Investigation of Electrode Cap Life Made of New Cu–Cr–Zr Copper Alloys with Scandium Addition Dedicated for Resistance Spot Welding of Galvanized Steel Sheets
Abstract
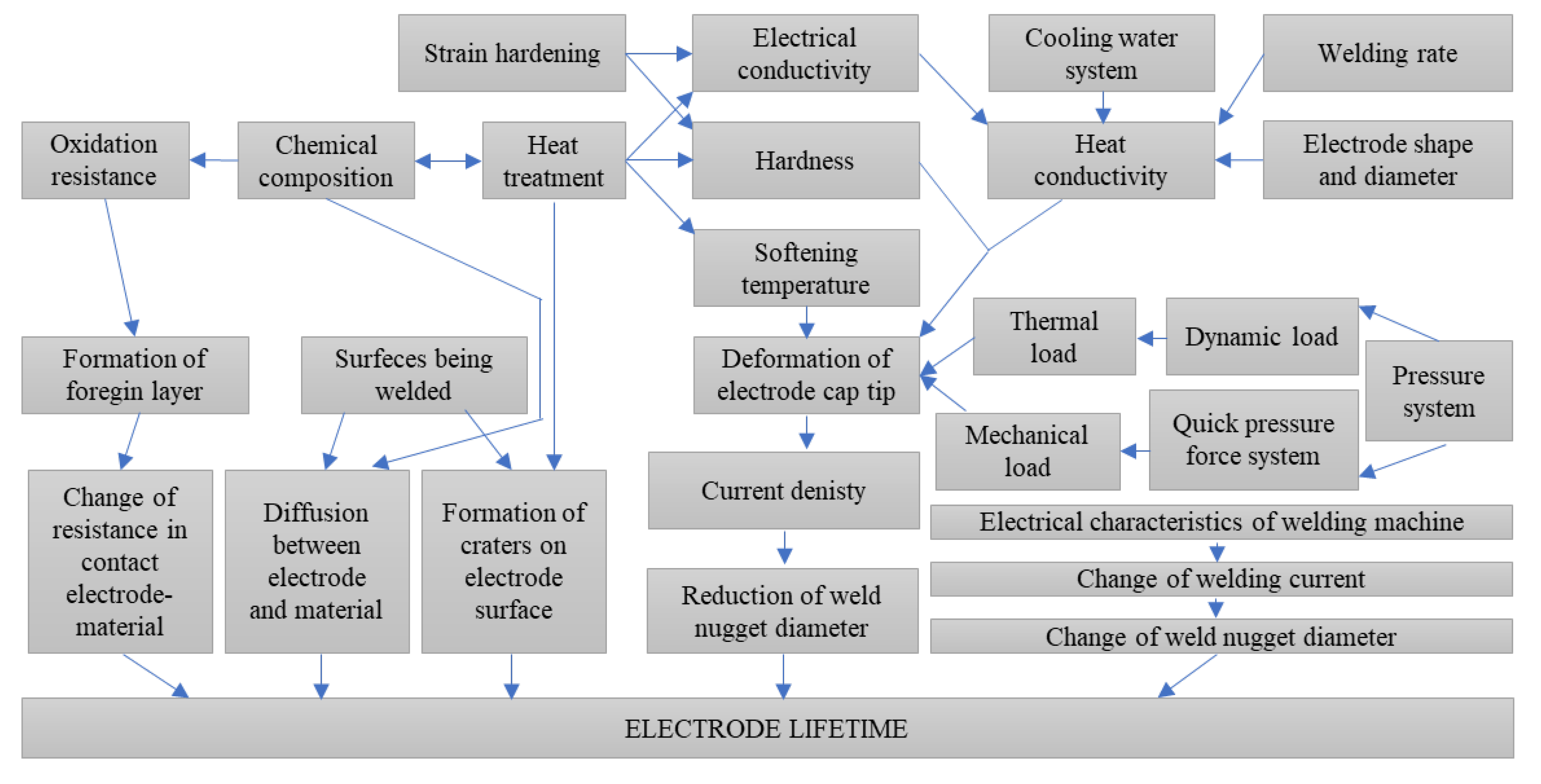
1. Introduction
2. Experimental Study
2.1. Materials and Methods
2.2. Electrode Life Tests
2.3. Structural and Mechanical Characterization
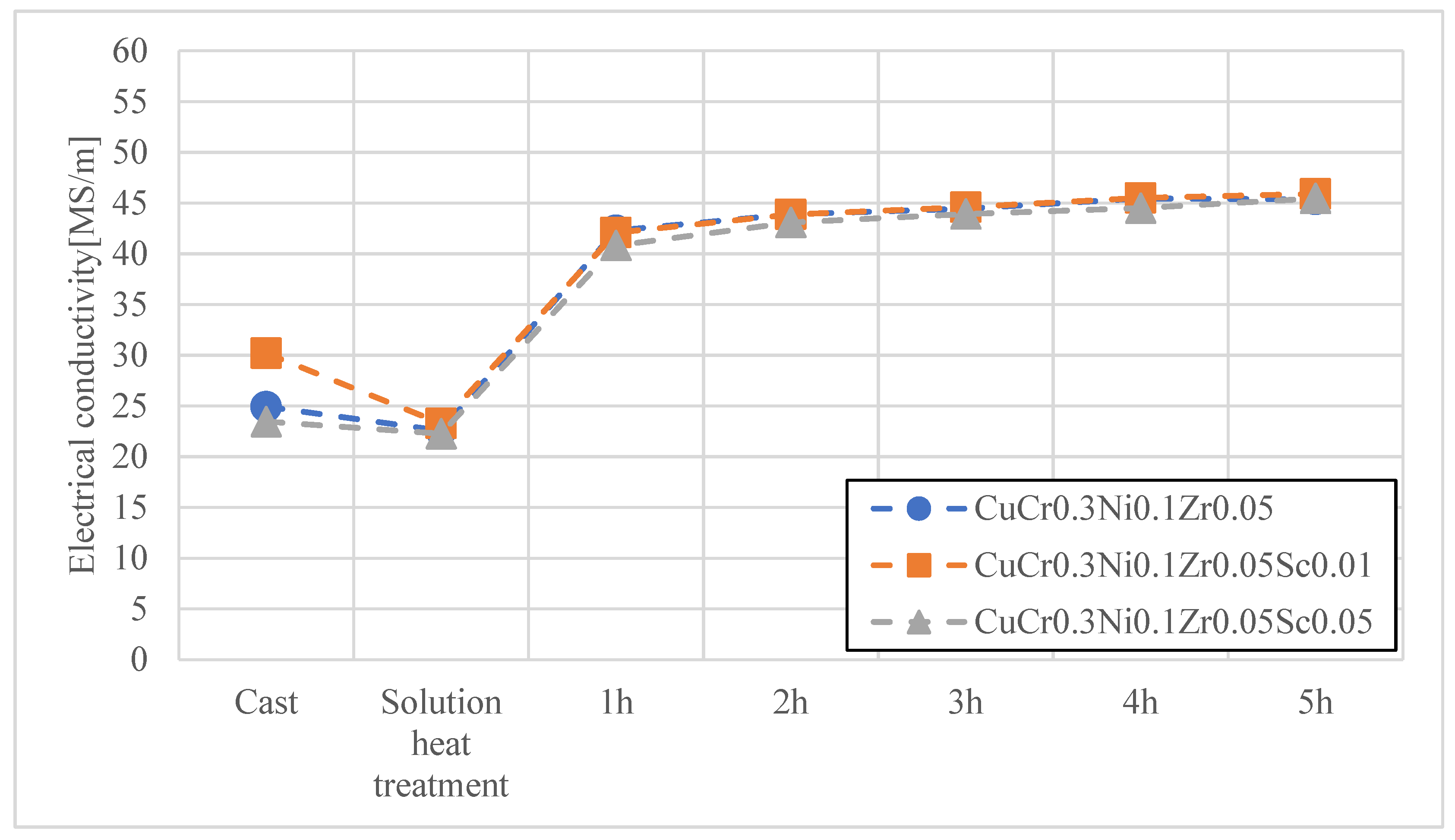
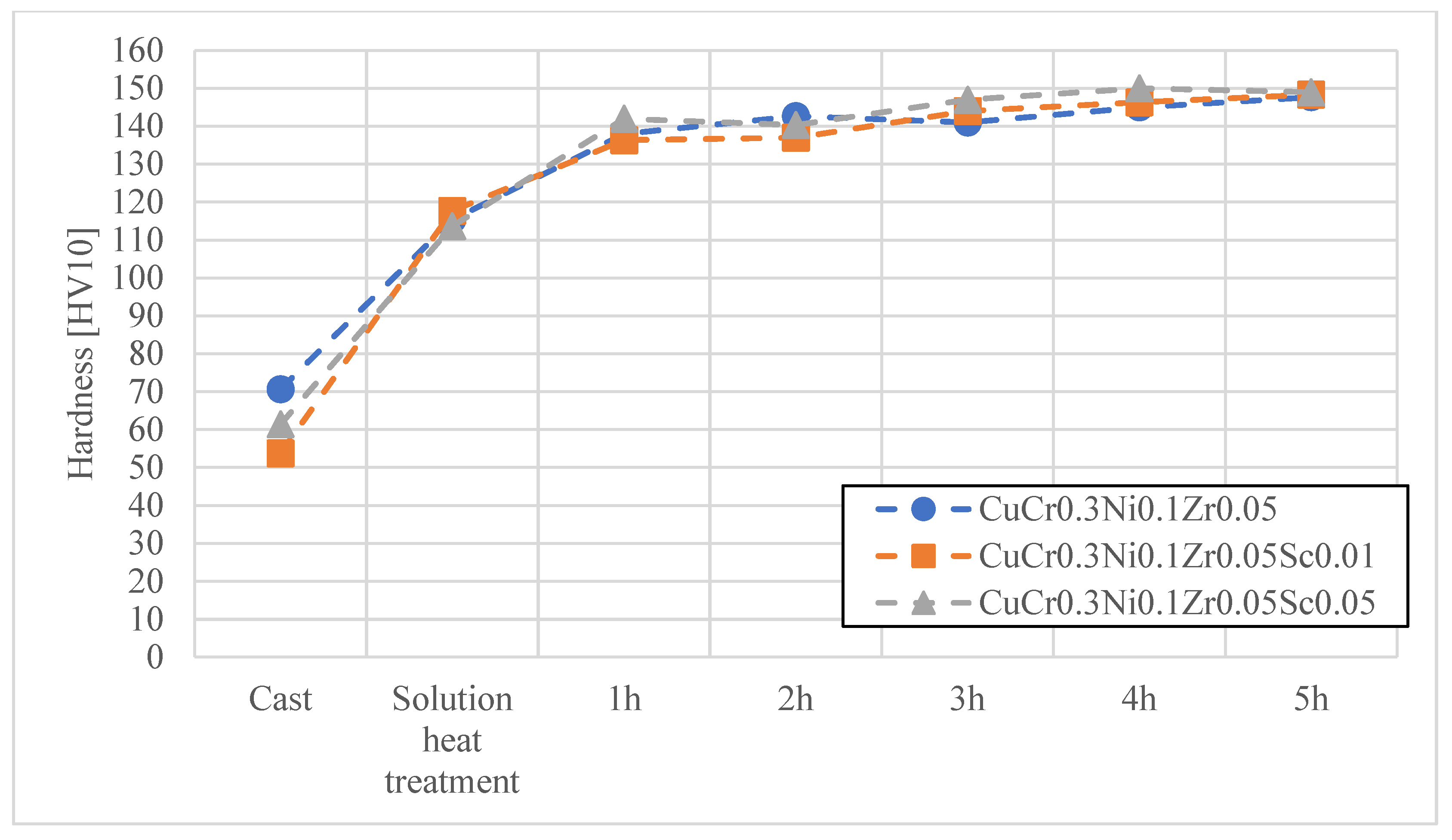
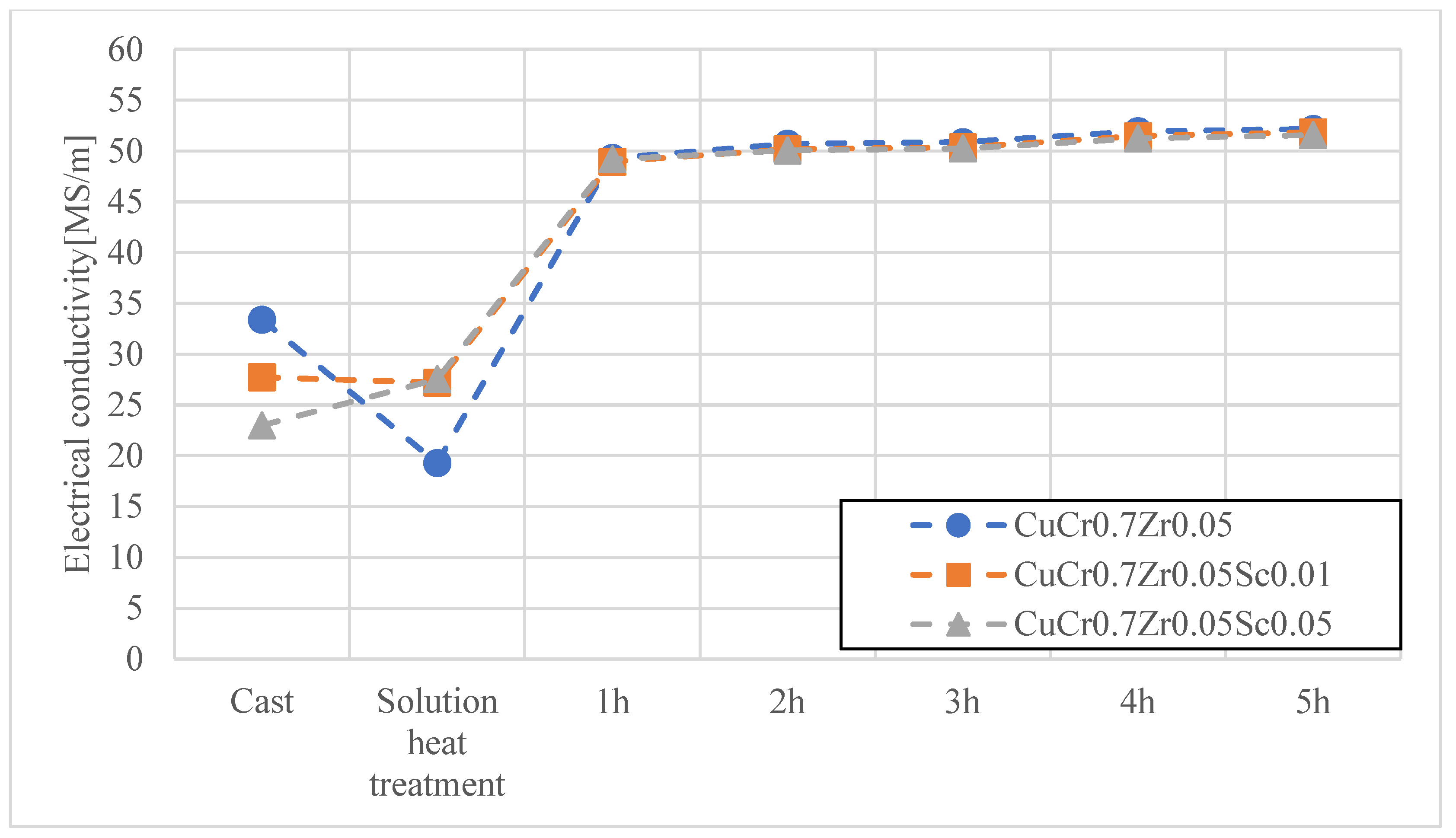
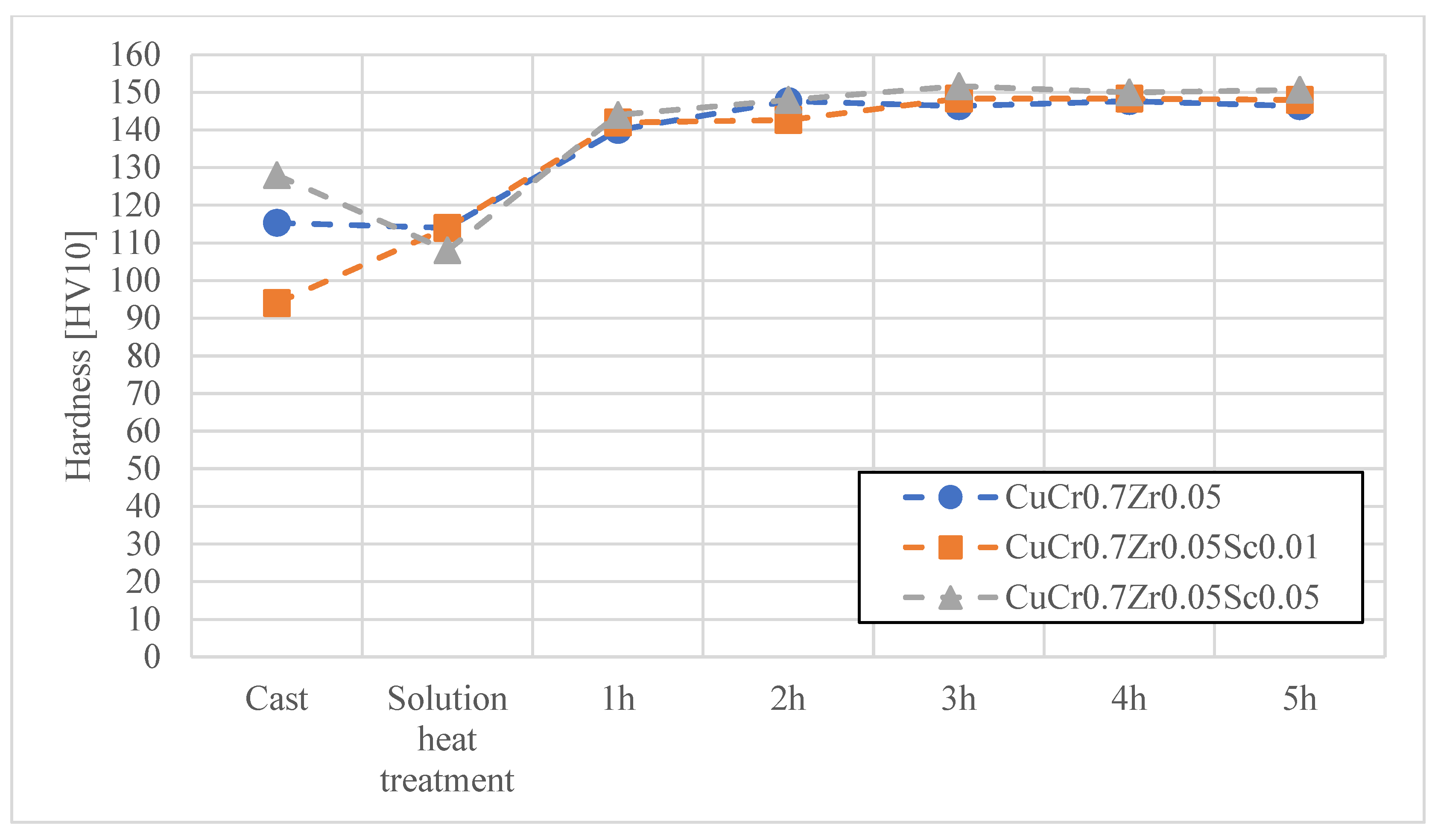
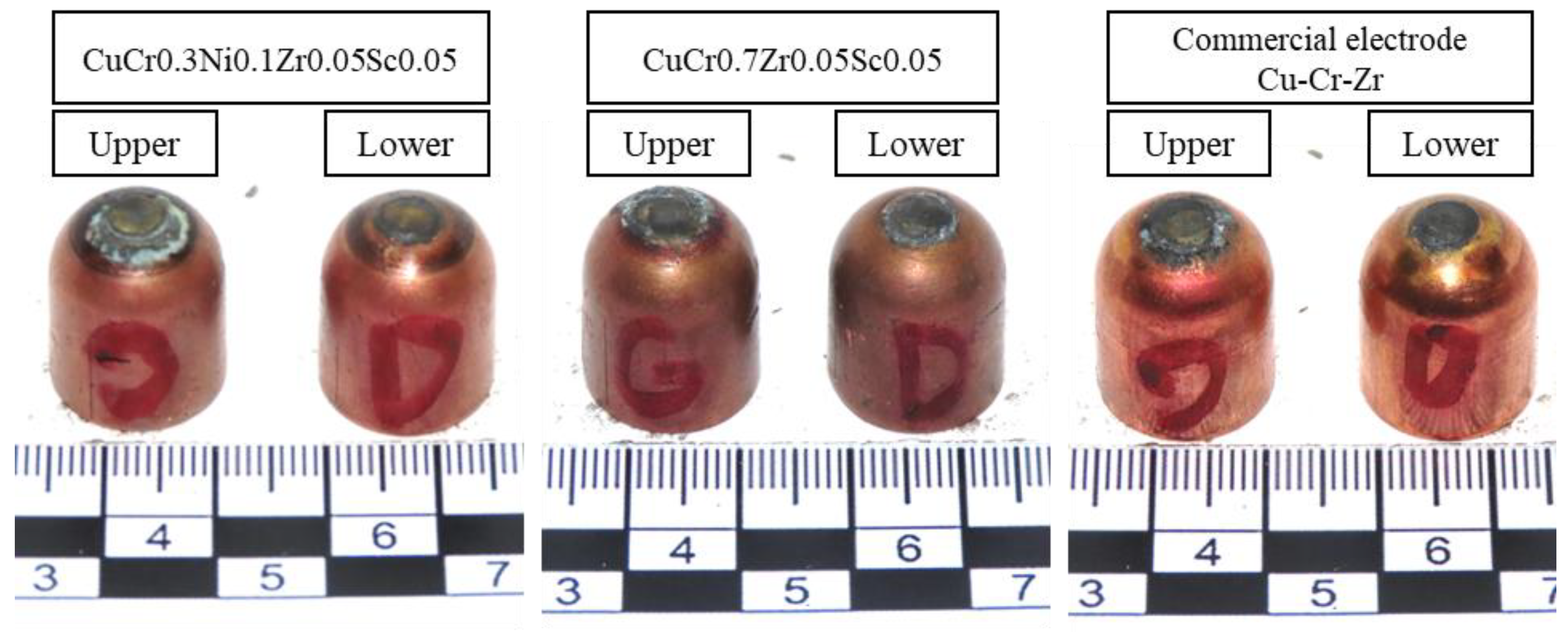
3. Results and Discussion
4. Conclusions
- The conducted research clearly confirmed that the modification of Cu–Cr–Zr and Cu–Cr–Ni–Zr alloys through controlled scandium (Sc) additions contributes to a significant improvement in the service properties of electrode caps used in the resistance spot welding (RSW) of galvanized steels. The applied alloying concept allows for increased electrode hardness with only a minimal reduction in electrical conductivity, remaining within acceptable limits for this type of application.
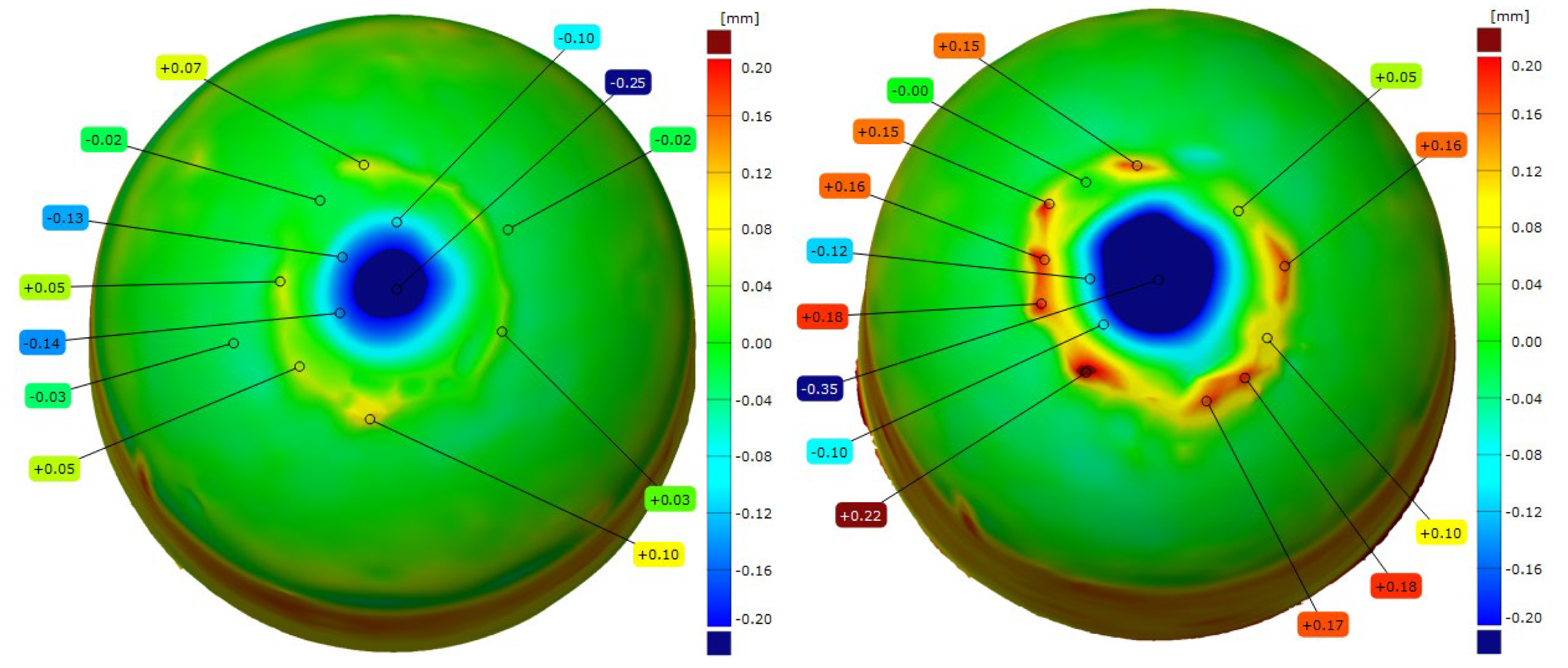
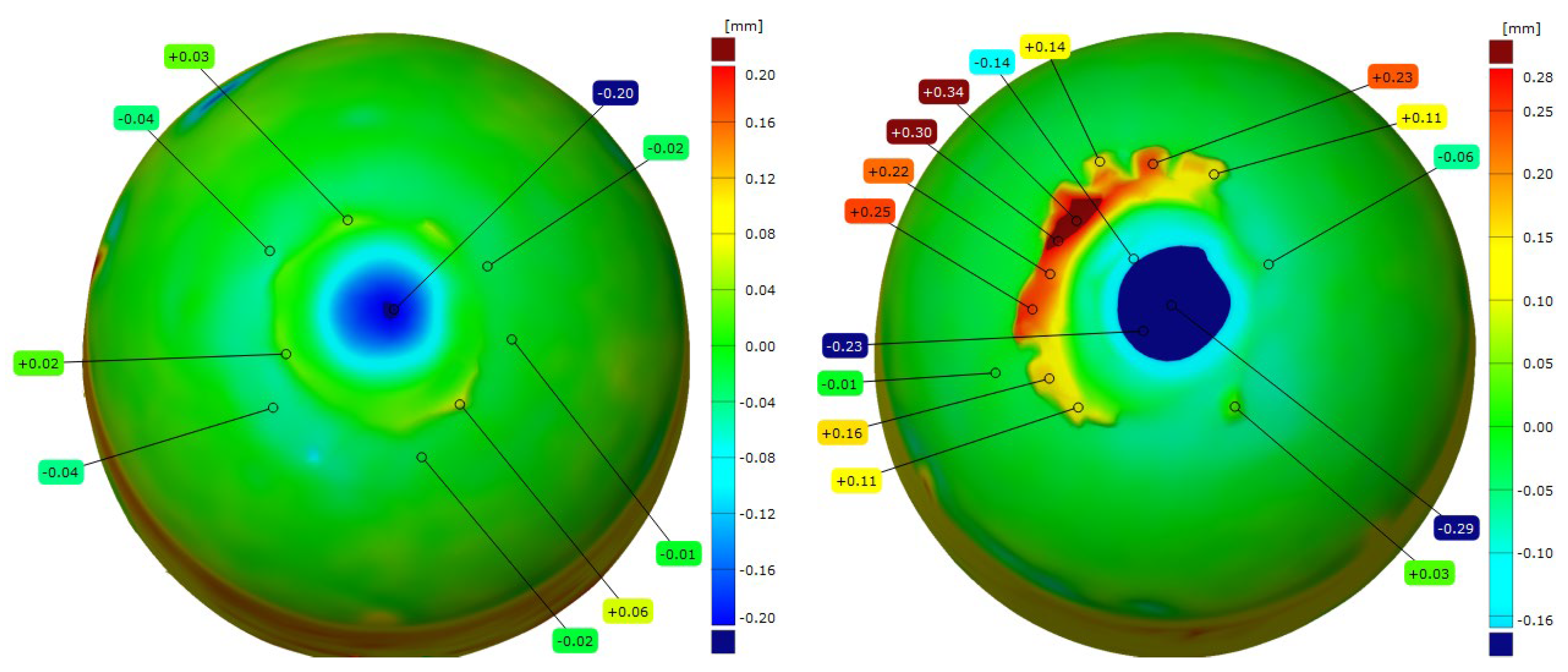
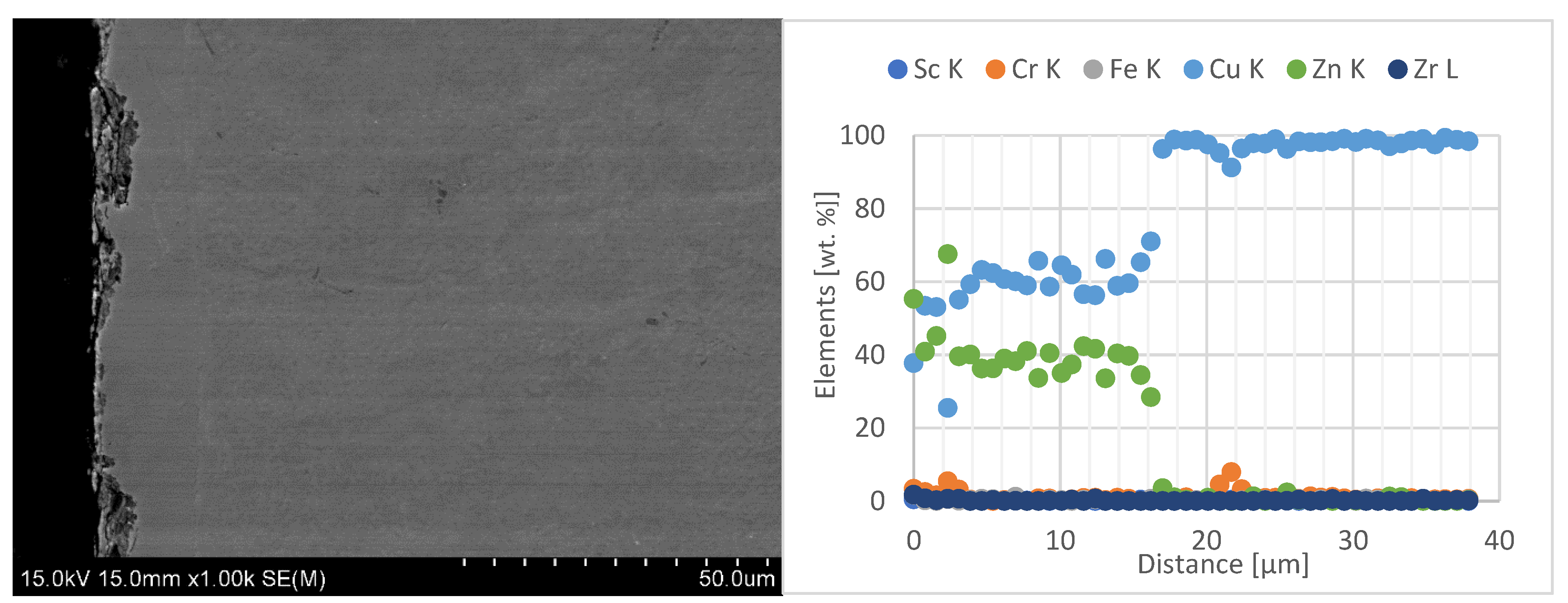
- Moreover, the developed alloy compositions effectively reduce the diffusion layer in the electrode material, which is a key factor leading to the degradation of the electrode face geometry and to accelerated wear during welding. Compared with commercial Cu–Cr–Zr electrodes, the newly developed Sc-modified electrodes exhibited about 40% reduction in the diffusion-layer-zone thickness and a distinct decrease in material loss from the electrode working surface, as confirmed by 3D geometry analysis.
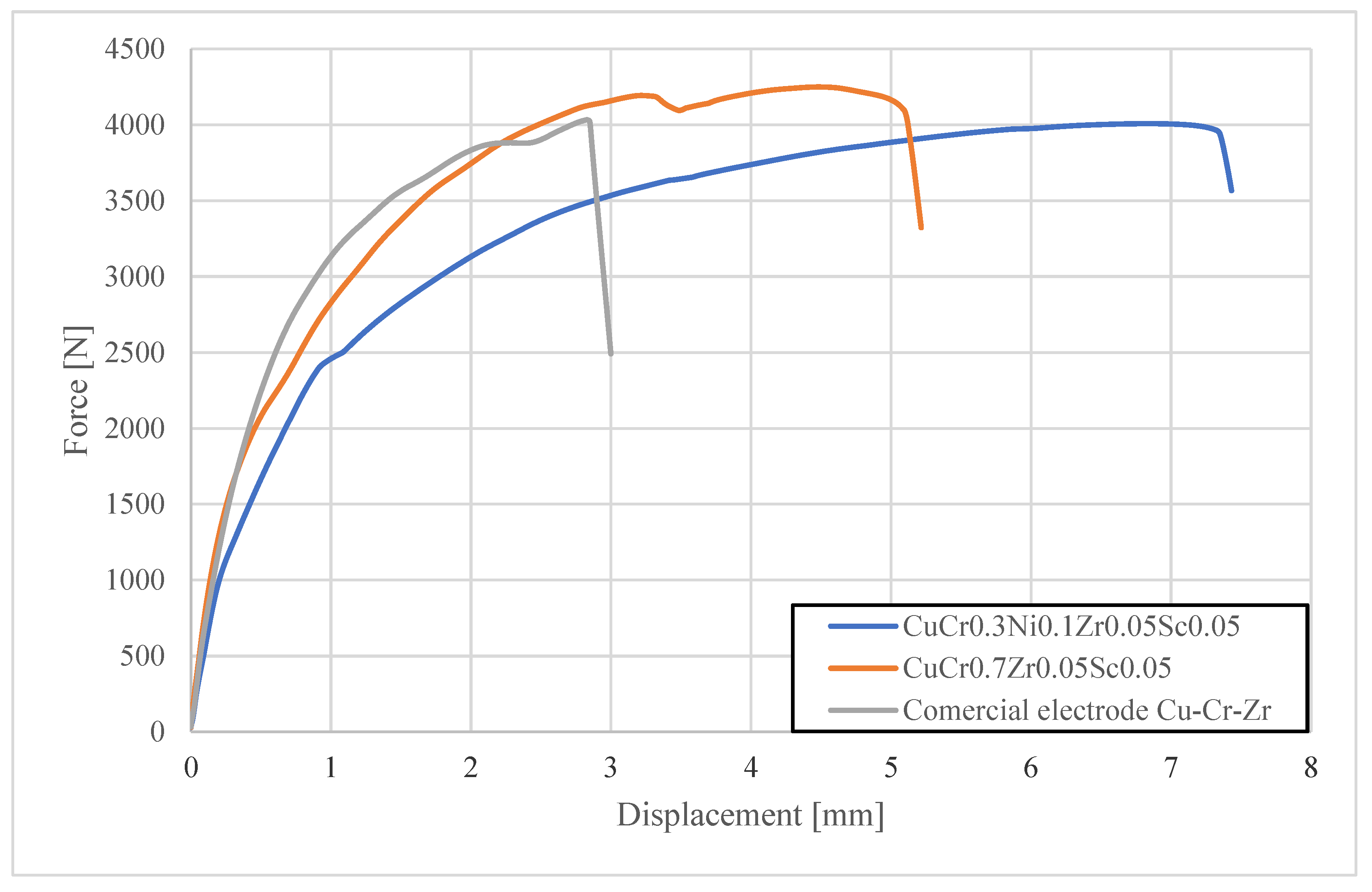
- Mechanical tests of the produced spot welds showed that the use of Sc-modified electrodes ensures high shear strength of welds, exceeding both the minimum normative requirements and the performance of joints made with commercial electrode caps after 500 welds.
- The results obtained are consistent with current global research trends focused on increasing electrode durability through microstructural and compositional modifications. However, it should be emphasized that the proposed solution, based on the modification of the chemical composition of the electrode material, offers a technological advantage over surface engineering methods by simplifying the manufacturing process and eliminating the need for additional technological treatments.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Economic and Market Report, Global and EU Auto Industry: Full Year 2024, ACEA. 2025. Available online: https://www.acea.auto/files/Economic_and_Market_Report-Full_year-2024.pdf (accessed on 26 October 2025).
- Akkaş, N. Welding Time Effect on Tensile-Shear Loading in Resistance Spot Welding of SPA-H Weathering Steel Sheets Used in Railway Vehicles. Acta Phys. Pol. A 2017, 131, 52–54. [Google Scholar] [CrossRef]
- Rdzawski, Z.; Kwaśniewski, P.; Głuchowski, W.; Łagoda, M.; Maleta, M.; Boczkal, S.; Franczak, K. Research on changes in microstructures and mechanical properties of Welding caps as a result of their usage during resistance spot welding process. Arch. Met. Mater. 2023, 68, 295–306. [Google Scholar] [CrossRef]
- Choudhari, R.; Adhaye, A.; Sulakhe, V.; Fegade, R. Recent Status of Research and Developments in Resistance Spot Welding. Int. J. Mech. Eng. 2025, 12, 1–27. [Google Scholar] [CrossRef]
- Başkaya, Ü.; Uzun, R.; Atapek, Ş.H.; Kılıç, Y.; Polat, Ş. Effect of coating type on electrode degradation and its life in resistance spot welding of a low carbon steel. Eng. Fail. Anal. 2024, 157, 107879. [Google Scholar] [CrossRef]
- Mazur, W.; Kyriakopoulos, A.; Bott, N.; West, D. Use of modified electrode caps for Surface quality welds in resistance spot welding. J. Manuf. Process. 2016, 22, 60–73. [Google Scholar] [CrossRef]
- Lin, H.; Hsu, C.; Lee, C.; Kuo, T.; Jeng, S. Effects of zinc layer thickness on Resistance spot Welding of galvanized mild steel. J. Mech. Work. Technol. 2018, 251, 205–213. [Google Scholar] [CrossRef]
- Sheikhi, M.; Valaee-Tale, M.; Mazaheri, Y.; Usefifar, G.R. Electrode lifetime in resistance spot welding of coated sheets: Experiments and modeling. Mater. Today Commun. 2024, 38, 107903. [Google Scholar] [CrossRef]
- Mikno, Z.; Bartnik, Z. Heating of electrodes during spot Resistance Welding in FEM calculations. Arch. Civ. Mech. Eng. 2016, 16, 86–100. [Google Scholar] [CrossRef]
- Song, S.; Shojaee, M.; Midawi, A.; Sherepenko, O.; Ghassemi-Armaki, H.; Biro, E. Influence of expulsion and heat extraction resulting from changes to electrode force on liquid metal embrittlement during resistance spot welding. J. Mater. Res. Technol. 2023, 23, 1458–1470. [Google Scholar] [CrossRef]
- Blondeau, R. Metallurgy and Mechanics of Welding—Processes and Industrail Applications; ISTE: London, UK, 2008. [Google Scholar]
- Panza, L.; De Maddis, M.; Spena, P.R. Use of electrode displacement signals for electrode degradation assessment in Resistance spot Welding. J. Manuf. Process. 2022, 76, 93–105. [Google Scholar] [CrossRef]
- Ghatei-Kalashami, A.; Zhang, S.; Shojaee, M.; Midawi, A.R.H.; Goodwin, F.; Zhou, N.Y. Failure behawior of Resistance spot welded advanced high strength steel: The role of Surface condition and initial microstructure. J. Mech. Work. Technol. 2022, 299, 117370. [Google Scholar]
- Mahmud, K.; Murugan, S.P.; Cho, Y.; Ji, C.; Nam, D.; Park, Y.-D. Geometrical degradation of electrode and liquid metal embrittlement cracking in resistance spot welding. J. Manuf. Process. 2021, 61, 334–348. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, D.; Han, L.; Li, Y. Optimised design of electrode morphology for novel dissimilar Resistance spot Welding of aluminium alloy and galvanised high strength steel. Mater. Des. 2015, 85, 461–470. [Google Scholar] [CrossRef]
- Zou, J.; Zhao, Q.; Chen, Z. Surface modified long-life electrode for Resistance spot Welding of Zn-coated steel. J. Mech. Work. Technol. 2009, 209, 4141–4146. [Google Scholar] [CrossRef]
- C1.1M/C1.1:2019; AWS Recommended Practices for Resistance Welding. American Welding Society: Doral, FL, USA, 2019.
- ISO 5182:2016(E); Resistance Welding—Materials for Electrodes and Ancillary Equipment. ISO: Geneva, Switzerland, 2016.
- Zhang, H.; Senkara, J. Resistance Welding, 2nd ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2012. [Google Scholar]
- Tu, J.P.; Qi, W.X.; Yang, Y.Z.; Liu, F.; Zhang, J.T.; Gan, G.Y.; Wang, N.Y.; Zhang, X.B.; Liu, M.S. Effect of aging treatment on the electrical sliding wear behawior of Cu-Cr-Zr alloy. Wear 2002, 249, 1021–1027. [Google Scholar] [CrossRef]
- Sarin, V.K.; Grant, N.J. Cu-Zr and Cu-Zr-Cr alloys produced from rapidly quenched powders. Met. Trans. 1972, 3, 875–878. [Google Scholar] [CrossRef]
- Batra, I.S.; Dey, G.K.; Kulkarni, U.D.; Banerjee, S. Microstructure and properties of a Cu-Cr-Zr alloy. J. Nucl. Mater. 2001, 299, 105–298. [Google Scholar] [CrossRef]
- Ping, L.; Shijie, D.; Zhixiong, X.; Anzhuo, Y.; Wei, Y. The effects of coating parameters on the quality of TiB2–TiC composite phase coating on the surface of Cu–Cr–Zr alloy electrode. Surf. Coat. Technol. 2014, 253, 132–138. [Google Scholar]
- Dong, S.J.; Zhou, Y. Effects of TiC composite coating on electrode degradation in microresistance welding of nickel-plated steel. Met. Mater. Trans. A 2003, 34, 1501–1511. [Google Scholar] [CrossRef]
- Cheng, L.; Shijie, D.; Xiang, X.; Norman, Z. Mass loss of copper alloy electrode during TiB2 coating by electrospark deposition. Surf. Coat. Technol. 2009, 203, 3333–3337. [Google Scholar]
- Franczak, K.; Kwaśniewski, P.; Kiesiewicz, G.; Zasadzinska, M.; Jurkiewicz, B.; Strzepek, P.; Rdzawski, Z. Research of mechanical and electrical properties of Cu-Sc and Cu-Zr alloys. Arch. Civ. Mech. Eng. 2020, 20, 28. [Google Scholar] [CrossRef]
- Lin, G.B.; Wang, Z.D.; Zhang, M.K.; Zhang, H.; Zhao, M. Heat treatment method for making high strength and conductivity Cu–Cr–Zr alloy. Mater. Sci. Technol. 2011, 27, 966–969. [Google Scholar] [CrossRef]
- Mishnev, R.; Shakhova, I.; Belyakov, A.; Kaibyshev, R. Deformation microstructures, strengthening mechanisms, and electrical conductivity in a Cu–Cr–Zr alloy. Mater. Sci. Eng. A 2015, 629, 29–40. [Google Scholar] [CrossRef]
- Lipinska, M.; Bazarnik, P.; Lewandowska, M. The electrical conductivity of CuCrZr alloy after SPD processing. In IOP Conference Series Materials Science and Engineering; IOP Publishing: Bristol, UK, 2014; Volume 63. [Google Scholar]
- Yan, F.; Chen, W.; Feng, P.; Dong, L.; Yang, T.; Ren, S.; Fu, Y. Microstructure evolution and enhanced properties of Cu–Cr–Zr alloys through synergistic effects of alloying, heat treatment and low-energy cyclic impact. J. Mater. Res. 2020, 35, 2746–2755. [Google Scholar] [CrossRef]
- Ostachowski, P.; Bochniak, W.; Łagoda, M.; Ziółkiewicz, S. Strength properties and structure of CuCrZr alloy subjected to low-temperature KOBO extrusion and heat treatment. Int. J. Adv. Manuf. Technol. 2019, 105, 5023–5044. [Google Scholar] [CrossRef]
- Davis, J.R. ASM Specialty Handbook: Copper and Copper Alloys; ASM International: Novelty, OH, USA, 2001. [Google Scholar]
- Franczak, K.; Sadzikowski, M.; Kwaśniewski, P.; Kiesiewicz, G.; Ściężor, W.; Kordaszewski, S. Research on Alloying Elements’ Influence on CuETP-Grade Copper’s Mechanical and Electrical Properties. Materials 2024, 17, 3020. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Y.; Yang, H.; Su, L.; Wang, C.; Tong, C.; Zhou, J.; Chen, J.; Wang, B. Effects of Si addition on properties and microstructure of CuCrZr alloy. J. Alloys Compd. 2022, 906, 164277. [Google Scholar] [CrossRef]
- Zhou, H.T.; Zhong, J.W.; Zhou, X.; Zhao, Z.K.; Li, Q.B. Microstructure and properties of Cu–1.0Cr–0.2Zr–0.03Fe alloy. Mater. Sci. Eng. A 2008, 498, 225–230. [Google Scholar] [CrossRef]
- Liu, P.; Kang, B.X.; Cao, X.G.; Huang, J.L.; Yen, B.; Gu, H.C. Aging precipitation and recrystallization of rapidly solidified Cu–Cr–Zr–Mg alloy. Mater. Sci. Eng. A 1999, 265, 262–267. [Google Scholar] [CrossRef]
- Chenna Krishna, S.; Sudarsana Rao, G.; Jha, A.K.; Pant, B.; Venkitakrishnan, P.V. Strengthening in high strength Cu-Cr-Zr-Ti alloy plates produced by hot rolling. Mater. Sci. Eng. A 2016, 674, 164–170. [Google Scholar] [CrossRef]
- Zhang, Y.; Volinsky, A.A.; Tran, H.T.; Chai, Z.; Liu, P.; Tian, B.; Liu, Y. Aging behavior and precipitates analysis of the Cu–Cr–Zr–Ce alloy. Mater. Sci. Eng. A 2016, 650, 248–253. [Google Scholar] [CrossRef]
- Zhang, Y.; Volinsky, A.A.; Tran, H.T.; Chai, Z.; Liu, P.; Tian, B. Effects of Ce Addition on High Temperature Deformation Behavior of Cu-Cr-Zr Alloys. J. Mater. Eng. Perform. 2015, 24, 3783–3788. [Google Scholar] [CrossRef]
- Franczak, K. Influence of heat treatment and plastic deformation on the mechanical and electrical properties of Cu-Sc alloys prepared by continuous casting process. Arch. Foundry Eng. 2025, 1, 11–120. [Google Scholar] [CrossRef]
- Franczak, K.; Kwaśniewski, P.; Kiesiewicz, G.; Sadzikowski, M.; Sciezor, W.; Kordaszewski, S.; Kuca, D. Research on the continuous casting process of CuCrZr alloys with the addition of scandium dedicated for Resistance Welding electrodes. Metalurgija 2022, 61, 631–633. [Google Scholar]
- Franczak, K.; Kwaśniewski, P.; Kiesiewicz, G.; Ściężor, W.; Sadzikowski, M.; Kordaszewski, S.; Noga, P. Research on zinc diffusion in the resistance spot welding process of galvanised steels using Cu-Sc and Cu-Zr alloy electrodes. Weld. World 2025, 61, 1–16. [Google Scholar] [CrossRef]
- ISO 5821:2009; Spot Welding Electrode Caps. ISO: Geneva, Switzerland, 2009.
- Franczak, K. Research on Cu-Sc Alloys Dedicated for Resistance Welding Electrodes. Ph.D. Thesis, AGH University of Krakow, Kraków, Poland, 2023. [Google Scholar]
- Vabai, B.; Sommer, C.; Szabo, M.; Toth, T.; Majlinger, K. Shear tension strength of resistance spot welded ultra high strength steel. Thin-Walled Struct. 2019, 142, 64–73. [Google Scholar] [CrossRef]






| Parameter | Value |
|---|---|
| Number of spot welds | 500 |
| Number of current pulses | 2 |
| Initial welding current | 6500 A |
| Welding current increase | 500 every 100 welds |
| Thickness of welded materials | 0.75 mm |
| Type of welded materials | DX53D |
| UTS of welded materials | 270 MPa |
| No. | Material | Elements [wt. %] | |||||
|---|---|---|---|---|---|---|---|
| Cr | Zr | Ni | Sc | Others | Cu (Rest) | ||
| 1 | CuCr0.7Zr0.05 | 0.68 | 0.06 | - | - | <0.01 | 99.25 |
| 2 | CuCr0.7Zr0.05Sc0.01 | 0.67 | 0.07 | - | 0.01 | <0.01 | 99.24 |
| 3 | CuCr0.7Zr0.05Sc0.05 | 0.68 | 0.06 | - | 0.06 | <0.01 | 99.19 |
| 4 | CuCr0.3Ni0.1Zr0.05 | 0.31 | 0.05 | 0.11 | - | <0.01 | 99.52 |
| 5 | CuCr0.3Ni0.1Zr0.05Sc0.01 | 0.3 | 0.06 | 0.11 | 0.01 | <0.01 | 99.51 |
| 6 | CuCr0.3Ni0.1Zr0.05Sc0.05 | 0.31 | 0.05 | 0.11 | 0.05 | <0.01 | 99.47 |
| Type of Electrode | Lower Electrode [mm] | Upper Electrode [mm] |
|---|---|---|
| CuCr0.3Zr0.1Ni0.1Sc0.05 | −0.25 | −0.35 |
| CuCr0.7Zr0.05Sc0.05 | −0.2 | −0.29 |
| Commercial electrode Cu-Cr-Zr | −0.27 | −0.45 |
| Type of Electrode | Average Value of Diffusion Layer [µm] | SD |
|---|---|---|
| CuCr0.3Zr0.1Ni0.1Sc0.05 | 8.28 | 0.8 |
| CuCr0.7Zr0.05Sc0.05 | 12.4 | 1.05 |
| Commercial electrode Cu-Cr-Zr | 19.9 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franczak, K.; Kwaśniewski, P.; Kiesiewicz, G.; Ściężor, W.; Sadzikowski, M.; Kordaszewski, S.; Micek, P.; Kuca, D.; Pestrak, R. Investigation of Electrode Cap Life Made of New Cu–Cr–Zr Copper Alloys with Scandium Addition Dedicated for Resistance Spot Welding of Galvanized Steel Sheets. Materials 2025, 18, 4950. https://doi.org/10.3390/ma18214950
Franczak K, Kwaśniewski P, Kiesiewicz G, Ściężor W, Sadzikowski M, Kordaszewski S, Micek P, Kuca D, Pestrak R. Investigation of Electrode Cap Life Made of New Cu–Cr–Zr Copper Alloys with Scandium Addition Dedicated for Resistance Spot Welding of Galvanized Steel Sheets. Materials. 2025; 18(21):4950. https://doi.org/10.3390/ma18214950
Chicago/Turabian StyleFranczak, Krystian, Paweł Kwaśniewski, Grzegorz Kiesiewicz, Wojciech Ściężor, Michał Sadzikowski, Szymon Kordaszewski, Piotr Micek, Damian Kuca, and Rafał Pestrak. 2025. "Investigation of Electrode Cap Life Made of New Cu–Cr–Zr Copper Alloys with Scandium Addition Dedicated for Resistance Spot Welding of Galvanized Steel Sheets" Materials 18, no. 21: 4950. https://doi.org/10.3390/ma18214950
APA StyleFranczak, K., Kwaśniewski, P., Kiesiewicz, G., Ściężor, W., Sadzikowski, M., Kordaszewski, S., Micek, P., Kuca, D., & Pestrak, R. (2025). Investigation of Electrode Cap Life Made of New Cu–Cr–Zr Copper Alloys with Scandium Addition Dedicated for Resistance Spot Welding of Galvanized Steel Sheets. Materials, 18(21), 4950. https://doi.org/10.3390/ma18214950





