Effect of Poly (Lactic Acid/ε-Caprolactone) Bilayer Membrane on Tooth Extraction Socket Wound Healing in a Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
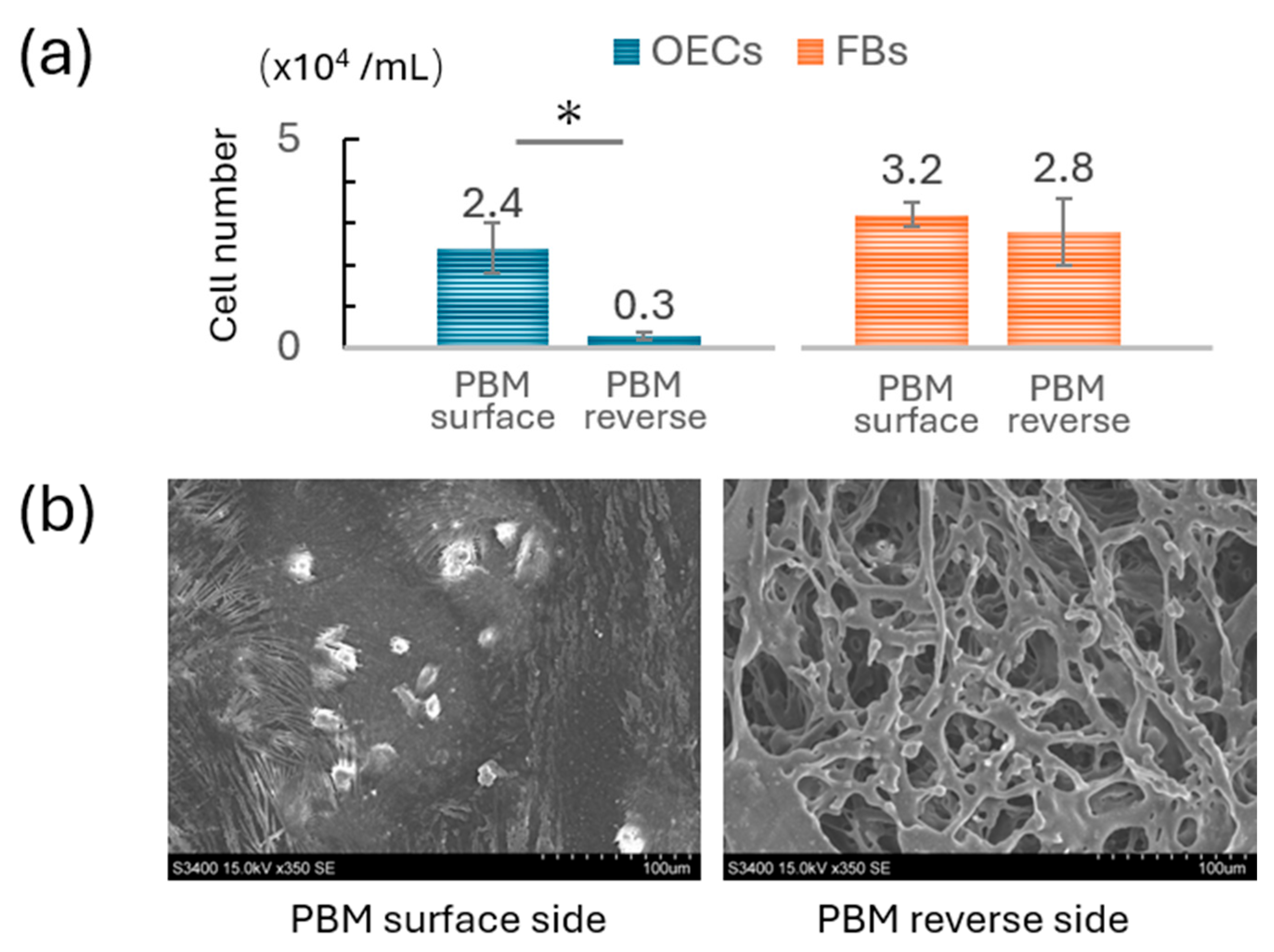
2.3. Scanning Electron Microscopy (SEM)
2.4. Membrane Permeability Test
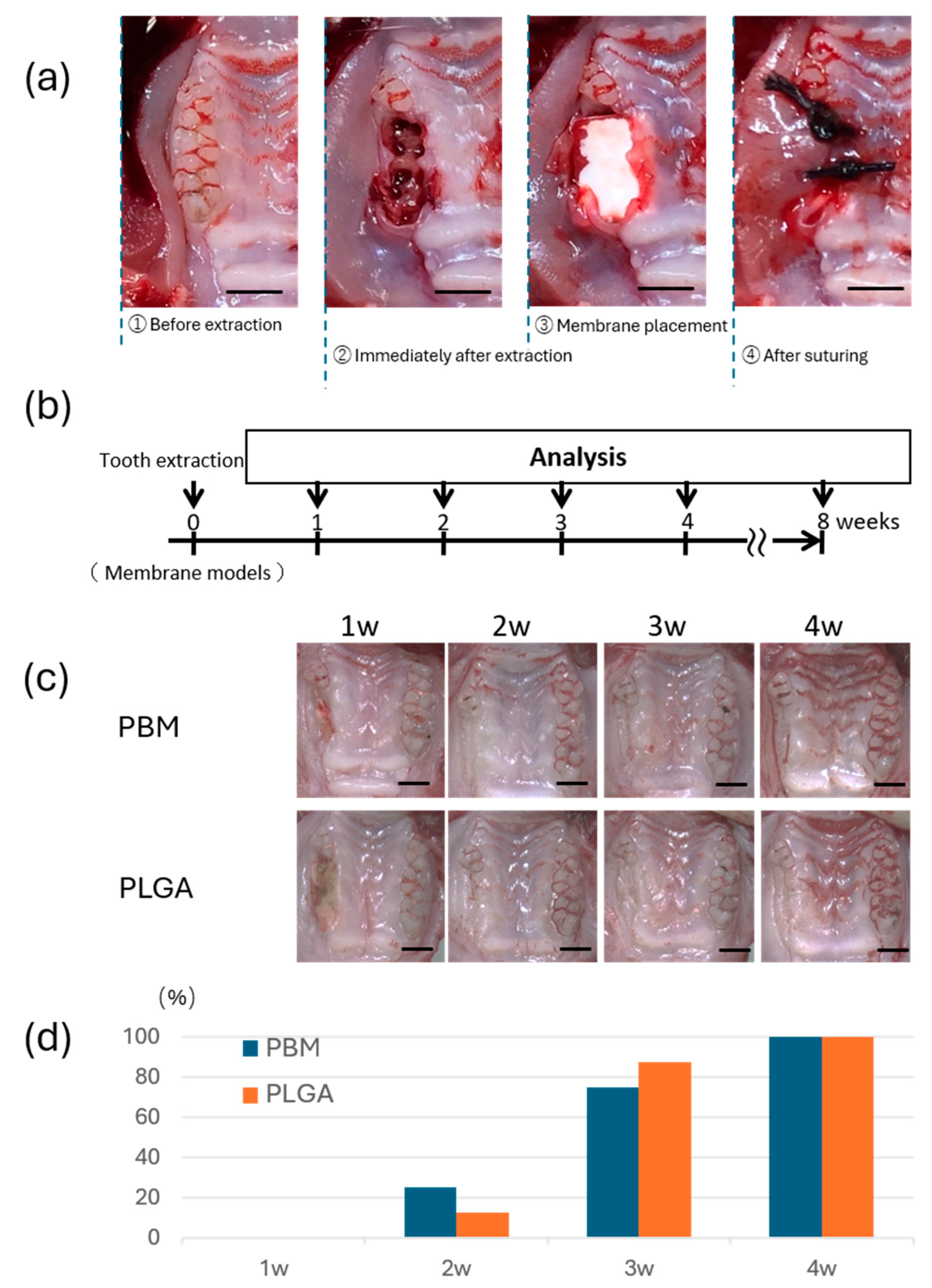
2.5. Establishment of the Rat Membrane Model
2.6. Euthanasia and Sample Preparation
2.7. Micro-Computed Tomography (Micro-CT) Analysis
2.8. Histological Analysis
2.9. Statistical Analysis
3. Results
3.1. Comparison of OEC and FB Adhesion on PBM Membrane
3.2. Permeability Differences Between Membranes
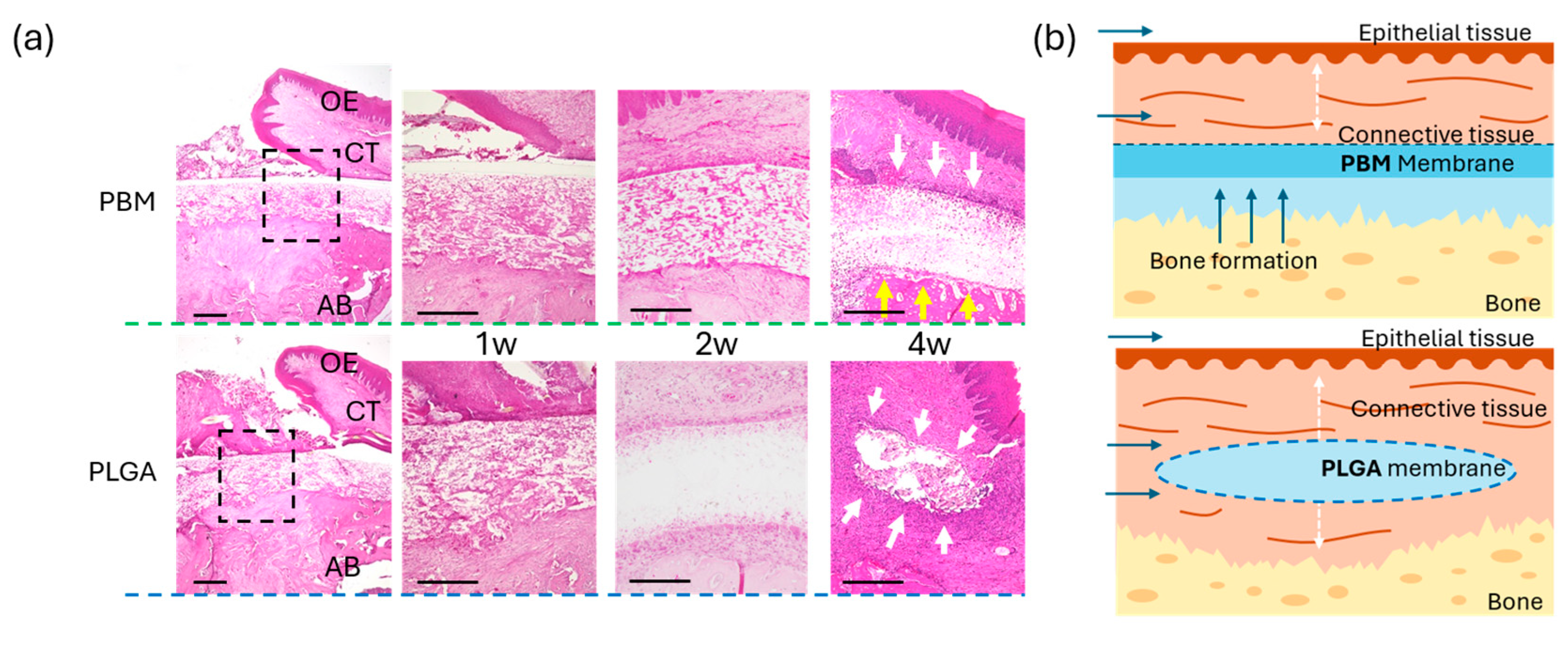
3.3. Mucosal Wound Healing over Membranes
3.4. Bone Formation Beneath the Membranes
4. Discussion
- Enhanced epithelial compatibility for early soft tissue sealing;
- Effective space maintenance for bone regeneration;
- Synthetic and fully resorbable, free from animal-derived components.
- Evaluate PBM performance in larger animal models with more complex defects;
- Investigate long-term space maintenance and bone maturation;
- Consider design improvements to enhance the barrier function of the reverse side.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PBM | Poly (L-lactic acid-co-ε-caprolactone) Bilayer Membrane |
| PLGA | Poly (lactic-co-glycolic acid) |
| GBR | Guided Bone Regeneration |
| GTR | Guided Tissue Regeneration |
| OECs | Oral Epithelial Cells |
| FBs | Fibroblasts |
| FBS | Fetal Bovine Serum |
| SEM | Scanning Electron Microscopy |
| Micro-CT | Micro-Computed Tomography |
| OCT | Optimal Cutting Temperature (compound) |
| HE | Hematoxylin and Eosin |
| OE | Oral Epithelium |
| CT | Connective Tissue |
References
- Benic, G.I.; Hämmerle, C.H. Horizontal bone augmentation by means of guided bone regeneration. Periodontol. 2000 2014, 66, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kerns, D.G. Mechanisms of guided bone regeneration: A review. Open Dent. J. 2014, 8, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J. Optimized bone grafting. Periodontol. 2000 2024, 94, 143–160. [Google Scholar] [CrossRef]
- Polimeni, G.; Xiropaidis, A.V.; Wikesjö, U.M. Biology and principles of periodontal wound healing/regeneration. Periodontol. 2000 2006, 41, 30–47. [Google Scholar] [CrossRef]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implant. Res. 2010, 21, 567–576. [Google Scholar] [CrossRef]
- Buser, D.; Dahlin, C.; Schenk, R.K. Guided Bone Regeneration in Implant Dentistry; Quintessence Books: New Malden, UK, 1994. [Google Scholar]
- Cardaropoli, G.; Araújo, M.; Lindhe, J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J. Clin. Periodontol. 2003, 30, 809–818. [Google Scholar] [CrossRef]
- Nyman, S.; Lindhe, J.; Karring, T.; Rylander, H. New attachment following surgical treatment of human periodontal disease. J. Clin. Periodontol. 1982, 9, 290–296. [Google Scholar] [CrossRef]
- Dahlin, C.; Linde, A.; Gottlow, J.; Nyman, S. Healing of bone defects by guided tissue regeneration. Plast. Reconstr. Surg. 1988, 81, 672–676. [Google Scholar] [CrossRef]
- Melcher, A.H. On the repair potential of periodontal tissues. J. Periodontol. 1976, 47, 256–260. [Google Scholar] [CrossRef]
- Faria-Almeida, R.; Astramskaite-Januseviciene, I.; Puisys, A.; Correia, F. Extraction Socket Preservation with or without Membranes, Soft Tissue Influence on Post Extraction Alveolar Ridge Preservation: A Systematic Review. J. Oral Maxillofac. Res. 2019, 10, e5. [Google Scholar] [CrossRef]
- Soldatos, N.K.; Stylianou, P.; Koidou, V.P.; Angelov, N.; Yukna, R.; Romanos, G.E. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. 2017, 48, 131–147. [Google Scholar] [CrossRef]
- Scantlebury, T.V. 1982–1992: A Decade of Technology Development for Guided Tissue Regeneration. J. Periodontol. 1993, 64 (Suppl. S11), 1129–1137. [Google Scholar] [CrossRef]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar] [CrossRef]
- Korzinskas, T.; Jung, O.; Smeets, R.; Stojanovic, S.; Najman, S.; Glenske, K.; Hahn, M.; Wenisch, S.; Schnettler, R.; Barbeck, M. In Vivo Analysis of the Biocompatibility and Macrophage Response of a Non-Resorbable PTFE Membrane for Guided Bone Regeneration. Int. J. Mol. Sci. 2018, 19, 2952. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers 2021, 13, 3868. [Google Scholar] [CrossRef] [PubMed]
- Radenković, M.; Alkildani, S.; Stoewe, I.; Bielenstein, J.; Sundag, B.; Bellmann, O.; Jung, O.; Najman, S.; Stojanović, S.; Barbeck, M. Comparative In Vivo Analysis of the Integration Behavior and Immune Response of Collagen-Based Dental Barrier Membranes for Guided Bone Regeneration (GBR). Membranes 2021, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Hoornaert, A.; d’Arros, C.; Heymann, M.F.; Layrolle, P. Biocompatibility, resorption and biofunctionality of a new synthetic biodegradable membrane for guided bone regeneration. Biomed. Mater. 2016, 11, 045012. [Google Scholar] [CrossRef]
- Lyu, C.; Shao, Z.; Zou, D.; Lu, J. Ridge Alterations following Socket Preservation Using a Collagen Membrane in Dogs. Biomed. Res. Int. 2020, 2020, 1487681. [Google Scholar] [CrossRef]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef]
- Sasaki, J.I.; Abe, G.L.; Li, A.; Thongthai, P.; Tsuboi, R.; Kohno, T.; Imazato, S. Barrier membranes for tissue regeneration in dentistry. Biomater. Investig. Dent. 2021, 8, 54–63. [Google Scholar] [CrossRef]
- Wang, H.L.; Boyapati, L. “PASS” principles for predictable bone regeneration. Implant. Dent. 2006, 15, 8–17. [Google Scholar] [CrossRef]
- Abe, G.L.; Sasaki, J.I.; Katata, C.; Kohno, T.; Tsuboi, R.; Kitagawa, H.; Imazato, S. Fabrication of novel poly(lactic acid/caprolactone) bilayer membrane for GBR application. Dent. Mater. 2020, 36, 626–634. [Google Scholar] [CrossRef]
- Abe, G.L.; Sasaki, J.I.; Tsuboi, R.; Kohno, T.; Kitagawa, H.; Imazato, S. Poly(lactic acid/caprolactone) bilayer membrane achieves bone regeneration through a prolonged barrier function. J. Biomed. Mater. Res. B Appl. Biomater. 2024, 112, e35365. [Google Scholar] [CrossRef]
- Watanabe, T.; Hasuike, A.; Wakuda, S.; Kogure, K.; Min, S.; Watanabe, N.; Sakai, R.; Chaurasia, A.; Arai, Y.; Sato, S. Resorbable bilayer membrane made of L-lactide-ε-caprolactone in guided bone regeneration: An in vivo experimental study. Int. J. Implant. Dent. 2024, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Atsuta, I.; Ayukawa, Y.; Furuhashi, A.; Narimatsu, I.; Kondo, R.; Oshiro, W.; Koyano, K. Epithelial sealing effectiveness against titanium or zirconia implants surface. J. Biomed. Mater. Res. A 2019, 107, 1379–1385. [Google Scholar] [CrossRef]
- Baumhammers, A.; Langkamp, H.H.; Matta, R.K.; Kilbury, K. Scanning electron microscopy of epithelial cells grown on enamel, glass and implant materials. J. Periodontol. 1978, 49, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Atsuta, I.; Narimatsu, I.; Ueda, N.; Takahashi, R.; Egashira, Y.; Zhang, J.Q.; Gu, J.Y.; Koyano, K.; Ayukawa, Y. Replacement Process of Carbonate Apatite by Alveolar Bone in a Rat Extraction Socket. Materials 2021, 14, 4457. [Google Scholar] [CrossRef]
- Adachi, N.; Ayukawa, Y.; Yasunami, N.; Furuhashi, A.; Imai, M.; Sanda, K.; Atsuta, I.; Koyano, K. Preventive effect of fluvastatin on the development of medication-related osteonecrosis of the jaw. Sci. Rep. 2020, 10, 5620. [Google Scholar] [CrossRef]
- Simion, M.; Maglione, M.; Iamoni, F.; Scarano, A.; Piattelli, A.; Salvato, A. Bacterial penetration through Resolut resorbable membrane in vitro. An histological and scanning electron microscopic study. Clin. Oral Implants Res. 1997, 8, 23–31. [Google Scholar] [CrossRef]
- Gabriela, L.A.; Tsuboi, R.; Kitagawa, H.; Sasaki, J.I.; Li, A.; Kohno, T.; Imazato, S. Poly(lactic acid/caprolactone) bilayer membrane blocks bacterial penetration. J. Periodontal Res. 2022, 57, 510–518. [Google Scholar] [CrossRef]
- Ku, Y.; Shim, I.K.; Lee, J.Y.; Park, Y.J.; Rhee, S.H.; Nam, S.H.; Park, J.B.; Chung, C.P.; Lee, S.J. Chitosan/poly(L-lactic acid) multilayered membrane for guided tissue regeneration. J. Biomed. Mater. Res. A 2009, 90, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Hürzeler, M.B.; Quiñones, C.R.; Morrison, E.C.; Caffesse, R.G. Treatment of peri-implantitis using guided bone regeneration and bone grafts, alone or in combination, in beagle dogs. Part 1: Clinical findings and histologic observations. Int. J. Oral Maxillofac. Implants 1995, 10, 474–484. [Google Scholar] [PubMed]
- Viateau, V.; Guillemin, G.; Calando, Y.; Logeart, D.; Oudina, K.; Sedel, L.; Hannouche, D.; Bousson, V.; Petite, H. Induction of a barrier membrane to facilitate reconstruction of massive segmental diaphyseal bone defects: An ovine model. Vet. Surg. 2006, 35, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Malchiodi, L.; Scarano, A.; Quaranta, M.; Piattelli, A. Rigid fixation by means of titanium mesh in edentulous ridge expansion for horizontal ridge augmentation in the maxilla. Int. J. Oral Maxillofac. Implant. 1998, 13, 701–705. [Google Scholar]
- Shido, R.; Ohba, S.; Tominaga, R.; Sumita, Y.; Asahina, I. A Prospective Study of the Assessment of the Efficacy of a Biodegradable Poly(l-lactic acid/ε-caprolactone) Membrane for Guided Bone Regeneration. J. Clin. Med. 2023, 12, 5994. [Google Scholar] [CrossRef]
- Dimitriou, R.; Mataliotakis, G.I.; Calori, G.M.; Giannoudis, P.V. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence. BMC Med. 2012, 10, 81. [Google Scholar] [CrossRef]
- Monje, A.; Pons, R.; Nart, J.; Miron, R.J.; Schwarz, F.; Sculean, A. Selecting biomaterials in the reconstructive therapy of peri-implantitis. Periodontol. 2000 2024, 94, 192–212. [Google Scholar] [CrossRef]
- Gottlow, J.; Nyman, S.; Karring, T.; Lindhe, J. New attachment formation as the result of controlled tissue regeneration. J. Clin. Periodontol. 1984, 11, 494–503. [Google Scholar] [CrossRef]
- Xue, S.; Tang, N.; Zhou, C.; Fang, S.; Haick, H.; Sun, J.; Wu, X. Anti-Wound Dehiscence and Antibacterial Dressing with Highly Efficient Self-Healing Feature for Guided Bone Regeneration Wound Closure. Adv. Healthc. Mater. 2024, 13, e2304128. [Google Scholar] [CrossRef]
- Abtahi, S.; Chen, X.; Shahabi, S.; Nasiri, N. Resorbable Membranes for Guided Bone Regeneration: Critical Features, Potentials, and Limitations. ACS Mater. Au 2023, 3, 394–417. [Google Scholar] [CrossRef]
- Furuhashi, A.; Rakhmatia, Y.D.; Ayukawa, Y.; Koyano, K. Titanium membrane layered between fluvastatin-loaded poly (lactic-co-glycolic) acid for guided bone regeneration. Regen. Biomater. 2022, 9, rbac061. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Tonda-Turo, C.; Ferreira, A.M.; Ciardelli, G. Polymeric membranes for guided bone regeneration. Biotechnol. J. 2011, 6, 1187–1197. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, B.; Xie, T.; Atsuta, I.; Narimatsu, I.; Jinno, Y.; Takahashi, A.; Imai, M.; Koyano, K.; Ayukawa, Y. Effect of Poly (Lactic Acid/ε-Caprolactone) Bilayer Membrane on Tooth Extraction Socket Wound Healing in a Rat Model. Materials 2025, 18, 4956. https://doi.org/10.3390/ma18214956
Ji B, Xie T, Atsuta I, Narimatsu I, Jinno Y, Takahashi A, Imai M, Koyano K, Ayukawa Y. Effect of Poly (Lactic Acid/ε-Caprolactone) Bilayer Membrane on Tooth Extraction Socket Wound Healing in a Rat Model. Materials. 2025; 18(21):4956. https://doi.org/10.3390/ma18214956
Chicago/Turabian StyleJi, Bin, Tingyu Xie, Ikiru Atsuta, Ikue Narimatsu, Yohei Jinno, Akira Takahashi, Mikio Imai, Kiyoshi Koyano, and Yasunori Ayukawa. 2025. "Effect of Poly (Lactic Acid/ε-Caprolactone) Bilayer Membrane on Tooth Extraction Socket Wound Healing in a Rat Model" Materials 18, no. 21: 4956. https://doi.org/10.3390/ma18214956
APA StyleJi, B., Xie, T., Atsuta, I., Narimatsu, I., Jinno, Y., Takahashi, A., Imai, M., Koyano, K., & Ayukawa, Y. (2025). Effect of Poly (Lactic Acid/ε-Caprolactone) Bilayer Membrane on Tooth Extraction Socket Wound Healing in a Rat Model. Materials, 18(21), 4956. https://doi.org/10.3390/ma18214956




