Prolonged Protection of Copper in Acidic Media Through the Synergistic Effect of Fat-Soluble Vitamins
Abstract
1. Introduction
2. Materials and Methods
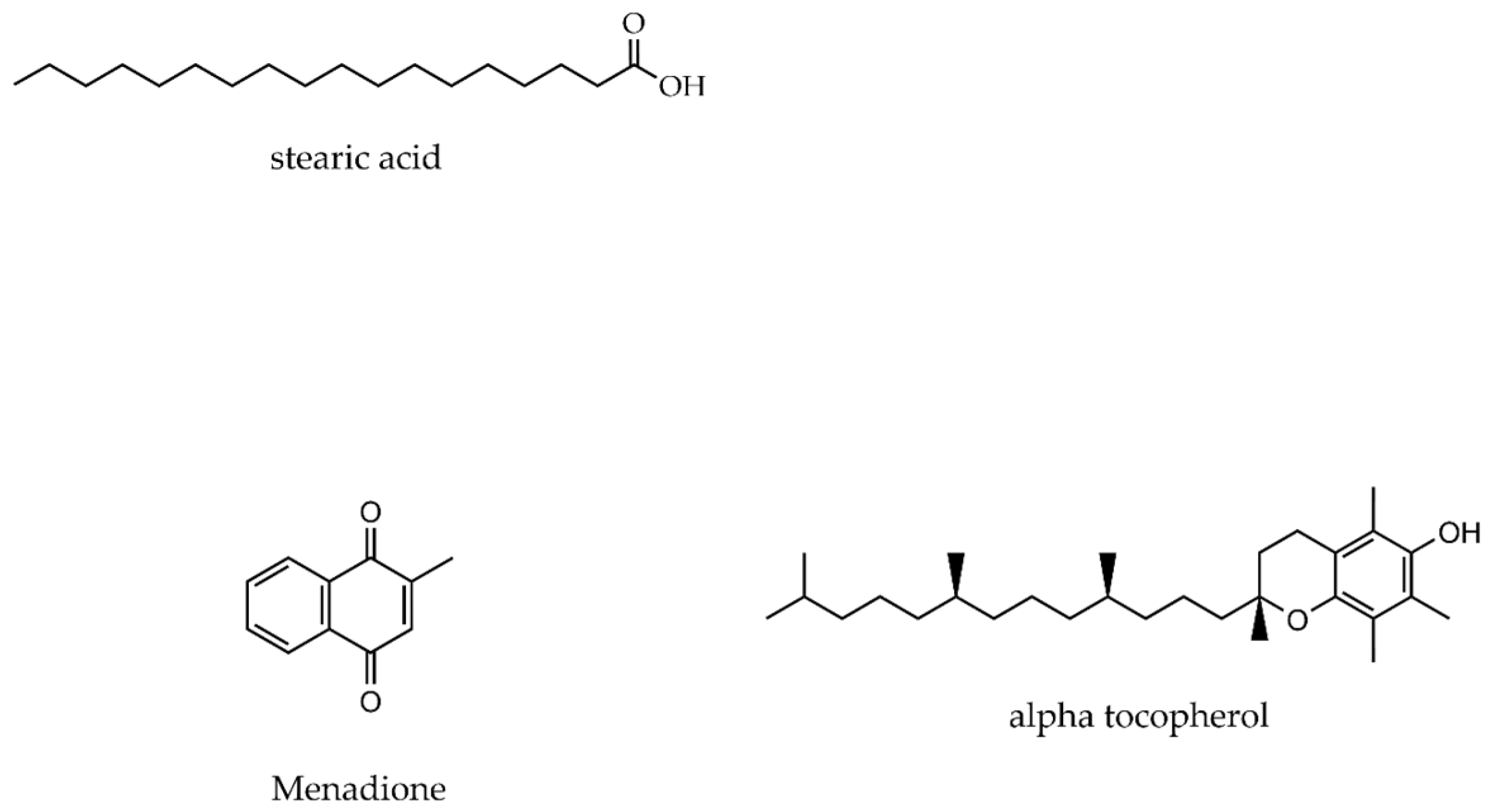
2.1. Materials and Chemicals
2.2. Instrumentation
- –
- Gamry 600™ potentiostat/galvanostat, Warminster, PA, USA, controlled by an electrochemical program.
- –
- Processing and analysis of experimental data using the following software programs: CorrView2, CorrWare, Zplot and ZView4 programs from Scribner Associates, Southern Pines, NC, USA (all version 2.80).
- –
- ATR-FTIR: SHIMADZU-IRAffinity-1, Shimadzu Europa GmbH, Duisburg, Germany.
- –
- Scanning Electron Microscope (SEM): JSM IT800SHL; Joel, Tokyo, Japan.
- –
- Goniometer: Data Physics OCA 25, Filderstadt, Germany.
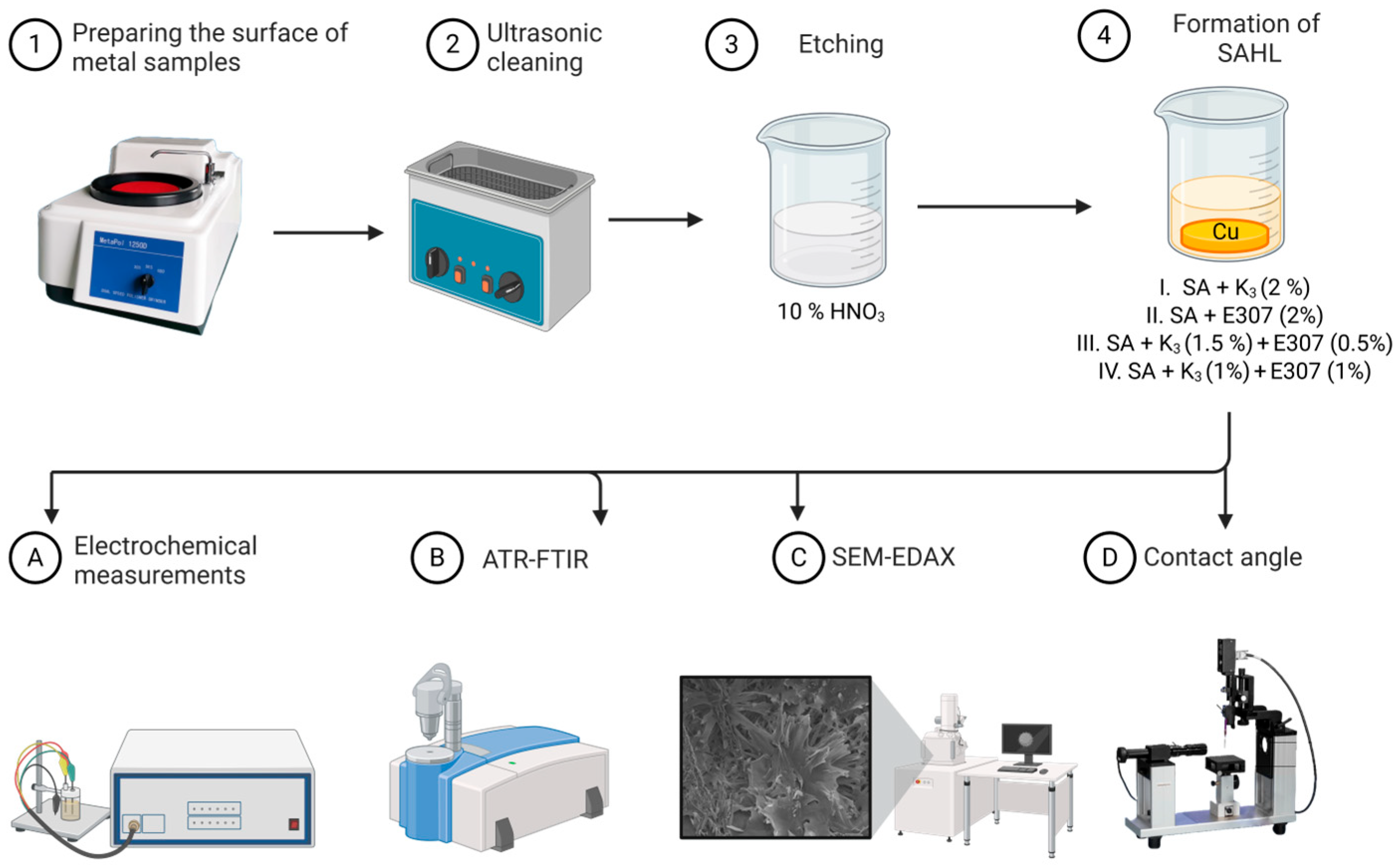
2.3. Pretreatment for the Electrode and Electrochemical Measurements
2.3.1. Contact Angle
2.3.2. ATR-FTIR Analysis
2.3.3. Surface Characterisation Using SEM/EDX
3. Results and Discussion
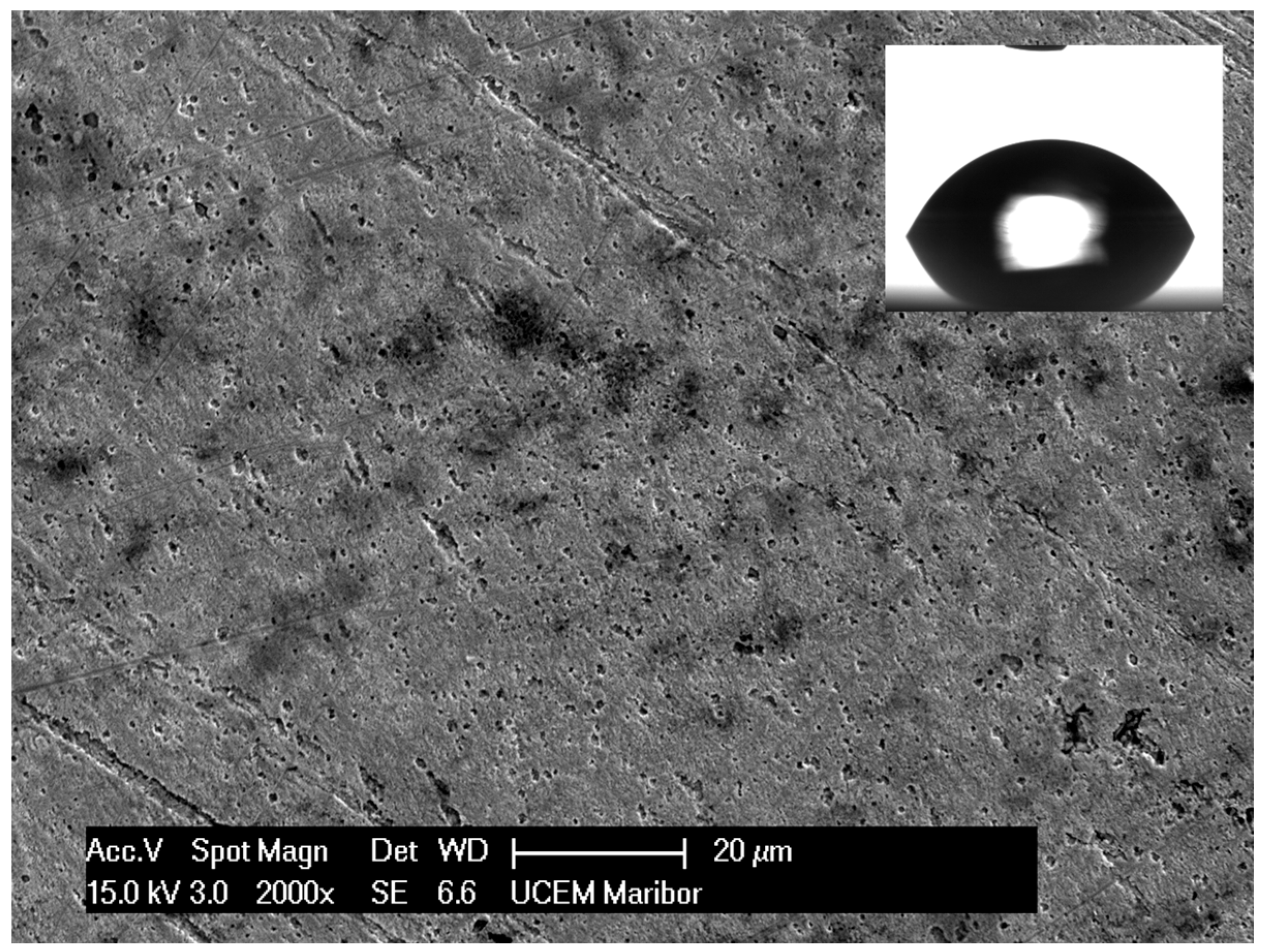
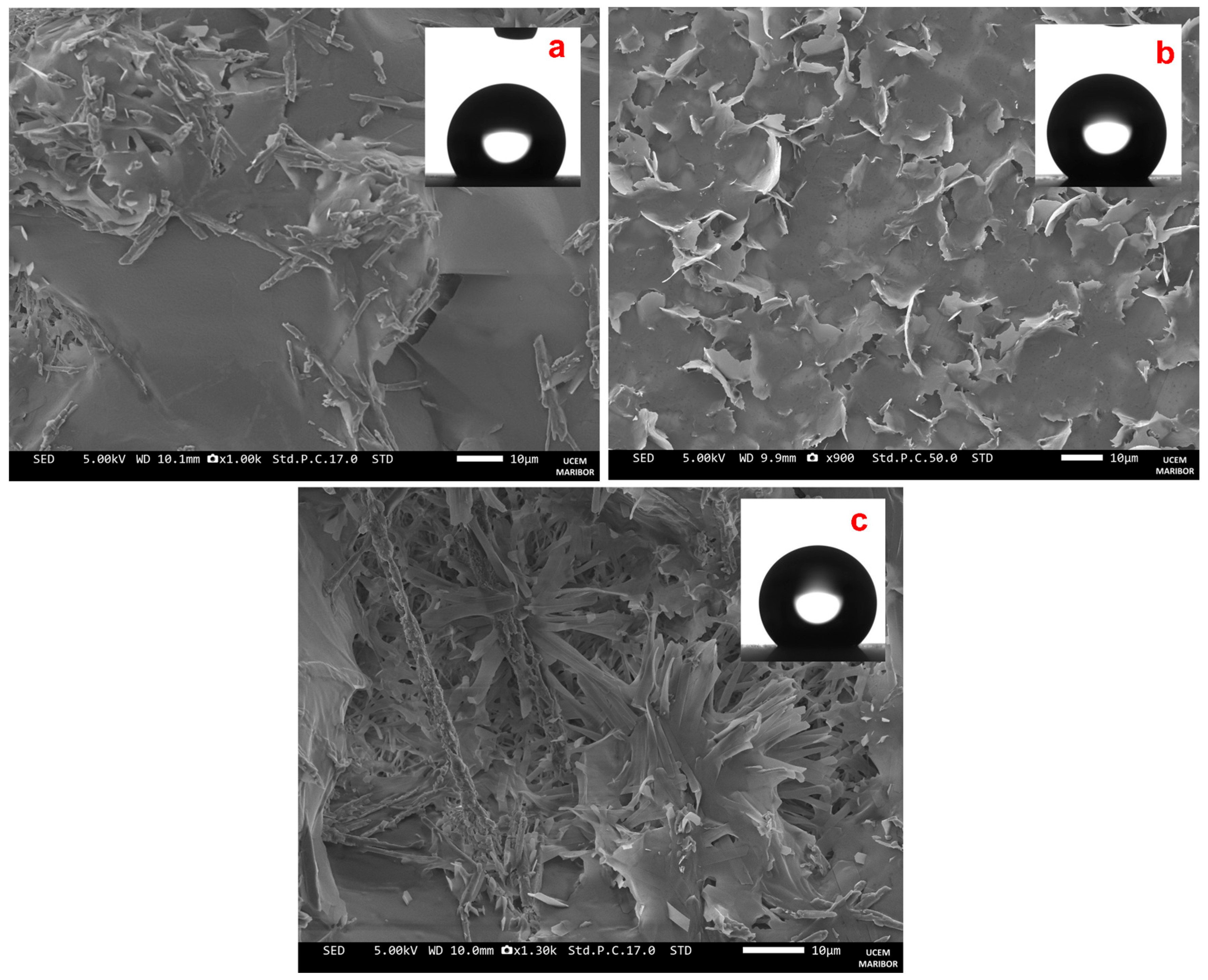
3.1. Wettability of a High-Level Hydrophobic Surface
3.2. Corrosion Resistance of SAHLs
3.2.1. Potentiodynamic Measurements (PDM)
3.2.2. EIS Measurements
Bare Surface
Copper Surface Modified with (SA + 2.0% K3)
Copper Surface Modified with (SA + 2.0% E307)
Copper Surface Modified with (SA + [1.5% K3 + 0.5% E307]) and (SA + [1.0% K3 + 1.0% E307])
- (SA + [1.5% K3 + 0.5% E307])
- (SA + [1.0% K3+ 1.0% E307])
3.3. Surface Analyses of the SAHLs on Copper
3.3.1. FTIR Analysis
3.3.2. SEM-EDAX Analyses
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Yuan, S.; Du, B.; Gui, Y.; Shen, M.; Wang, J.; Li, Y. Study on Deterioration Mechanism of Mechanical Properties and Damage Constitutive Model of Limestone Concrete Composite Materials under Chemical Corrosion. Constr. Build. Mater. 2025, 493, 143–292. [Google Scholar] [CrossRef]
- Sitzia, F.; Lisci, C.; Mirão, J. The Interaction between Rainwater and Polished Building Stones for Flooring and Cladding—Implications in Architecture. J. Build. Eng. 2022, 52, 104–495. [Google Scholar] [CrossRef]
- Chen, J.; Pan, X.; Nie, H.Y.; Kobe, B.; Bergendal, E.; Lilja, C.; Behazin, M.; Shoesmith, D.W.; Noël, J.J. Topographical and Statistical Studies of the Corrosion Damage underneath a Sulfide Film Formed on a Cu Surface. Corros. Sci. 2025, 248, 112–801. [Google Scholar] [CrossRef]
- Jia, Z.; Yang, Y.; He, Z.; Ma, H.; Ji, F. Mechanical Test Study on Corroded Marine High Performance Steel under Cyclic Loading. Appl. Ocean Res. 2019, 93, 101–942. [Google Scholar] [CrossRef]
- Singh, U.K.; Ramanathan, A.L.; Subramanian, V. Groundwater Chemistry and Human Health Risk Assessment in the Mining Region of East Singhbhum, Jharkhand, India. Chemosphere 2018, 204, 501–513. [Google Scholar] [CrossRef]
- Gece, G. Drugs: A Review of Promising Novel Corrosion Inhibitors. Corros. Sci. 2011, 53, 3873–3898. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, S.; Tao, B.; Tan, B.; Xiang, B.; Tian, W.; Chen, S. Two Novel Drugs as Bio-Functional Inhibitors for Copper Performing Excellent Anticorrosion and Antibacterial Properties. Colloids Surf. B Biointerfaces 2020, 190, 110–898. [Google Scholar] [CrossRef]
- Xu, Q.; Hou, D.; Zhang, H.; Wang, P.; Wang, M.; Wu, D.; Liu, C.; Ding, Z.; Zhang, M.; Xin, Z.; et al. Understanding the Effect of Vitamin B3, B6 and C as a Corrosion Inhibitor on the Ordinary Portland Cement Hydration: Experiments and DFT Study. Constr. Build. Mater. 2022, 331, 127–294. [Google Scholar] [CrossRef]
- Ahmed, R.A. Investigation of Corrosion Inhibition of Vitamins B1and C on Mild Steel in 0.5 m HCl Solution: Experimental and Computational Approach. Orient. J. Chem. 2016, 32, 295–304. [Google Scholar] [CrossRef]
- Nurdin, I.; Fuadi, A.; Suhendrayatna, S. Investigation of Ascorbic Acid as Environment-Friendly Corrosion Inhibitor of Low Carbon Steel in Marine Environment. IOP Conf. Ser. Mater. Sci. Eng. 2019, 536, 012108. [Google Scholar]
- Fuchs-Godec, R.; Pavlović, M.G.; Tomić, M.V. The Inhibitive Effect of Vitamin-C on the Corrosive Performance of Steel in HCl Solutions. Int. J. Electrochem. Sci. 2013, 8, 1511–1519. [Google Scholar] [CrossRef]
- Fuchs-Godec, R.; Pavlović, M.G.; Tomić, M.V. The Inhibitive Effect of Vitamin-C on the Corrosive Performance of Steel in HCl Solutions—Part II. Int. J. Electrochem. Sci. 2015, 10, 10502–10512. [Google Scholar] [CrossRef]
- Kasatkin, V.E.; Dorofeeva, V.N.; Kasatkina, I.V.; Korosteleva, I.G.; Kornienko, L.P.; Andreev, N.N.; Gedvillo, I.A.; Zhmakina, A.S. Ascorbic acid as a corrosion inhibitor of steel in chloride-containing solutions of calcium hydroxide. Int. J. Corros. Scale Inhib. 2022, 11, 727–751. [Google Scholar] [CrossRef]
- Souza, B.D.F.; Lage, M.R.; dos Santos, A.O.; de Sousa, F.F.; Gester, R.; Stoyanov, S.R.; Andrade-Filho, T. Ascorbic Acid, Ascorbate, and Dehydroascorbic Acid as Green Corrosion Inhibitors: A Computational Investigation. Corros. Mater. Degrad. 2024, 5, 615–623. [Google Scholar] [CrossRef]
- Argiz, C.; Arroyo, C.; Bravo, A.; Moragues, A.; Andrade, C.; Bolzoni, F. L-Ascorbic Acid as an Efficient Green Corrosion Inhibitor of Steel Rebars in Chloride Contaminated Cement Mortar. Materials 2022, 15, 8005. [Google Scholar] [CrossRef]
- Pranjoto, H.; Anggorowati, A.A.; Joewono, A.; Suratno, L.; Candra, A. The inhibitive effect of vitamin B2, B6 and vitamin C on the copper corrosion. E3S Web Conf. 2024, 475, 04003. [Google Scholar] [CrossRef]
- Manjunath, V.; Badhe, R.V.; McCoy, M.; Rynne, J.; Bhatti, A.; Segu, A.; Oral, E.; Jacobs, J.J.; Chastain, P.; Bijukumar, D.; et al. The Role of Vitamin E in Hip Implant-Related Corrosion and Toxicity: Initial Outcome. J. Mech. Behav. Biomed. Mater. 2021, 123, 104–769. [Google Scholar] [CrossRef]
- Fuchs-Godec, R.; Zerjav, G. Corrosion Resistance of High-Level-Hydrophobic Layers in Combination with Vitamin E—(α-Tocopherol) as Green Inhibitor. Corros. Sci. 2015, 97, 7–16. [Google Scholar] [CrossRef]
- Fuchs-Godec, R. Flower-like Superhydrophobic Surfaces Fabricated on Stainless Steel as a Barrier against Corrosion in Simulated Acid Rain. Materials 2022, 15, 7104. [Google Scholar] [CrossRef] [PubMed]
- Fuchs-Godec, R. A Synergistic Effect between Stearic Acid and (+)-α-Tocopherol as a Green Inhibitor on Ferritic Stainless Steel Corrosion Inhibition in 3.0% NaCl Solution. Coatings 2021, 11, 971. [Google Scholar] [CrossRef]
- Fuchs-Godec, R.; Tomic, M.V.; Pavlovic, M.G. Effect of α-Tocopherol as a Green Inhibitor on Chloride-Induced Corrosion of Steel. Int. J. Electrochem. Sci. 2019, 14, 10396–10409. [Google Scholar] [CrossRef]
- Aloysius, A.; Ramanathan, R.; Christy, A.; Baskaran, S.; Antony, N. Experimental and Theoretical Studies on the Corrosion Inhibition of Vitamins—Thiamine Hydrochloride or Biotin in Corrosion of Mild Steel in Aqueous Chloride Environment. Egypt. J. Pet. 2018, 27, 371–381. [Google Scholar] [CrossRef]
- Hippolyte, C.N.; Serge, B.Y.; Sagne, A.; Creus, J.; Albert, T. Nicotinamide Inhibition Properties for Copper Corrosion in 3.5% NaCl Solution: Experimental and Theorical Investigations. Mater. Sci. Chem. Eng. 2018, 6, 100–121. [Google Scholar] [CrossRef]
- Lovander, M.D.; Lyon, J.D.; Parr, D.L.; Wang, J.; Parke, B.; Leddy, J. Critical Review—Electrochemical Properties of 13 Vitamins: A Critical Review and Assessment. J. Electrochem. Soc. 2018, 165, G18–G49. [Google Scholar] [CrossRef]
- Kwiatkowski, H.; Krakowiak, S.; Gaweł, Ł. Vitamin B9 as a New Eco-Friendly Corrosion Inhibitor for Copper in 3.5% NaCl Solution. J. Ind. Eng. Chem. 2025, 142, 282–292. [Google Scholar] [CrossRef]
- Kesari, P.; Udayabhanu, G. Investigation of Vitamin B12 as a Corrosion Inhibitor for Mild Steel in HCl Solution through Gravimetric and Electrochemical Studies. Ain Shams Eng. J. 2023, 14, 101920. [Google Scholar] [CrossRef]
- Liu, C.; Wu, H.; Qiang, Y.; Zhao, H.; Wang, L. Design of Smart Protective Coatings with Autonomous Self-Healing and Early Corrosion Reporting Properties. Corros. Sci. 2021, 184, 109355. [Google Scholar] [CrossRef]
- Feng, L.; Wang, J.; Shi, X.; Chai, C. Superhydrophobic Copper Surface with Mechanical, Chemical, and UV Durability along with Corrosion Resistance and Self-Cleaning Effect. Appl. Phys. A Mater. Sci. Process. 2019, 125, 261. [Google Scholar] [CrossRef]
- Barati Darband, G.; Aliofkhazraei, M.; Khorsand, S.; Sokhanvar, S.; Kaboli, A. Science and Engineering of Superhydrophobic Surfaces: Review of Corrosion Resistance, Chemical and Mechanical Stability. Arab. J. Chem. 2020, 13, 1763–1802. [Google Scholar] [CrossRef]
- Li, X.; Yang, W.; Liu, Y.; Gong, Y.; Zhang, R.; Li, Q.; Wang, B.; Guo, Z. Improvement of corrosion resistance of H59 brass through fabricating superhydrophobic surface using laser ablation and heating treatment. Corros. Sci. 2021, 180, 109186. [Google Scholar] [CrossRef]
- Cong, Q.; Qin, X.; Chen, T.; Jin, J.; Liu, C.; Wang, M. Research Progress of Superhydrophobic Materials in the Field of Anti-/De-Icing and Their Preparation: A Review. Materials 2023, 16, 5151. [Google Scholar] [CrossRef] [PubMed]
- Kravanja, G.; Godec, R.F.; Rozman, M.; Rudolf, R.; Ivanič, A. Biomimetic Superhydrophobic Concrete with Enhanced Anticorrosive, Freeze Thaw, and Deicing Resistance. Adv. Eng. Mater. 2022, 24, 2101445. [Google Scholar] [CrossRef]
- Han, Z.; Huang, X.; Chen, J.; Chen, J.; Zhou, H. Study on the Compounding of Cysteine Modified Schiff Base and Decanoic Acid as Corrosion Inhibitors for Bronze with Patina. Surf. Interfaces 2024, 46, 103996. [Google Scholar] [CrossRef]
- Sajadinia, Z.; Arabian, D.; Charmi, M. Production of a Green Organic Multifunctional Anticorrosion Coating Composed of Stearic Acid and Nanoparticles for Concrete Using a Simple and Cost-Effective Method. Prog. Org. Coat. 2025, 207, 109389. [Google Scholar] [CrossRef]
- Wang, L.; Wen, J.; Ma, X.; Wang, S.; Zhong, H.; Cao, Z.; Li, L. Synergistic Effect of Al3+ on In-Situ Constructing a Stearic Acid Anti-Corrosion Coating for Galvanized Steel. Chem. Eng. Sci. 2025, 305, 121073. [Google Scholar] [CrossRef]
- Wang, T.; Li, P.; Guo, Y.; Xu, Y.; Kou, W.; Li, G.; Lian, J. Enhanced Corrosion Resistance of Calcium Carbonate Coatings on Magnesium Alloy via Simple Stearic Acid Treatment. J. Magnes. Alloys 2025, 13, 1602–1616. [Google Scholar] [CrossRef]
- Qi, S.; Zhu, J. Cerium-Based Coating Modified by Stearic Acid on Phytic Acid Conversion Surface of AZ91D Magnesium Alloy: Preparation and Performance. Mater. Today Commun. 2025, 46, 112663. [Google Scholar] [CrossRef]
- Amamra, S.; Kaabi, I.; Arrar, L.; Baghiani, A.; Hamla, M.; Aouni, S.I.; Lakikza, I.; Boublia, A.; Ernst, B.; Raish, M.; et al. Thymus Vulgaris Extract: A Green Approach to Antioxidant Efficacy, Antibacterial Action, and Corrosion Inhibition. J. Environ. Chem. Eng. 2025, 13, 116067. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, A. In-Depth Analysis of Corrosion-Resistance and Dynamic Stability of Superhydrophobic Copper Developed by a Green Approach. Mater. Today Commun. 2023, 36, 106744. [Google Scholar] [CrossRef]
- Yang, H.; Dong, Y.; Li, X.; Gao, Y.; He, W.; Liu, Y.; Mu, X.; Zhao, Y.; Fu, W.; Wang, X.; et al. Development of a Mechanically Robust Superhydrophobic Anti-Corrosion Coating Using Micro-HBN/Nano-Al2O3 with Multifunctional Properties. Ceram. Int. 2025, 51, 491–505. [Google Scholar] [CrossRef]
- Ma, R.; Li, R.; Niu, Q.; Zeng, Y.; Li, J.; Bai, S.; Cheng, Y. Preparation of Superhydrophobic Surfaces Based on Copper Mesh Substrates and Its Application Performance. ACS Omega 2023, 8, 45616–45625. [Google Scholar] [CrossRef]
- Rahul, N.; Park, B.; Pradhan, S.K.; Sung, H.E.; Jeong, I.H.; Yun, Y.S.; Oh, M.S. Scalable Engineering of Superhydrophobic Copper Surfaces with Enhanced Corrosion Resistance by Combined Nanostructuring and Chemical Vapor Deposition. Materials 2025, 18, 3981. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, M.; Li, H. Fabrication of Durable Superhydrophobic Surfaces with a Mesh Structure and Drag Reduction by Chemical Etching Technology. Coatings 2025, 15, 402. [Google Scholar] [CrossRef]
- Jiang, F.; Song, T.; Kuang, Y.; Wu, H. Facile Fabrication of a Flower-like Superhydrophobic Copper Surface with Superior Corrosion Resistance. Int. J. Mater. Res. 2024, 115, 540–545. [Google Scholar] [CrossRef]
- Scendo, M. The Effect of Purine on the Corrosion of Copper in Chloride Solutions. Corros. Sci. 2007, 49, 373–390. [Google Scholar] [CrossRef]
- Antonijevic, M.M.; Petrovic, M.B. Copper corrosion inhibitors—A review. Int. J. Electrochem. Sci. 2008, 3, 1–28. [Google Scholar] [CrossRef]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical impedance spectroscopy—A tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef]
- Mei, B.A.; Munteshari, O.; Lau, J.; Dunn, B.; Pilon, L. Physical Interpretations of Nyquist Plots for EDLC Electrodes and Devices. J. Phys. Chem. C 2018, 122, 194–206. [Google Scholar] [CrossRef]
- Alizadeh Razin, A.; Ramezanzadeh, B.; Yari, H. Detecting and Estimating the Extent of Automotive Coating Delamination and Damage Indexes after Stone Chipping Using Electrochemical Impedance Spectroscopy. Prog. Org. Coat. 2016, 92, 95–109. [Google Scholar] [CrossRef]
- Hayashibara, H.; Tada, E.; Nishikata, A. Monitoring the Early Stage of Degradation of Epoxy-Coated Steel for Ballast Tank by Electrochemical Impedance Spectroscopy. Mater. Trans. 2017, 58, 1687–1694. [Google Scholar] [CrossRef]
- Dong, Z.; Ter-Ovanessian, B.; Abe, H.; Mary, N.; Watanabe, Y.; Normand, B. Design of an EIS-Based Sensor for Indirect Non-Invasive Corrosion Inspection. Corros. Sci. 2025, 254, 113029. [Google Scholar] [CrossRef]
- Feliu, S. Electrochemical Impedance Spectroscopy for the Measurement of the Corrosion Rate of Magnesium Alloys: Brief Review and Challenges. Metals 2020, 10, 775. [Google Scholar] [CrossRef]
- Asperti, D.; Cabrini, M.; Lorenzi, S.; Rosace, G.; Omrani, A.; Pastore, T. Electrochemical Impedance Spectroscopy Analysis of Organic Epoxy Coatings Reinforced with Nano Clay. Materials 2024, 17, 3028. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Liu, Y.; Yin, X.; Shi, J.; Dilger, K. Electrochemical Behavior and Interfacial Delamination of a Polymer-Coated Galvanized Steel System in Acid Media. ACS Omega 2021, 6, 20331–20340. [Google Scholar] [CrossRef]
- de Paula, A.S.; Aroeira, B.M.; de Oliveira Souza, L.H.; da Cruz, A.C.; Fedel, M.; da Silva, B.P.; Cotting, F. Influence of Organic Coating Thickness on Electrochemical Impedance Spectroscopy Response. Coatings 2024, 14, 285. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Q.; Wang, N.; Zheng, X. Fabrication of Superhydrophobic Green Surfaces with Good Self-Cleaning, Chemical Stability and Anti-Corrosion Properties. J. Mater. Sci. 2019, 54, 13006–13016. [Google Scholar] [CrossRef]
- Fu, T.; Tang, X.; Cai, Z.; Zuo, Y.; Tang, Y.; Zhao, X. Correlation Research of Phase Angle Variation and Coating Performance by Means of Pearson’s Correlation Coefficient. Prog. Org. Coat. 2020, 139, 105459. [Google Scholar] [CrossRef]
- Liu, J.; Gong, G.; Yan, C. EIS Study of Corrosion Behaviour of Organic Coating/Dacromet Composite Systems. Electrochim. Acta 2005, 50, 3320–3332. [Google Scholar]
- Kashkovskiy, R.; Strelnikova, K.; Fedotova, A. Application of Electrochemical Impedance Spectroscopy to Study Hydrogen Sulphide Corrosion of Steel and Its Inhibition: A review. Corros. Eng. Sci. Technol. 2019, 54, 493–515. [Google Scholar] [CrossRef]
- Ramírez-González, J.; Sinclair, D.C.; West, A.R. Impedance and Dielectric Spectroscopy of Functional Materials: A Critical Evaluation of the Two Techniques. J. Electrochem. Soc. 2023, 170, 116504. [Google Scholar] [CrossRef]
- Ekerenam, O.O.; Ikeuba, A.I.; Njoku, C.N.; Njoku, D.I.; Emori, W.; Nwokolo, I.K.; Etim, I.I.N.; Okonkwo, B.O.; Udoh, I.I.; Daniel, E.F.; et al. Advancements in Corrosion Studies and Protective Measures for Copper and Copper-Based Alloys in Varied Environmental Conditions. Results Eng. 2025, 26, 105257. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Z.; Li, Z.; Wu, Y.; Wei, W.; Wang, Y.; Xu, J.; Ding, M. Stable Cu+/Cu2+ Species Derived from in-Situ Growing Cu-S-V Bonds in CuVxS Electrocatalysts Enables High Efficiency CO2 Electroreduction to Methanol. Appl. Catal. B 2024, 358, 124445. [Google Scholar] [CrossRef]
- Benzarti, Z.; Arrousse, N.; Serra, R.; Cruz, S.; Bastos, A.; Tedim, J.; Salgueiro, R.; Cavaleiro, A.; Carvalho, S. Copper Corrosion Mechanisms, Influencing Factors, and Mitigation Strategies for Water Circuits of Heat Exchangers: Critical Review and Current Advances. Corros. Rev. 2024, 43, 429–455. [Google Scholar] [CrossRef]
- She, X.; Peng, J.; Qiang, Y.; Zhou, Y.; Zhang, S. Recent advances in protective technologies against copper corrosion. J. Mater. Sci. Technol. 2024, 201, 75–94. [Google Scholar] [CrossRef]
- Rončević, I.Š.; Vladislavić, N.; Buzuk, M. Surface Modifications of the Biodegradable Magnesium Based Implants with Self-Assembled Monolayers Formed by T-Bag Method. Acta. Chim. Slov. 2018, 65, 698–708. [Google Scholar] [CrossRef]
- Hassan, G.S. Menadione. In Profiles of Drug Substances, Excipients and Related Methodology; Academic Press Inc.: London, UK, 2013; Volume 38, pp. 227–313. [Google Scholar]
- Xing, G.; Miller, C.J.; Ninh Pham, A.; Jones, A.M.; Waite, T.D. Oxidant Generation Resulting from the Interaction of Copper with Menadione (Vitamin K3)—A Model for Metal-Mediated Oxidant Generation in Living Systems. J. Inorg. Biochem. 2018, 188, 38–49. [Google Scholar] [CrossRef]
- Guk, D.A.; Krasnovskaya, O.O.; Zyk, N.V.; Beloglazkina, E.K. Convenient Synthesis of 2-Thioimidazolone/Menadione Conjugates via a Two-Step Sequence Starting with Direct Amination of Menadione. SynOpen 2020, 4, 38–43. [Google Scholar] [CrossRef]
- Gaikwad, M.N.; Gaikwad, S.T.; Rajbhoj, A.S. Potentiometric study of vitamin K3 complexes with transition metals in methanol–water and acetonitrile–water medium. Int. J. ChemTech Res. 2012, 4, 1392–1395. [Google Scholar]
- Boyatzis, S.C.; Fragkos-Livanios, L.; Giannoulaki, M.; Filopoulou, A. Infrared Spectroscopy Reveals the Reactivity of Fatty Acids on Copper Surfaces and Its Implications for Cultural Heritage Objects. Herit. Sci. 2023, 11, 23. [Google Scholar] [CrossRef]
- Jadhav, S.A. Self-Assembled Monolayers (SAMs) of Carboxylic Acids: An Overview. Cent. Eur. J. Chem. 2011, 9, 369–378. [Google Scholar] [CrossRef]
- Jahanmardi, R.; Saberi, M.; Fathi, M. Preparation of Thymolphthalein Stearate and Appraisement of Its Efficacy as an Antioxidant for Polypropylene. Front. Chem. Res. 2020, 2, 2020–2024. [Google Scholar]
- Silva, S.D.; Rosa, N.F.; Ferreira, A.E.; Boas, L.V.; Bronze, M.R. Rapid Determination of α-Tocopherol in Vegetable Oils by Fourier Transform Infrared Spectroscopy. Food Anal. Methods 2009, 2, 120–127. [Google Scholar] [CrossRef]
- Singh, G.; Sachdeva, R.; Rai, B.; Saini, G.S.S. Structure and Vibrational Spectroscopic Study of Alpha-Tocopherol. J. Mol. Struct. 2017, 1144, 347–354. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Huck, C.W.; Mazurek, S.; Czarnecki, M.A. Anharmonicity and Spectra-Structure Correlations in MIR and NIR Spectra of Crystalline Menadione (Vitamin K3). Molecules 2021, 26, 6779. [Google Scholar] [CrossRef]
- Parvate, S.; Dixit, P.; Chattopadhyay, S. Superhydrophobic Surfaces: Insights from Theory and Experiment. J. Phys. Chem. B 2020, 124, 1323–1360. [Google Scholar] [CrossRef]
- Bhosale, S.V.; Al Kobaisi, M.; Jadhav, R.W.; Jones, L.A. Flower-Like Superstructures: Structural Features, Applications and Future Perspectives. Chem. Rec. 2021, 21, 257–283. [Google Scholar] [CrossRef] [PubMed]
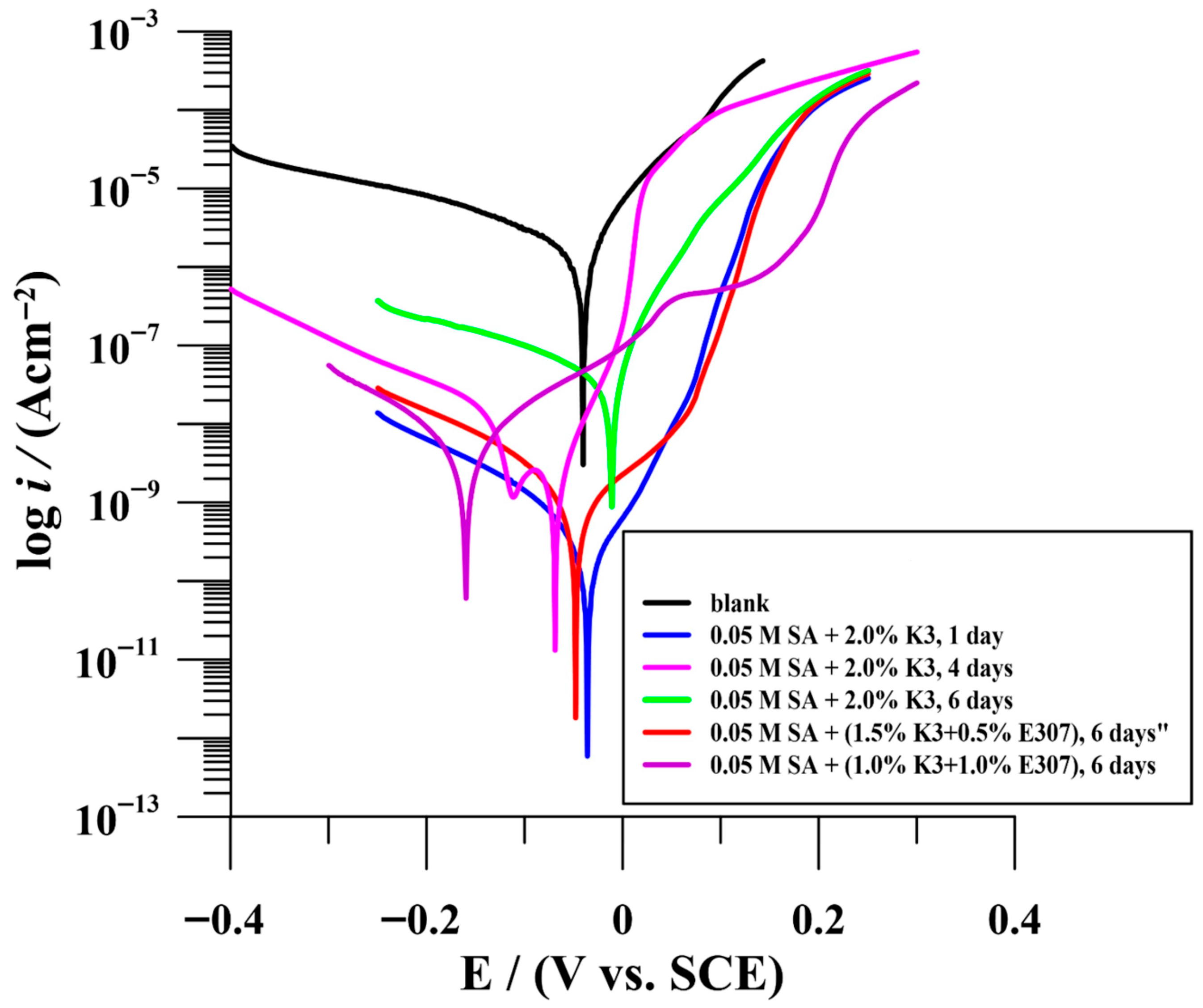
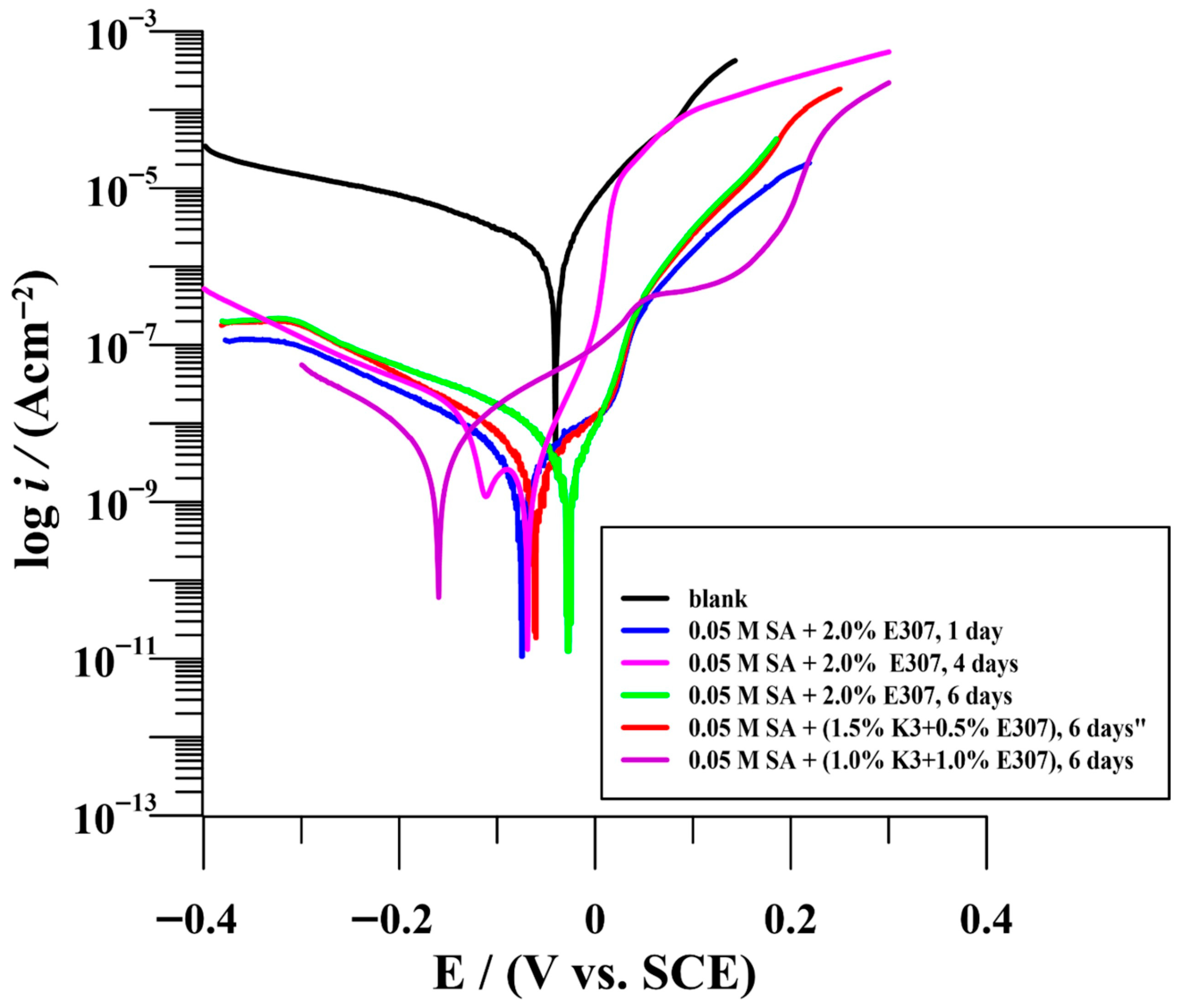
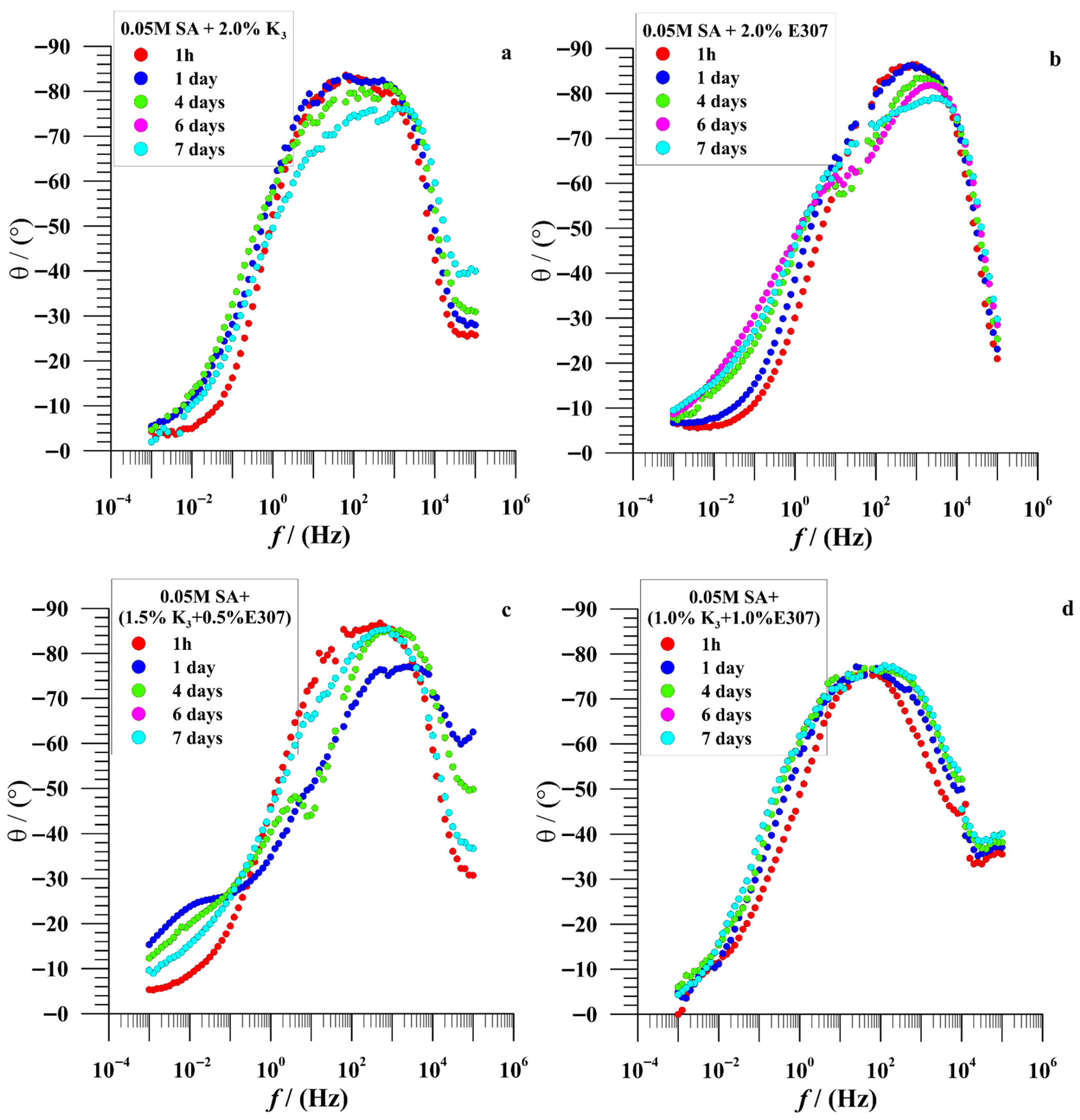
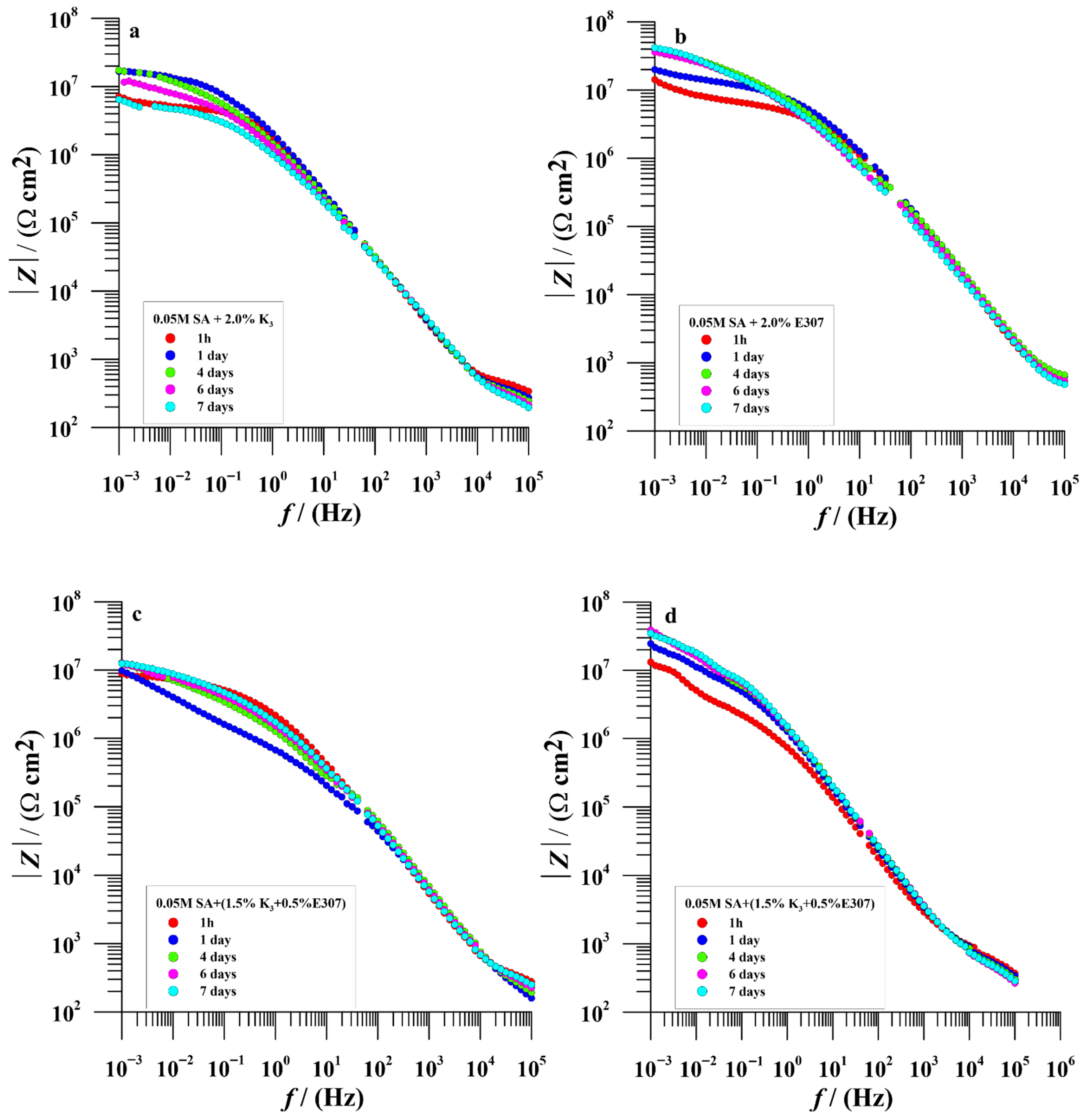
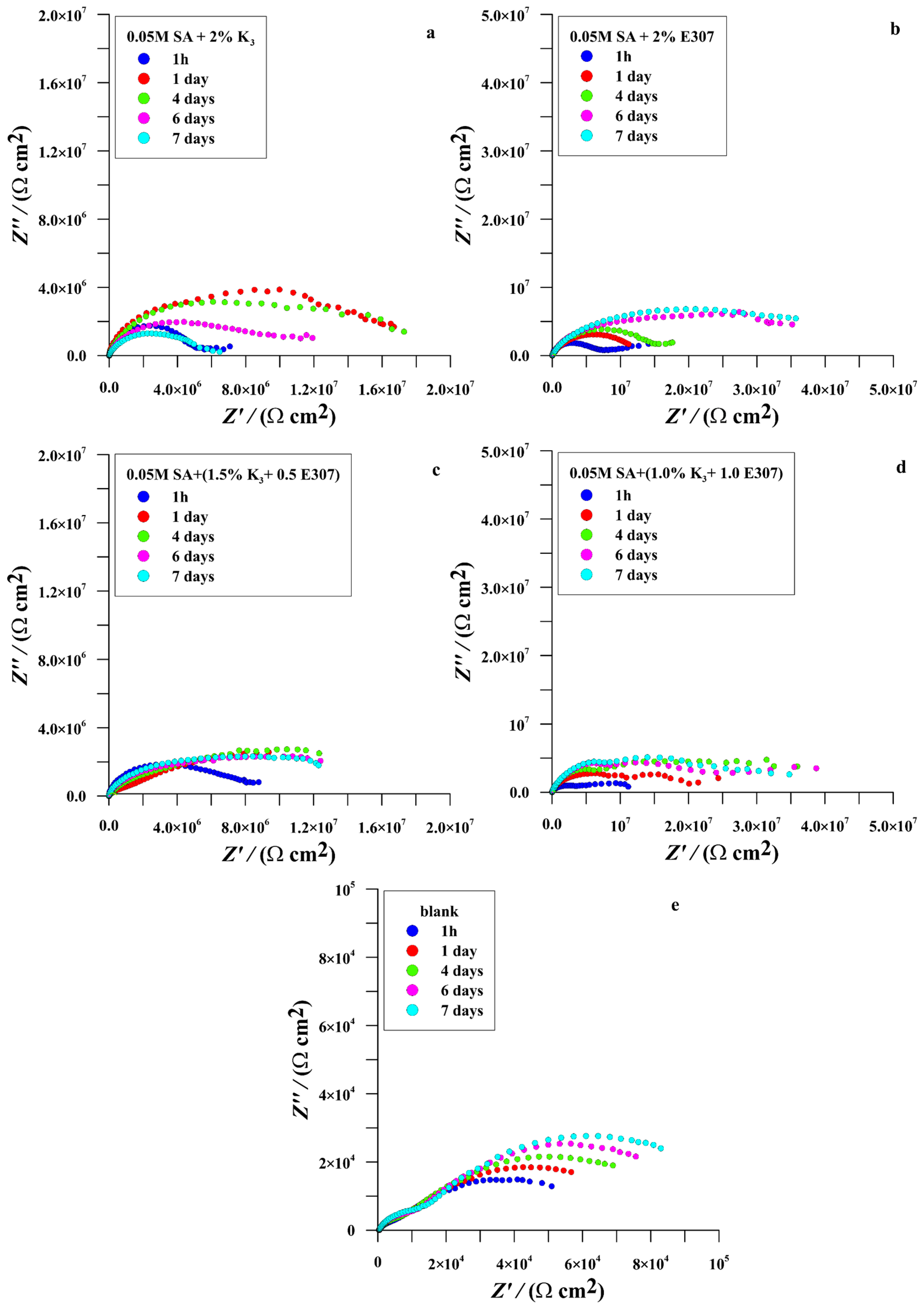
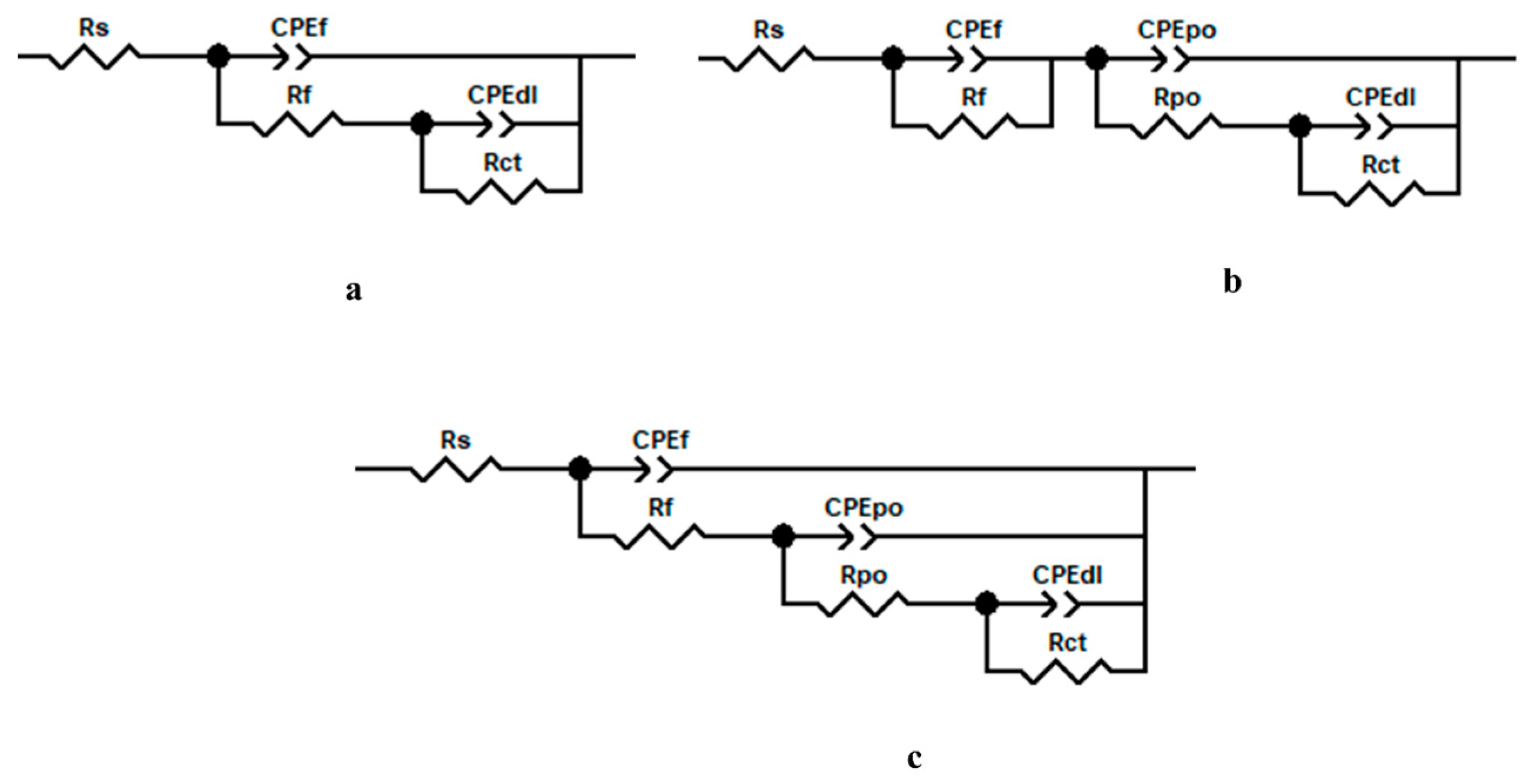
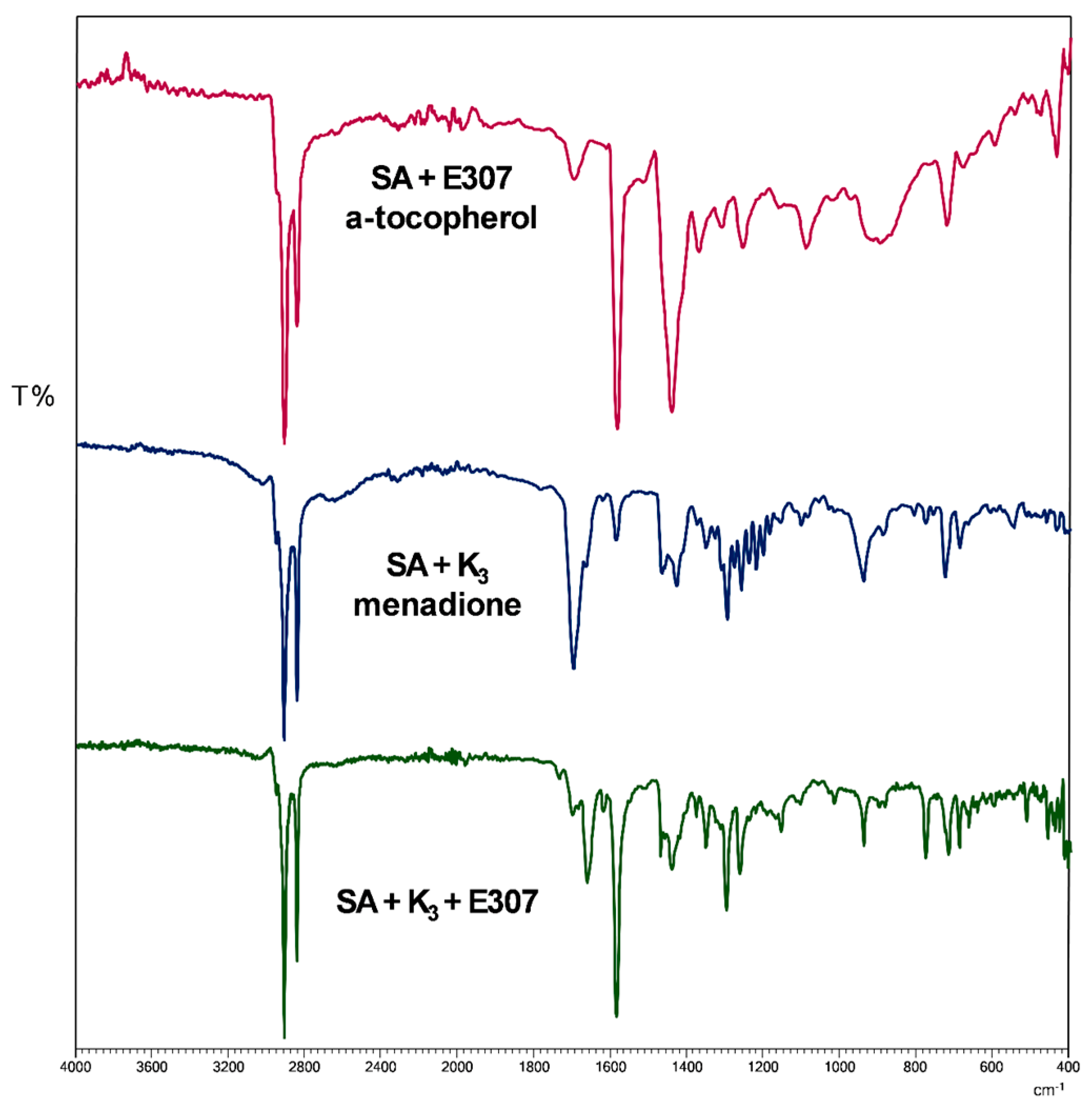
| Time (Day) | icorr (nAcm−2) | Ecorr (V/SCE) | Rp (MΩcm2) | bc (mV/dec) | ba (mV/dec) | % ηicorr | % ηRp | |
|---|---|---|---|---|---|---|---|---|
| A | 1731.76 | −0.044 | 0.10 | 290.03 | 81.60 | |||
| 1 d | 0.10 | −0.028 | 19.721 | 61.62 | 41.65 | 99.99 | 99.55 | |
| B | 4 d | 1.00 | −0.045 | 16.416 | 75.92 | 86.93 | 99.94 | 99.47 |
| 6 d | 31.72 | −0.019 | 9.162 | 163.75 | 59.27 | 98.17 | 99.05 | |
| 7 d | 43.82 | −0.011 | 4.330 | 223.15 | 40.26 | 97.47 | 97.99 | |
| 1 d | 2.99 | −0.078 | 7.481 | 131.86 | 153.11 | 99.83 | 98.84 | |
| C | 4 d | 3.84 | −0.051 | 9.171 | 123.04 | 88.83 | 99.78 | 99.05 |
| 6 d | 6.75 | −0.018 | 34.146 | 171.14 | 56.51 | 99.61 | 99.74 | |
| 7 d | 8.45 | −0.013 | 35.006 | 179.62 | 46.26 | 99.51 | 99.75 | |
| D | 6 d | 4.94 | −0.069 | 13.573 | 138.61 | 41.52 | 99.71 | 99.36 |
| E | 6 d | 2.47 | −0.162 | 34.481 | 149.27 | 62.33 | 99.86 | 99.75 |
| Time (Hour) (Day) | Rs (kΩcm2) | Rf (kΩcm2) | nf | Cf (nFcm−2) | Rpo (MΩcm2) | npo | Cpo (nFcm−2) | Rct (MΩcm2) | nct | Cdl (μFcm−2) | Rp-EIS (MΩcm2) | % ηRp-EIS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 1 h | 0.199 | 1.511 | 0.869 | 2672.00 | / | / | / | 0.067 | 0.493 | 3297.91 | 0.069 | |
| 1 d | 0.191 | 3.000 | 0.661 | 2306.00 | / | / | / | 0.080 | 0.478 | 999.85 | 0.083 | ||
| 4 d | 0.175 | 3.665 | 0.680 | 1879.00 | / | / | / | 0.096 | 0.510 | 287.54 | 0.100 | ||
| 6 d | 0.161 | 6.305 | 0.705 | 1550.00 | / | / | / | 0.103 | 0.569 | 63.71 | 0.109 | ||
| 7 d | 0.152 | 8.821 | 0.706 | 1708.00 | / | / | / | 0.120 | 0.598 | 96.72 | 0.129 | ||
| B | 1 h | 0.199 | 0.205 | 0.999 | 9.40 | 0.367 | 0.946 | 57.20 | 7.068 | 0.528 | 6.66 | 7.435 | 99.08 |
| 1 d | 0.191 | 0.166 | 0.998 | 10.80 | 3.909 | 0.946 | 62.80 | 14.003 | 0.535 | 5.79 | 17.912 | 99.54 | |
| 4 d | 0.175 | 0.165 | 0.999 | 10.90 | 4.003 | 0.946 | 63.20 | 13.474 | 0.513 | 5.60 | 17.477 | 99.43 | |
| 6 d | 0.161 | 0.138 | 0.998 | 10.40 | 0.045 | 0.910 | 81.30 | 11.494 | 0.532 | 6.12 | 11.539 | 99.05 | |
| 7 d | 0.152 | 0.137 | 0.999 | 10.90 | 0.035 | 0.921 | 97.10 | 6.486 | 0.435 | 7.92 | 6.521 | 98.03 | |
| C | 1 h | 0.497 | 1.705 | 0.982 | 9.40 | 6.791 | 0.997 | 40.30 | 2.313 | 0.596 | 6.87 | 9.104 | 99.25 |
| 1 d | 0.491 | 1.710 | 0.979 | 8.60 | 7.473 | 0.883 | 13.90 | 5.235 | 0.543 | 1.63 | 12.709 | 99.35 | |
| 4 d | 0.395 | 0.801 | 0.948 | 8.30 | 8.346 | 0.997 | 21.50 | 10.437 | 0.505 | 2.75 | 18.783 | 99.47 | |
| 6 d | 0.380 | 0.724 | 0.973 | 7.60 | 13.180 | 0.982 | 29.10 | 25.821 | 0.502 | 2.10 | 39.001 | 99.72 | |
| 7 d | 0.359 | 0.579 | 0.962 | 8.90 | 10.244 | 0.983 | 31.30 | 31.481 | 0.591 | 1.09 | 41.725 | 99.69 | |
| D | 1 h | 0.304 | 0.175 | 0.999 | 10.10 | 0.107 | 0.840 | 22.70 | 9.039 | 0.428 | 9.47 | 9.146 | 99.25 |
| 1 d | 0.288 | 0.285 | 0.999 | 7.50 | 0.483 | 0.858 | 26.60 | 13.328 | 0.542 | 5.25 | 13.811 | 99.40 | |
| 4 d | 0.260 | 0.399 | 0.999 | 8.40 | 0.771 | 0.879 | 13.90 | 13.549 | 0.490 | 4.37 | 14.317 | 99.30 | |
| 6 d | 0.198 | 0.401 | 0.939 | 25.00 | 0.782 | 0.858 | 19.40 | 13.569 | 0.675 | 5.41 | 14.351 | 99.24 | |
| 7 d | 0.171 | 0.451 | 0.939 | 36.80 | 1.140 | 0.857 | 18.80 | 12.419 | 0.578 | 2.66 | 13.559 | 99.05 | |
| 1 h | 0.418 | 0.504 | 0.999 | 7.70 | 1.829 | 0.496 | 15.60 | 11.801 | 0.532 | 9.32 | 13.631 | 99.50 | |
| 1 d | 0.388 | 0.566 | 0.999 | 7.60 | 5.776 | 0.682 | 13.20 | 18.234 | 0.473 | 7.94 | 24.011 | 99.66 | |
| E | 4 d | 0.376 | 0.602 | 0.991 | 5.50 | 7.576 | 0.680 | 8.90 | 29.217 | 0.495 | 5.16 | 36.794 | 99.73 |
| 6 d | 0.373 | 0.686 | 0.961 | 7.80 | 9.095 | 0.671 | 11.30 | 31.082 | 0.545 | 5.24 | 40.178 | 99.73 | |
| 7 d | 0.300 | 0.692 | 0.930 | 7.70 | 8.815 | 0.586 | 57.20 | 31.099 | 0.511 | 3.05 | 40.606 | 99.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuchs-Godec, R. Prolonged Protection of Copper in Acidic Media Through the Synergistic Effect of Fat-Soluble Vitamins. Materials 2025, 18, 5107. https://doi.org/10.3390/ma18225107
Fuchs-Godec R. Prolonged Protection of Copper in Acidic Media Through the Synergistic Effect of Fat-Soluble Vitamins. Materials. 2025; 18(22):5107. https://doi.org/10.3390/ma18225107
Chicago/Turabian StyleFuchs-Godec, Regina. 2025. "Prolonged Protection of Copper in Acidic Media Through the Synergistic Effect of Fat-Soluble Vitamins" Materials 18, no. 22: 5107. https://doi.org/10.3390/ma18225107
APA StyleFuchs-Godec, R. (2025). Prolonged Protection of Copper in Acidic Media Through the Synergistic Effect of Fat-Soluble Vitamins. Materials, 18(22), 5107. https://doi.org/10.3390/ma18225107






