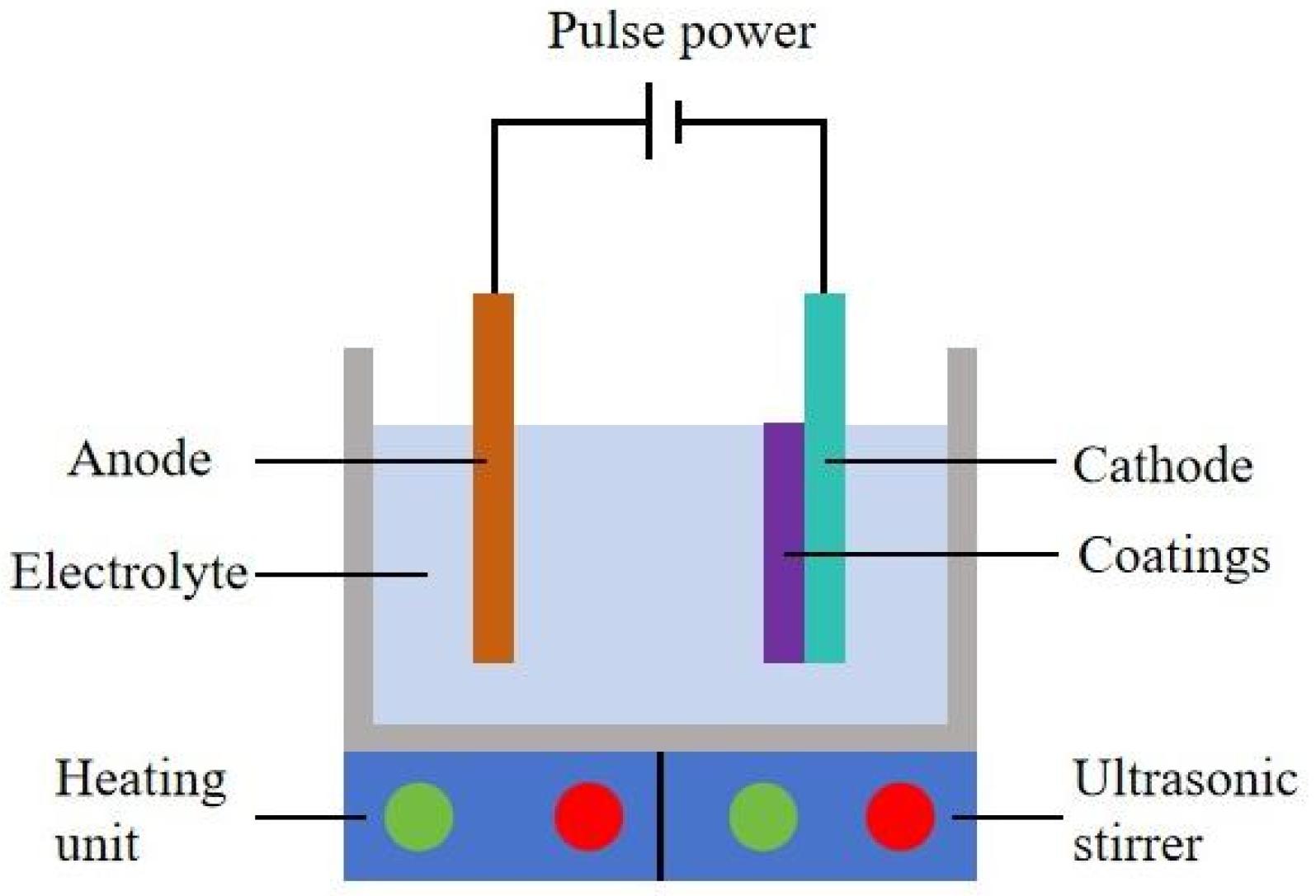
2.1. Preparation
2.2. Characterization
3. Results and Discussion
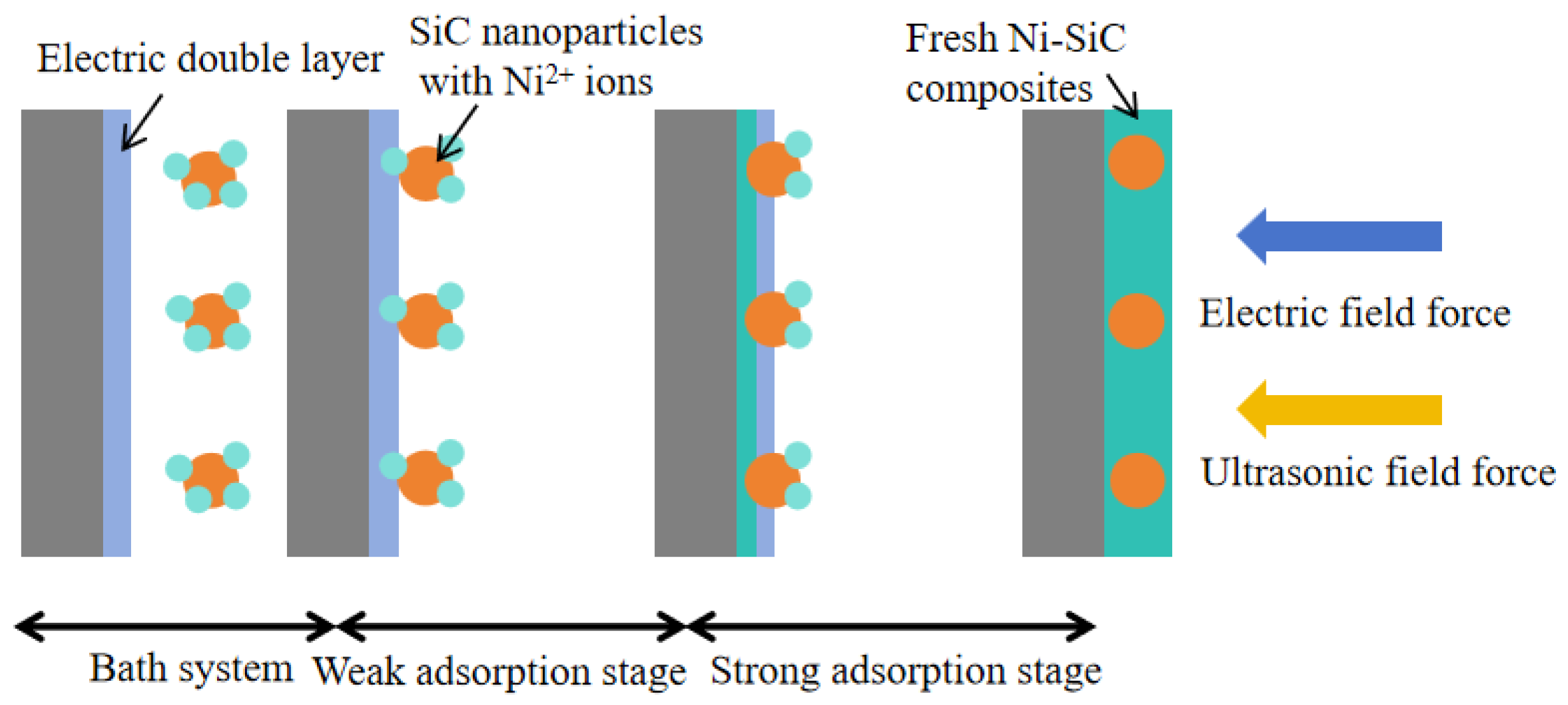
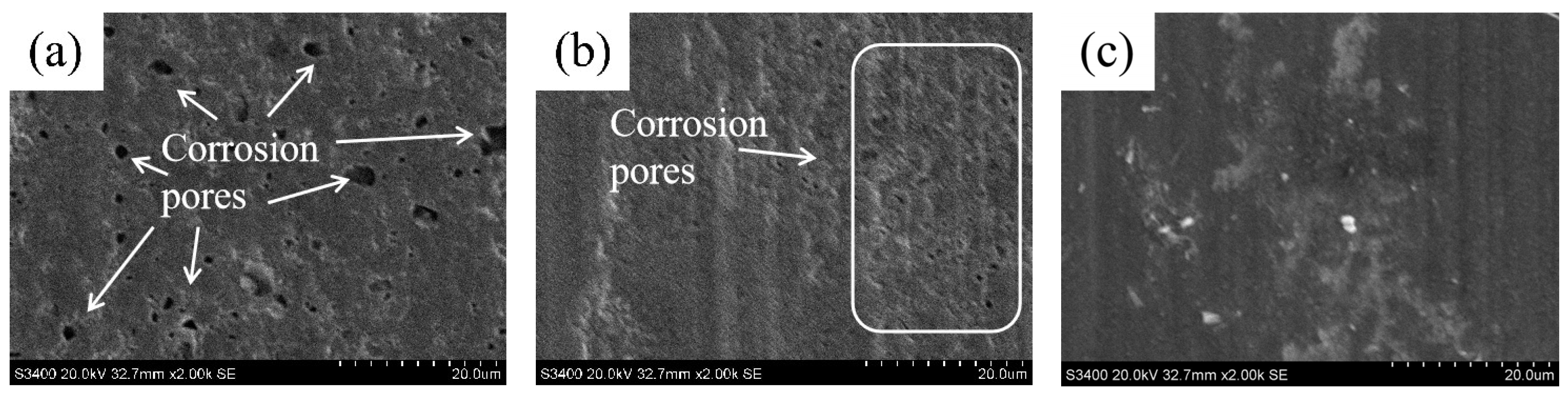
3.1. Surface Morphology Investigation
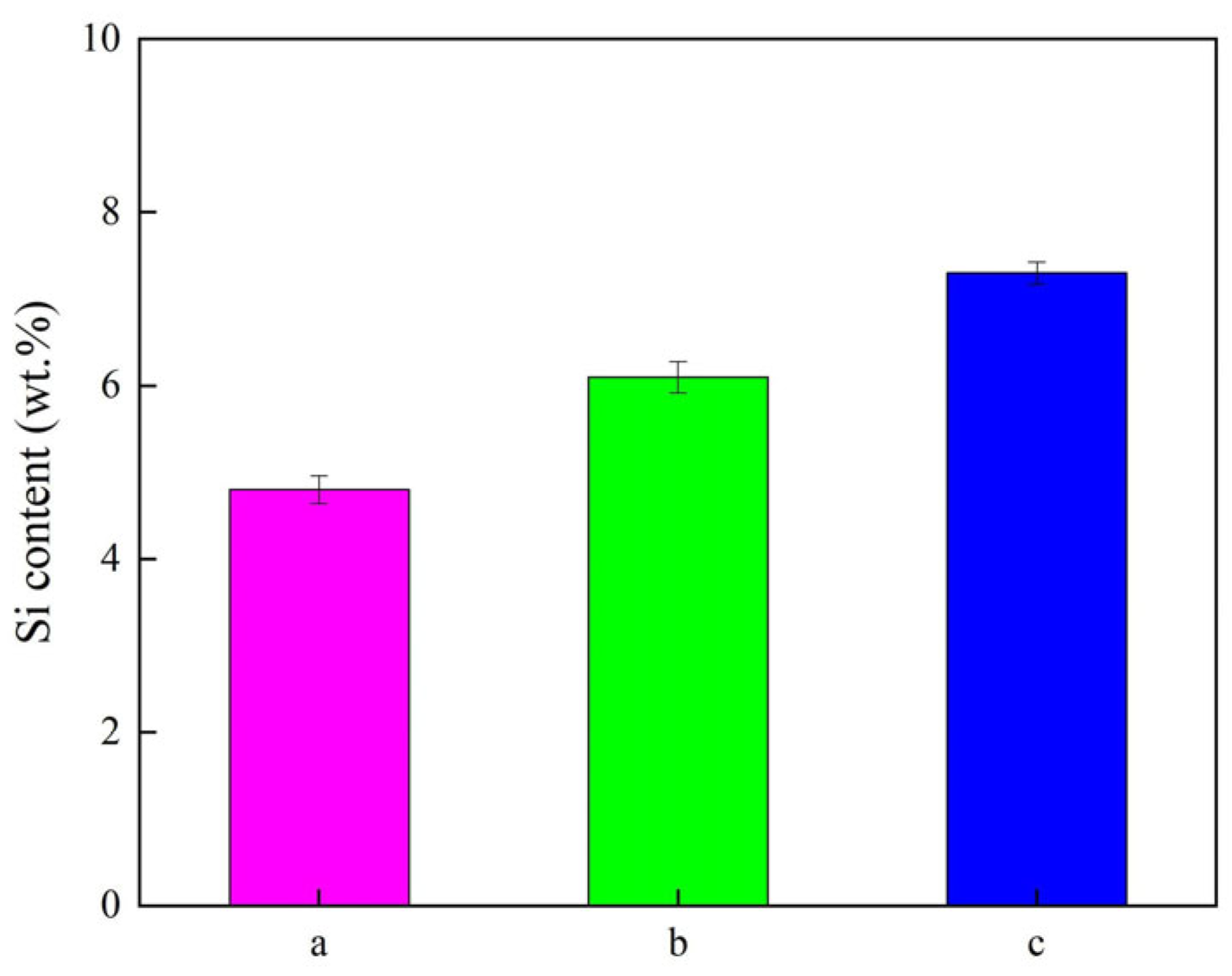
3.2. Element Content Detection
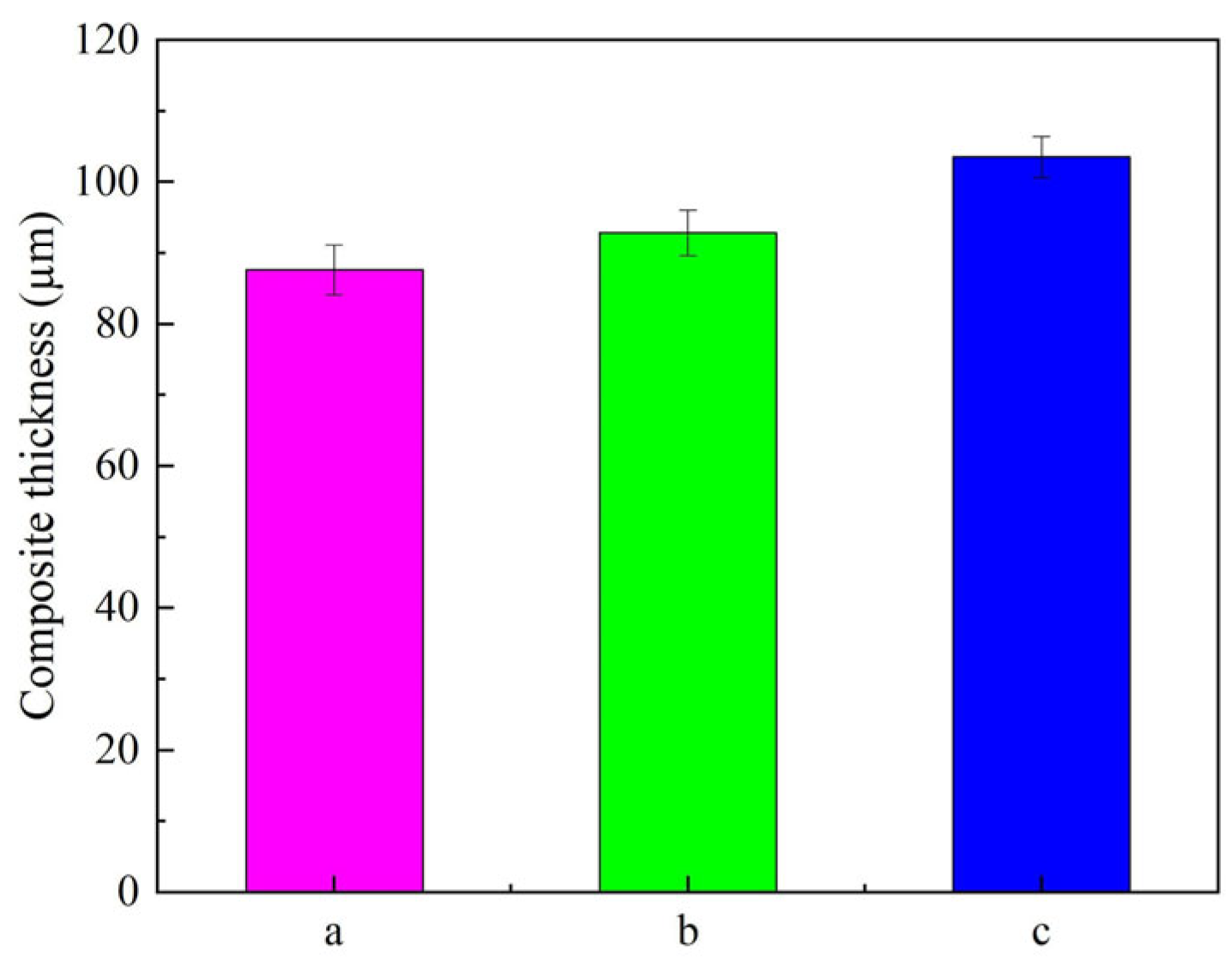
3.3. Composites Thickness Analysis
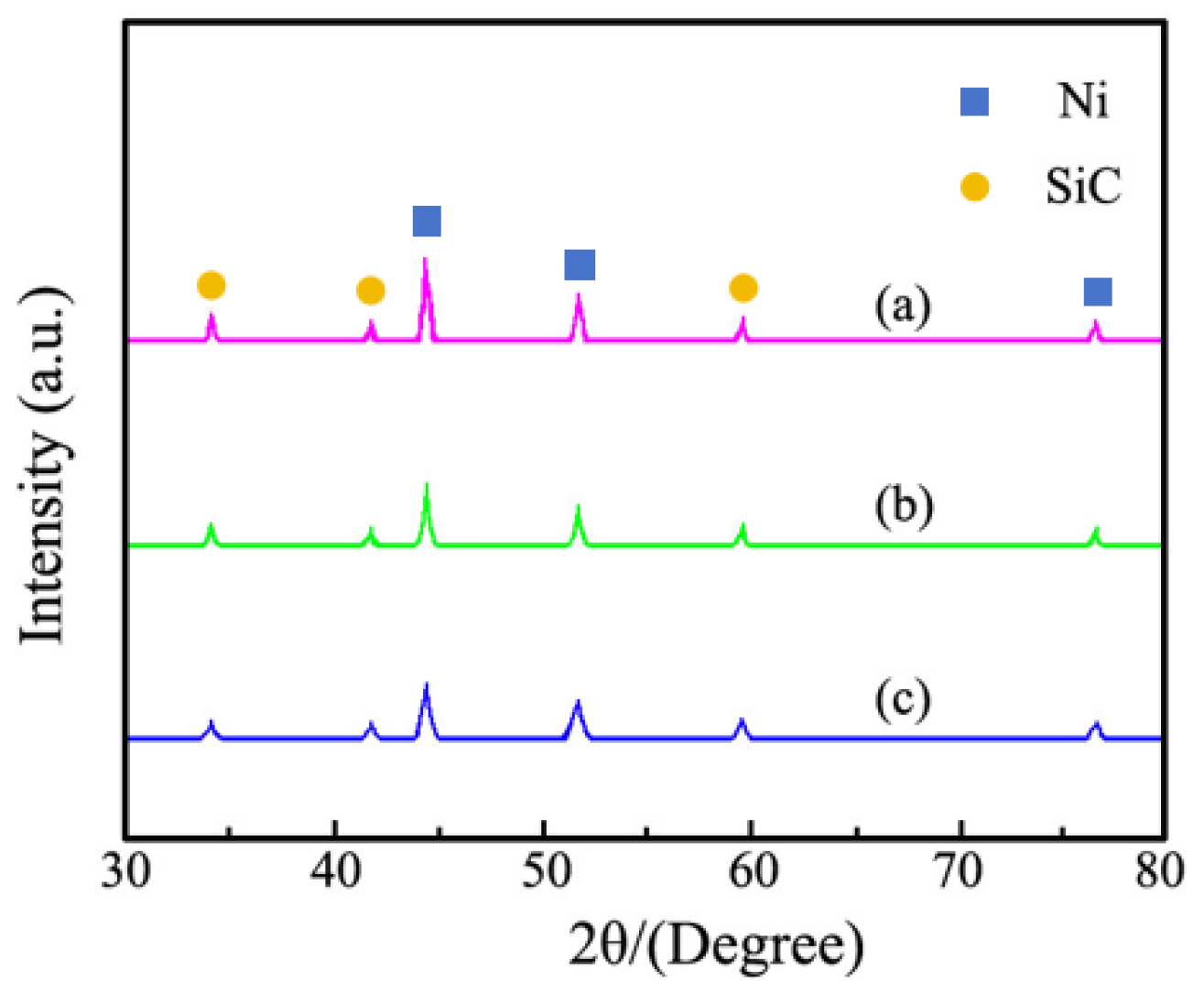
3.4. XRD Pattern Examination
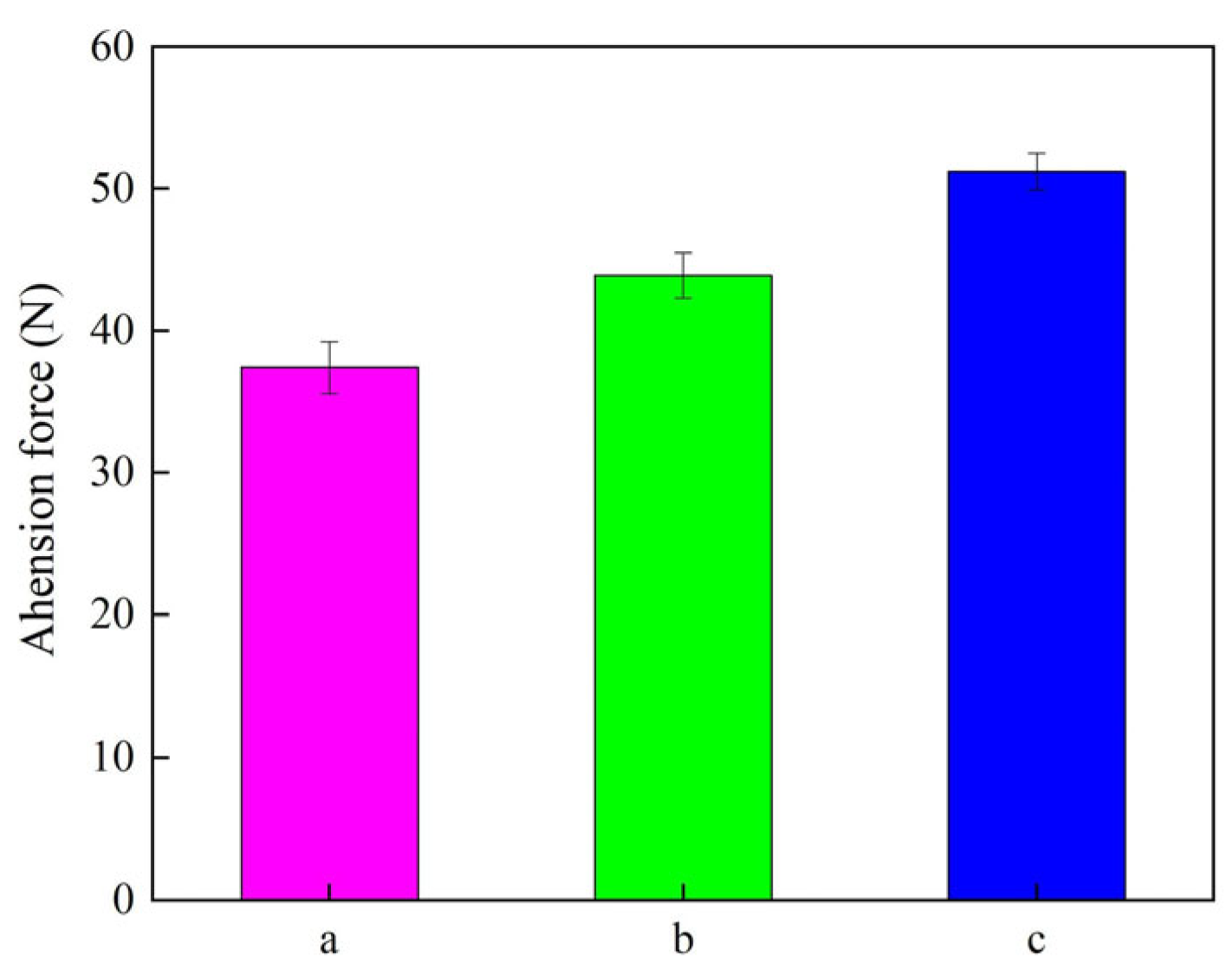
3.5. Adhesion Force Detection
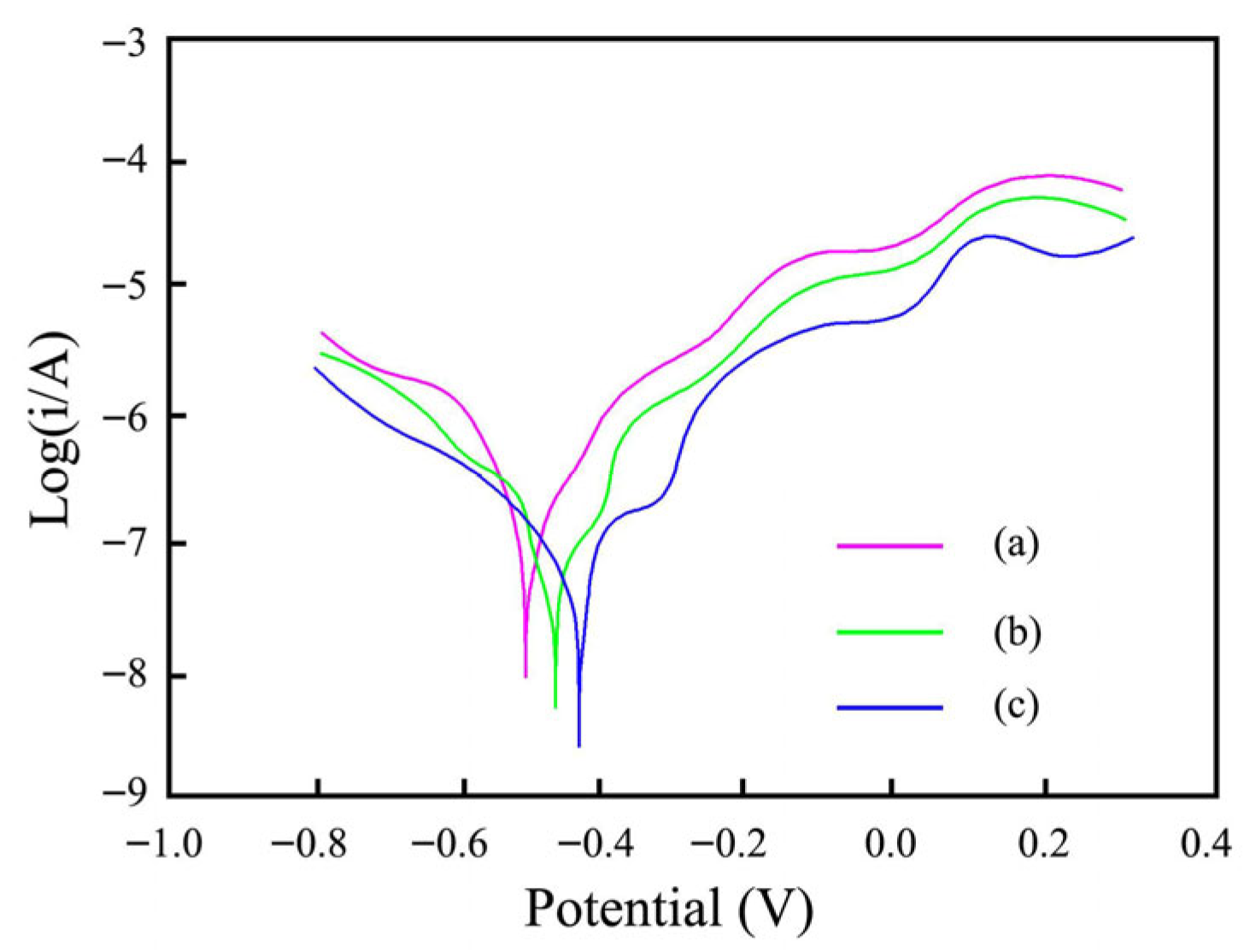
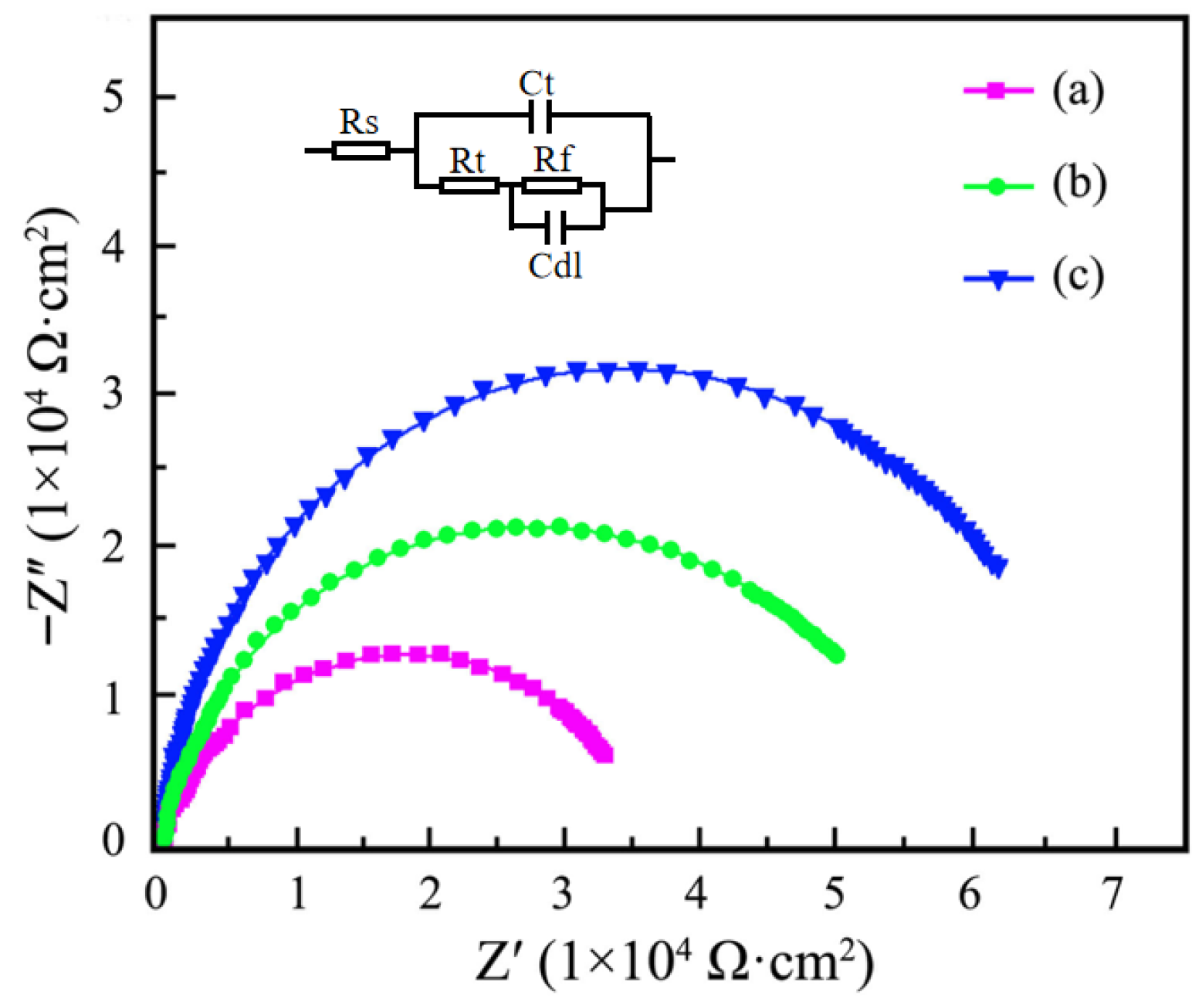
3.6. Anti-Corrosion Performance Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ulloa-Rojas, J.; Colombo, J.; Wilches, J.; Almazan, J. Influence of anchoring on the seismic fragility of buckling in liquid storage tanks considering soil-tank interaction: A case study for wine storage tanks. Dyna 2024, 99, 1–7. [Google Scholar] [CrossRef]
- Liu, F.P.; Ma, J.D.; Ye, Z.Z.; Wang, L.J.; Sun, Y.; Yu, J.X.; Qin, Y.L.; Zhang, D.L.; Cai, W.A.; Li, H. Cross-Scale Reliability Analysis Framework for LNG Storage Tanks Considering Concrete Material Uncertainty. J. Mar. Sci. Eng. 2024, 12, 276. [Google Scholar] [CrossRef]
- Cui, L.F.; Zhu, L.J.; Lyu, Y.; Sun, J.G.; Wu, Y.J. Effect of Flexible Tank Wall on Seismic Response of Horizontal Storage Tank. Processes 2024, 12, 1633. [Google Scholar] [CrossRef]
- Shin, H.K.; Ha, S.K. A Review on the Cost Analysis of Hydrogen Gas Storage Tanks for Fuel Cell Vehicles. Energies 2023, 16, 5233. [Google Scholar] [CrossRef]
- Qin, D.; Ping, Y.; Xu, G.; Deng, J. Mechanisms and modeling of low cycle fatigue crack propagation in a pressure vessel steel Q345. Int. J. Fatigue 2016, 89, 2–10. [Google Scholar] [CrossRef]
- Kan, H.M.; Feng, X.J.; Wei, X.D.; Zhang, N.; Wang, X.Y.; Long, H.B. Effects of Surfactants SDS and CTAB on Ni-SiC Deposition. J. Wuhan Univ. Technol. 2018, 33, 836–842. [Google Scholar] [CrossRef]
- Liu, H.Z.; Wang, Z.L.; Wang, Y.K.; Li, H.C. Self-induced electrical discharge machining of Ni-Al2O3 functionally graded materials. Int. J. Adv. Manuf. Tech. 2016, 83, 587–594. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, D.; Ma, Z.G.; Deng, Z.J.; Li, Z.; Drummer, D.; Jiang, B.Y. Pulse current electroforming of Ni-PTFE nanocomposite mold insert with long-lifetime and anti-adhesive properties. Electrochim. Acta 2024, 491, 144308. [Google Scholar] [CrossRef]
- Renner, P.; Raut, A.; Liang, H. High-Performance Ni-SiC Coatings Fabricated by Flash Heating. Lubricants 2022, 10, 42. [Google Scholar] [CrossRef]
- Huang, P.C.; Hou, K.H.; Hong, J.J.; Liu, M.H.; Wang, G.L. Study of fabrication and wear properties of Ni-SiC composite coatings on A356 aluminum alloy. Wear 2021, 477, 203772. [Google Scholar] [CrossRef]
- Yu, X.L.; Jiang, K.D.; Zhu, Z.G. Influence of Jet Rate on the Structures and Corrosion Resistances of Jet Pulse Electrodeposited Ni-SiC Nanocoatings. J. Mater. Eng. Perform. 2025, 34, 16523–16534. [Google Scholar] [CrossRef]
- Renner, P.; Liang, H. Optimization of the corrosion performance of Ni-SiC coatings through flash heating. Surf. Coat. Technol. 2022, 447, 128848. [Google Scholar] [CrossRef]
- Wang, S.Q.; Xie, F.Q.; Wu, X.Q. Effect of SiC Contents on Wear Resistance Performance of Electro-Codeposited Ni-SiC Composite Coatings. Coatings 2024, 14, 1224. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, F.Q.; Wu, X.Q.; Zhao, W.D.; Chen, X. A novel plating apparatus for electrodeposition of Ni-SiC composite coatings using circulating-solution co-deposition technique. J. Alloys Compd. 2017, 699, 366–377. [Google Scholar] [CrossRef]
- Xu, B.K.; He, Q.C.; Wang, Y.Q.; Yin, X.M. Hollow porous Ni@SiC nanospheres for enhancing electromagnetic wave absorption. Ceram. Int. 2023, 49, 21335–21345. [Google Scholar] [CrossRef]
- Liu, T.X.; Li, H.X.; Xiao, Z.M. Microstructures and performances of Ni-SiC coatings manufactured by laser cladding deposition. Int. J. Electrochem. Sci. 2023, 18, 100030. [Google Scholar] [CrossRef]
- Owoseni, T.A.; Baiamonte, L.; Dumm, T.; Bjorklund, S.; Joshi, S. Microstructural characteristics and wear behaviour of HVAF sprayed Ni-SiC composite coatings. Ceram. Int. 2025, 51, 24281–24296. [Google Scholar] [CrossRef]
- Liu, J.G.; Fang, X.T.; Zhu, C.Y.; Xing, X.; Cui, G.; Li, Z.L. Fabrication of superhydrophobic coatings for corrosion protection by electrodeposition: A comprehensive review. Colloid. Surf. A 2020, 607, 125498. [Google Scholar] [CrossRef]
- Torabinejad, V.; Aliofkhazraei, M.; Assareh, S.; Allahyarzadeh, M.H.; Rouhaghdam, A.S. Electrodeposition of Ni-Fe alloys, composites, and nano coatings-A review. J. Alloys Compd. 2017, 691, 841–859. [Google Scholar] [CrossRef]
- Prado, L.H.; Virtanen, S. Cu-MoS2 Superhydrophobic Coating by Composite Electrodeposition. Coatings 2020, 10, 238. [Google Scholar] [CrossRef]
- Cao, M.Y.; Yue, Y.H.; Guo, X.; Wang, B.J. Effects of SiC Particle Concentration on the Ultrasonic-Assisted Jet Electrodeposited Ni-SiC Nanocoatings. Int. J. Electrochem. Sci. 2022, 17, 220113. [Google Scholar] [CrossRef]
- Loganathan, A.; Kamdi, Z.; Ainuddin, A.R.; Husin, R.; Ibrahim, A.; Salleh, M.A.A.M. Microstructure Characterisation, Hardness, and Corrosion Behaviour of Nickel-Silicon Carbide Electrodeposition Coating with the Influence of Ultrasonic Agitation. Arch. Metall. Mater. 2024, 69, 859–863. [Google Scholar] [CrossRef]
- Zhang, W.W.; Mei, T.Y.; Li, B.; Yang, L.; Du, S.S.; Miao, Y.C.; Chu, H.Q. Effect of current density and agitation modes on the structural and corrosion behavior of Ni/diamond composite coatings. J. Mater. Res. Technol. 2021, 12, 1473–1485. [Google Scholar] [CrossRef]
- He, T.; He, Y.; Li, H.; Fan, Y.; Yang, Q.B.; He, Z. A comparative study of effect of mechanical and ultrasound agitation on the properties of pulse electrodeposited Ni-W/MWCNTs composite coatings. J. Alloys Compd. 2018, 743, 63–72. [Google Scholar] [CrossRef]
- Chen, Y.L.; Li, H.G.; Zhu, J.C.; Fang, C.; Li, Z.Q.; Jiang, W. Fabrication of Ni-TiN nanocomposite coatings by ultrasonic assisted jet electrodeposition. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2024, 238, 1507–1518. [Google Scholar] [CrossRef]
- Li, B.S.; Mei, T.Y.; Chu, H.Q.; Wang, J.J.; Du, S.S.; Miao, Y.C.; Zhang, W.W. Ultrasonic-assisted electrodeposition of Ni/diamond composite coatings and its structure and electrochemical properties. Ultrason. Sonochem. 2021, 73, 105475. [Google Scholar] [CrossRef]
- Wang, H.; Liu, H.J.; He, Y.; Ma, C.Y.; Li, L.Z. Ni-SiC Composite Coatings with Good Wear and Corrosion Resistance Synthesized Via Ultrasonic Electrodeposition. J. Mater. Eng. Perform. 2021, 30, 1535–1544. [Google Scholar] [CrossRef]
- Zhang, H.B.; Wang, J.D.; Chen, S.X.; Wang, H.; He, Y.; Ma, C.Y. Ni-SiC composite coatings with improved wear and corrosion resistance synthesized via ultrasonic electrodeposition. Ceram. Int. 2021, 47, 9437–9446. [Google Scholar] [CrossRef]
- Cheng, Q.; Yao, Z.J.; Zhang, F.; Zhang, S.S.; Oleksander, M. Microstructure and tribological property of Ni-MoS2 composite coatings prepared by ultrasonic and mechanical stirring electrodeposition. Mater. Res. Experss 2019, 6, 126434. [Google Scholar] [CrossRef]
- Li, B.S.; Zhang, W.W. Facile synthesis and electrochemical properties of a novel Ni-B/TiC composite coating via ultrasonic-assisted electrodeposition. Ultrason. Sonochem. 2020, 61, 104837. [Google Scholar] [CrossRef]
- Li, H.G.; Chen, Y.L.; Zhu, F.K.; Fang, C.; Li, J.J.; Jiang, W. Fabrication and characterization of Ni-Co-TiN/CeO2 composite coating by ultrasonic vibration-assisted jet electrodeposition. Colloid. Surf. A 2024, 687, 133526. [Google Scholar] [CrossRef]
- Kharitonov, D.S.; Kasach, A.A.; Sergievich, D.S.; Wrzesinska, A.; Bobowska, I.; Darowicki, K.; Zielinski, A.; Ryl, J.; Kurilo, I.I. Ultrasonic-assisted electrodeposition of Cu-Sn-TiO2 nanocomposite coatings with enhanced antibacterial activity. Ultrason. Sonochem. 2021, 75, 105593. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Fang, C.; Li, H.G.; Li, Z.Q.; He, G.Q.; Jiang, W. Overall performance of Ni-Co-WC nanocomposite coatings prepared by ultrasonic vibration-assisted scanning jet electrodeposition. Surf. Coat. Tech. 2024, 477, 13062. [Google Scholar] [CrossRef]
- Yang, Z.G.; Yi, S.J.; Wang, Y.; Zhao, S.X.; Shi, W. Study on Characteristics and Microstructure of Ni-AlN Thin Coatings Prepared via Different Electrodeposition Techniques. Int. J. Electrochem. Sci. 2022, 17, 220226. [Google Scholar] [CrossRef]
- Pinate, S.; Eriksson, F.; Leisner, P.; Zanella, C. Effects of SiC particles codeposition and ultrasound agitation on the electrocrystallisation of nickel-based composite coatings. J. Mater. Sci. 2021, 56, 18463–18476. [Google Scholar] [CrossRef]
- Xia, F.F.; Yan, P.; Ma, C.Y.; Wang, B.J.; Liu, Y. Effect of different heat-treated temperatures upon structural and abrasive performance of Ni-TiN composite nanocoatings. J. Mater. Res. Technol. 2023, 27, 2874–2881. [Google Scholar] [CrossRef]
- Cao, M.Y.; Tian, D.H.; Guo, X.; Li, W. Optimization of Plating Parameters and Properties of Ultrasonic-Assisted Jet-Electrodeposited Ni-W-Al2O3 Nanocomposite Coatings. Int. J. Mol. Sci. 2025, 26, 2404. [Google Scholar] [CrossRef]
- Ji, R.J.; Han, K.; Jin, H.; Li, X.P.; Liu, Y.H.; Liu, S.G.; Dong, T.C.; Cai, B.P.; Cheng, W.H. Preparation of Ni-SiC nano-composite coating by rotating magnetic field-assisted electrodeposition. J. Manuf. Process 2020, 57, 787–797. [Google Scholar] [CrossRef]
- Li, C.Y.; Xia, F.F.; Yao, L.M.; Li, H.X.; Jia, X. Investigation of the mechanical properties and corrosion behaviors of Ni-BN-TiC layers constructed via laser cladding technique. Ceram. Int. 2023, 49, 6671–6677. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Shen, Z.Y.; Wu, H.B.; Li, L.Q.; Fu, X.Q. Study on Preparation of Superhydrophobic Ni-Co Coating and Corrosion Resistance by Sandblasting-Electrodeposition. Coatings 2020, 10, 1164. [Google Scholar] [CrossRef]
- Li, C.Y.; Xia, F.F.; Ma, C.Y.; Li, Q. Research on the Corrosion Behavior of Ni-SiC Nanocoating Prepared Using a Jet Electrodeposition Technique. J. Mater. Eng. Perform. 2021, 30, 6336–6344. [Google Scholar] [CrossRef]
- Nasri, K.; Ahmed, N.A.; Issaadi, H.K.; Hamzaoui, A.H.; Aliouane, N.; Djermoune, A.; Chassigneux, C.; Eyraud, M. Electrodeposition of hydrophobic Ni-Co-TiO2 nanocomposite coatings on XC70 mild steels for improving corrosion resistance. J. Indian. Chem. Soc. 2025, 102, 101544. [Google Scholar] [CrossRef]
- Safavi, M.S.; Rasooli, A.; Sorkhabi, F.A. Electrodeposition of Ni-P/Ni-Co-Al2O3 duplex nanocomposite coatings: Towards improved mechanical and corrosion properties. Trans. IMF 2020, 98, 320–327. [Google Scholar] [CrossRef]
- Pang, S.K.; Jiang, K.D.; Zhu, Y.W. Simulation and properties of jet-electrodeposited Ni-W-SiC coatings. J. Nanoparticle Res. 2025, 27, 203. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Wang, L.B.; Qin, Z.B.; Wang, C.X.; Xu, Z.; Jiang, C.H.; Ji, V. The Roles of Ti Particles in Improving the Corrosion Resistance of Electrochemically Assembled Ni-Ti Composite Coatings. Corrosion 2017, 73, 1107–1118. [Google Scholar] [CrossRef]


| Operation Parameters | Specific |
|---|---|
| Current density (A/dm2) | 5 |
| Plating temperature (°C) | 48 |
| Electrodeposition time (min) | 50 |
| NiSO4 (g/L) | 170 |
| NiCl2 (g/L) | 25 |
| pH value | 4.7 |
| SiC concentration | 5 |
| CTAB (mg/L) | 50 |
| H3BO3 (g/L) | 40 |
| Ni–SiC Composites | Average Corrosive Weight Loss | Corrosion Rate (mg·h−1) |
|---|---|---|
| Prepared at 0 W | 7.9 mg | 0.11 |
| Prepared at 60 W | 6.3 mg | 0.09 |
| Prepared at 120 W | 4.2 mg | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiang, L.; Luo, L.; Cao, M.; Guo, X.; Li, C.; Gao, H. Preparation and Anti-Corrosion Performance Investigation of Ni–SiC Composites Produced at Different Ultrasonic Powers. Materials 2025, 18, 5177. https://doi.org/10.3390/ma18225177
Qiang L, Luo L, Cao M, Guo X, Li C, Gao H. Preparation and Anti-Corrosion Performance Investigation of Ni–SiC Composites Produced at Different Ultrasonic Powers. Materials. 2025; 18(22):5177. https://doi.org/10.3390/ma18225177
Chicago/Turabian StyleQiang, Lei, Limei Luo, Mengyu Cao, Xue Guo, Chaoyu Li, and Hao Gao. 2025. "Preparation and Anti-Corrosion Performance Investigation of Ni–SiC Composites Produced at Different Ultrasonic Powers" Materials 18, no. 22: 5177. https://doi.org/10.3390/ma18225177
APA StyleQiang, L., Luo, L., Cao, M., Guo, X., Li, C., & Gao, H. (2025). Preparation and Anti-Corrosion Performance Investigation of Ni–SiC Composites Produced at Different Ultrasonic Powers. Materials, 18(22), 5177. https://doi.org/10.3390/ma18225177






