1. Introduction
Bone defects that exceed the intrinsic healing capacity of the body often require grafting. Autografts and allografts are still considered the clinical gold standard, yet their limited supply, donor site morbidity, and risk of immune reactions have prompted the search for synthetic alternatives [
1,
2,
3]. Synthetic bone substitutes offer unlimited availability, consistent quality, and the possibility of precise adaptation to individual defect geometries [
4,
5]. Despite extensive research efforts, no material currently fulfills all the requirements of an ideal bone substitute. Such a material should combine biocompatibility, osteoconductivity, and controlled degradability to support bone ingrowth and to be gradually replaced by newly formed, mechanically competent tissue [
5,
6,
7].
Among synthetic materials, calcium phosphate cements (CPCs) are well established due to their chemical similarity to the mineral phase of natural bone, which accounts for their excellent biocompatibility and osteoconductivity [
6,
7]. Despite these advantages, CPCs are brittle and exhibit low mechanical strength [
8]. In addition, their slow resorption often leads to the persistence of residual material for extended periods, which can impede complete remodeling and increase the risk of inflammatory reactions and fractures [
9,
10].
To overcome these limitations, magnesium phosphate cements (MPCs) were developed as a new class of bioresorbable bone substitutes [
11,
12]. These materials combine improved solubility with higher mechanical stability compared to pure CPCs. Moreover, the release of magnesium ions (Mg
2+) has been shown to enhance osteoblast proliferation, inhibit osteoclast activity and promote angiogenesis, resulting in accelerated degradation and improved osseointegration [
13,
14,
15,
16]. However, purely magnesium phosphate (MgP)-based systems may degrade too rapidly, causing temporary loss of mechanical integrity before sufficient bone formation occurs [
17].
To combine these positive effects of both materials, calcium–magnesium phosphate cements (CMPCs) were introduced, combining the favorable biological activity of MPCs with the structural stability of CPCs. These materials show adjustable degradation rates, high mechanical strength, and excellent biocompatibility, making them promising candidates for resorbable bone substitutes [
18,
19].
Recent advances in additive manufacturing have further expanded the potential of CMPCs and MPCs. The three-dimensional (3D) powder printing process allows the cost-efficient production of patient-specific scaffolds with tailored porosity and architecture [
20,
21]. Their inherent microporosity (typically > 30 vol. %) promotes nutrient diffusion, cell adhesion, and vascularization [
22,
23]. Optimal osteoconduction occurs at pore diameters of 100–500 µm, whereas higher porosity reduces compressive strength. Therefore, bone scaffolds should ideally exhibit compressive strengths between 2 and 12 MPa, comparable to cancellous bone [
14,
24]. Post-processing techniques are essential for tuning the mechanical and degradation behavior of 3D-printed CMPCs and MPCs. Alkaline post-treatment with diammonium hydrogen phosphate (DAHP) promotes the formation of struvite, markedly increasing compressive strength from approximately 1.3–2.8 MPa to about 10 MPa, as demonstrated in studies by Vorndran et al. [
5,
25]. At the same time, the higher solubility of magnesium-substituted phases allows for better synchronization between scaffold degradation and bone regeneration compared with pure calcium phosphate materials as also confirmed in vitro [
18,
26].
Kowalewicz et al. [
27] confirmed that the degradation rate and new bone formation depend heavily on the type of post-treatment. While CMPCs treated with alkaline post-treatment showed faster degradation, materials treated with acidic post-treatment showed more uniform osseointegration.
Kanter et al. [
28] investigated the degradation mechanisms and bone regeneration capacity of struvite-forming MPC pastes in a sheep model, which showed complete degradation and trabecular bone replacement within 10 months. Kaiser et al. [
17] expanded on these findings by optimizing the degradation rate of MPCs by adjusting the powder-to-liquid ratio. In a partially loaded sheep model, partial degradation of the cements and simultaneous new bone formation were demonstrated after four months.
Hemmerlein et al. [
29] compared 3D-printed MPC and CMPC wedges in a partially loaded rabbit tibia model and found faster degradation for alkaline-treated MPCs than for CMPCs, corresponding to differences in their post-treatment products.
The findings of these studies highlight that the interplay between material chemistry (Ca:Mg ratio), post-treatment conditions, and pore architecture is decisive for tailoring degradation kinetics, ion exchange, and mechanical performance to achieve balanced bone regeneration. Although individual MPC and CMPC scaffolds have been widely investigated regarding their mechanical strength, porosity, degradation, and osteointegration, systematic comparisons of alternative MgP-rich formulations, such as Mg275d and Mg3d, remain to be conducted. In particular, there is a need for qualitative and quantitative characterization of their degradation behavior and the associated osteoregeneration in vivo. This study addresses this gap by investigating 3D powder-printed scaffolds that underwent alkaline post-treatment with defined pore architecture in an unloaded rabbit femoral condyle defect model. The investigated scaffold materials included Mg3d, composed of magnesium phosphate (Mg3(PO4)2), and Mg275d, a calcium-substituted magnesium phosphate (Ca0·25Mg2·75(PO4)2). Tricalcium phosphate (TCP) was used as a clinically established calcium phosphate reference material and served as the control in this study.
2. Materials and Methods
2.1. Production and Characterization of the Scaffold
The experimental methods used in this study are based on the protocols described by Kowalewicz et al. [
19] and adapted to the specific requirements of the materials used.
2.1.1. Production of the Scaffolds
Production of the cements was based on the protocols described in the preliminary studies [
18,
26]. Cements with the chemical composition Ca
xMg
3−x(PO
4)
2 with x = 0 (Mg3d), x = 0.25 (Mg275d) und x = 3 (TCP) were produced from calcium hydrogen phosphate (CaHPO
4, J.T. Baker, Phillipsburg, NJ, USA), calcium carbonate (CaCO
3, Merck, Darmstadt, Germany), magnesium hydrogen phosphate (MgHPO
4-3H
2O, Alfa Aesar, Kandel, Germany) and magnesium hydroxide (Mg(OH)
2, VWR International GmbH, Darmstadt, Germany) in molar ratios corresponding to (
Table 1).
The raw materials were sintered at 1100–1400 °C, ground and then sieved to a particle size of <355 µm. The cylindrical scaffolds (h = 5.1 mm, Ø = 4.2 mm) were 3D-printed using a 3D powder printer (Z-Corporation, Burlington, MA, USA) mixed with 4 wt% hydroxypropyl methylcellulose. After printing, the scaffolds were dedusted, cellulose was burned out at 500 °C for 2 h and sintered. In the case of Ca
0.25Mg
2.75(PO
4)
2 sintering was carried out at 1100 °C, while Mg
3(PO
4)
2 was sintered at 1200 °C. Ca
3(PO
4)
2 was sintered for 4 h at 1350 °C and in a further sintering process to convert α- to ß-tricalcium phosphate for 4 h at 1000 °C. After the sintering process, the Mg3d and Mg275d scaffolds underwent alkaline post-treatment. They were stored for 24 h in 3.5 M (NH
4)
2HPO
4 solution. Sintered TCP scaffolds were used without post-treatment and served as clinically established reference material. Scaffolds (
Figure 1) were washed, dried and γ-sterilized (>25 kGy, BBF Sterilisationsservice GmbH, Kernen, Germany) prior to implantation.
2.1.2. Analysis of Chemical Composition
The chemical composition of the scaffolds was investigated by X-ray diffractometry (D8 Advance, Bruker Corporations, Karlsruhe, Germany) in combination with Rietveld analysis.
The phases were identified using DIFFRAC.EVA V.5.1.0.5. software (Bruker Corporations, Billerica, MA, USA). The quantitative phase analysis was performed based on the diffractograms using TOPAS V6 software (Bruker Corporations, Billerica, MA, USA).
2.1.3. Compressive Strength of the Scaffolds and Young’s Modulus
The compressive strength test was carried out in accordance with the specifications of ISO standard 13175-3:2012 [
30]. The compressive strength was determined for each material using ten samples that had been stored for 24 h at 37 °C in phosphate-buffered saline (PBS). The measurements were performed using a testing machine (Z010, Zwick GmbH, Ulm, Germany) equipped with a 10 kN load cell. A small preload of 1 N was applied to eliminate gaps between specimen and the testing platens. The test speed was 1 mm/min. Young’s modulus was calculated from the stress–strain slope.
2.1.4. Porosity of the Scaffolds
The porosity of the materials was determined by three measurements per group. The Pascal 140/440 porosimeter (Thermo Fisher Scientific Inc., Waltham, MA, USA) was used for this purpose. The measurements were taken within a pressure range of 0.01 kPa to 400 MPa. The data was evaluated using SOLID software (SOLver of Intrusion Data Ver. 1.6.5, Thermo Fisher Scientific Inc., Waltham, MA, USA).
2.1.5. Energy Dispersive X-Ray (EDX) and Scanning Electron Microscopy (SEM) Prior to Implantation
The scaffolds were fixed in 4% buffered formalin solution, then dehydrated in an ascending alcohol series (Roth, Karlsruhe, Germany) and degreased with xylene (Roth, Karlsruhe, Germany). They were then embedded in a methyl methacrylate-based plastic system (Technovit® 9100, Heraeus Kulzer, Wehrheim, Germany). A central thin section (4 µm) was prepared using a rotary microtome (RM2255 Leica, Wetzlar, Germany) and mounted on glass slides coated with ponal-poly-L-lysine (Glaswarenfabrik Karl Hecht, Sondheim, Germany). The samples were smoothed, dried and fixed in a slide press at 37 °C.
For SEM analysis, the samples were coated with platinum layer and examined using a field emission electron microscope (Crossbeam CB 340, Zeiss, Oberkochen, Germany). The chemical phase distribution was analysed using EDX (INCA Energy 350 AzTec Advanced System, Oxford Instruments, Abingdon, UK) at an acceleration voltage of 5–10 keV. The pore structure and the chemical elements magnesium (Mg), calcium (Ca) and phosphate (P) were qualitatively evaluated.
2.2. In Vivo Study
This study was approved by the government of Upper Bavaria in accordance with paragraph 8 of the Animal Welfare Act (approval number Az. ROB 55.2-2532.Vet_02-19-64). A total of 36 adult female Zimmermann rabbits (ZIKA rabbits, Asamhof, Kissing, Germany) aged 6.1 months on average and weighing 4.3 kg on average were included in the study. The rabbits were housed individually in climate-controlled facilities (15–21 °C, 45–65% humidity, 12:12 h light–dark cycle) in cages equipped with a drinking bottle, feed bowl, hay rack, a shelter, and enrichment. A 14-day acclimatization period was observed, and animals received controlled group exercise (daily 10 min, twice weekly 60 min). The rabbits were divided into three observation groups (6, 12, and 24 weeks, n = 12 animals/group). Each rabbit received two scaffolds, one in each lateral femoral condyle. Three scaffold types (Mg3d, Mg275d, TCP) were investigated, with each type implanted 24 times in total (n = 8 per material and time group).
Surgery
One hour before surgery, the animals were given an antibiotic (enrofloxacin 10 mg/kg, Enrobactin
®, CP-Pharma GmbH, Burgdorf, Germany) and analgesic treatment (meloxicam 0.3 mg/kg, Rheumocam
®, Albrecht GmbH, Aulendorf, Germany) orally. The operation was performed under general anaesthesia, which was induced with medetomidine (0.25 mg/kg i.m., Dorbene vet
®, Zoetis Deutschland GmbH, Berlin, Germany) and ketamine (15 mg/kg i.m., Anesketin
®, Albrecht GmbH, Aulendorf, Germany). The animals were then endotracheally intubated, the surgical site shaved and prepared aseptically. Anaesthesia was maintained with isoflurane (1.5–2 vol%, with simultaneous oxygen supply of 1 L/min). Throughout the operation, the rabbits received fentanyl (10 µg/kg/h i.v., Fentadon
®, Albrecht GmbH, Aulendorf, Germany) via a syringe pump (Perfusor
® compact, B. Braun AG, Melsungen, Germany) for analgesia. The surgical procedures were performed as described by Kowalewicz et al. [
19,
27]. The scaffold was implanted precisely into a drill hole defect in the spongy area of the lateral femoral condyle (
Figure 2). Immediately after surgery, in vivo microcomputed tomography (µCT) was performed on both hind limbs and X-rays were taken in two projections. Postoperatively, the animals received buprenorphine (20 µg/kg i.v., Bupresol
®, CP-Pharma GmbH, Burgdorf, Germany). The antibiotic enrofloxacin (10 mg/kg, Enrobactin
®, p.o., CP-Pharma GmbH, Burgdorf, Germany) and the analgesic meloxicam (0.3 mg/kg p.o., Rheumocam
®, Albrecht GmbH, Aulendorf, Germany) were administered to the animals once daily for five days after the operation. In the first two weeks after surgery, the animals received close daily clinical examination, encompassing evaluations of overall condition, appetite and hydration, gastrointestinal and urinary output, body weight fluctuations, and any evidence of pain, lameness, or wound-healing disorders. In the following weeks, the animals were clinically examined every other day. After the respective trial period (6, 12, or 24 weeks), the animals were euthanized with pentobarbital (200–230 mg/kg, Narkodorm
®, CP-Pharma GmbH, Burgdorf, Germany) in accordance with animal welfare guidelines after sedation with propofol (5 mg/kg, Narcofol
®, CP-Pharma GmbH, Burgdorf, Germany). The femora were obtained, and any attached soft tissue was carefully removed.
2.3. X-Ray Examinations
Immediately after surgery and at fixed intervals (every 2 weeks until week 12, then every 4 weeks), the knee with the implanted scaffolds were X-rayed in two projections (cranio-caudal (cr/cd) and medio-lateral (m/l)) using an X-ray machine (Multix Select DR, Siemens GmbH, Erlangen, Germany). For this purpose, the animals were sedated with medetomidine (0.25 mg/kg i.m., Dorbene vet®, Zoetis Deutschland GmbH, Berlin, Germany) and ketamine (115 mg/kg–0.5 kg i.m., Anesketin®, Albrecht GmbH, Aulendorf, Germany). The settings 54.9 kV and 4.5 mAs were used for the images. The images were evaluated using DicomPACS vet software (Ver. 8.3.20, Oehm und Rehbein GmbH, Rostock, Germany). Both projections were evaluated independently by two examiners for visibility of the scaffold.
2.4. In Vivo µCT Examinations
After the X-ray examination the still sedated animals were in a supine position with their hind limbs extended and supplied with oxygen (2 L/min) via a laryngeal mask (v-gel® rabbit, Docsinnovet Ltd., London, UK). The µCT scans from the knee joint space to the proximal femoral condyles were performed using the XtremeCT (Scanco Medical, Zurich, Switzerland). The settings used were 68 kV voltage, 1000/180° projections, 200 ms integration time and voxel size 30.3 µm. All µCT scans were displayed and calculated using the µCT Evaluation Program V6.6:403 software (Scanco Medical, Zurich, Switzerland). The original scans were rotated along the longitudinal axis of the scaffolds, thereby displaying the scaffold cross-section.
2.4.1. Quantitative Evaluation of In Vivo μCT Scans
In order to distinguish the scaffold from the surrounding bone tissue, a separate threshold (Th) was determined for each material based on the gray values of six implanted scaffolds immediately after surgery, as described in the literature [
19]. The thresholds were 103 for Mg3d, 121 for Mg275d, 219 for TCP and 142 for cancellous bone at the same location.
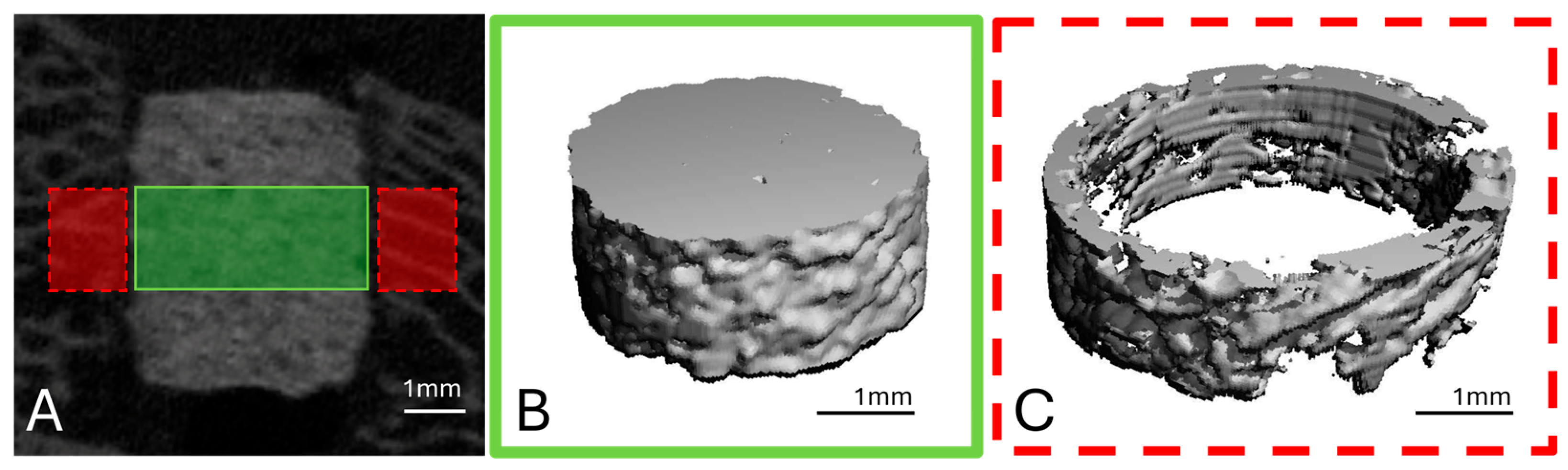
To analyse the scaffold volume (SV), a region of interest (ROI) was defined in the middle third of the scaffold height in the form of a cylinder with a diameter of 140 voxels (=4.24 mm) and a height of 60 slices (=1.82 mm), based on established methods [
19] (
Figure 3A,B).
To examine the scaffold environment, a hollow cylinder (inner diameter: 144 voxels = 4.36 mm; outer diameter: 180 voxels = 5.45 mm; height: 60 slices) was placed around the previously defined ROI (
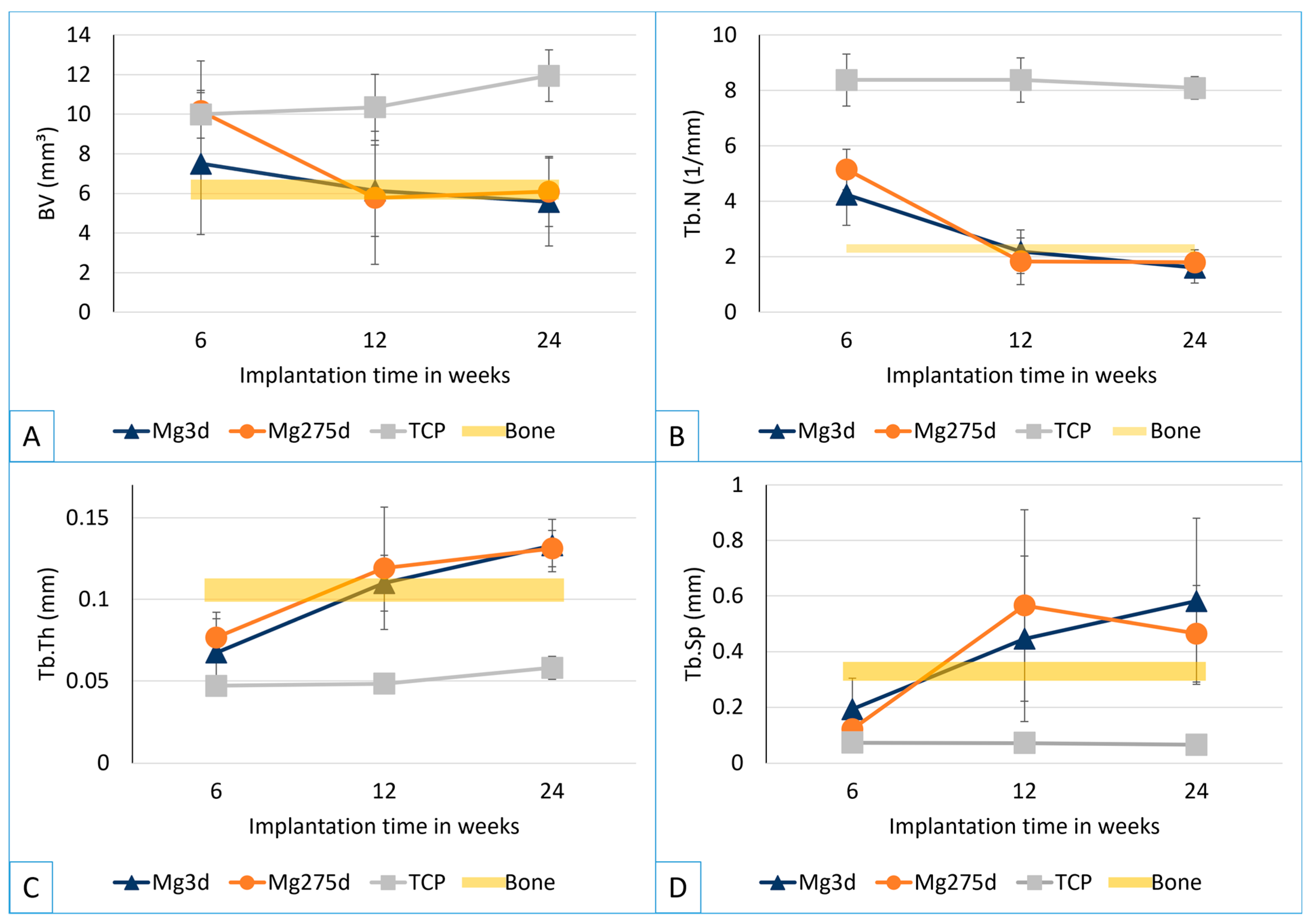
Figure 3A,C). Here, the bone volume (BV), trabecular number (Tb.N), trabecular thickness (Tb.Th) and trabecular spacing (Tb.Sp) were determined. All parameters were evaluated using the µCT Evaluation Program V6.6 software (Scanco Medical, Zurich, Switzerland).
2.4.2. Semiquantitative Evaluation of In Vivo μCT Scans
The semiquantitative evaluation was performed on two levels. To obtain a cross-sectional view of the scaffolds with the surrounding cancellous bone, the original scan was reoriented using the µCT Evaluation Program V6.6 software (Scanco Medical, Zurich, Switzerland). For a more accurate assessment of the degradation process, an established semi-quantitative scoring system was used [
19] (
Table S1). The following parameters were evaluated by two independent observers in both levels: (1) position in the bone; (2) scaffold–bone contact; (3) distinguishability from bone tissue; (4) degradation behaviour in the medullary and cortical bone; (5) loss of cylindrical shape; (6) formation of a radiolucent reconstruction zone (area within the scaffold volume characterized by a significantly lower gray value). Score values from 0 to 2 were assigned for each parameter.
2.5. Micro-Computed Tomography 80 (µCT80)
The distal lateral femoral condyles were separated from the prepared femurs using a diamond band saw (cut-grinder, Walter Messner GmbH, Oststeinbek, Germany) and fixed in 4% formalin solution. They were then dehydrated in an ascending ethanol series (50%, 70%, 80%, 90%, 96% and 100%). The samples were then embedded in methyl methacrylate-based plastic resin (Technovit® 9100, Heraeus Kulzer, Wehrheim, Germany) and, after complete curing, scanned in the even higher resolution µCT80 (Scanco Medical, Zurich, Switzerland) with the settings 70 kV voltage, 600 ms integration time and 10 µm voxel size. The scans were displayed in the same way as the in vivo scans using the µCT Evaluation Program V6.6:403 software (Scanco Medical, Zurich, Switzerland). Manual contouring of the scaffolds and rotation made the cross-section of the original implantation area visible.
2.5.1. Quantitative Analysis of μCT80
For quantitative evaluation, the threshold for cancellous bone (146–272) was determined based on the gray value of eight samples per material group. The BV and bone structure parameters (Tb.N, Tb.Th, Tb.Sp) were analysed in the cylindrical ROI in the middle third of the scaffold area (h = 182 voxels = 1.82 mm; Ø = 424 voxels = 4.24). As a reference for intact cancellous bone, the parameters were determined at the same location in four explanted lateral femoral condyles of adult Zimmermann rabbits with intact femurs.
2.5.2. Semiquantitative Analysis of μCT80
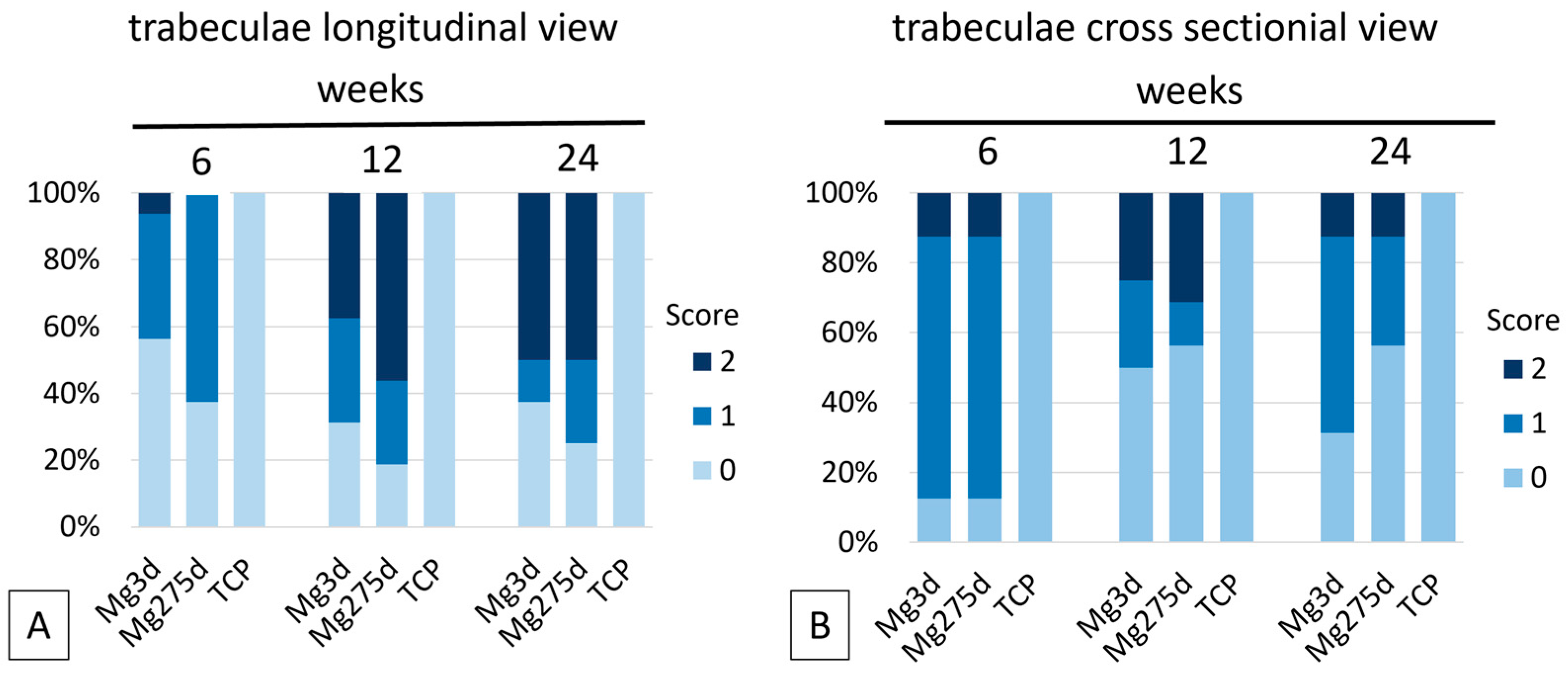
For the semi-quantitative analysis, the entire scaffold area was evaluated by two independent observers in the cross sectional and longitudinal views using a modified scoring system [
19]. Scores from 0 to 2 were assigned for the presence of scaffold material and the distribution of trabeculae in the longitudinal and cross sectional view (
Table S2).
2.6. Histological Examination
From the bone scaffold complexes embedded in Technovit 9100, a central thick section of approx. 70–100 µm was created from the cross-section of the implantation region using the Donath separation thin section technique [
31] with a diamond saw (Cut Grinder, Walter Messner, Oststeinbek, Germany) and a grinding machine (Lap Grinder, Walter Messner, Oststeinbek, Germany) and then stained with 0.1% toluidine blue (Waldeck, Münster, Germany). The longitudinal axis of the cylinder was perpendicular to the cut surface.
The histological sections were evaluated by two observers using a light microscope (Zeiss Axio Imager 2, Carl Zeiss Microscopy GmbH, Jena, Germany), the Zeiss Axio Cam Mrc digital camera and the Zeiss ZEN 3.0 software (Carl Zeiss Microscopy GmbH, Jena, Germany) in accordance with the study by Kowalewicz et al. [
19].
2.6.1. Quantitative Analysis of the Tissue (Histomorphometry)
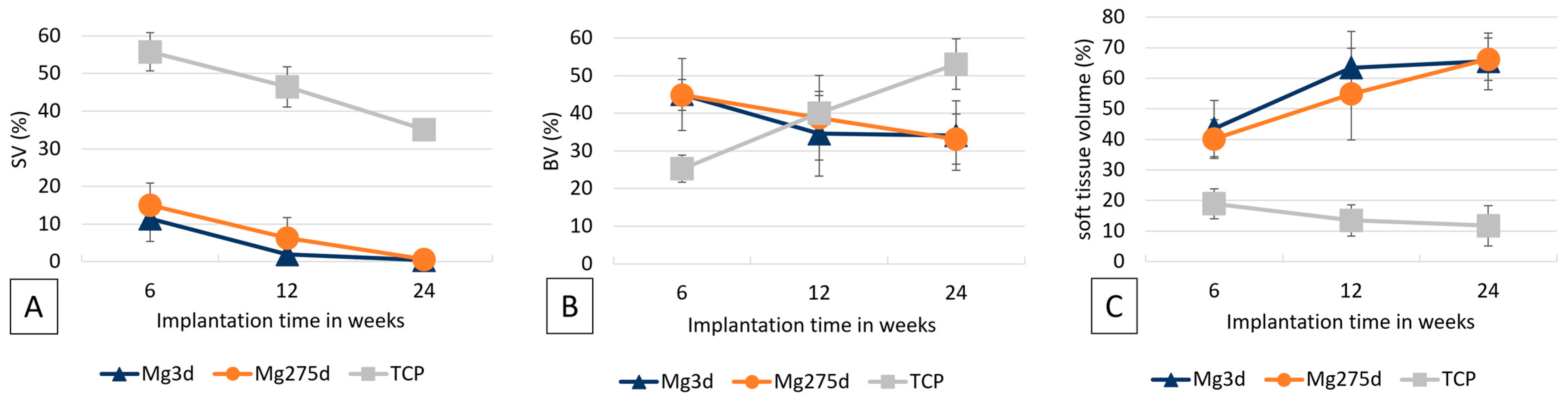
Images of the cross sections were taken at 20× magnification from the stained thick sections. A circular ROI (Ø = 1060 pixels = approx. 4.2 mm) was placed centrally around the original implantation area. The percentage areas of scaffold material, newly formed bone, and soft tissue (granulation tissue, bone marrow) were quantified and analysed using the Intellesis module of the Zeiss ZEN 3.0 software(Carl Zeiss Microscopy GmbH, Jena, Germany).
2.6.2. Semiquantitative Analysis of Histological Sections
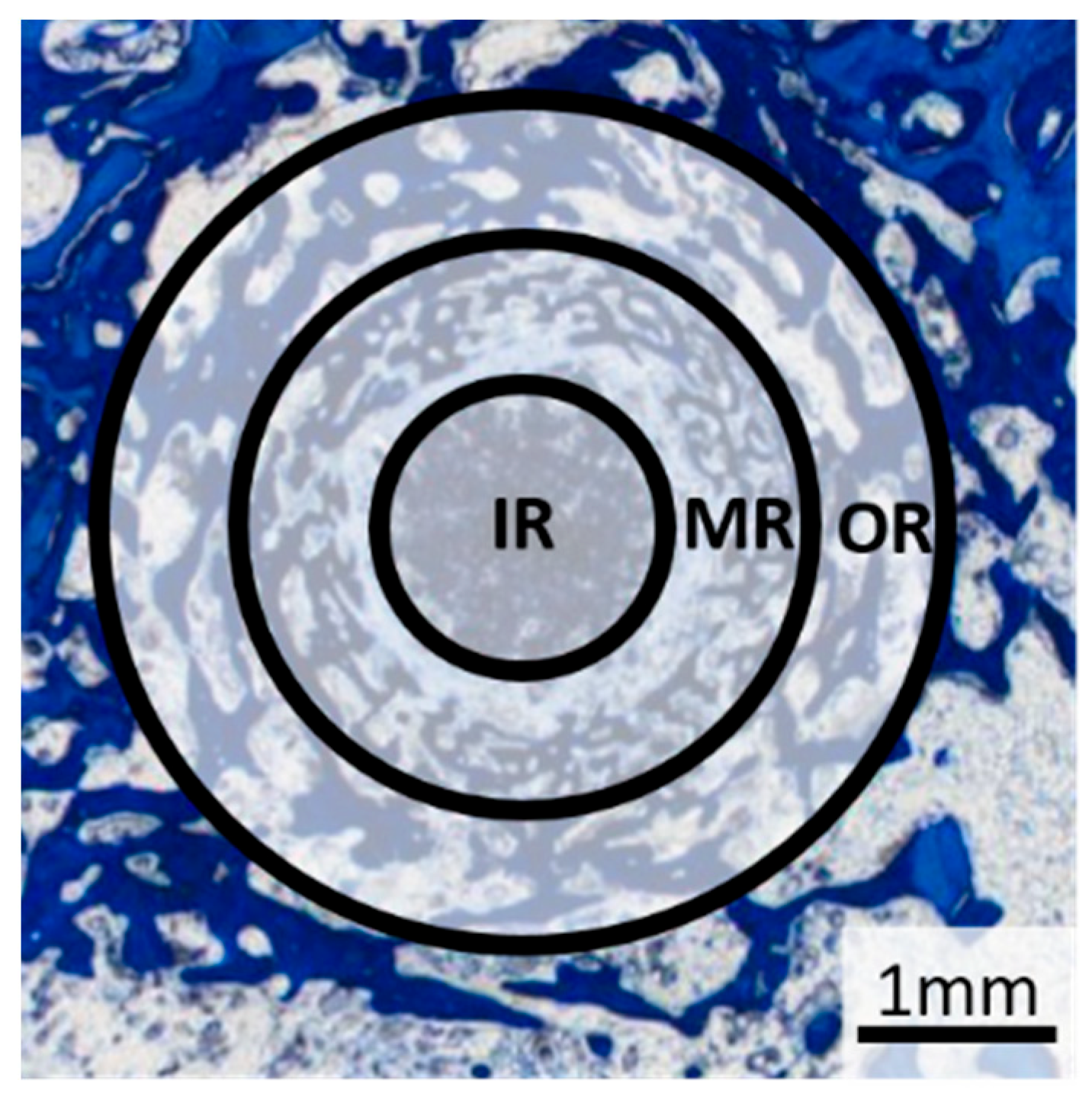
A 25× magnification was used to evaluate the tissue types. Based on the method described by von Doernberg et al. [
32] the scaffold region was divided into three zones, each corresponding to one third of the scaffold diameter (inner ring (IR): Ø = 1.41 mm, middle ring (MR): Ø = 2.83 mm, outer ring (OR): Ø = 4.24 mm) (
Figure 4). The tissue in each zone was analysed according to the criteria of previous studies [
19,
32,
33].
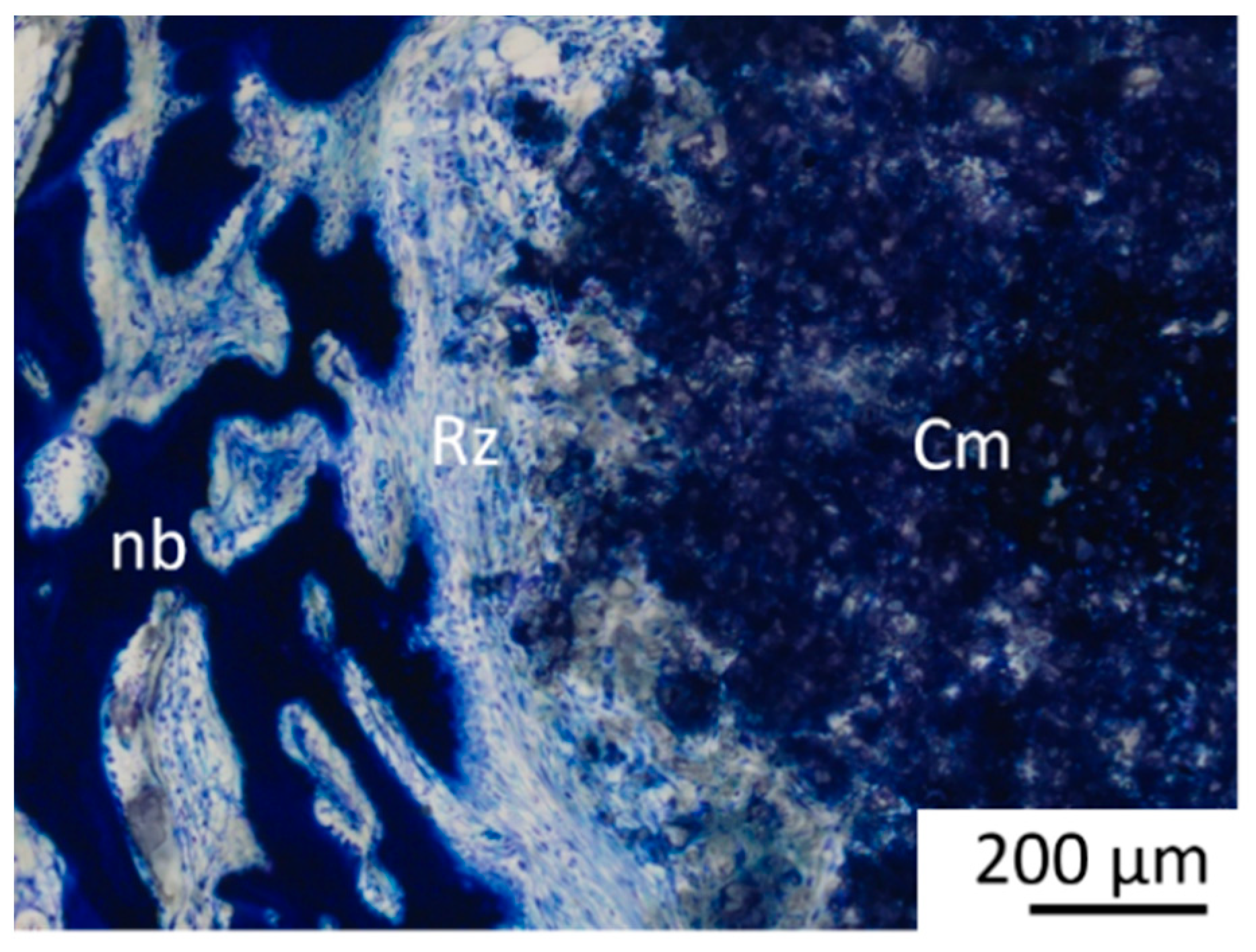
The scaffold material, young bone tissue (thin, dark blue trabeculae), mature bone tissue (light blue lamellar structure) and the reconstruction zone (cell-rich zone consisting of fibroblasts, macrophages and erythrocyte accumulations) were evaluated according to the area fraction per zone (IR, MR and OR) using scores from 0 to 3: 0% (score 0), 1–25% (score 1), 26–50% (score 2) and over 50% (score 3). Scaffold material surrounded by bone was assessed as follows: no scaffold surrounded by bone (score 0), 1–25% of the scaffold material surrounded (score 1), 26–50% of the scaffold material surrounded, all of the scaffold material surrounded by bone (score 3).
Cell assessment was performed in one field of view per zone at 100× magnification. Adipocytes, precursor cells, macrophages, and osteoblasts were quantified using a semi-quantitative scale (score 0 = none, score 1 = 1–5 cells, score 2 = 6–10 cells, score 3 = >10 cells). Multinucleated cells, including foreign body giant cells (FBGCs) and osteoclast-like cells, were identified based on histological morphology and location (at bone or material surfaces) and evaluated according to the same criteria. Vascular supply was analyzed by the number of erythrocyte accumulations, while osteoid formation was graded as absent (score 0), sporadic (score 1), thin layer on trabeculae/scaffold (score 2), or thick layer on trabeculae/scaffold (score 3).
2.7. SEM and EDX Analysis of Scaffold–Bone Composite
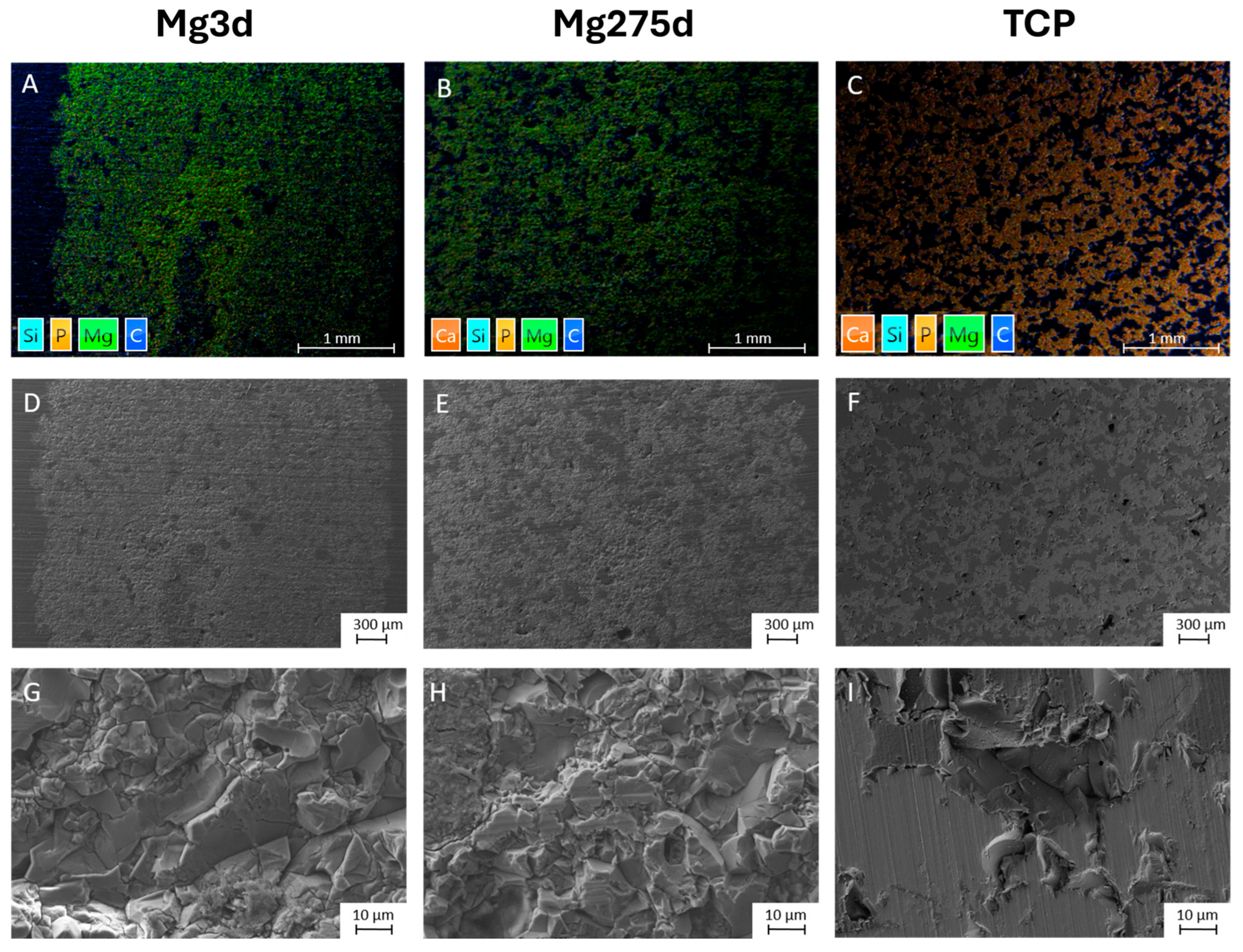
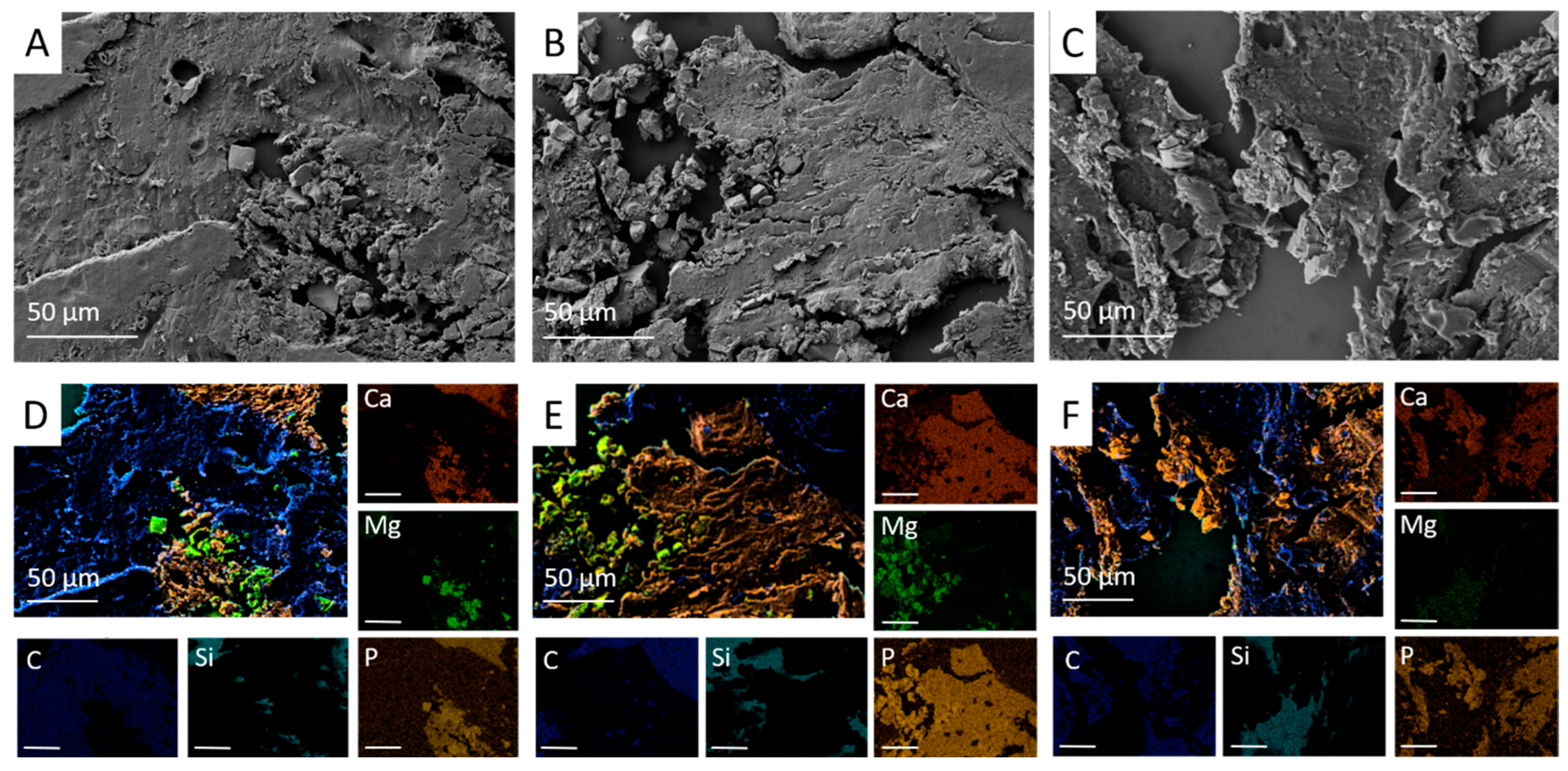
For SEM and EDX analysis, a central unstained thin section of the bone-plastic block (4 µm; n = 2 per material and time group) was prepared according to the methods described in
Section 2.1.5. The scaffold centre was defined based on the ROIs determined during the histological examination and examined at 28×, 500× and 1000× magnification. The SEM images were used to assess osseointegration and the structure in the implantation area. In the EDX, the presence of material particles was determined based on the distribution of magnesium (Mg), calcium (Ca) and phosphorus (P) ions using EDX.
2.8. Statistics
The sample size (n = 8 per material and time point) was determined in accordance with the biometric planning that was approved by the animal experiment authorization, allowing reliable estimation of effect sizes and confidence intervals for exploratory analysis. All statistical evaluations were performed exploratively at a 5% significance level. The statistical analysis of the compressive strength and porosity measurements was performed using 1-way ANOVA analysis and Tukey test (Origin 7G, OriginLab Corporation, Northampton, MA, USA). The in vivo data was evaluated using SPSS Statistics 26 (IBM Company, Armonk, NY, USA). The Shapiro–Wilk test was performed to determine the normal distribution of the quantitative data. Normally distributed data were evaluated using analysis of variance (ANOVA followed by Tukey’s post hoc test). For semi-quantitative and non-normally distributed data, the Kruskal–Wallis test was used, followed by adjustment of the significance values using the Bonferroni correction. Values with p ≤ 0.05 were considered statistically significant.
4. Discussion
The present study evaluated the degradation, osseointegration, and osteoregenerative capacity of 3D powder-printed magnesium phosphate-based scaffolds (Mg3d and Mg275d) in comparison with TCP as a reference. Both MgP-containing scaffolds exhibited excellent biocompatibility, centripetal degradation that was significantly faster than TCP, and bone formation progressing in synchrony with scaffold resorption. No major differences were found between Mg3d and Mg275d regarding degradation or bone regeneration, indicating that moderate differences in Mg content do not substantially affect biological performance. These findings are consistent with previous CMPC and MPC investigations in rabbits and large animals [
17,
19,
29], confirming the strong osteoconductive response and favorable degradation profile of MgP-based ceramics.
The initial compressive strength of both Mg3d and Mg275d scaffolds exceeded that of TCP and was within the range of cancellous bone [
18,
34]. This improvement has been attributed to the phase transformation from farringtonite to struvite during DAHP post-treatment [
27,
35]. Similar strength increases were described for other 3D-printed MPC scaffolds [
35,
36]. Hemmerlein et al. [
29] likewise confirmed adequate stability of 3D-printed CMPC wedges under partial load, emphasizing that the degradation rate must still ensure mechanical integrity until sufficient bone bridging occurs. The measured Young’s moduli in this study (0.21–1.35 GPa) indicate that the tested Ca–Mg–P formulations match the mechanical stiffness of cancellous bone, reducing the risk of stress-shielding. However, since this modulus is substantially lower than cortical bone (≈10–25 GPa), so applications requiring high load-bearing capacity will require metallic reinforcement during the time of bone regeneration. Nevertheless, as no post-implantation mechanical testing was performed, potential changes in mechanical properties during in vivo degradation remain unknown and should be addressed in future studies.
The surface roughness determined for the scaffolds in this study, which is attributed to fused ceramic particles, is a hallmark of 3D powder-printed scaffolds [
5,
26,
37]. This characteristic facilitates a substantial surface area, thereby promoting cell adhesion and early osteointegration [
23,
38].
In the present study, Mg3d and Mg275d exhibited high open porosity comparable to TCP. Although alkaline post-treatment can slightly reduce porosity via struvite precipitation [
27,
39], this effect was minimal in the current scaffolds. High porosity supports cell migration and vascularization but compromises mechanical [
40]. A comparable porosity range was found optimal for balancing stability and degradation in CMPC and MPC systems [
29].
The excellent clinical compatibility observed for the Mg3d, Mg275d, and TCP scaffolds in this study aligns well with prior research on Mg3d-based bone substitutes in various forms [
15,
41]. Only two rabbits experienced transient mild lameness and minor wound healing issues, which were unequivocally not attributable to the scaffold materials. Moreover, the cellular examination consistently showed no significant inflammatory reaction, further reinforcing the favorable in vivo biocompatibility of the tested materials.
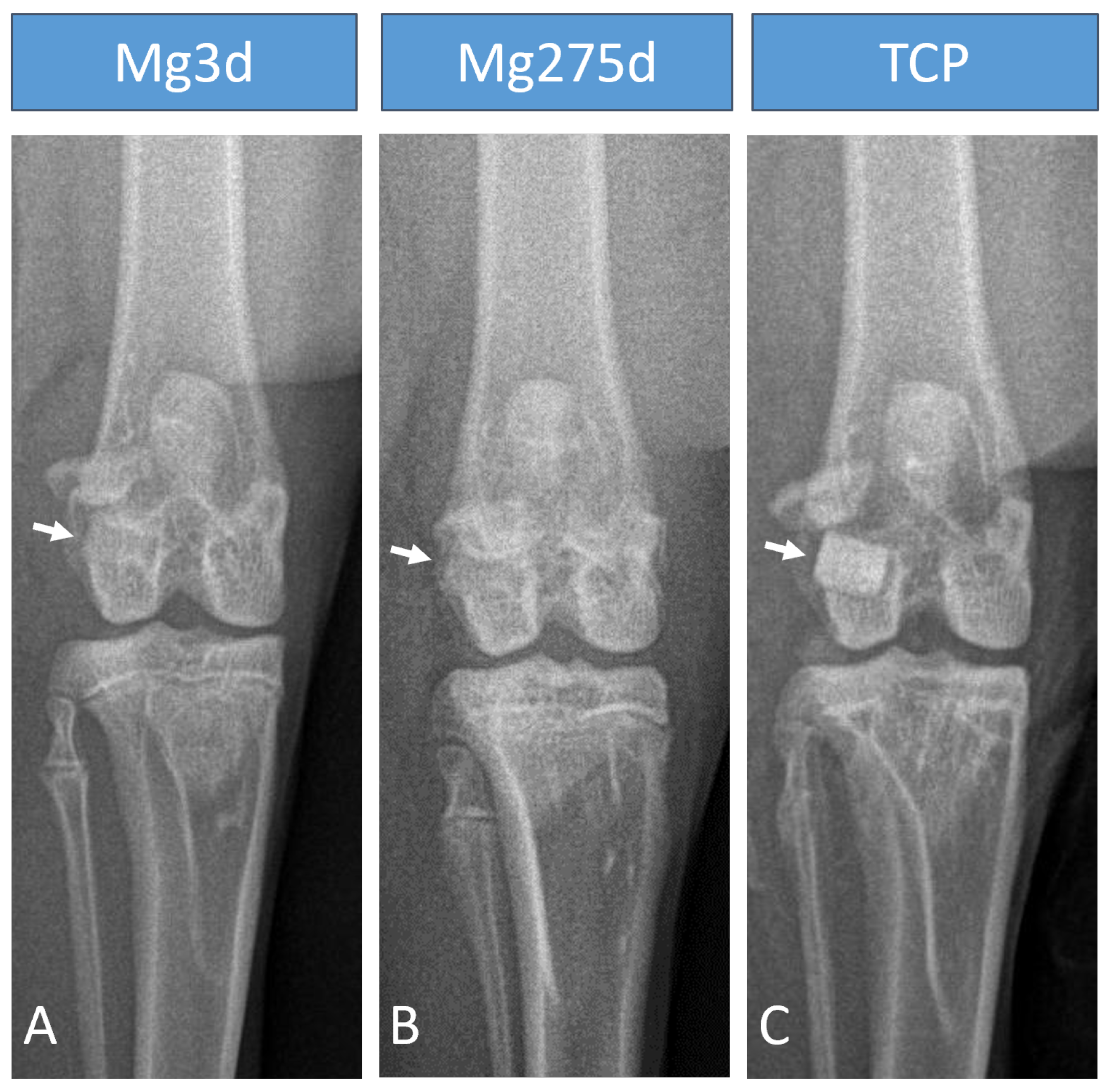
The X-ray examination immediately after surgery showed that Mg3d and Mg275d had similar radiopacity to the surrounding bone, with Mg3d being the least visible. In contrast, TCP could be clearly distinguished from the surrounding bone due to its high X-ray density [
42]. Although this limited long-term radiological monitoring, the rapid loss of visibility indicated accelerated degradation. Similar findings have been reported in other in vivo studies, which also observed reduced visibility and faster degradation of CMPC scaffolds compared to TCP [
19,
41].
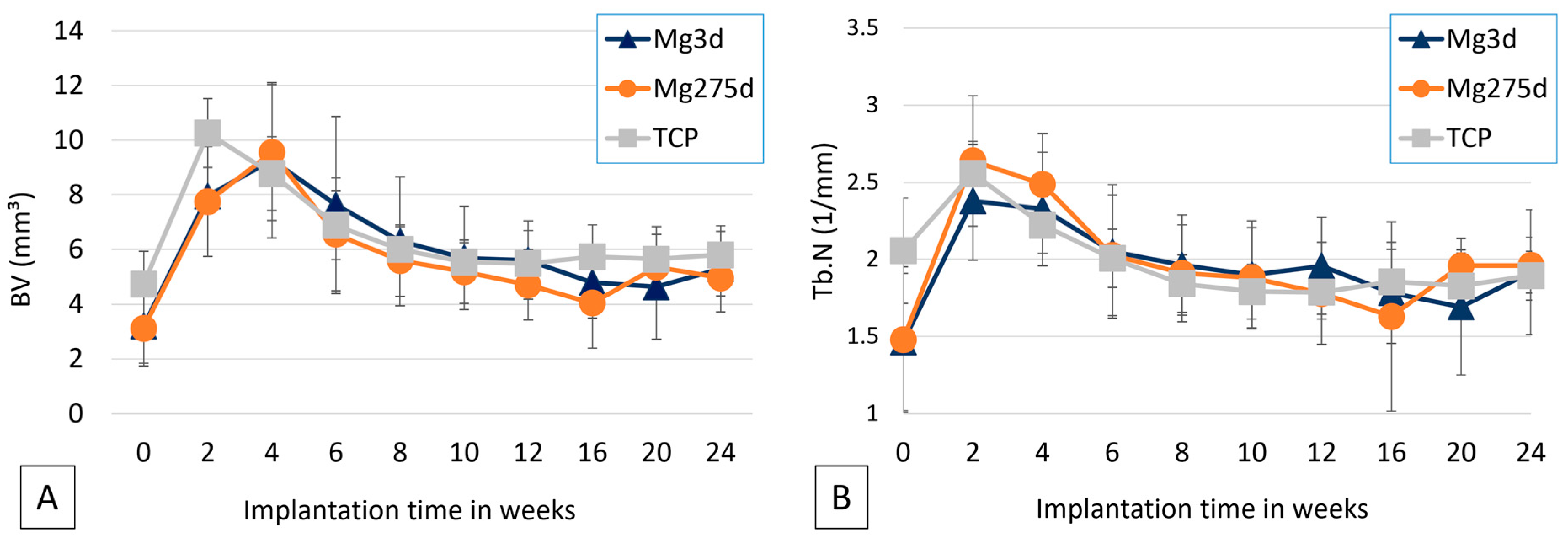
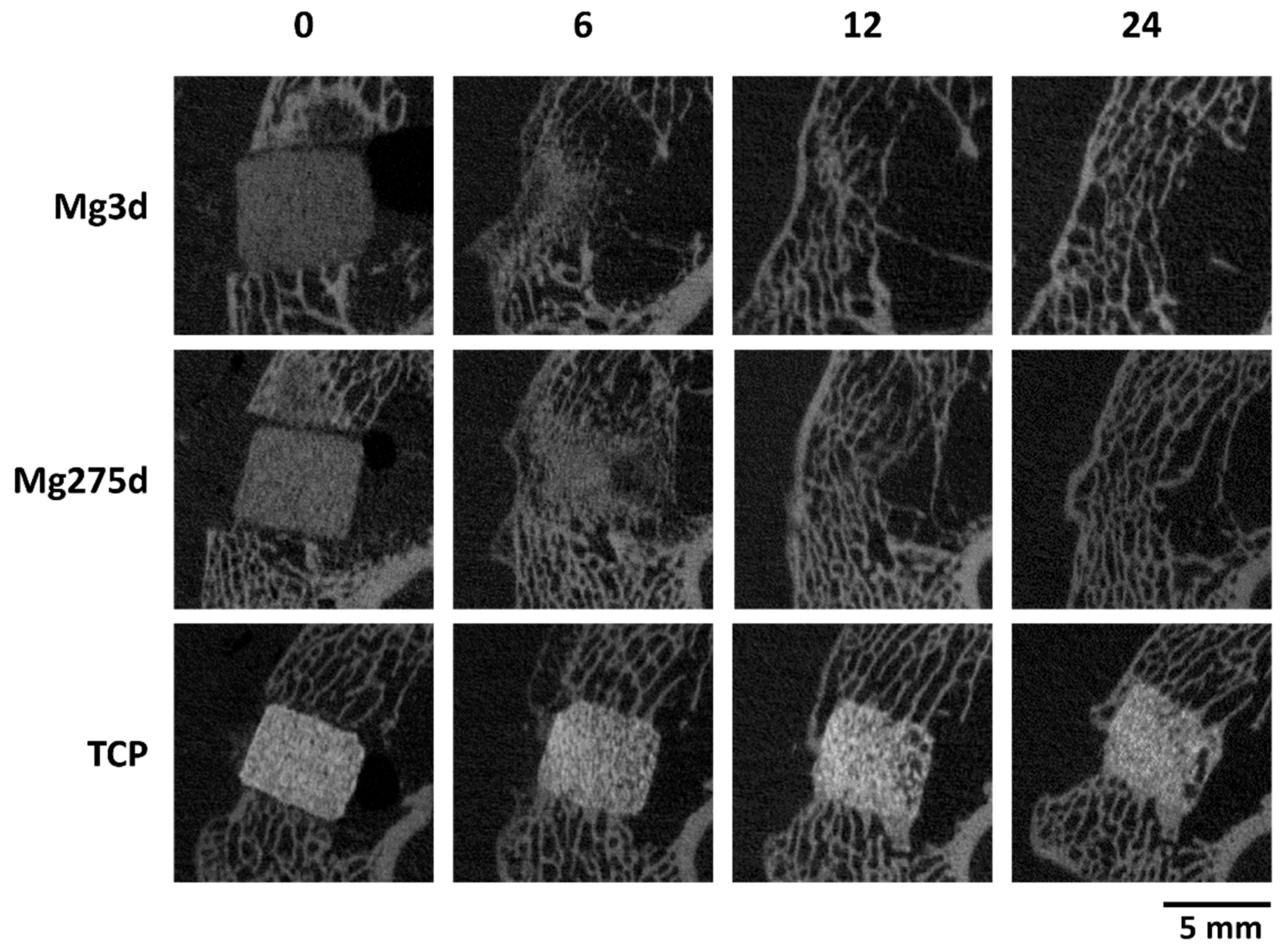
µCT analyses confirmed these results, revealing a significantly faster volume loss for Mg3d and Mg275d than TCP from week 4 onwards. Degradation of the MgP-based scaffolds initiated at the periphery and progressed centripetally, leading to an early loss of the cylindrical structure. In contrast, TCP degraded slowly and uniformly, maintaining its geometry until the end of the study. By week 12, Mg3d and Mg275d were barely distinguishable in µCT, although histology and EDX still revealed small remnants, indicating that most degradation occurred within the first 12 weeks. TCP degraded considerably more slowly, consistent with earlier findings [
42,
43]. Kowalewicz et al. [
19] demonstrated almost complete degradation of alkaline-treated MPC scaffolds by week 24, and Kaiser et al. [
17] observed similar rapid degradation of struvite-based pastes in vivo.
The accelerated degradation of Mg3d and Mg275d can be attributed mainly to the high solubility of their constituent phases. Stanfieldite (0.361 g/L) and struvite (0.2 g/L) dissolve markedly faster than farringtonite (0.0022 g/L) and the TCP phases α-TCP (0.0025 g/L) and β-TCP (0.0005 g/L) [
12,
18]. Similar mechanisms were described by Kaiser et al. [
17], who reported rapid dissolution of struvite and K-struvite cements in partially loaded sheep tibiae, with up to 63% mass loss after 4 months. As shown in the study by Kanter et al. [
10], the highly soluble phases (struvite and stanfieldite) dissolve first, while the loose farringtonite fragments remain. As a result, the scaffolds quickly lose stability [
44].
Moreover, degradation in the present study proceeded more rapidly near the bone marrow cavity, where higher vascularization and metabolic activity accelerated ion exchange and dissolution. This region-dependent pattern was more pronounced for Mg3d and Mg275d than for TCP and agrees with prior findings [
19,
45,
46,
47].In addition to chemical dissolution, histology showed macrophages, FBGCs and osteoclast-like cells indicating active cell-mediated resorption. Their number decreased as Mg3d and Mg275d degraded, while remaining relatively constant in TCP samples. The appearance of macrophages is considered a typical reaction to cement in rapidly degrading cements [
34]. They play an important role in the homeostasis of bone regeneration and are also discussed as osteoclast precursor cells [
34]. According to Sheikh et al. [
48], the fusion of macrophages into FBGCs is a reaction to the biomaterial. They are formed in the presence of large, difficult-to-degrade particles that cannot be phagocytosed by individual macrophages. Although Gefel et al. [
26] reported only chemical dissolution of 3D-printed magnesium phosphate scaffolds composed of struvite and newberyite without osteoclastic resorption in vitro, the present in vivo results demonstrated the presence of osteoclast-like cells at the implantation site, suggesting that cellular mechanisms may contribute to scaffold remodeling under physiological conditions. Such osteoclastic involvement has also been proposed in other in vivo studies [
19,
28].
These observations support the notion that degradation of MgP-based scaffolds is governed by a synergistic interplay of physicochemical dissolution and cellular resorption. The release of Mg
2+ and phosphate ions may further promote macrophage activation and osteoclast differentiation. A comparable mixed degradation behavior was also reported for 3D-printed CMPC and MPC wedges in partially loaded rabbit tibiae by Hemmerlein et al. [
29], where the presence of FBGCs and osteoclasts coincided with advanced scaffold resorption and early trabecular bone formation.
Minor fluctuations in quantitative µCT data at later time points likely resulted from segmentation artefacts due to similar radiodensity of new bone and residual scaffold. Consequently, µCT may have slightly overestimated residual volume, whereas histology provided more accurate quantification, confirming the overall degradation trend [
10,
19,
49].
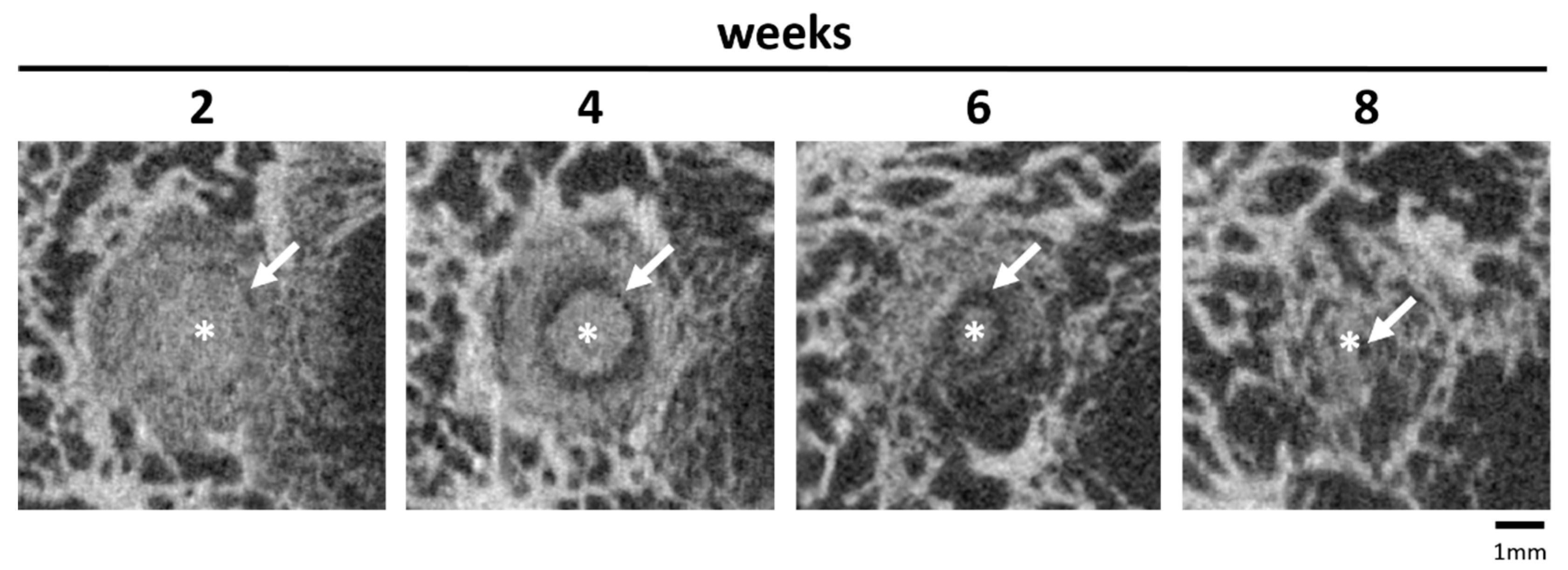
A characteristic feature was the formation of a cell-rich reconstruction zone composed of fibroblasts, macrophages, and osteoprogenitor cells. This zone migrated centripetally, drawing a dense network of newly formed trabeculae with it, until it had completely disappeared after 10 (Mg275d) or 12 (Mg3d) weeks at the latest. Similar transient reconstruction zones were described by Kowalewicz et al. [
19] for Mg225d scaffolds and by Kaiser et al. [
17] for struvite-based MPCs in load-bearing defects. Additionally, cell-rich reconstruction zones of a comparable nature have also been described in rapidly degrading CPCs [
42,
50]. There is probably a connection between the reconstruction zone and the degradation rate, as it occurs more frequently in faster-degrading cements [
17,
19]. In addition, the formation of the reconstruction zone may have been favoured by the type of post-treatment. By placing the scaffolds in the DAHP solution, it is possible that the phase transition from farringtonite to struvite occurred most strongly in the peripheral areas of the scaffolds. Due to the higher solubility of struvite compared to farringtonite, the scaffolds degraded more quickly in the peripheral area, making room for migrating cells. The associated high cell activity in the reconstruction zone indicates an active tissue reaction. This zone supports the decomposition of cement and promotes the migration of osteoprogenitor cells and osteoblasts, which contributes to the formation of new bone tissue [
17,
48].
The faster degradation of Mg3d and Mg275d compared with TCP and the formation of a transient reconstruction zone are promising features, yet their clinical relevance depends on how well degradation and new bone formation are synchronized. The interplay between degradation and bone regeneration is of central importance for the mechanical performance of bioresorbable scaffolds. Too rapid degradation may result in premature loss of stability as fibrous tissue forms around the implant [
17,
51], whereas excessively slow degradation can delay complete integration and replacement by native bone, potentially promoting foreign-body reactions or chronic inflammation [
52].
Histological and µCT80 analyses of the present study revealed material-specific regeneration patterns among the tested materials. In Mg3d and Mg275d scaffolds, bone formation occurred mainly outside the reconstruction zone, forming dense trabecular networks, while TCP exhibited homogeneous bone ingrowth directly onto the scaffold surface, consistent with previous findings by Kowalewicz et al. [
19]. All scaffolds showed an early increase in bone volume at week 6 compared with native bone, confirming their osteostimulatory potential, as also documented for other MPC-, CMPC-, and TCP-based materials [
15,
19,
28,
41].
The release of Mg
2+ ions during scaffold resorption likely enhanced osteogenesis and angiogenesis [
15,
41,
53]. Rapid bone formation in the peri-implant region further indicates high biocompatibility [
15,
16,
41,
53]. An additional osteoinductive effect may have been triggered by mechanical stimulation during drilling, as described previously [
54].
Spatially, MgP-based scaffolds showed greater trabecular formation near the cortex and less in the marrow region, similar to the results reported by Kowalewicz et al. [
19] for faster-degrading Mg225d scaffolds. These observations suggest that the rapid degradation of the Mg3d and Mg275d scaffolds in the medullary region could impair their osteoconductive effect. The slower degradation of TCP, on the other hand, preserves sufficient scaffold structure to serve as a guide for bone growth even in the medullary region [
19]. However, it should be noted that even in the physiological state, there is less bone tissue in the medullary region, which could further explain the lower trabecular formation in this area.
Between weeks 6 and 24, Mg3d and Mg275d scaffolds exhibited a gradual transition from woven to lamellar bone, with trabecular parameters approaching physiological values. Few osteoblasts or osteoclast-like cells were detected by week 24, indicating completion of bone maturation [
19,
55]. TCP, in contrast, still contained osteoid and active cells, suggesting delayed remodeling.
Although bone ingrowth was clearly demonstrated, no biomechanical testing of the bone–scaffold composites was performed. Subsequent investigations should therefore include mechanical analyses of explanted specimens to better assess the functional stability and clinical relevance of the regenerated bone. During degradation, macrophages and osteoclast-like cells actively contributed to material resorption, a typical response to bioresorbable magnesium phosphate ceramics [
19,
34,
48].
While this study clearly demonstrates the material-specific osseointegration and degradation behavior of the scaffolds in an unloaded rabbit model, the lack of physiological loading limits direct transferability to clinical conditions. To address this, upcoming experiments should include mechanically more demanding settings and extended observation periods beyond 24 weeks. Evidence from long-term investigations in large animal models demonstrated that complete remodeling and cortical consolidation of magnesium phosphate cements may require up to 9–12 months [
10,
17,
28]. These findings underline the importance of prolonged evaluation to verify the long-term stability and functional performance of regenerated bone in load-bearing applications.
As an exploratory and orientation study, the present work was designed to estimate effect sizes, assess variability, and identify general trends between the different materials. The sample size was determined according to the 3Rs principle, ensuring ethical use of animals while providing meaningful preliminary data. To substantiate the observed tendencies and detect more subtle differences between materials and time points, future studies with larger cohorts will be necessary.