AlOOH-Coated Glass Fiber-Reinforced Composites for Pipeline Rehabilitation: Enhancement of Interfacial Adhesion and Durability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
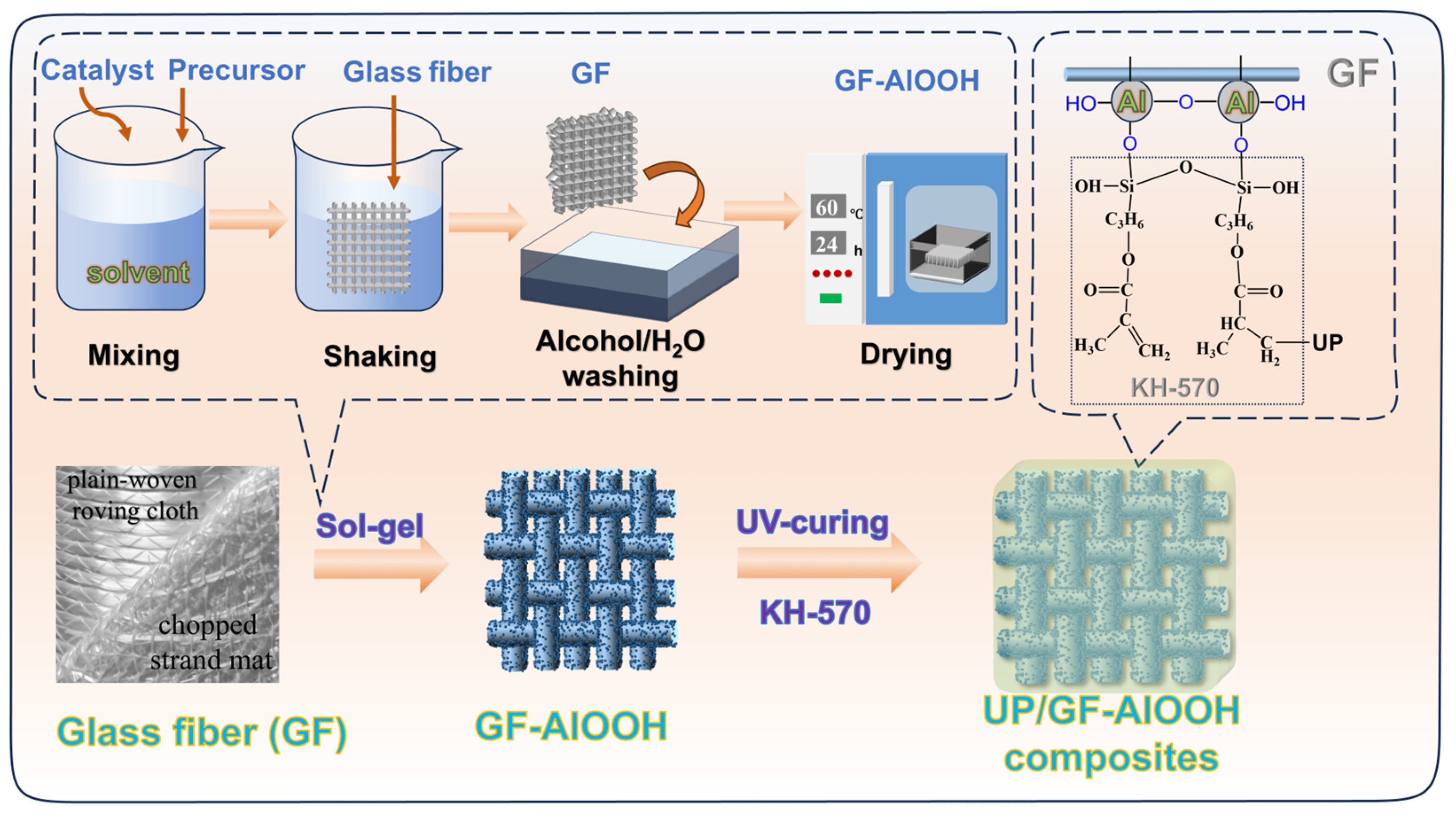
2.2. Synthesis of Glass Fiber Coated with AlOOH (GF-AlOOH)
2.3. Preparation of UP/GF Composites
2.4. Materials Characterization
3. Results and Discussion
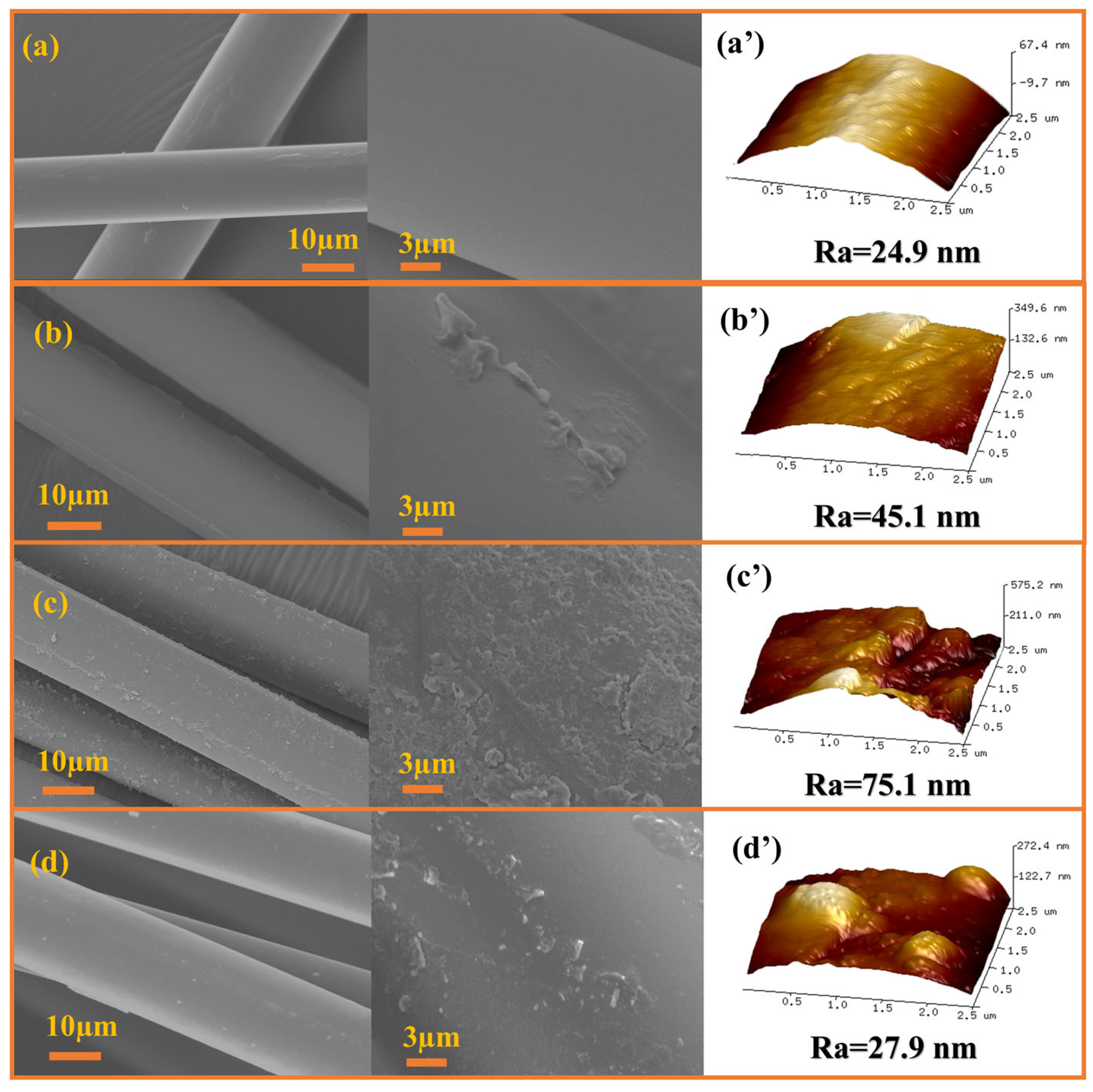
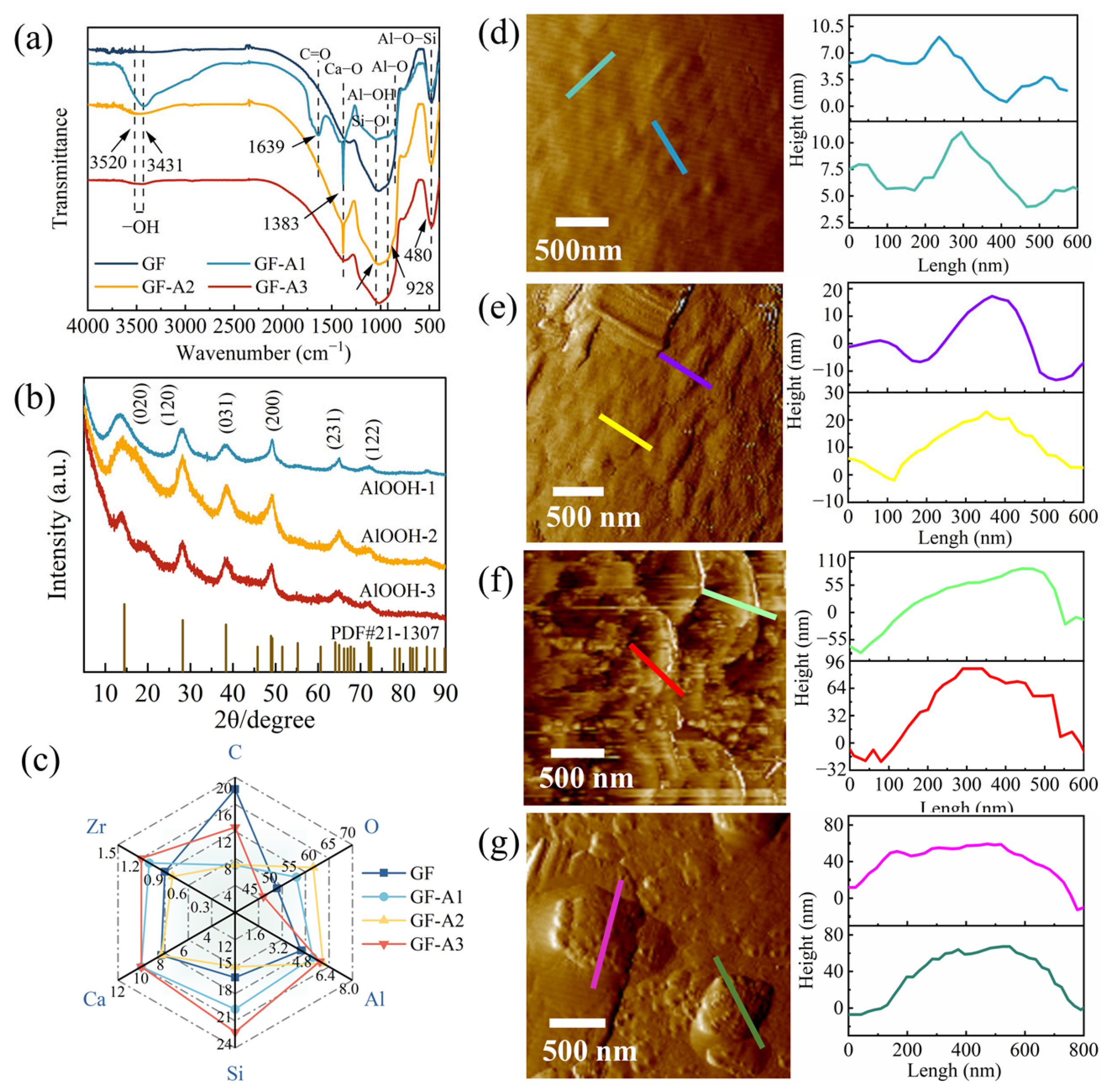
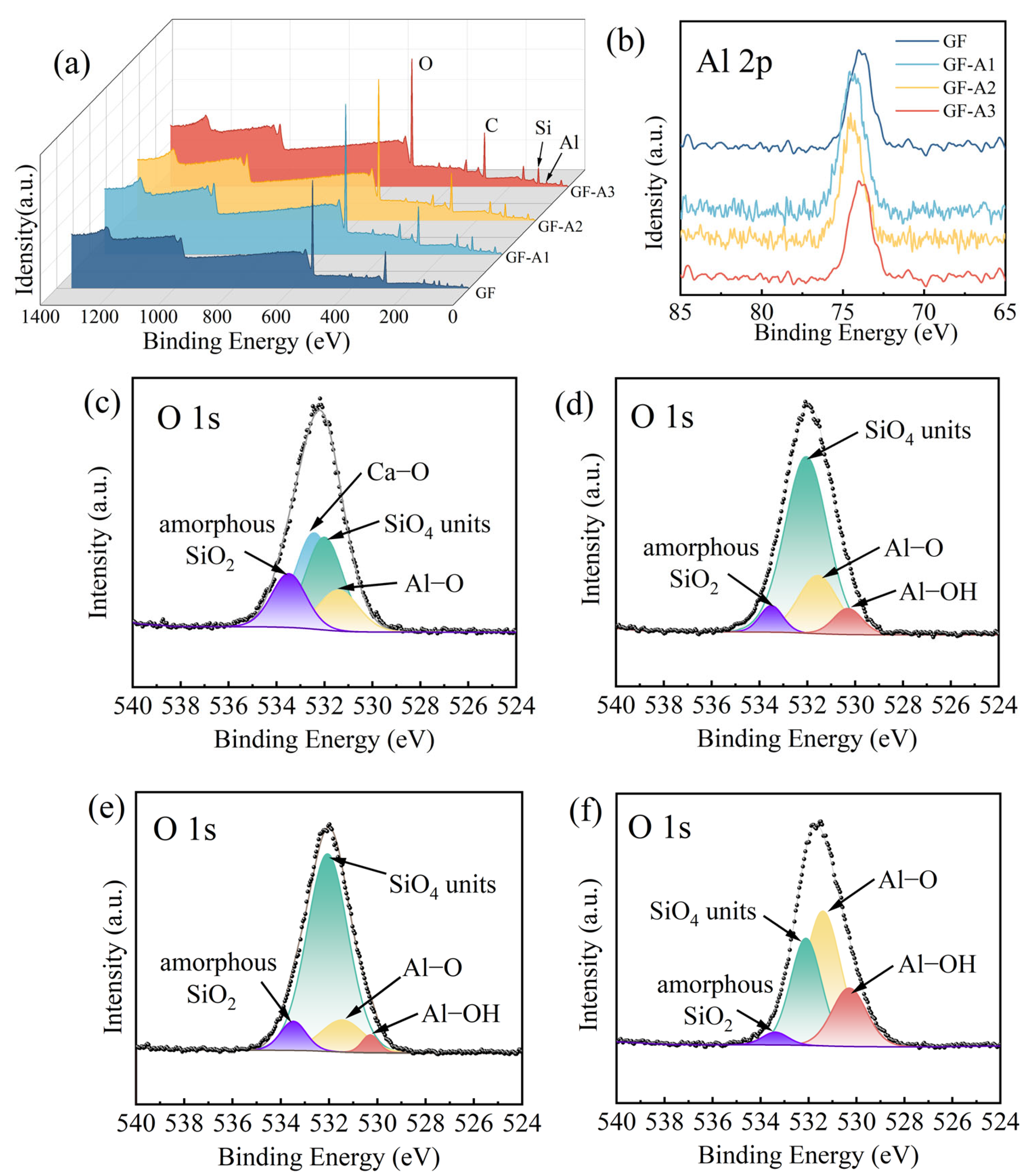
3.1. Grafting Efficiency and Composition of GF-AlOOH
3.2. Surfaces Wettability and Energetics of GF-AlOOH
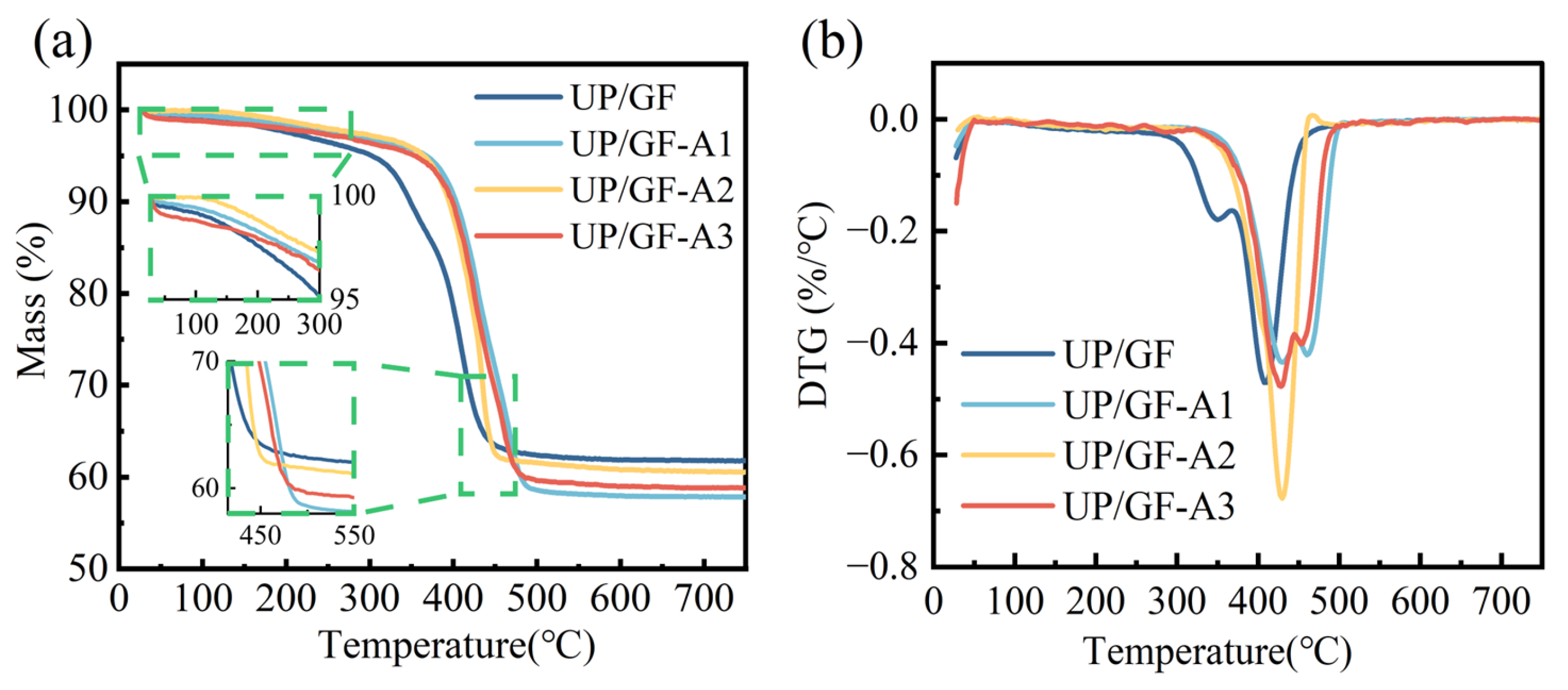
3.3. Thermal Behavior of UP/GF-AlOOH Composites
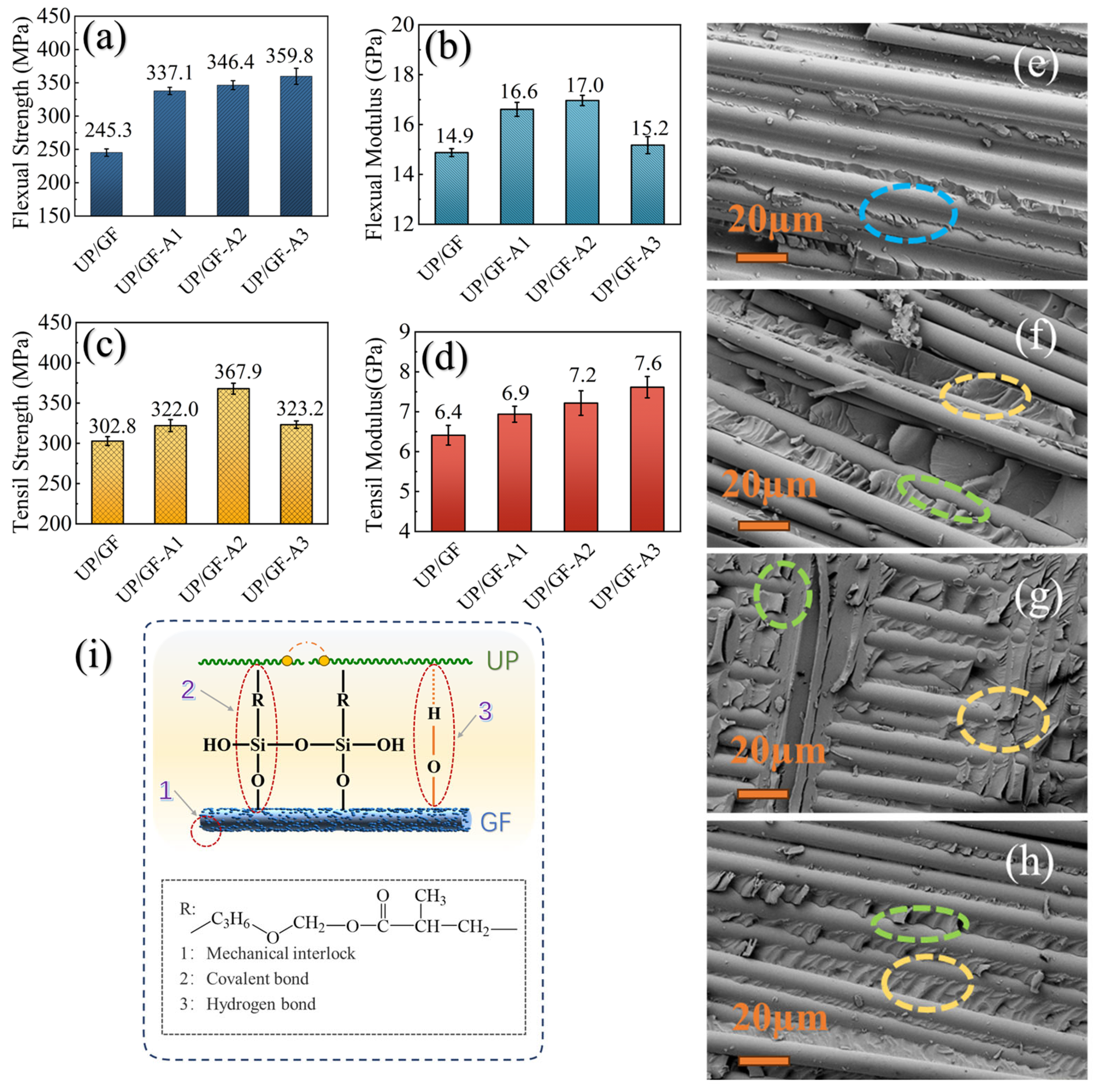
3.4. Mechanical Properties of UP/GF-AlOOH Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GF | Glass fiber |
| UP | unsaturated polyester resin |
| UV–CIPP | Ultraviolet-cured-in-place pipe |
| AlOOH | Boehmite |
| BPO | Benzoil peroxide |
References
- Sapuan, S.M.; Aulia, H.S.; Ilyas, R.A.; Atiqah, A.; Dele-Afolabi, T.T.; Nurazzi, M.N.; Supian, A.B.M.; Atikah, M.S.N. Mechanical Properties of Longitudinal Basalt/Woven-Glass-Fiber-reinforced Unsaturated Polyester-Resin Hybrid Composites. Polymers 2020, 12, 2211. [Google Scholar] [CrossRef]
- Shahid, A.T.; Silvestre, J.D.; Hofmann, M.; Garrido, M.; Correia, J.R. Life cycle assessment of an innovative bio-based unsaturated polyester resin and its use in glass fibre reinforced bio-composites produced by vacuum infusion. J. Clean. Prod. 2024, 441, 140906. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Y.; Duan, J.; Xie, K.; Li, J.; An, Q.; Wang, X. Hygrothermal Effect on GF/VE and GF/UP Composites: Durability Performance and Laboratory Assessment. Polymers 2024, 16, 632. [Google Scholar] [CrossRef]
- Ranjeth Kumar Reddy, T.; Subba Rao, T.; Padma Suvarna, R. Studies on thermal characteristics of cow dung powder filled glass–polyester hybrid composites. Compos. B Eng. 2014, 56, 670–672. [Google Scholar] [CrossRef]
- Khorasani, M.A.M.; Sahebian, S.; Zabett, A. Effects of toughened polyester on fatigue behavior of glass fiber reinforced polyester composite for wind turbine blade. Polym. Compos. 2021, 42, 70–82. [Google Scholar] [CrossRef]
- Wang, C.; Guo, L.; Xia, Y.; Zhang, C.; Sang, X.; Xu, C.; Zhu, G.; Ji, H.; Zhao, P.; Fang, H.; et al. Flexural performance and damage evolution of multiple fiberglass-reinforced UV-CIPP composite materials—A view from mechanics and energy release. J. Mater. Res. Technol. 2024, 29, 3317–3339. [Google Scholar] [CrossRef]
- Malik, R.; Parida, R.K.; Parida, B.N.; Nayak, N.C. Two-dimensional Ti3C2Tx (MXene)-multiwalled carbon nanotubes reinforced ethyl methyl acrylate/ethylene octene copolymer binary blend hybrid nanocomposites with enhanced thermal and dielectric properties. Polym. Compos. 2023, 45, 1534–1550. [Google Scholar] [CrossRef]
- Bosseler, B.; Homann, D.; Brüggemann, T.; Naismith, I.; Rubinato, M. Quality assessment of CIPP lining in sewers: Crucial knowledge acquired by IKT and research gaps identified in Germany. Tunn. Undergr. Space Technol. 2024, 143, 105425. [Google Scholar] [CrossRef]
- Malik, M.S.; Wolfahrt, M.; Domínguez Pardo, J.J.; Bublitz, D.; Schlögl, S. Prospects in the application of a frontally curable epoxy resin for cured-in-place-pipe rehabilitation. J. Appl. Polym. Sci. 2023, 141, e55024. [Google Scholar] [CrossRef]
- Wen, J.; Zhang, C.; Xia, Y.; Wang, C.; Sang, X.; Fang, H.; Wang, N. UV/thermal dual-cured MWCNTs composites for pipeline rehabilitation: Mechanical properties and damage analysis. Constr. Build. Mater. 2024, 450, 138602. [Google Scholar] [CrossRef]
- Yan, F.; Zhou, Q.; Xu, Y.; Wang, G.; Li, G.; Ma, C.; Su, G.; Zhan, X.; Liu, L. Learning from nature: Constructing “rigid-soft” structure on carbon fibers surface by self-assembly to improve the performance of epoxy composites. Compos. A Appl. Sci. Manuf. 2024, 176, 107888. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Han, X.; Li, X.; Huyan, C.; Li, J.; Liu, D.; Chen, F. Glass fiber/epoxy composites with improved interfacial adhesion by using cross-linking sizing agent. Polym. Compos. 2023, 45, 1737–1748. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, Y.; Qian, C.; Dai, H.; Fu, Y.; Dong, Y. Interfacial modification between glass fiber and polypropylene using a novel waterborne amphiphilic sizing agent. Compos. B Eng. 2022, 241, 110029. [Google Scholar] [CrossRef]
- Fang, H.; Jia, F.; Chen, S.; Zhang, W.; Huang, L.; Wu, F.; Chen, H.; Lin, D.; Jia, J. Significant enhancement of mechanical properties for glass fiber fabric-reinforced polyamide 6 composites by improving interfacial bonding. Polym. Compos. 2024, 45, 7541–7550. [Google Scholar] [CrossRef]
- Cheng, S.; Li, M.; Li, N.; Ren, Z.; Hao, H.; Liu, J.; Wen, J.; Wang, B.; Feng, J.; Hu, F.; et al. Interfacial properties of PPBES/CF composites reinforced by ammonified carbon fibers through thiol-ene click reaction. Polymer 2023, 283, 126251. [Google Scholar] [CrossRef]
- Saeedifar, M.; Ahangar, M. Augmented interlaminar fracture toughness of nanofibers-interleaved composite laminates via plasma treatment. J. Mater. Sci. 2024, 59, 9291–9302. [Google Scholar] [CrossRef]
- Luo, N.; Zhong, H.; Yang, M.; Yuan, X.; Fan, Y.B. Modifying glass fiber surface with grafting acrylamide by UV-grafting copolymerization for preparation of glass fiber reinforced PVDF composite membrane. J. Environ. Sci. 2016, 39, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Che, C.; Zhu, X.; Dashtbozorg, B.; Li, X.; Dong, H.; Jenkins, M.J. Enhancing the bond strength between glass fibre reinforced polyamide 6 and aluminium through μPlasma surface modification. Appl. Surf. Sci. 2024, 657, 159734. [Google Scholar] [CrossRef]
- Cao, Z.; Hao, T.; Wang, P.; Zhang, Y.; Cheng, B.; Yuan, T.; Meng, J. Surface modified glass fiber membranes with superior chemical and thermal resistance for O/W separation. Chem. Eng. J. 2017, 309, 30–40. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Cheng, S.; Hu, T.; Lu, F.; Li, X.; Zhang, C. Synthetic of phenolic resin@SiO2 containing PAO6 to reinforce the tribological properties of PTFE/aramid fabric liner composites. J. Mater. Res. Technol. 2024, 31, 157–164. [Google Scholar] [CrossRef]
- Feng, X.; Shao, L.; Qiu, Y.; Tian, W.; Tao, F.; Zhu, C. Interfacial engineering of glass fiber-reinforced vinyl ester resin composites: Using polydopamine-SiO2 for property enhancement. Polym. Compos. 2024, 45, 6861–6871. [Google Scholar] [CrossRef]
- Xiao, Z.; Yu, S.; Li, Y.; Ruan, S.; Kong, L.B.; Huang, Q.; Huang, Z.; Zhou, K.; Su, H.; Yao, Z.; et al. Materials development and potential applications of transparent ceramics: A review. Mater. Sci. Eng. R Rep. 2020, 139, 100518. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Mao, J.; Liu, C.; Zhang, L.; Cheng, Y.; Du, C.; Tanaka, T. Dielectric strength of glass fibre fabric reinforced epoxy by nano-Al2O3. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 1086–1094. [Google Scholar] [CrossRef]
- Nayak, R.K. Influence of seawater aging on mechanical properties of nano-Al2O3 embedded glass fiber reinforced polymer nanocomposites. Constr. Build. Mater. 2019, 221, 12–19. [Google Scholar] [CrossRef]
- Asi, O. Mechanical Properties of Glass-Fiber Reinforced Epoxy Composites Filled with Al2O3 Particles. J. Reinf. Plast. Compos. 2009, 28, 2861–2867. [Google Scholar] [CrossRef]
- Ba, Z.; Luo, H.; Cui, J.; Guo, Z. Mechanically robust and environmentally stable Al2O3/KH550 densified bamboo structural materials. Ind. Crops Prod. 2024, 211, 118201. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, X.; Rong, X.; Zhang, X.; Zhao, D.; He, C.; Zhao, N. Macro- and meso-mechanic investigations on the mechanical properties of heterostructured Al matrix composites featuring intragranular reinforcement. Mater. Res. Lett. 2024, 12, 408–416. [Google Scholar] [CrossRef]
- Zhong, H.; Hu, H.; Ni, B.; Guo, Y.; Luo, Z.; Zhao, T.; Zhang, B.-X. Silica sol nanoparticles hybridized allyl phenolic resins for improving mechanical and thermal performance. Polymer 2022, 254, 125052. [Google Scholar] [CrossRef]
- Hassani Rad, S.J.; Haghighi, M.; Alizadeh Eslami, A.; Rahmani, F.; Rahemi, N. Sol–gel vs. impregnation preparation of MgO and CeO2 doped Ni/Al2O3 nanocatalysts used in dry reforming of methane: Effect of process conditions, synthesis method and support composition. Int. J. Hydrogen Energy 2016, 41, 5335–5350. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.; Liao, X.; Shi, B. Preparation of highly active and reusable heterogeneous Al2O3–Pd catalysts by the sol–gel method using bayberry tannin as stabilizer. Res. Chem. Intermed. 2012, 38, 1609–1618. [Google Scholar] [CrossRef]
- Amiri, S.; Rahimi, A. Hybrid nanocomposite coating by sol–gel method: A review. Iran. Polym. J. 2016, 25, 559–577. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, W.; Wang, C. Characterization and tribological investigation of sol-gel Al2O3 and doped Al2O3 films. J. Eur. Ceram. Soc. 2002, 22, 2869–2876. [Google Scholar] [CrossRef]
- Li, J.; Pan, Y.; Xiang, C.; Ge, Q.; Guo, J. Low temperature synthesis of ultrafine α-Al2O3 powder by a simple aqueous sol–gel process. Ceram. Int. 2006, 32, 587–591. [Google Scholar] [CrossRef]
- Mat Rozi, N.; Hamid, H.A.; Hossain, M.S.; Khalil, N.A.; Ahmad Yahaya, A.N.; Syimir Fizal, A.N.; Haris, M.Y.; Ahmad, N.; Zulkifli, M. Enhanced Mechanical and Thermal Properties of Modified Oil Palm Fiber-Reinforced Polypropylene Composite via Multi-Objective Optimization of In Situ Silica Sol-Gel Synthesis. Polymers 2021, 13, 3338. [Google Scholar] [CrossRef]
- Ma, L.; Liu, H.; Wen, X.; Szymańska, K.; Mijowska, E.; Hao, C.; Tang, T.; Lei, Q. Polyhydric SiO2 coating assistant to graft organophosphorus onto glass fabric for simultaneously improving flame retardancy and mechanical properties of epoxy resin composites. Compos. B Eng. 2022, 243, 110176. [Google Scholar] [CrossRef]
- Chengxin, Z.; Feng, C.; Yang, X.; Zhihang, P. Thermal shock resistance of Al2O3/SiO2 composites by sol-gel. Ceram. Int. 2019, 45, 11270–11274. [Google Scholar] [CrossRef]
- GB/T 2577-2005; Test method for resin content of glass fiber reinforced plastics. Standards Press of China: Beijing, China, 2005.
- Pedrazzoli, D.; Pegoretti, A. Expanded graphite nanoplatelets as coupling agents in glass fiber reinforced polypropylene composites. Compos. A Appl. Sci. Manuf. 2014, 66, 25–34. [Google Scholar] [CrossRef]
- Pedrazzoli, D.; Pegoretti, A. Silica nanoparticles as coupling agents for polypropylene/glass composites. Compos. Sci. Technol. 2013, 76, 77–83. [Google Scholar] [CrossRef]
- GB/T 1449-2005; Fibre-reinforced plastic composites—Determination of flexural properties. Standards Press of China: Beijing, China, 2005.
- GB/T 1447-2005; Fiber-reinforced plastics composites—Determination of tensile properties. Standards Press of China: Beijing, China, 2005.
- Zhu, S.; Qian, Y.; Hassan, E.A.M.; Shi, R.; Yang, L.; Cao, H.; Zhou, J.; Ge, D.; Yu, M. Enhanced interfacial interactions by PEEK-grafting and coupling of acylated CNT for GF/PEEK composites. Compos. Commun. 2020, 18, 43–48. [Google Scholar] [CrossRef]
- Li, G.Z.; Zhang, S.; Yu, H.; Liu, G.; Wang, W.; Chen, G.; Wang, J.; Wan, W.; Lu, Q.; Chen, H.; et al. Enhancing the mechanical properties of basalt fiber/nylon 6 composites by surface roughening and hydrogen bonding interaction. Polym. Compos. 2024, 45, 7801–7810. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Cattaneo, A.S.; Mustarelli, P. Al2O3·2SiO2 powders synthesized via sol–gel as pure raw material in geopolymer preparation. J. Am. Ceram. Soc. 2017, 100, 1919–1927. [Google Scholar] [CrossRef]
- Todea, M.; Muresan-Pop, M.; Simon, V.; Vulpoi, A.; Simon, S. Synthesis and characterization of composite SiO2–Al2O3–Fe2O3 core–shell microspheres. J. Sol-Gel Sci. Technol. 2020, 96, 395–404. [Google Scholar] [CrossRef]
- Ghadimi, Z.; Esfahani, H.; Mazaheri, Y. Control on nanostructured quaternary Ti–Al–O–B composite synthesized via electrospinning method, from nanoparticles to nanowhiskers. J. Sol-Gel Sci. Technol. 2021, 98, 127–137. [Google Scholar] [CrossRef]
- Irie, Y.; Tanaka, H.; Kinoshita, K.; Kishida, S. Studies on interface of Cu/Al and Al/SiO2/Si. Procedia Eng. 2016, 141, 144–151. [Google Scholar] [CrossRef]
- Hu, S.; Han, P.; Meng, C.; Yu, Y.; Han, S.; Wang, H.; Wei, G.; Gu, Z. Comparative study of different bonding interactions on the interfacial adhesion and mechanical properties of MXene-decorated carbon fiber/epoxy resin composites. Compos. Sci. Technol. 2024, 245, 110352. [Google Scholar] [CrossRef]
- Hu, S.; Han, P.; Zhang, S.; Wang, J.; Meng, C.; Wei, G.; Gu, Z. Layer-by-layer assembling MXene /poly(3-glycidyloxypropyldimethoxymethylsilane) hierarchical structure onto carbon fibers for high-performance carbon fiber/epoxy composites. Compos. Sci. Technol. 2023, 241, 110149. [Google Scholar] [CrossRef]
- Laoubi, K.; Hamadi, Z.; Ahmed Benyahia, A.; Serier, A.; Azari, Z. Thermal behavior of E-glass fiber-reinforced unsaturated polyester composites. Compos. B Eng. 2014, 56, 520–526. [Google Scholar] [CrossRef]
- Suchitra, M.; Vinay, B.K.; Parameshwara, S.; Umashankar, M.; Panchami, S.V. Effect of Combining Nano- and Microfillers for the Assessment of Thermal Class of Glass Fiber-Reinforced Epoxy Composites for Outdoor Insulation. IEEE Trans. Dielectr. Electr. Insul. 2023, 30, 2896–2904. [Google Scholar] [CrossRef]
- ASTM F1216-22; Standard Practice for Rehabilitation of Existing Pipelines and Conduits by the Inversion and Curing of a Resin-Impregnated Tube. ASTM: West Conshohocken, PA, USA, 2022.
- GB/T 41666.4-2024; Plastics piping systems for renovation of underground non-pressure drainage and sewerage networks-Part 4: Lining with cured-in-place pipes. Standards Press of China: Beijing, China, 2024.
- T/CECS 559-2018; Specification for water and drainage pipeline rehabilitation using cured-in-place-pipe method. Planning Press: Beijing, China, 2018.
- Zheng, H.; Zhang, W.; Li, B.; Zhu, J.; Wang, C.; Song, G.; Wu, G.; Yang, X.; Huang, Y.; Ma, L. Recent advances of interphases in carbon fiber-reinforced polymer composites: A review. Compos. B Eng. 2022, 233, 109639. [Google Scholar] [CrossRef]
- Chen, G.; Li, A.; Liu, H.; Huang, S.; Zhang, Z.; Liu, W.; Zha, C.; Li, B.; Wang, Z. Mechanical and dynamic properties of resin blend and composite systems: A molecular dynamics study. Compos. Struct. 2018, 190, 160–168. [Google Scholar] [CrossRef]
- Huang, Y.L.; Xue, D.S.; Zhou, P.H.; Ma, Y.; Li, F.S. α-Fe–Al2O3 nanocomposites prepared by sol–gel method. Mater. Sci. Eng. A 2003, 359, 332–337. [Google Scholar] [CrossRef]
- Niero, D.F.; Montedo, O.R.K.; Bernardin, A.M. Synthesis and characterization of nano α-alumina by an inorganic sol–gel method. Mater. Sci. Eng. B 2022, 280, 115690. [Google Scholar] [CrossRef]
- Yabuki, M.; Takahashi, R.; Sato, S.; Sodesawa, T.; Ogura, K. Silica–alumina catalysts prepared in sol–gel process of TEOS with organic additives. Phys. Chem. Chem. Phys. 2002, 4, 4830–4837. [Google Scholar] [CrossRef]
- Suhasinee Behera, P.; Bhattacharyya, S.; Sarkar, R. Effect of citrate to nitrate ratio on the sol-gel synthesis of nanosized α-Al2O3 powder. Ceram Int. 2017, 43, 15221–15226. [Google Scholar] [CrossRef]

| Sample | Precursor | Solvent System | Reaction Condition | Catalyst |
|---|---|---|---|---|
| GF-A1 | aluminum isopropoxide | Isopropanol | 85–95 °C | nitric acid |
| GF-A2 | Al(NO3)3·9H2O | H2O | room temperature | NaOH |
| GF-A3 | Al(NO3)3·9H2O and citric acid | H2O | 75 °C | ammonia solution |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, M.; Yan, X.; Wu, C.; Wang, K. AlOOH-Coated Glass Fiber-Reinforced Composites for Pipeline Rehabilitation: Enhancement of Interfacial Adhesion and Durability. Materials 2025, 18, 4887. https://doi.org/10.3390/ma18214887
Du M, Yan X, Wu C, Wang K. AlOOH-Coated Glass Fiber-Reinforced Composites for Pipeline Rehabilitation: Enhancement of Interfacial Adhesion and Durability. Materials. 2025; 18(21):4887. https://doi.org/10.3390/ma18214887
Chicago/Turabian StyleDu, Mengfei, Xilai Yan, Chuandong Wu, and Ke Wang. 2025. "AlOOH-Coated Glass Fiber-Reinforced Composites for Pipeline Rehabilitation: Enhancement of Interfacial Adhesion and Durability" Materials 18, no. 21: 4887. https://doi.org/10.3390/ma18214887
APA StyleDu, M., Yan, X., Wu, C., & Wang, K. (2025). AlOOH-Coated Glass Fiber-Reinforced Composites for Pipeline Rehabilitation: Enhancement of Interfacial Adhesion and Durability. Materials, 18(21), 4887. https://doi.org/10.3390/ma18214887









