Adsorptive Removal of Arsenite and Cobalt by Commercial Sorbents
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Metal Quantification and Data Analysis
3. Results
3.1. Characterization of Sorbents
3.2. Sorption Kinetics
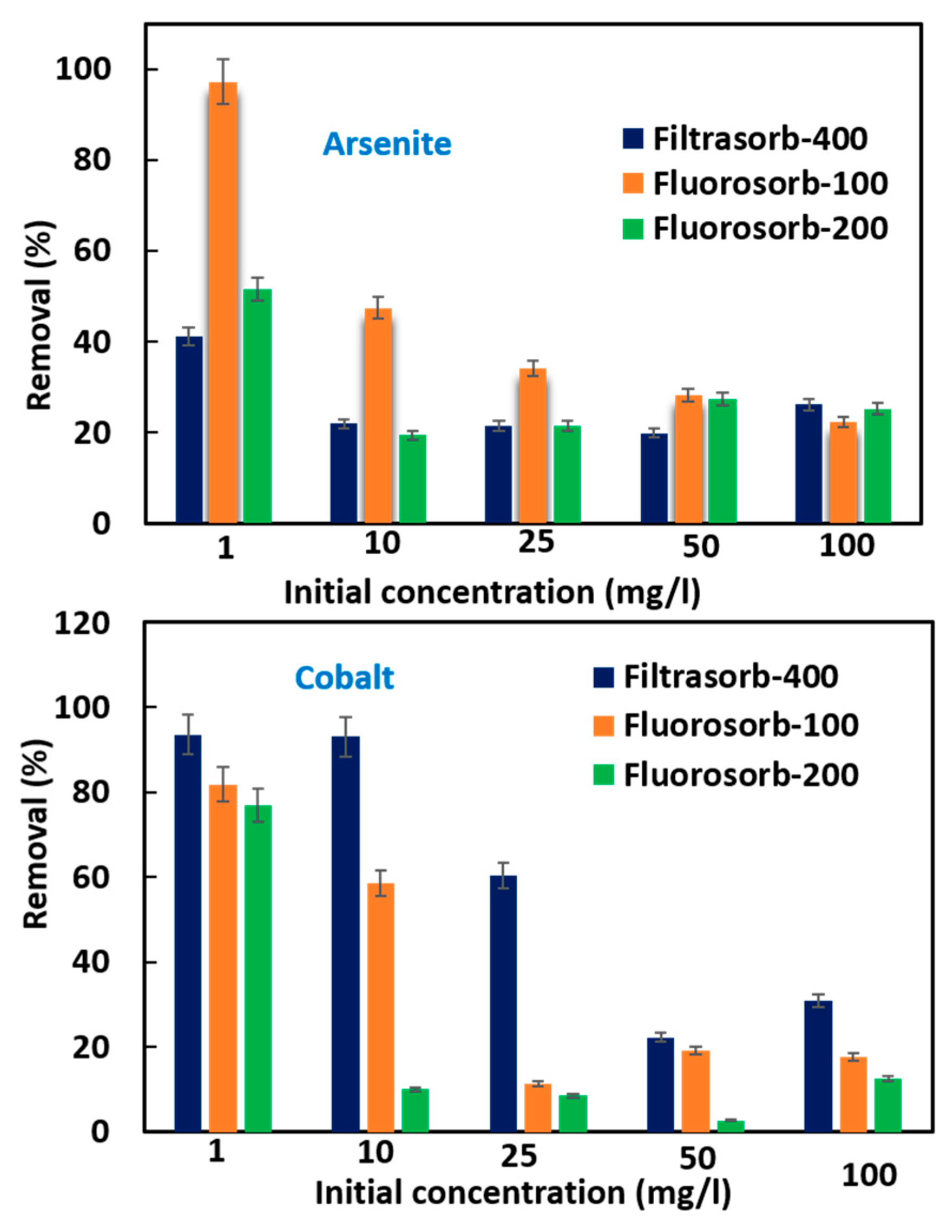
3.3. Effect of Contaminant Concentration on Removal Efficiency
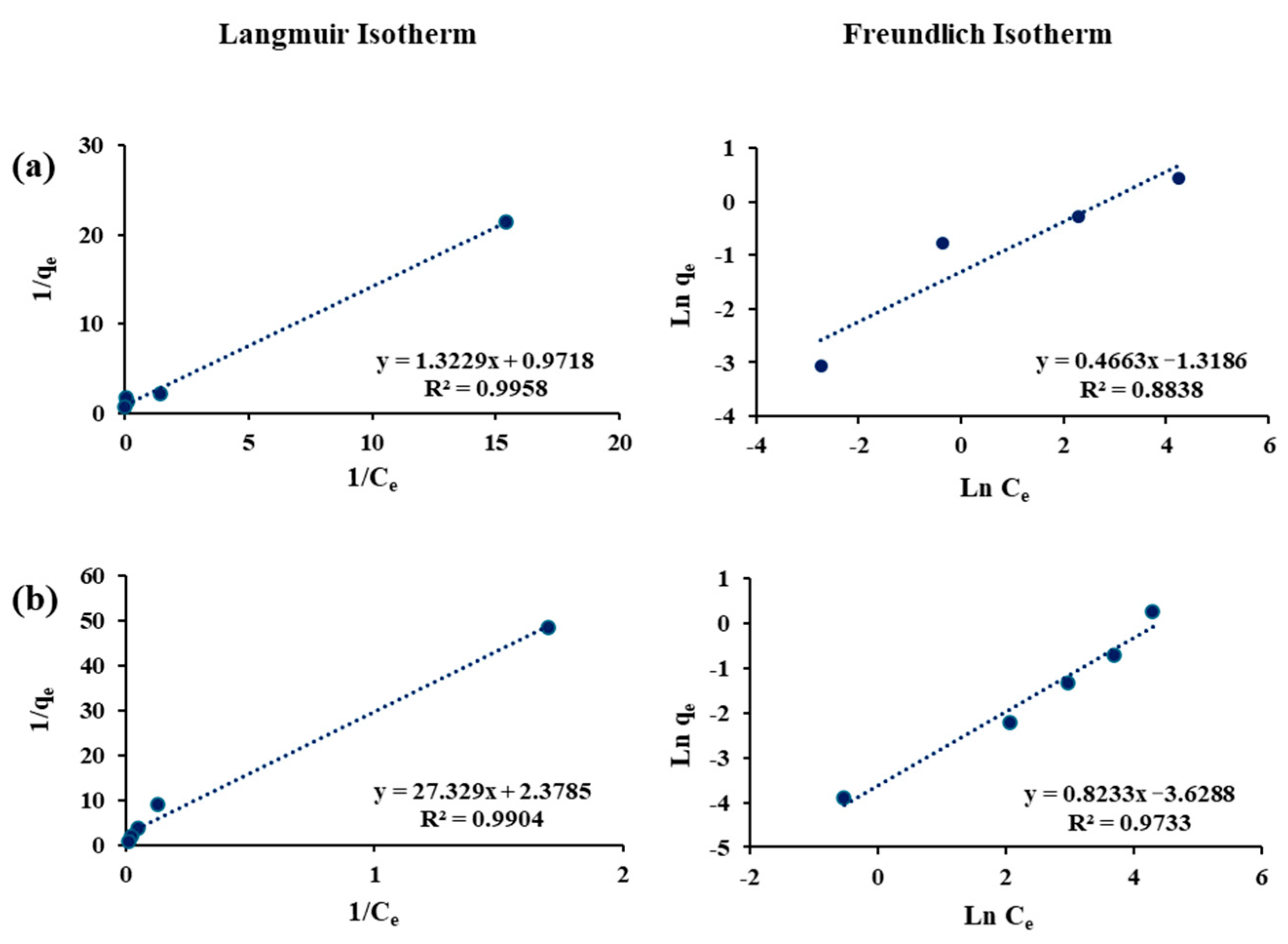
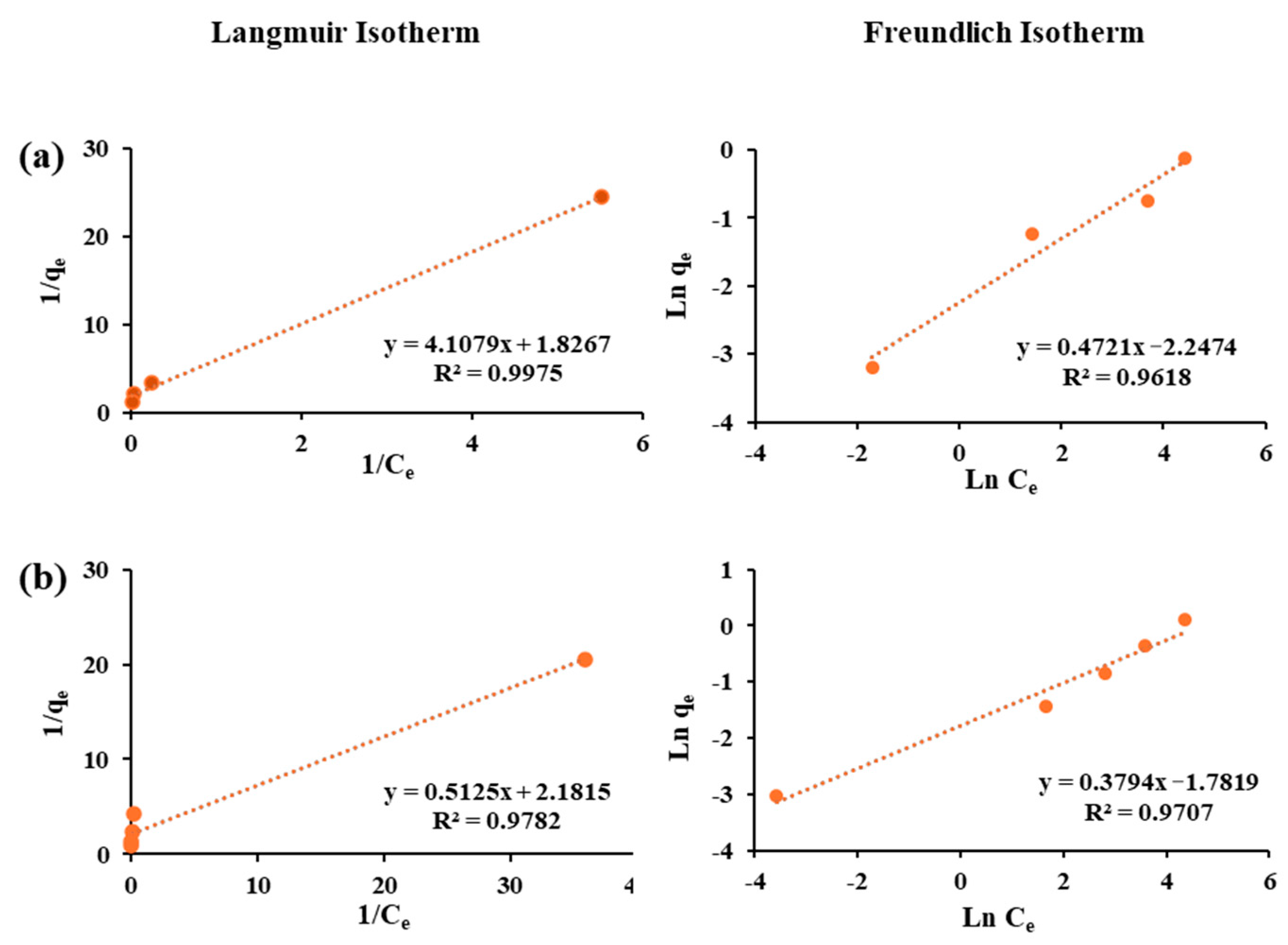
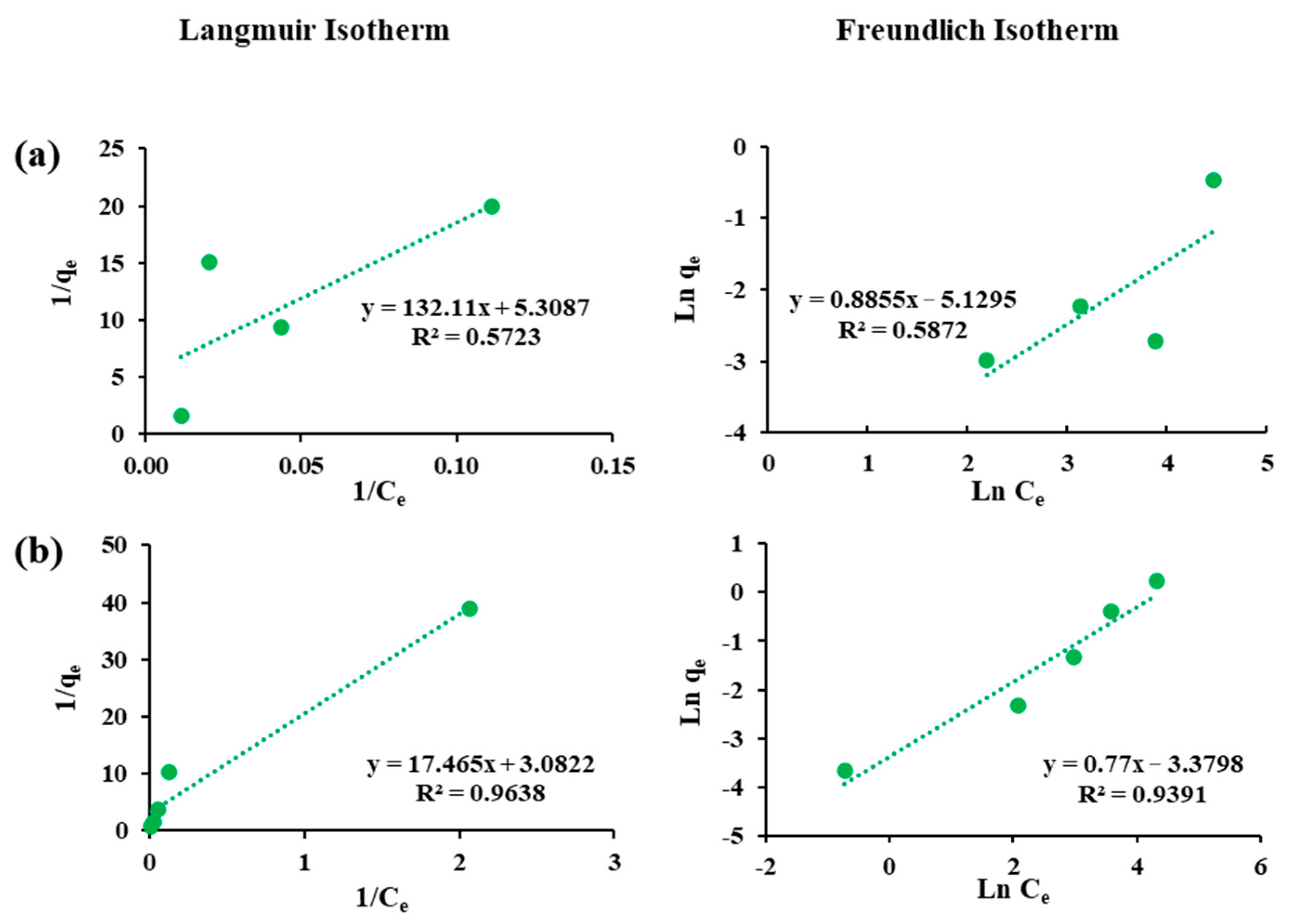
3.4. Adsorption Isotherms
3.5. Desorption Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FS-200 | Fluorosorb-200 |
| FS-100 | Fluorosorb-100 |
| F-400 | Filtrasorb-400 |
| PRB | Permeable reactive barrier |
| BET | Brunauer–Emmett–Teller |
| FE-SEM | Field-emission Scanning Electron Microscopy |
| XRD | X-ray Diffraction |
| BJH | Barrett–Joyner–Halenda |
| PFO | Pseudo-first-order |
| PSO | Pseudo-second-order |
| As(III) | Arsenite |
| Co(II) | Cobalt |
| EDX | Energy-dispersive X-ray spectroscopy |
| GAC | Granular activated carbon |
| ICP-OES | inductively coupled plasma–optical emission spectroscopy |
References
- Sharma, M.; Kant, R.; Sharma, A.K.; Sharma, A.K. Exploring the Impact of Heavy Metals Toxicity in the Aquatic Ecosystem. Int. J. Energy Water Resour. 2025, 9, 267–280. [Google Scholar] [CrossRef]
- Tavallali, A.; Mousavi, S.M.; Rabbee, M.F.; Lai, C.W.; Rahman, M.M.; Chiang, W.-H. Ionic Liquids-Based Technologies as a Sustainable Agent for Removing Heavy Metals and Organic Pollutants for Water Purification: A Review. J. Water Process Eng. 2025, 71, 107367. [Google Scholar] [CrossRef]
- Tavallali, A.; Mousavi, S.M.; Hashemi, S.A.; Lai, C.W.; Chiang, W.-H.; Bahrani, S. Ionic Liquid–Supported Nanoparticles for Gas-Sensing Applications. In Ionic Liquid-Based Technologies for Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2022; pp. 331–345. [Google Scholar]
- Abad, S.S.A.M.K.; Javidan, P.; Baghdadi, M.; Mehrdadi, N. Green Synthesis of Pd@ Biochar Using the Extract and Biochar of Corn-Husk Wastes for Electrochemical Cr (VI) Reduction in Plating Wastewater. J. Env. Chem. Eng. 2023, 11, 109911. [Google Scholar] [CrossRef]
- Diyabalanage, S.; Manthrirathne, M.; Lakmali, M.; Jayasinghe, R.; Werahera, M.; Chandrajith, R. Exposure to Mercury and Toxic Trace Elements in Waste Gold Extraction in Sri Lanka: A Study on an under-Recognised Occupational Health Hazard. Environ. Res. 2025, 285, 122597. [Google Scholar] [CrossRef]
- Sadee, B.A.; Zebari, S.M.S.; Galali, Y.; Saleem, M.F. A Review on Arsenic Contamination in Drinking Water: Sources, Health Impacts, and Remediation Approaches. RSC Adv. 2025, 15, 2684–2703. [Google Scholar] [CrossRef] [PubMed]
- Zehhaf, A.; Benyoucef, A.; Quijada, C.; Taleb, S.; Morallon, E. Algerian Natural Montmorillonites for Arsenic (III) Removal in Aqueous Solution. Int. J. Environ. Sci. Technol. 2015, 12, 595–602. [Google Scholar] [CrossRef]
- Rathi, B.S.; Kumar, P.S. Application of Adsorption Process for Effective Removal of Emerging Contaminants from Water and Wastewater. Environ. Pollut. 2021, 280, 116995. [Google Scholar] [CrossRef]
- Edition, F. Guidelines for Drinking-Water Quality. WHO Chron. 2011, 38, 104–108. [Google Scholar]
- Mosoarca, G.; Vancea, C.; Popa, S.; Boran, S. Adsorption, Bioaccumulation and Kinetics Parameters of the Phytoremediation of Cobalt from Wastewater Using Elodea Canadensis. Bull. Environ. Contam. Toxicol. 2018, 100, 733–739. [Google Scholar] [CrossRef]
- Suhasini, I.P.; Sriram, G.; Asolekar, S.R.; Sureshkumar, G.K. Biosorptive Removal and Recovery of Cobalt from Aqueous Systems. Process Biochem. 1999, 34, 239–247. [Google Scholar] [CrossRef]
- Proctor, N.H.; Hughes, J.P.; Hathaway, G.J. Proctor and Hughes’ Chemical Hazards of the Workplace; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Benalia, M.C.; Youcef, L.; Bouaziz, M.G.; Achour, S.; Menasra, H. Removal of Heavy Metals from Industrial Wastewater by Chemical Precipitation: Mechanisms and Sludge Characterization. Arab. J. Sci. Eng. 2022, 47, 5587–5599. [Google Scholar] [CrossRef]
- Dabrowski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective Removal of the Heavy Metal Ions from Waters and Industrial Wastewaters by Ion-Exchange Method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Luo, C.; Tian, T.; Feng, R.; Li, A.-K.; Sun, S.-P.; Cao, X.-L. Comparative Evaluation of Acid-Resistant Nanofiltration Membranes for Heavy Metal Removal in Acidic Wastewater. Desalination 2024, 576, 117349. [Google Scholar] [CrossRef]
- Javidan, P.; Baghdadi, M.; Torabian, A.; Goharrizi, B.A. A Tailored Metal–Organic Framework Applicable at Natural PH for the Removal of 17α-Ethinylestradiol from Surface Water. Desalination Water Treat. 2022, 264, 259–269. [Google Scholar] [CrossRef]
- Satyam, S.; Patra, S. Innovations and Challenges in Adsorption-Based Wastewater Remediation: A Comprehensive Review. Heliyon 2024, 10, e29573. [Google Scholar] [CrossRef]
- Sakr, M.; El Agamawi, H.; Klammler, H.; Mohamed, M.M. A Review on the Use of Permeable Reactive Barriers as an Effective Technique for Groundwater Remediation. Groundw. Sustain. Dev. 2023, 21, 100914. [Google Scholar] [CrossRef]
- Naidu, R.; Bekele, D.N.; Birke, V. Permeable Reactive Barriers: Cost-Effective and Sustainable Remediation of Groundwater. In Permeable Reactive Barrier: Sustainable Groundwater Remediation; CRC Press: Boca Raton, FL, USA, 2014; pp. 1–23. [Google Scholar]
- Alkhaldi, H.; Alharthi, S.; Alharthi, S.; AlGhamdi, H.A.; AlZahrani, Y.M.; Mahmoud, S.A.; Amin, L.G.; Al-Shaalan, N.H.; Boraie, W.E.; Attia, M.S. Sustainable Polymeric Adsorbents for Adsorption-Based Water Remediation and Pathogen Deactivation: A Review. RSC Adv. 2024, 14, 33143–33190. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Liu, J. Surface Modification of Coconut Shell Based Activated Carbon for the Improvement of Hydrophobic VOC Removal. J. Hazard. Mater. 2011, 192, 683–690. [Google Scholar] [CrossRef]
- Mehrdad, A.; Samadiani, N.; Poormoosa, L. Effect of Temperature and Hydrochloric Acid on the Intrinsic Viscosity of Poly (Acrylic Acid) in Aqueous Solutions. J. Mol. Liq. 2013, 187, 177–182. [Google Scholar] [CrossRef]
- Pasciucco, E.; Pasciucco, F.; Castagnoli, A.; Iannelli, R.; Pecorini, I. Removal of Heavy Metals from Dredging Marine Sediments via Electrokinetic Hexagonal System: A Pilot Study in Italy. Heliyon 2024, 10, e27616. [Google Scholar] [CrossRef]
- Asere, T.G.; Stevens, C.V.; Du Laing, G. Use of (Modified) Natural Adsorbents for Arsenic Remediation: A Review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Joudiazar, S.; Satpathy, A.; Fernando, E.; Rahmati, R.; Kim, J.; de Falco, G.; Datta, R.; Sarkar, D. Removal of Per- and Polyfluoroalkyl Substances Using Commercially Available Sorbents. Materials 2025, 18, 1299. [Google Scholar] [CrossRef]
- Zeng, C.; Atkinson, A.; Sharma, N.; Ashani, H.; Hjelmstad, A.; Venkatesh, K.; Westerhoff, P. Removing Per- and Polyfluoroalkyl Substances from Groundwaters Using Activated Carbon and Ion Exchange Resin Packed Columns. AWWA Water Sci. 2020, 2, e1172. [Google Scholar] [CrossRef]
- Yin, C.Y.; Aroua, M.K.; Daud, W.M.A.W. Review of Modifications of Activated Carbon for Enhancing Contaminant Uptakes from Aqueous Solutions. Sep. Purif. Technol. 2007, 52, 403–415. [Google Scholar] [CrossRef]
- Ghosh, S.; Igwegbe, C.A.; Malloum, A.; Elmakki, M.A.E.; Onyeaka, H.; Fahmy, A.H.; Aquatar, M.O.; Ahmadi, S.; Alameri, B.M.; Ghosh, S. Sustainable Technologies for Removal of Arsenic from Water and Wastewater: A Comprehensive Review. J. Mol. Liq. 2025, 427, 127412. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; van Hullebusch, E.D.; Amrane, A.; Rtimi, S. Mechanisms and Adsorption Capacities of Biochar for the Removal of Organic and Inorganic Pollutants from Industrial Wastewater. Int. J. Environ. Sci. Technol. 2021, 18, 3273–3294. [Google Scholar] [CrossRef]
- Bilici Baskan, M.; Hadimlioglu, S. Removal of Arsenate Using Graphene Oxide-Iron Modified Clinoptilolite-Based Composites: Adsorption Kinetic and Column Study. J. Anal. Sci. Technol. 2021, 12, 22. [Google Scholar] [CrossRef]
- Singh, S.; Naik, T.S.S.K.; Basavaraju, U.; Khan, N.A.; Wani, A.B.; Behera, S.K.; Nath, B.; Bhati, S.; Singh, J.; Ramamurthy, P.C. A Systematic Study of Arsenic Adsorption and Removal from Aqueous Environments Using Novel Graphene Oxide Functionalized UiO-66-NDC Nanocomposites. Sci. Rep. 2022, 12, 15802. [Google Scholar] [CrossRef]
- Zhang, Q.; Hou, Q.; Huang, G.; Fan, Q. Removal of Heavy Metals in Aquatic Environment by Graphene Oxide Composites: A Review. Environ. Sci. Pollut. Res. 2020, 27, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.K. A Review on the Adsorption of Heavy Metals by Clay Minerals, with Special Focus on the Past Decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Crini, G.; Badot, P.-M. Sorption Processes and Pollution: Conventional and Non-Conventional Sorbents for Pollutant Removal from Wastewaters; Presses Univ. Franche-Comté: Besançon Cedex, France, 2010; ISBN 2848673044. [Google Scholar]
- Zhu, R.; Zhu, J.; Ge, F.; Yuan, P. Regeneration of Spent Organoclays after the Sorption of Organic Pollutants: A Review. J. Environ. Manag. 2009, 90, 3212–3216. [Google Scholar] [CrossRef]
- Guégan, R. Organoclay Applications and Limits in the Environment. Comptes Rendus. Chim. 2019, 22, 132–141. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, C.; Yang, L.; Chen, J.P. Cerium Oxide Modified Activated Carbon as an Efficient and Effective Adsorbent for Rapid Uptake of Arsenate and Arsenite: Material Development and Study of Performance and Mechanisms. Chem. Eng. J. 2017, 315, 630–638. [Google Scholar] [CrossRef]
- Kushwaha, R.; Singh, R.S.; Mohan, D. Comparative Study for Sorption of Arsenic on Peanut Shell Biochar and Modified Peanut Shell Biochar. Bioresour. Technol. 2023, 375, 128831. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Kan, A.T.; Chen, W.; Tomson, M.B. PH-Dependent Effect of Zinc on Arsenic Adsorption to Magnetite Nanoparticles. Water Res. 2010, 44, 5693–5701. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Kaddour, S.; Trari, M. Kinetic and Equilibrium Studies of Cobalt Adsorption on Apricot Stone Activated Carbon. J. Ind. Eng. Chem. 2014, 20, 745–751. [Google Scholar] [CrossRef]
- Manohar, D.M.; Noeline, B.F.; Anirudhan, T.S. Adsorption Performance of Al-Pillared Bentonite Clay for the Removal of Cobalt (II) from Aqueous Phase. Appl. Clay Sci. 2006, 31, 194–206. [Google Scholar] [CrossRef]
- Dabbagh, R.; Moghaddam, Z.A.; Ghafourian, H. Removal of Cobalt (II) Ion from Water by Adsorption Using Intact and Modified Ficus Carica Leaves as Low-Cost Natural Sorbent. Desalination Water Treat. 2016, 57, 19890–19902. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Colloid and Capillary Chemistry Translated from the 3rd German Edition by H. Stafford Hatfield; EP Dutton and Company: New York, NY, USA, 1922. [Google Scholar]
- Ayub, A.; Raza, Z.A.; Majeed, M.I.; Tariq, M.R.; Irfan, A. Development of Sustainable Magnetic Chitosan Biosorbent Beads for Kinetic Remediation of Arsenic Contaminated Water. Int. J. Biol. Macromol. 2020, 163, 603–617. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, F.; Zhai, T.; Yu, J.; Wang, C.; Liu, Z.; Liu, Z.; Gao, Y.; Yang, M. Removal of PFOA from Water by Activated Carbon Adsorption: Influence of Pore Structure. J. Environ. Chem. Eng. 2024, 12, 113923. [Google Scholar] [CrossRef]
- Walcarius, A.; Etienne, M.; Lebeau, B. Rate of Access to the Binding Sites in Organically Modified Silicates. 2. Ordered Mesoporous Silicas Grafted with Amine or Thiol Groups. Chem. Mater. 2003, 15, 2161–2173. [Google Scholar] [CrossRef]
- Mandal, B.K.; Suzuki, K.T. Arsenic Round the World: A Review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Gibb, H.J.; Howe, P. Cobalt and Inorganic Cobalt Compounds; World Health Organization: Geneva, Switzerland, 2006; ISBN 9241530693.
- Muñiz, G.; Fierro, V.; Celzard, A.; Furdin, G.; Gonzalez-Sánchez, G.; Ballinas, M.L. Synthesis, Characterization and Performance in Arsenic Removal of Iron-Doped Activated Carbons Prepared by Impregnation with Fe (III) and Fe (II). J. Hazard. Mater. 2009, 165, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Z.; Zhou, J.; Sun, T.; Liang, Z. Enhanced Adsorption of As (III) on Chemically Modified Activated Carbon Fibers. Appl. Water Sci. 2019, 9, 41. [Google Scholar] [CrossRef]
- Almasri, D.A.; Rhadfi, T.; Atieh, M.A.; McKay, G.; Ahzi, S. High Performance Hydroxyiron Modified Montmorillonite Nanoclay Adsorbent for Arsenite Removal. Chem. Eng. J. 2018, 335, 1–12. [Google Scholar] [CrossRef]
- Omer, O.S.; Hussein, B.H.M.; Hussein, M.A.; Mgaidi, A. Mixture of Illite-Kaolinite for Efficient Water Purification: Removal of As (III) from Aqueous Solutions. Desalin. Water Treat. 2017, 79, 273–281. [Google Scholar] [CrossRef]
- Ceban, I.; Lupascu, T.; Mikhalovsky, S.; Nastas, R. Adsorption of Cobalt and Strontium Ions on Plant-Derived Activated Carbons: The Suggested Mechanisms. C 2023, 9, 71. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S. Sen Adsorption of Co (II) from Aqueous Medium on Natural and Acid Activated Kaolinite and Montmorillonite. Sep. Sci. Technol. 2007, 42, 3391–3418. [Google Scholar] [CrossRef]
- Das, B.; Devi, R.R.; Umlong, I.M.; Borah, K.; Banerjee, S.; Talukdar, A.K. Arsenic (III) Adsorption on Iron Acetate Coated Activated Alumina: Thermodynamic, Kinetics and Equilibrium Approach. J. Environ. Health Sci. Eng. 2013, 11, 42. [Google Scholar] [CrossRef]
- Zama, E.F.; Li, G.; Tang, Y.-T.; Reid, B.J.; Ngwabie, N.M.; Sun, G.-X. The Removal of Arsenic from Solution through Biochar-Enhanced Precipitation of Calcium-Arsenic Derivatives. Environ. Pollut. 2022, 292, 118241. [Google Scholar] [CrossRef] [PubMed]
- Conte, N.; Díez, E.; Almendras, B.; Gómez, J.M.; Rodríguez, A. Sustainable Recovery of Cobalt from Aqueous Solutions Using an Optimized Mesoporous Carbon. J. Sustain. Metall. 2023, 9, 266–279. [Google Scholar] [CrossRef]


| Adsorbents | Pollutants | pH | Removal (%) | qmax (mg/g) | References |
|---|---|---|---|---|---|
| Cerium oxide modified AC | As(III) | 5 | 90 | 36.7 | [37] |
| Modified peanut shell biochar | As(III) | 6 | 86 | 1.92 | [38] |
| Magnetite nanoparticles | As(III) | 8 | 95 | 1.63 | [39] |
| Apricot stone activated carbon | Co(II) | 9 | - | 111 | [40] |
| Al-pillared bentonite clay | Co(II) | 6 | 38.6 | [41] | |
| Modified Ficus carica leaves | Co(II) | 6 | 57 | 33.9 | [42] |
| Adsorbents | Adsorbates | Pseudo-First-Order | Pseudo-Second-Order | |||
|---|---|---|---|---|---|---|
| R2 | K1 (h−1) | R2 | qe | K2 | ||
| Filtrasorb-400 | As(III) | 0.86 | 0.97 | 0.90 | 1.43 | 0.65 |
| Co(II) | 0.88 | 1.36 | 0.96 | 1.38 | 1.31 | |
| Fluorosorb-100 | As(III) | 0.76 | 0.05 | 0.93 | 0.39 | 2.72 |
| Co(II) | 0.88 | 0.04 | 0.70 | 0.35 | 1.48 | |
| Fluorosorb-200 | As(III) | 0.76 | 0.03 | 0.71 | 0.33 | 1.92 |
| Co(II) | 0.81 | 0.04 | 0.65 | 0.36 | 1.24 | |
| Sorbents | Adsorbates | Langmuir Constants | Freundlich Constants | ||||
|---|---|---|---|---|---|---|---|
| R2 | R2 | n | |||||
| Filtrasorb-400 | As(III) | 0.99 | 0.42 | 0.09 | 0.97 | 1.20 | 0.03 |
| Co(II) | 0.99 | 1.00 | 0.73 | 0.88 | 2.10 | 0.27 | |
| Fluorosorb-100 | As(III) | 0.97 | 0.46 | 4.30 | 0.97 | 2.60 | 0.17 |
| Co(II) | 0.99 | 0.55 | 0.44 | 0.96 | 2.10 | 0.11 | |
| Fluorosorb-200 | As(III) | 0.97 | 0.32 | 0.04 | 0.58 | 1.13 | 0.01 |
| Co(II) | 0.57 | 0.19 | 0.17 | 0.93 | 1.30 | 0.03 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joudiazar, S.; Yadav, S.; Zhang, Z.; Satpathy, A.; Fernando, E.; Rahmati, R.; Kim, J.; Datta, R.; Sarkar, D. Adsorptive Removal of Arsenite and Cobalt by Commercial Sorbents. Materials 2025, 18, 5133. https://doi.org/10.3390/ma18225133
Joudiazar S, Yadav S, Zhang Z, Satpathy A, Fernando E, Rahmati R, Kim J, Datta R, Sarkar D. Adsorptive Removal of Arsenite and Cobalt by Commercial Sorbents. Materials. 2025; 18(22):5133. https://doi.org/10.3390/ma18225133
Chicago/Turabian StyleJoudiazar, Sevda, Sushma Yadav, Zhiming Zhang, Anshuman Satpathy, Eustace Fernando, Roxana Rahmati, Junchul Kim, Rupali Datta, and Dibyendu Sarkar. 2025. "Adsorptive Removal of Arsenite and Cobalt by Commercial Sorbents" Materials 18, no. 22: 5133. https://doi.org/10.3390/ma18225133
APA StyleJoudiazar, S., Yadav, S., Zhang, Z., Satpathy, A., Fernando, E., Rahmati, R., Kim, J., Datta, R., & Sarkar, D. (2025). Adsorptive Removal of Arsenite and Cobalt by Commercial Sorbents. Materials, 18(22), 5133. https://doi.org/10.3390/ma18225133











