In Situ Synthesis of Porous SnO2/SnS2@PC Anode Material with High Capacity Using Calcium Carbonate as Template for Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
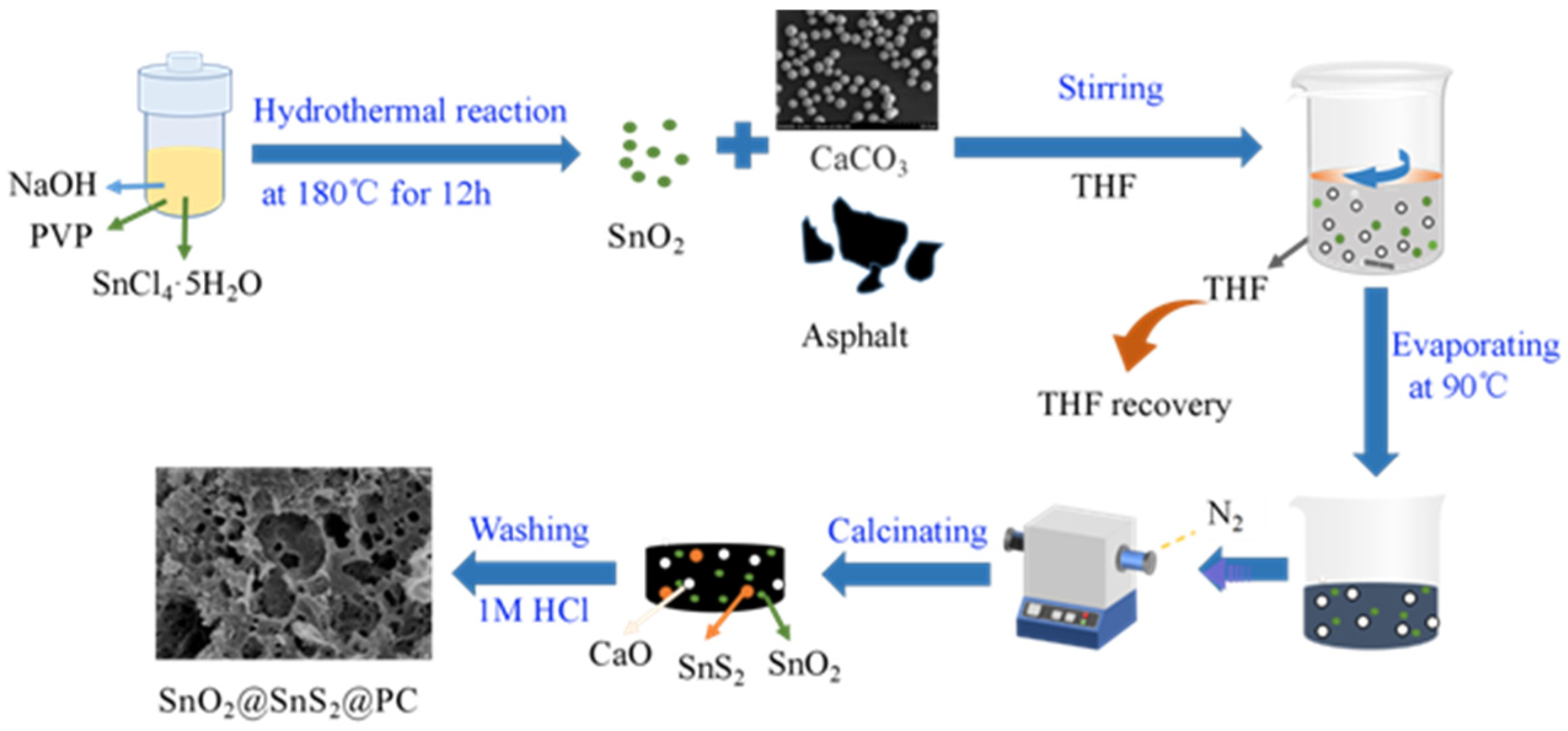
2.2. Synthesis of SnO2/SnS2@PC Composite
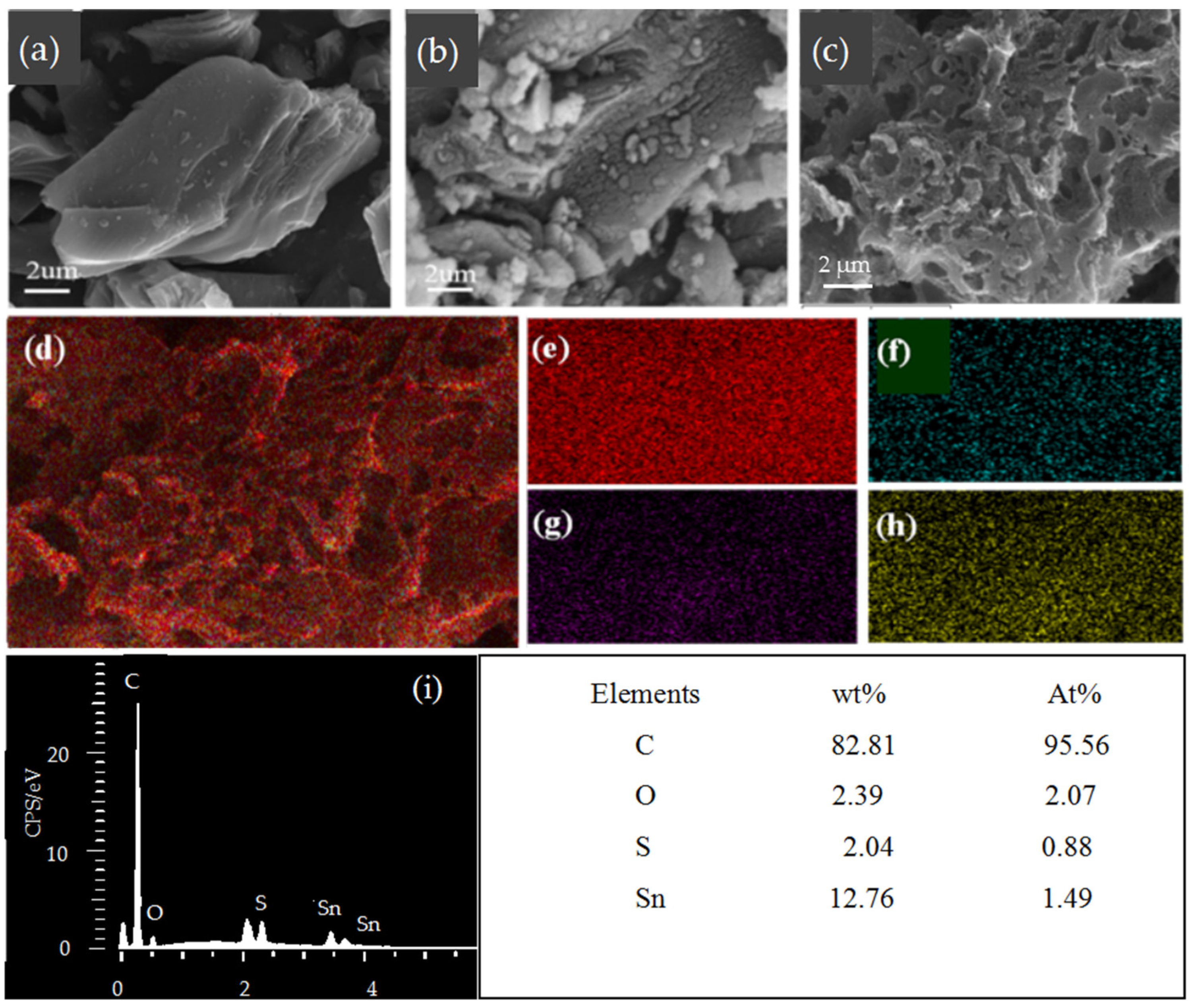
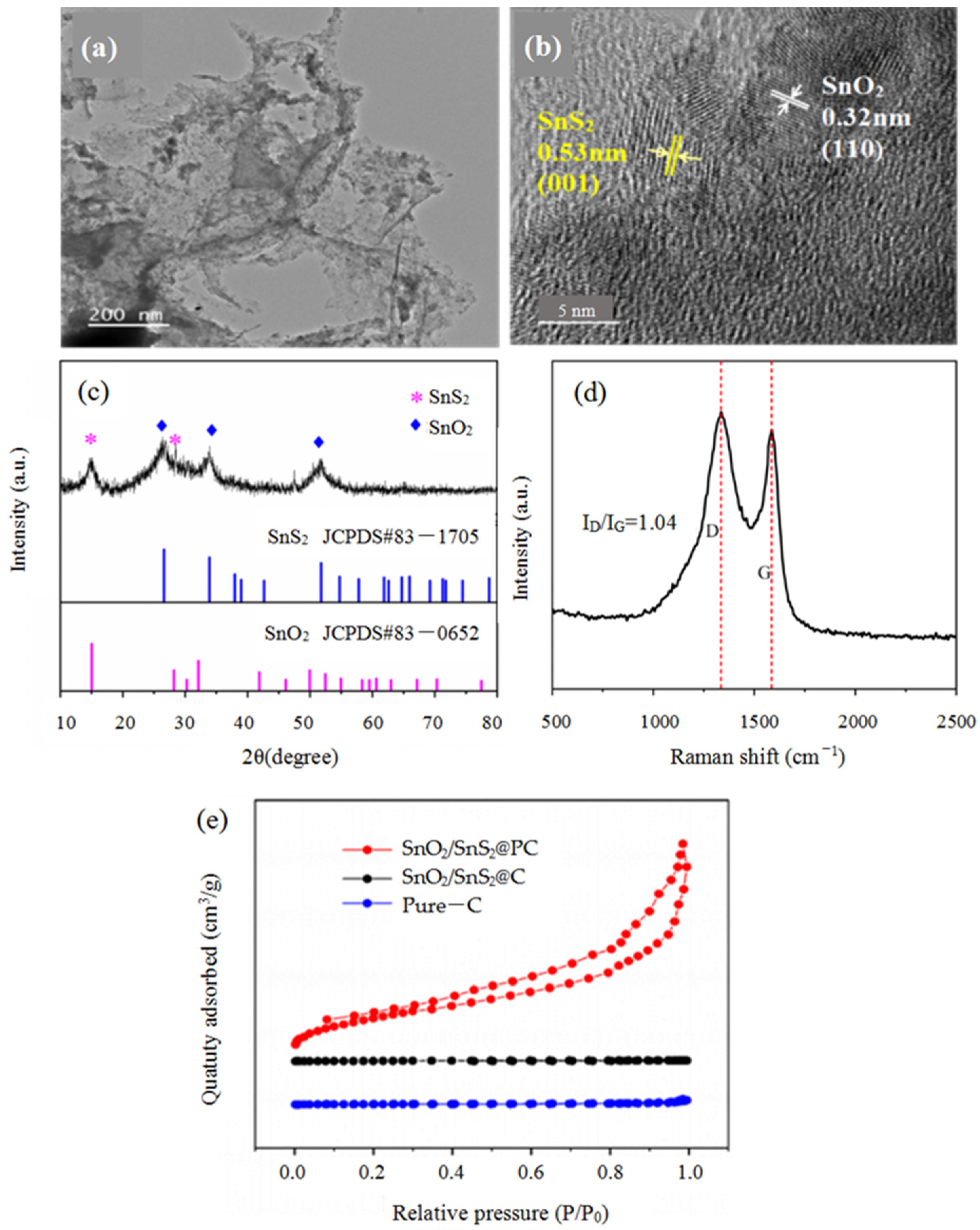
2.3. Characterization
2.4. Electrochemical Measurements
3. Results
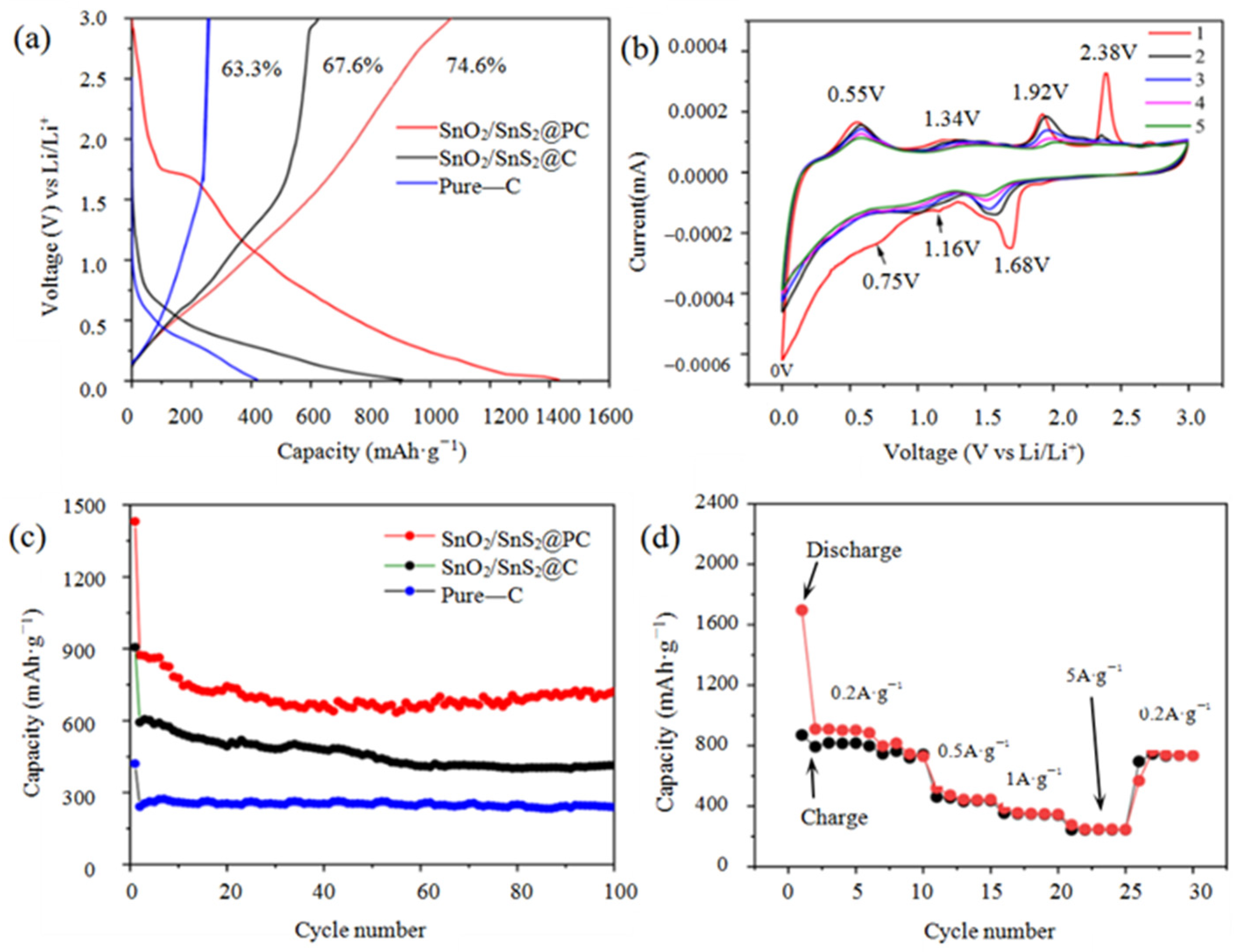
Electrochemical Properties of SnO2/SnS2@PC Composite
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Z.; Bao Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.; Liao, B.Y.; Liu, P. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 2444–2451. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as anode materials: Fundamental mechanism, recent progress, and advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Abdelrahman, N.S.; Lalwani, S.; Hong, S.H.Y.; Choi, D.S.; Kim, J.K.; Almarzooqi, F. Nanocarbon materials for lithium-ion battery anodes: Review. J. Energy Storage 2025, 130, 117350. [Google Scholar] [CrossRef]
- Li, P.; Kim, H.; Myung, S.T.; Sun, Y. Diverting exploration of silicon anode into the practical way: A review focused on silicon-graphite composite for lithium-ion batteries. Energy Storage Mater. 2021, 35, 550–576. [Google Scholar] [CrossRef]
- Sui, Y.; Zhou, J.; Li, Y. Recent advances in black-phosphorus-based materials for electrochemical energy storage. Mater. Today 2021, 42, 117–136. [Google Scholar] [CrossRef]
- Wang, S.; Qu, C.; Wen, J.; Wang, C.; Wang, C.; Ma, X.; Yang, Y.; Huang, G.; Sun, H.; Xu, S. Progress of transition metal sulfides used as the lithium-ion battery anodes. Mater. Chem. Front. 2023, 7, 2779–2808. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, Y.; Kim, H.; Kim, K. Synergistic effect of graphene nano perforation on the reversibility of the conversion reaction of a SnO2/nano perforated graphene composite. Chem. Eng. J. 2021, 417, 128542. [Google Scholar] [CrossRef]
- Li, H.; Zhang, B.; Wang, X.; Zhang, J.; An, T.; Ding, Z.; Yu, W.; Tong, H. Heterostructured SnO2-SnS2@C embedded in nitrogen-doped graphene as a robust anode material for lithium-ion batteries. Front. Chem. 2019, 7, 339. [Google Scholar] [CrossRef]
- Syum, Z.; Venugopal, B.; Sabbah, A.; Billo, T.; Chou, T.; Wu, H.; Chen, L.; Chen, K. Superior lithium-ion storage performance of hierarchical tin disulfide and carbon nanotube-carbon cloth composites. J. Power Sources 2021, 482, 228923. [Google Scholar] [CrossRef]
- Cheng, Y.; Xie, H.; Zhou, L.; Shi, B.; Guo, L.; Huang, J. In-situ-phase transformation of SnS2/CNTs composite from SnO2/CNTs for high-performance lithium-ion battery anode. Appl. Sur. Sci. 2021, 566, 150645. [Google Scholar] [CrossRef]
- Zuo, Y.; Xu, X.; Zhang, C.; Ma, X.; Qian, L.; Lan, Y.; Han, Q.; Li, J.; Wang, X.; Zhang, R. The elaborate interface design of SnS2/SnO2@C/rGO nanocomposite as a high-performance anode for lithium-ion batteries. Electrochim. Acta 2020, 349, 136369. [Google Scholar] [CrossRef]
- Hassan, A.S.; Moyer, K.; Ramachandran, B.R.; Wick, C.D. Comparison of storage mechanisms in RuO2, SnO2, and SnS2 for lithium-ion battery anode materials. J. Phys. Chem. 2016, 120, 2036–2046. [Google Scholar]
- Li, R.; Miao, C.; Yu, L.; Zhang, M.; Xiao, W. Novel self-assembled SnO2@SnS2 hybrid microspheres as potential anode materials for lithium-ion batteries. Mater. Lett. 2022, 72, 127851. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Li, Y.; Zhang, Y.; Dong, Y.; Li, D.; Zhang, J. Construction of uniform SnS2/ZnS heterostructure nanosheets embedded in graphene for advanced lithium-ion batteries. J. Alloys Comp. 2020, 820, 153147. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. Fabrication of Li4Ti5O12(LTO) as anode material for Li-ion batteries. Micromachines 2024, 15, 310. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Senthamaraikannan, T.G.; Sahoo, G.; Baasanjav, E.; Kim, J.; Jeong, S. Hierarchically nanofibers embedded with NiMnS nanocrystals as anode for high-performance lithium-ion batteries: Experimental and theoretical studies. Chem. Eng. J. 2024, 481, 148578. [Google Scholar] [CrossRef]
- Mahmud, S.T.; Mia, R.; Mahmud, S.; Sha, S.; Zhang, R.; Deng, Z.; Yanilmaz, M.; Luo, L.; Zhu, J. Recent developments of Tin (II) sulfide/carbon composites for achieving high-performance lithium-ion batteries: A Critical Review. Nanomater 2022, 12, 1246. [Google Scholar] [CrossRef]
- Geng, P.; Zheng, S.; Tang, H.; Zhu, R.; Zhang, L.; Chao, S.; Xue, H.; Pang, H. Transition metal sulfides based on graphene for electrochemical energy storage. Adv. Energy Mater. 2018, 8, 1703259. [Google Scholar] [CrossRef]
- Wang, L.B.; Hu, X.L. Recent Advances in Porous Carbon Materials for Electrochemical Energy Storage. Chem. Asian J. 2018, 13, 1518–1529. [Google Scholar] [CrossRef]
- Li, Y.; Yang, W.; Tu, Z.; Che, S.; Xu, X.; Liu, H.; Huang, G.; Li, Y. In-situ bonding with sulfur in petroleum pitch to synthesize transition metal (Mn, Mo, Fe, or Co)-based/carbon composites for superior lithium storage. Carbon 2021, 182, 700–710. [Google Scholar] [CrossRef]
- Xing, B.; Zeng, H.; Huang, G.; Jia, J.; Yuan, R.; Zhang, C.; Sun, Q.; Cao, Y.; Chen, Z.; Liu, B. Magnesium citrate induced growth of noodle-like porousgraphitic carbons from coal tar pitch for high-performance lithium-ion batteries. Electrochim. Acta 2021, 376, 138043. [Google Scholar] [CrossRef]
- Vadivazhagan, M.; Shakkeel, N.K.; Nallathamby, K. Demonstration of biocarbon added NiS porous nanospheres as a potential anode for lithium-ion batteries. Energy Fuels 2021, 35, 8991–9000. [Google Scholar] [CrossRef]
- Guan, L.; Hu, H.; Teng, X.L.; Zhu, Y.; Zhang, Y.; Chao, H.; Yang, H.; Wang, X.; Wu, M. Templating synthesis of porous carbons for energy-related applications: A review. New Carbon Mater. 2022, 37, 25–45. [Google Scholar] [CrossRef]
- Xing, B.; Zhang, C.; Liu, Q.; Zhang, C.; Huang, G.; Guo, H.; Cao, J.; Cao, Y.; Yu, J.; Chen, Z. Green synthesis of porous graphitic carbons from coal tar pitch templated by nano-CaCO3 for high-performance lithium-ion batteries. J. Alloys Comp. 2019, 795, 91–102. [Google Scholar] [CrossRef]
- Liang, C.; Yang, H.; Yu, K.; Jin, W. Sunflower seed husk-derived submicron carbon spheres and SnO2 nanoparticles composite used as an anode for high-performance lithium-ion batteries. Diam. Relat. Mater. 2021, 116, 108392. [Google Scholar] [CrossRef]
- Huang, J.; Chen, J.; Ma, L.; Liu, Q.; Wang, M.; Liao, L.; Rujiralai, T.; Xu, L. In-situ coupling SnS with nitrogen-doped porous carbon for boosting Li-storage in lithium-ion batteries and capacitors. Electrochim. Acta 2021, 365, 137350. [Google Scholar] [CrossRef]
- Huang, J.; Dai, Q.; Wu, Q.; Ren, H.; Lu, X.; Gu, C.; Zhang, Y.; Joo, S. Preparation of hollow SnO2@N-C nanospheres for high-performance lithium-ion battery. J. Electroanal. Chem. 2022, 922, 116741. [Google Scholar] [CrossRef]
- Gao, X.; Tang, Z.; Meng, M.; Yu, Q.; Zhu, Y.; Shen, S.; Yang, J. Synthesis of crumpled SnO2/rGO nanocomposites with 2D-in-3D structure and high performance. Mater. Chem. Phys. 2020, 253, 123298. [Google Scholar] [CrossRef]
- Zhang, R.; Fang, T.; Ni, L.; Zhu, Y.; Shen, Y.; Xie, A.; Lao, L. SnO2/Bi2O3/NF heterojunction with ordered macro/mesopore structure as an advanced binder-free anode for lithium-ion batteries. J. Electroanal. Chem. 2022, 907, 115894. [Google Scholar] [CrossRef]
- Yan, S.; Li, K.; Lin, Z.; Song, H.; Jiang, T.; Wu, J.; Shi, Y. Fabrication of a reversible SnS2/RGO nanocomposite for high-performance lithium storage. RSC Adv. 2016, 6, 32414–32421. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Y.; Meng, W.; Xie, Y.; Zhang, J.; He, C.; Zhao, D. Nanoplates-assembled SnS2 nanoflowers with carbon coating anchored on reduced graphene oxide for high-performance Li-ion batteries. Appl. Sur. Sci. 2021, 539, 148283. [Google Scholar] [CrossRef]
- Chen, X.; Lu, H.; Lei, Y.; Zhang, J.; Xiao, F.; Wang, R.; Xie, P.; Xu, J. Expanded graphite confined SnO2 as an anode for lithium-ion batteries with low average working potential and enhanced rate capability. J. Mater. Sci. Tech. 2022, 107, 165–171. [Google Scholar] [CrossRef]
- Divya, M.; Praneetha, S.; Le, Y.; Aravindan, V. Next-generation Li-ion capacitor with high energy and high power by limiting alloying-intercalation process using SnO2@Gra.phite composite as battery type electrode. Compos. Part B Eng. 2022, 230, 109487. [Google Scholar] [CrossRef]
- Ji, J.; Kun, F.; Zou, Z.; Cao, C.; Yang, J.; Huang, Y.; Lin, J.; Zhang, J.; Liu, Z.; Guo, Z.; et al. Enhanced Li storage through highly hybridized networks of self-assembled SnS2/rGO aerogels. J. Alloys Comp. 2020, 828, 154192. [Google Scholar] [CrossRef]
- Li, Y.; Lin, K.; Qin, X.; Zhen, K.; Liu, Y.; Xia, Y.; Lv, F.; Zhu, H.; Li, B. A nanoscale interlayer void design enabling high-performance SnO2-carbon anodes. Carbon 2021, 183, 486–494. [Google Scholar] [CrossRef]
- Wang, W.; Guo, S.; Zhang, P.; Zhou, J.; Yang, Y.; Wang, W.; Xu, X.; Chen, F.; Chen, L. Polypyrrole-wrapped SnS2 vertical nanosheet arrays grown on three-dimensional nitrogen-doped porous graphene for high-performance lithium and sodium storage. ACS Appl. Energy Mater. 2021, 4, 11101–11111. [Google Scholar] [CrossRef]
- Huang, S.F.; Wang, M.; Jia, P.; Wang, B.; Zhang, J.; Zhao, F. N-graphene motivated SnO2@SnS2 heterostructure quantum dots for high-performance lithium/sodium storage. Energy Storage Mater. 2019, 20, 225–233. [Google Scholar] [CrossRef]
- Hui, Y.; Wei, Z.; Qing, Y.; Zhao, J.; Li, Y.; Xie, Y. The fabrication of hierarchical porous nano-SnO2@carbon@humic acid ternary composite for enhanced capacity and stability as anode material for lithium-ion battery. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129560. [Google Scholar]
- Xu, W.; Xie, Z.; Cui, X.; Zhao, K.; Zhang, L.; Dietrich, G.; Dooley, K.; Wang, Y. Hierarchical graphene-encapsulated hollow SnO2@SnS2 nanostructures with enhanced lithium storage capability. ACS Appl. Mater. Interface 2015, 7, 33–41. [Google Scholar] [CrossRef]
- Zheng, M.; Pan, Q.; Gong, F.; Li, C. The facile mechanical stripping of black phosphorus and hybriding with graphite to improve the performance of graphite-based anode material for lithium ion battery. Diam. Relat. Mater. 2023, 136, 109982. [Google Scholar] [CrossRef]
- Chen, F.; Shi, D.; Yang, M.; Jiang, H.; Shao, Y.; Wang, S.; Zhang, B.; Shen, J.; Wu, Y.; Hao, X. Novel designed MnS-MoS2 heterostructure for fast and stable Li/Na storage: Insights into the advanced mechanism attributed to phase engineering. Adv. Funct. Mater. 2021, 31, 2007132. [Google Scholar] [CrossRef]


| Samples | STotal (m2·g−1) | SMic (m2·g−1) | SMec (m2·g−1) | VMac (cm3·g−1) | VMec (cm3·g−1) | VTotal (cm3·g−1) |
|---|---|---|---|---|---|---|
| Pure-C | 2.3 | 0.1 | 2.2 | 0 | 0.001 | 0.001 |
| SnO2/SnS2@C | 3 | 0.4 | 2.6 | 0 | 0.005 | 0.005 |
| SnO2/SnS2@PC | 190 | 17 | 173 | 0.006 | 0.380 | 0.386 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Li, C.; Zheng, M.; Li, Y.; Gong, F. In Situ Synthesis of Porous SnO2/SnS2@PC Anode Material with High Capacity Using Calcium Carbonate as Template for Lithium-Ion Batteries. Materials 2025, 18, 4987. https://doi.org/10.3390/ma18214987
Chen W, Li C, Zheng M, Li Y, Gong F. In Situ Synthesis of Porous SnO2/SnS2@PC Anode Material with High Capacity Using Calcium Carbonate as Template for Lithium-Ion Batteries. Materials. 2025; 18(21):4987. https://doi.org/10.3390/ma18214987
Chicago/Turabian StyleChen, Wen, Chunling Li, Mengyang Zheng, Yanlin Li, and Fuzhong Gong. 2025. "In Situ Synthesis of Porous SnO2/SnS2@PC Anode Material with High Capacity Using Calcium Carbonate as Template for Lithium-Ion Batteries" Materials 18, no. 21: 4987. https://doi.org/10.3390/ma18214987
APA StyleChen, W., Li, C., Zheng, M., Li, Y., & Gong, F. (2025). In Situ Synthesis of Porous SnO2/SnS2@PC Anode Material with High Capacity Using Calcium Carbonate as Template for Lithium-Ion Batteries. Materials, 18(21), 4987. https://doi.org/10.3390/ma18214987





