An Enhancement in the Magnetocaloric Effect in a Composite Powder Based on Lanthanum Manganites
Abstract
1. Introduction
2. Materials and Methods
2.1. The Synthesis of Manganites and Preparation of the Composite
2.2. Crystal Structure and Microstructure
2.3. Magnetic and Magnetocaloric Properties
3. Results and Discussion
3.1. Crystal Structure
3.2. Particle Morphology and Chemical Composition
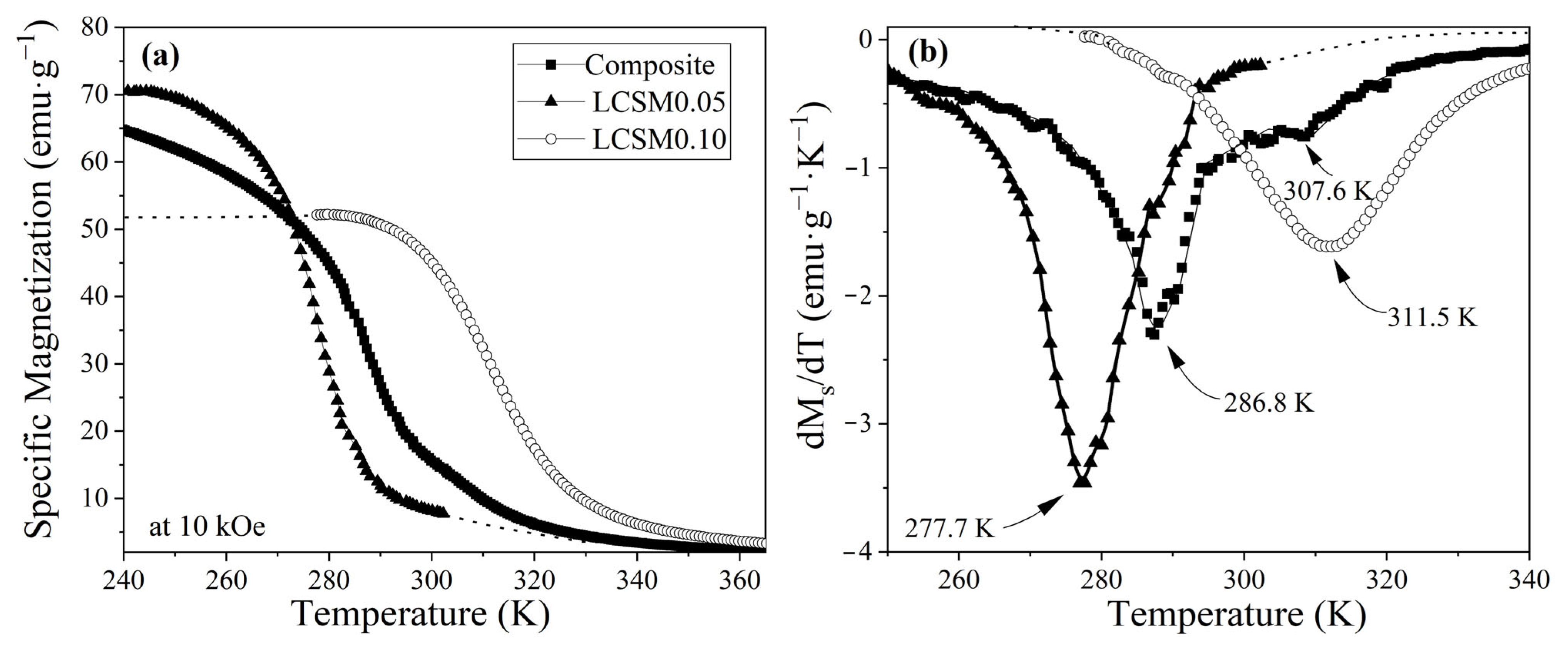
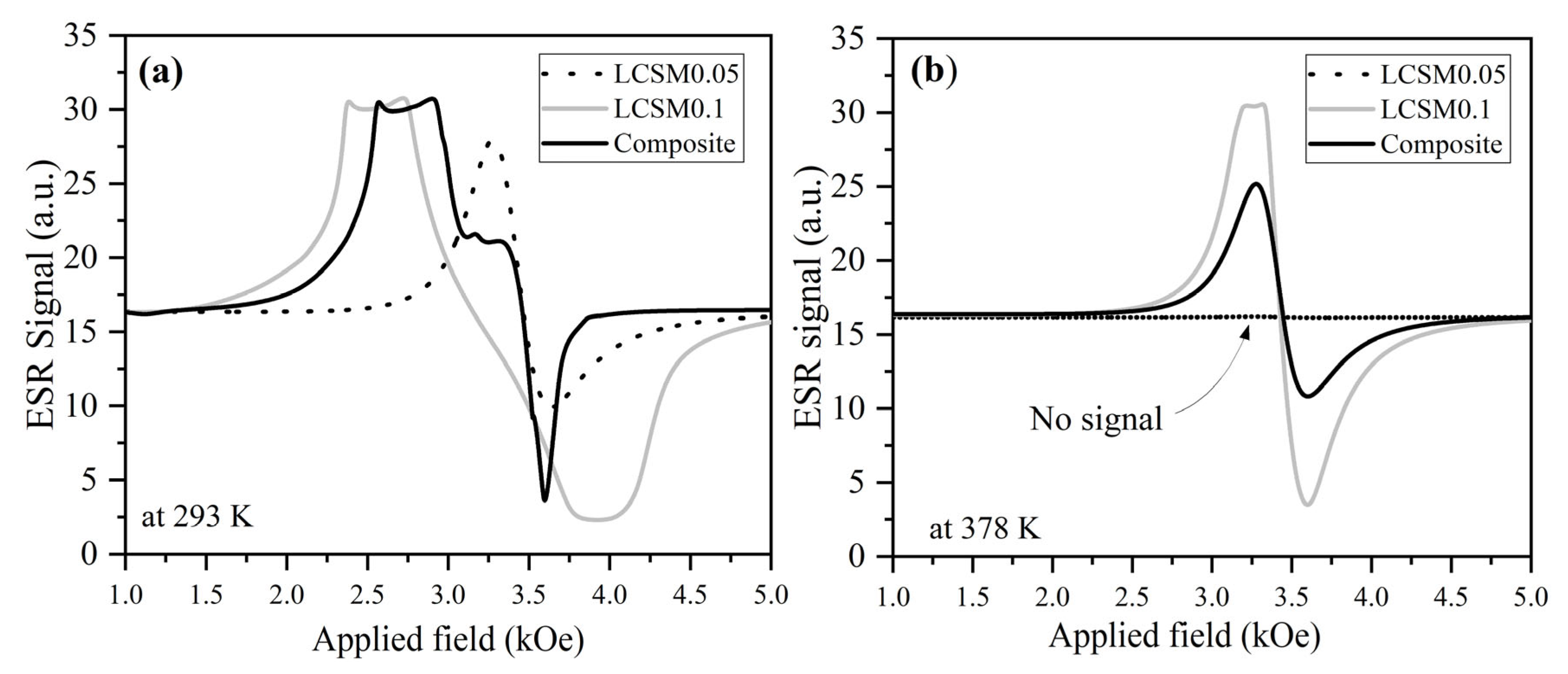
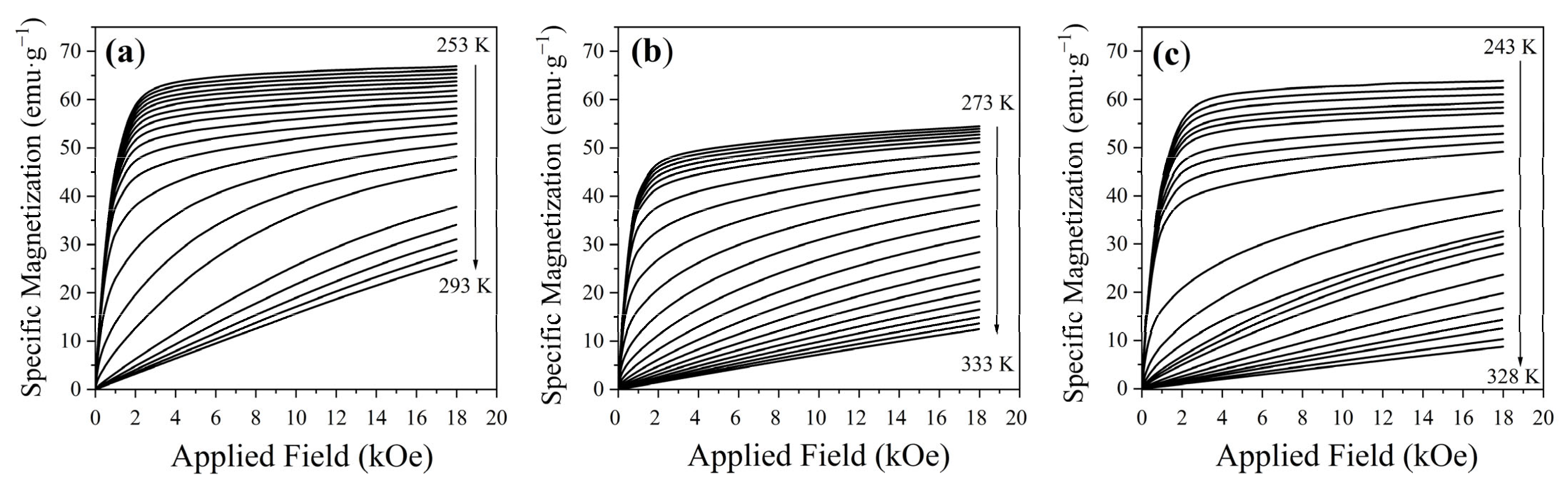
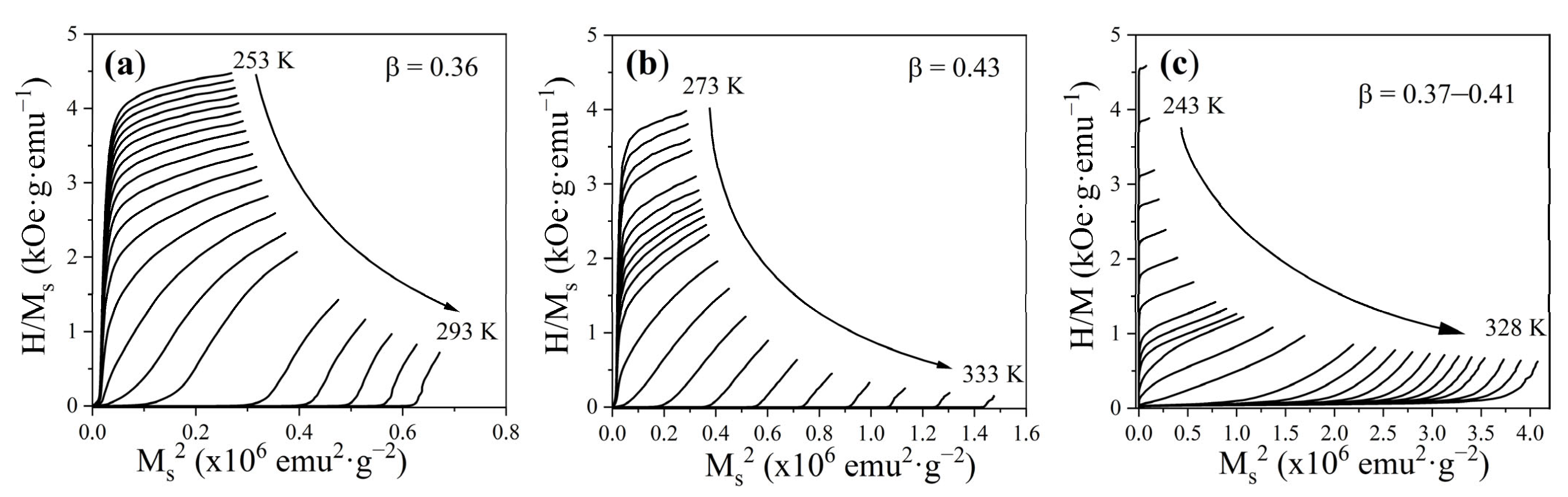
3.3. Magnetic Properties
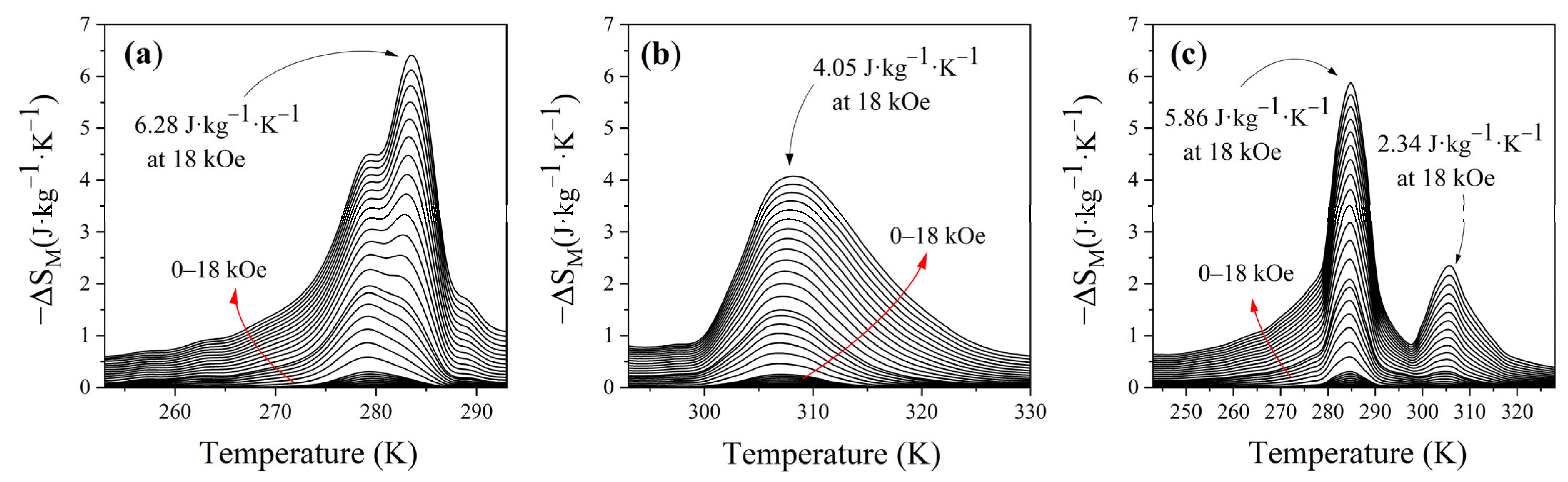
3.4. Magnetocaloric Properties: ΔS, OTW, and RCP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, Y.; Miao, X.; Qian, F.; Xu, F.; Caron, L. A brief review of microstructure design in transition metal-based magnetocaloric materials. J. Phys. Condens. Matter 2024, 36, 503001–503023. [Google Scholar] [CrossRef]
- Taboada, C.A.; Sánchez, F.; Pedro, F.; Cortés, C.A.; Betancourt, J.A.; Ramírez, M.; Bolarín, A.M. Large magnetocaloric effect near to room temperature in Sr-doped La0.7Ca0.3MnO3. J. Magn. Magn. Mater. 2020, 496, 165887–165898. [Google Scholar] [CrossRef]
- Biswal, H.; Singh, V.; Nath, R.; Sahu, J.R. Magnetic properties and near-room-temperature large magnetocaloric effect in (La1−xBix)0.67Ba0.33MnO3 (x = 0–0.3) ceramics. Mater. Res. Bull. 2021, 133, 111030–111037. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, J.; Shao, C.; Zhu, Y.; Zhu, F.; Chen, Y.; Cheng, Y.; Gu, W.; Li, H.; Liu, Z.; et al. Structural and magnetic properties of La0.80−xEuxNa0.20MnO3 perovskite manganites with enhanced magnetocaloric effect by appropriate Eu-doping content. Ceram. Int. 2025, 51, 21856–21866. [Google Scholar] [CrossRef]
- Bolarín, A.M.; Taboada, C.A.; Cortés, C.A.; Sánchez-De Jesús, F. Tuning the magnetocaloric properties of lanthanum–strontium manganite by rare-earth Nd3+ doping. J. Therm. Anal. Calorim. 2024, 149, 12665–12674. [Google Scholar] [CrossRef]
- Taboada, C.A.; Bolarín, A.M.; Pedro, F.; Cortés, C.A.; Sánchez, F. Role of Gd3+ on the magnetocaloric properties of lanthanum-strontium manganite. J. Magn. Magn. Mater. 2023, 570, 170542–170552. [Google Scholar] [CrossRef]
- Akça, G.; Kiliç, S.; Ekicibil, A. Magnetic and magnetocaloric properties of 0.5La0.7Ca0.2Sr0.1MnO3/0.5La0.7Te0.3MnO3 composite. Cumhur. Sci. J. 2020, 41, 144–151. [Google Scholar] [CrossRef]
- Maalam, K.E.; Balli, M.; Habouti, S.; Dietze, M.; Hamedoun, M.; Hlil, E.K.; Es-Souni, M.; El Kenz, A.; Benyoussef, A.; Mounkachi, O. Composite (La0.45Nd0.25) Sr0.3MnO3/5CuO materials for magnetic refrigeration applications. J. Magn. Magn. Mater. 2018, 449, 25–32. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, X.; Li, S. Effects of copper doping on the structure, electrical and low-field magnetoresistance properties of (1 − x)La0.67Sr0.33MnO3/xCu (x = 0−0.15) composite coatings. Ceram. Int. 2017, 43, 10026–10031. [Google Scholar] [CrossRef]
- Jeddi, M.; Gharsallah, H.; Bekri, M.; Dhahri, E.; Hlil, E.K. Improvement of magnetocaloric properties around room temperature in (1−x) La0.6Ca0.4MnO3/(x)La0.6Sr0.4MnO3 (0 ≤ x ≤ 1) composite system. Phase Transit. 2020, 93, 311–322. [Google Scholar] [CrossRef]
- Sellami, E.; Regaieg, Y.; Cheikhrouhou, W.; Koubaa, M.; Cheikhrouhou, A.; Njah, N. Magnetic and magnetocaloric properties of La0.65Ca0.35MnO3/La0.7Ca0.2Ba0.1MnO3 and La0.65Ca0.35MnO3/Pr0.5Sr0.5MnO3 composite manganites. J. Supercond. Nov. Magn. 2015, 28, 3121–3126. [Google Scholar] [CrossRef]
- Akça, G.; Çetin, S.K.; Ekicibil, A. Composite xLa0.7Ca0.2Sr0.1MnO3/(1 − x)La0.7Te0.3MnO3 materials: Magnetocaloric properties around room temperature. J. Mater. Sci. Mater. Electron. 2020, 31, 6796–6808. [Google Scholar] [CrossRef]
- M’nassri, R.; Nofal, M.M.; De Rango, P.; Chniba, N. Magnetic entropy table-like shape and enhancement of refrigerant capacity in La1.4Ca1.6Mn2O7–La1.3Eu0.1Ca1.6Mn2O7 composite. RSC Adv. 2019, 9, 14916–14927. [Google Scholar] [CrossRef]
- Yigiter, N.; Pektas, M.; Kolat, V.S.; Izgi, T.; Bayri, N.; Gencer, H.; Atalay, S. Structural, magnetic, and magnetocaloric properties of (1 − x)La0.7Ca0.3MnO3/(x)La0.7Ag0.3MnO3 composites. J. Mater. Sci. Mater. Electron. 2022, 33, 4721–4735. [Google Scholar] [CrossRef]
- Bisht, P.; Meenakshi; Gaur, A.; Mahato, R.N. Structural and magnetocaloric properties (0.75) La0.7Ca0.3MnO3/(0.25) La0.84Sr0.16MnO3 nanocomposite. Physica B Condens. Matter 2021, 619, 413215–413224. [Google Scholar] [CrossRef]
- Kılıç, S.; Akça, G.; Aslan, M.S.; Ekicibil, A. Room-temperature magnetocaloric properties of yLa0.67Ca0.27Sr0.06MnO3/1−yLa0.7Sr0.3Mn0.95Cu0.05O3 composites. J. Therm. Anal. Calorim. 2024, 149, 4441–4451. [Google Scholar] [CrossRef]
- Boubekri, A.; Elmaddahi, Z.; Jarmoumi, Y.; Gueddouch, K.; Farchakh, A.; Hafidi, M.E. A phenomenological approach for predicting magnetic and magnetocaloric properties in the (La2MnNiO6)x/(La2MnCoO6)1−x composite. J. Supercond. Nov. Magn. 2024, 37, 1401–1410. [Google Scholar] [CrossRef]
- Bouhani, H.; Endichi, A.; Kumar, D.; Copie, O.; Zaari, H.; David, A.; Fouchet, A.; Prellier, W.; Mounkachi, O.; Balli, M.; et al. Engineering the magnetocaloric properties of PrVO3 epitaxial oxide thin films by strain effects. Appl. Phys. Lett. 2020, 117, 072402–072407. [Google Scholar] [CrossRef]
- Ezaami, A.; Sellami, E.; Cheikhrouhou, W.; Cheikhrouhou, A. Enhanced magnetocaloric properties with a tunable Curie temperature in (1 − x)La0.55Ca0.35MnO3/xLa0.7Ca0.2Sr0.1MnO3 (0 ≤ x ≤ 1). J. Mater. Sci. Mater. Electron. 2017, 28, 16741–16746. [Google Scholar] [CrossRef]
- Bolarín, A.M.; Taboada, C.A.; Cortés, C.A.; Rosales, O.; Torres, G.; Sánchez, F. Effect of high-energy ball milling on magnetocaloric properties of La0.7Ca0.2Sr0.1MnO3. Appl. Phys. A 2020, 126, 369–379. [Google Scholar] [CrossRef]
- Pękała, M.; Pękała, K.; Drozd, V.; Staszkiewicz, K.; Fagnard, J.F.; Vanderbemden, P. Magnetocaloric and transport study of poly- and nanocrystalline composite manganites La0.7Ca0.3MnO3/La0.8Sr0.2MnO3. J. Appl. Phys. 2012, 112, 023906–023914. [Google Scholar] [CrossRef]
- Chen, Z.; Han, J.; Jiang, J.; Liang, X.; Wang, H.; Wang, Y.; Wang, Z.; Pan, Z.; Sun, J.; Ma, J.; et al. Obtained large room-temperature TCR of La0.67Ca0.25Sr0.08MnO3 ceramics by optimizing sintering temperature. Ceram. Int. 2024, 50, 34680–34688. [Google Scholar] [CrossRef]
- El Amrani, K.; Moubark, Y.; Ben Ali, M.; Toulemonde, O. Magnetocaloric effect in Nd0.7Sr0.3MnO3:CuO composites. In Proceedings of the 24th IIR International Congress of Refrigeration, Yokohama, Japan, 16–22 August 2015. [Google Scholar]
- Giri, S.K.; Dahal, R.H.; Casey, J.; Neupane, D.; Pathak, A.K.; Karna, S.; Mishra, S.R. Effect of metal-oxide phase on the magnetic and magnetocaloric properties of La0.7Ca0.3MnO3 – MO (MO = CuO, CoO, NiO) composite. Magnetochemistry 2022, 8, 163–182. [Google Scholar]
- Kılıç, S.; Akça, G.; Mehmet, S.A.; Ahmet, E. Large magnetocaloric effect in La-based manganite composites near room temperature. J. Therm. Anal. Calorim. 2022, 147, 3095–3104. [Google Scholar] [CrossRef]
- Linh, D.C.; Viet Chinh, N.T.; Dung, N.T.; Bau, L.V.; Duc, N.H.; Manh, D.H.; Thanh, T.D. Critical behavior and room temperature magnetocaloric effect of La-doped Pr0.7Sr0.3MnO3 compounds. Phys. B Condens. Matter. 2023, 661, 414945–414953. [Google Scholar] [CrossRef]
- Fkhar, L.; Lamouri, R.; Mahmoud, A.; Boschini, F.; Hamedoun, M.; Ez-Zahraouy, H.; Benyoussef, A.; Hlil, E.K.; Ait Ali, M.; Mounkachi, O. Enhanced magnetic and magnetocaloric properties of La0.45Nd0.25Sr0.3MnO3/CuO composite. J. Supercond. Nov. Magn. 2020, 33, 2543–2549. [Google Scholar] [CrossRef]
- Alonso, J.A.; Martínez-Lope, M.J.; Casais, M.T.; Fernández-Díaz, M.T. Evolution of the Jahn–Teller distortion of MnO6 octahedra in RMnO3 perovskites (R = Pr, Nd, Dy, Tb, Ho, Er, Y): A neutron diffraction study. Inorg. Chem. 2000, 39, 917–923. [Google Scholar] [CrossRef]
- Qin, J.; Wen, Z.; Ma, B.; Wu, Z.; Lv, Y.; Yu, J.; Zhao, Y. Microstructure and magnetic properties of novel high-entropy perovskite ceramics (Gd0.2La0.2Nd0.2Sm0.2Y0.2)MnO3. J. Magn. Magn. Mater. 2024, 597, 172010–172021. [Google Scholar] [CrossRef]
- Jin, S.; Li, H.; Chu, K.; Yu, X.; Guan, X.; Pu, X.; Gu, X.; Liu, X. High room-temperature TCR of La0.7(K0.25Sr0.05) MnO3:xAg2O composites obtained at optimized Ag2O ratio. J. Alloys Compd. 2021, 873, 159762–159773. [Google Scholar] [CrossRef]
- Krawczyk, P.A.; Jurczyszyn, M.; Pawlak, J.; Salamon, W.; Baran, P.; Kmita, A.; Gondek, Ł.; Sikora, M.; Kapusta, C.; Strączek, T.; et al. High-entropy perovskites as multifunctional metal oxide semiconductors: Synthesis and characterization of (Gd0.2Nd0.2La0.2Sm0.2Y0.2)CoO3. ACS Appl. Electron. Mater. 2020, 2, 3211–3220. [Google Scholar] [CrossRef]
- Qin, J.; Wen, Z.; Ma, B.; Wu, Z.; Yu, J.; Tang, L.; Lu, T.; Zhao, Y. Effect of A-site rare-earth ions on structure and magnetic properties of novel (Ln0.2Gd0.2La0.2Nd0.2Sm0.2)MnO3 (Ln = Eu, Ho, Yb) high-entropy perovskite ceramics. Ceram. Int. 2024, 50, 26040–26048. [Google Scholar] [CrossRef]
- Ezaami, A.; Chaaba, I.; Cheikhrouhou-Koubaa, W.; Cheikhrouhou, A.; Hlil, E.K. Enhancement of magnetocaloric properties around room temperature in (1 − x) La0.7Ca0.25Sr0.05MnO3/xLa0.7Ca0.2Sr0.1MnO3 (0 ≤ x ≤ 1). J. Alloys Compd. 2018, 735, 2331–2335. [Google Scholar] [CrossRef]
- Banerjee, B.K. On a generalised approach to first and second order magnetic transitions. Phys. Lett. 1964, 12, 16–17. [Google Scholar] [CrossRef]
- Koroleva, L.I.; Zashchirinskiĭ, D.M.; Khapaeva, T.M.; Gurskiĭ, L.I.; Kalanda, N.A.; Trukhan, V.M.; Szymczak, R.; Krzumanska, B. Effect of oxygen deficiency on the magnetic, electrical, magnetoelectric, and magnetoelastic properties of La1−xSrxMnO3−δ manganites. Phys. Solid State 2008, 50, 2298–2302. [Google Scholar] [CrossRef]
- Halder, P.; Mukadam, M.D.; Yusuf, S.M. Direct measurement of the magnetocaloric effect in Gd based alloy. AIP Conf. Proc. 2020, 2265, 18–22. [Google Scholar] [CrossRef]
- Bolarín, A.M.; Pedro, F.; Rosales, O.; Sánchez, F. Synergistic magnetocaloric effect in composite materials based on mixed doped lanthanum manganites. J. Supercond. Nov. Magn. 2024, 37, 469–472. [Google Scholar] [CrossRef]
- Anwar, M.S.; Ahmed, F.; Danish, R.; Koo, B.H. Impact of Co3O4 phase on the magnetocaloric effect and magnetoresistance in La0.7Sr0.3MnO3/Co3O4 and La0.7Ca0.3MnO3/Co3O4 ceramic composites. Ceram. Int. 2014, 40, 15471–15476. [Google Scholar] [CrossRef]
- Jeddi, M.; Gharsallah, H.; Bekri, M.; Dhahri, E.; Hlil, E.K. Structural, magnetic and magnetocaloric properties of 0.75La0.6Ca0.4MnO3/0.25La0.6Sr0.4MnO3 nanocomposite manganite. RSC Adv. 2018, 8, 28649–28656. [Google Scholar] [CrossRef]
- Das, K.; Roy Chowdhury, R.; Midda, S.; Sen, P.; Das, I. Magnetocaloric effect study of Pr0.67Ca0.33MnO3 − La0.67Sr0.33MnO3 nanocomposite. J. Magn. Magn. Mater. 2018, 449, 304–307. [Google Scholar] [CrossRef]
- Wang, G.F.; Zhao, Z.R.; Li, H.L.; Zhang, X.F. Enhancement of refrigeration capacity and table-like magnetocaloric effect in La0.8Ca0.2MnO3/ La0.8K0.2MnO3 nanocrystalline composite. Ceram. Int. 2015, 41, 10061–10067. [Google Scholar] [CrossRef]
- Toby, B.H. R factors in Rietveld analysis: How good is good enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Causa, M.T.; Moreno, N.O.; Tovar, M.; Levy, P. High-temperature spin dynamics in CMR manganites: ESR and magnetization. Phys. Rev. B 1998, 58, 3233–3238. [Google Scholar] [CrossRef]
- Autret, C.; Gervais, M.; Gervais, F.; Raimboux, N.; Simon, P. Signature of ferromagnetism, antiferromagnetism, charge ordering and phase separation by electron paramagnetic resonance study in rare earth manganites, Ln1−xAxMnO3 (Ln = rare earth, A = Ca, Sr). Solid State Sci. 2024, 6, 815–824. [Google Scholar] [CrossRef]
- Siqueira, G.O.; Belardi, R.M.; Almeida, P.H.; da Silva, C.L.; Brant, M.C.; Matencio, T.; Domingues, R.Z. Determination of the Mn3+/Mn4+ ratio in La1−xSrxMnO3 ± d powders. J. Alloys Compd. 2012, 521, 50–54. [Google Scholar] [CrossRef]
- Sakka, A.; M’nassri, R.; Nofal, M.M.; Mahjoub, S.; Cheikhrouhou-Koubaa, W.; Chniba-Boudjada, N.; Oumezzine, M.; Cheikhrouhou, A. Structure, magnetic and field dependence of magnetocaloric properties of Pr0.5RE0.1Sr0.4MnO3 (RE = Eu and Er). J. Magn. Magn. Mater. 2020, 514, 167158–167172. [Google Scholar] [CrossRef]
- Mahjoub, S.; M’nassri, R.; Baazaoui, M.; Hlil, E.K.; Oumezzine, M. Tuning magnetic and magnetocaloric properties around room temperature via chromium substitution in La0.65Nd0.05Ba0.3MnO3 system. J. Magn. Magn. Mater. 2019, 481, 29–38. [Google Scholar] [CrossRef]
- Griffith, L.D.; Mudryk, Y.; Slaughter, J.; Pecharsky, V.K. Material-based figure of merit for caloric materials. J. Appl. Phys. 2018, 123, 034902–034911. [Google Scholar] [CrossRef]
- Ho, T.A.; Dang, N.T.; Phan, T.L.; Yang, D.S.; Lee, B.W.; Yu, S.C. Magnetic and magnetocaloric properties in La0.7Ca0.3−xNaxMnO3 exhibiting first-order and second-order magnetic phase transitions. J. Alloys Compd. 2016, 676, 305–312. [Google Scholar] [CrossRef]
- Diana, I.P.; Vera, D.S.; Nataliya, I.C.; Vyacheslav, S.R.; Yulia, A.A.; Alexey, N.T.; Bernard, F.; Lorenzo, S.; Moulay, T.S. The structural and magnetic features of perovskite oxides La1–xSrxMnO3+δ (x = 0.05, 0.10, 0.20) depending on the strontium doping content and heat treatment. Ceram. Int. 2023, 49, 10774–10786. [Google Scholar]
- Diana, P.; Vera, S.; Oxana, R.; Bernard, F.; Moulay, T.S. In situ high temperature XRD study of Sr-doped ceramics La0.95Sr0.05MnO3+δ. Solid State Commun. 2021, 336, 114401–114405. [Google Scholar]
- Ferreira, M.C.; Pimentel, B.; Andrade, V.; Zverev, V.; Gimaev, R.R.; Pomorov, A.S.; Pyatakov, A.; Alekhina, Y.; Komlev, A.; Makarova, L. Understanding the Dependence of Nanoparticles Magnetothermal Properties on Their Size for Hyperthermia Applications: A Case Study for La-Sr Manganites. Nanomaterials 2021, 11, 1826. [Google Scholar] [CrossRef]


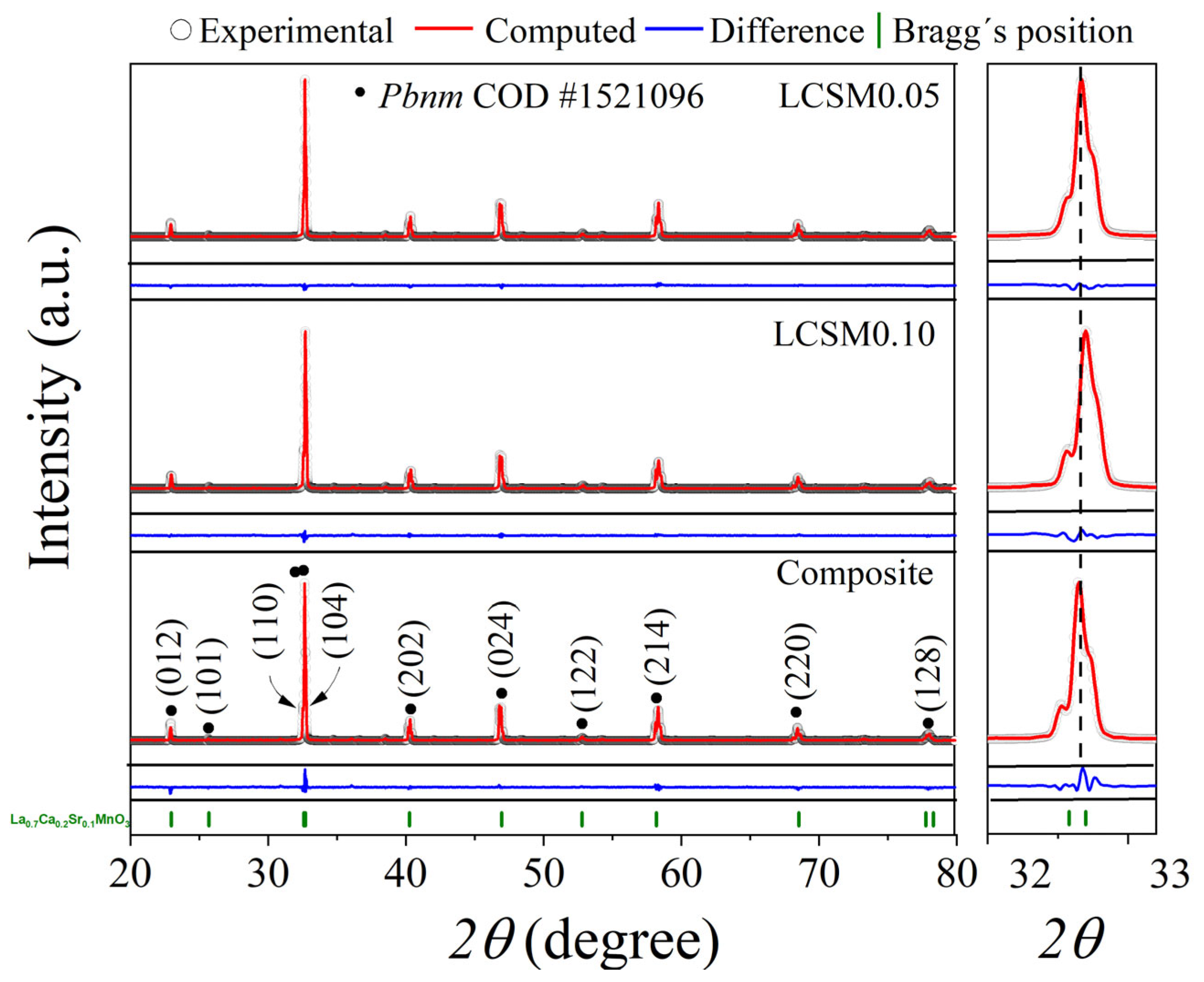

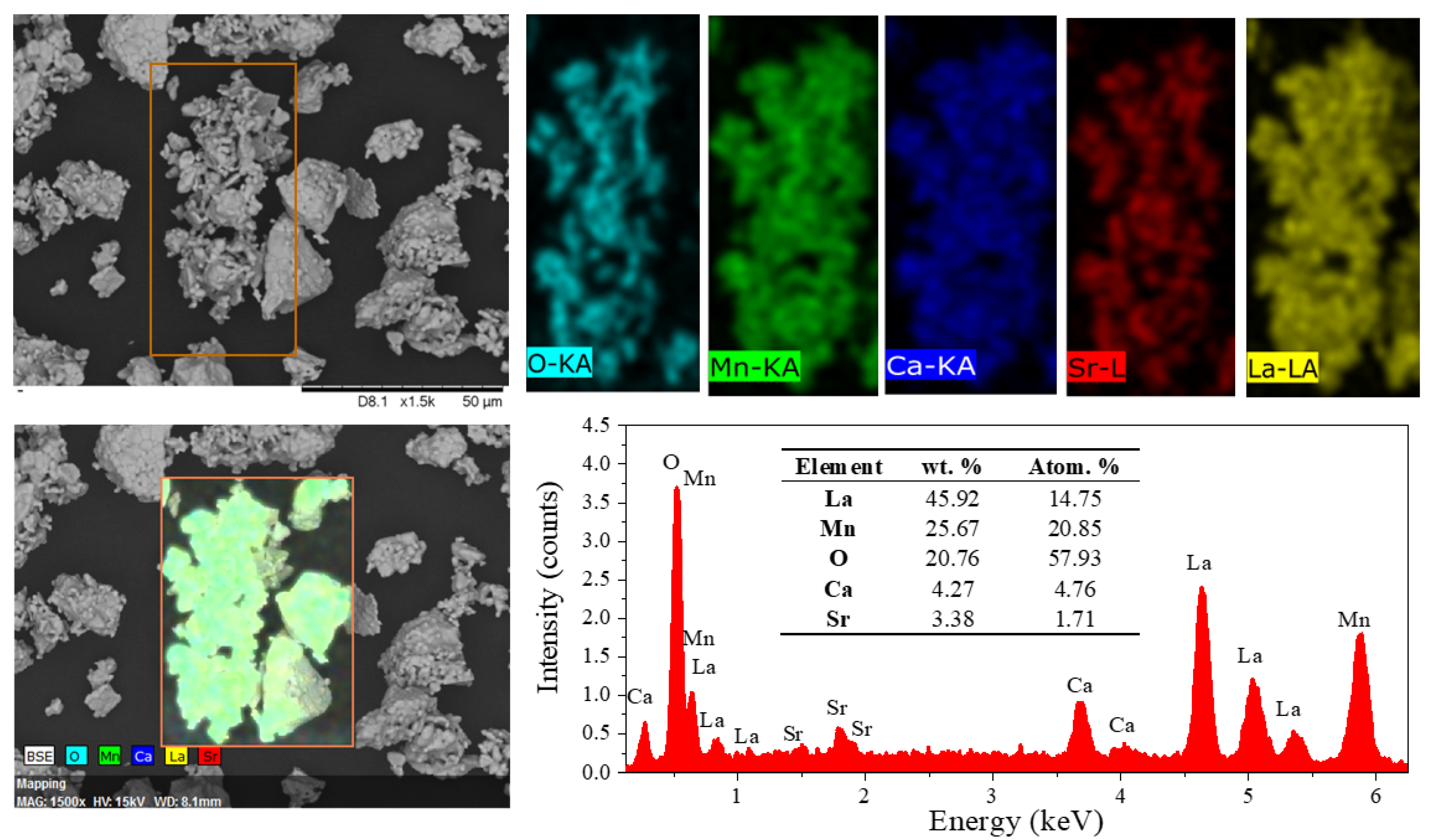

| Sample | Phase/Space Group | Phase (wt. %) | D (nm) | Microstrain (Adim) | Lattice Parameter (Å) | Goodness of Fit | |||
|---|---|---|---|---|---|---|---|---|---|
| a | b | c | Rwp | χ2 | |||||
| LCSM0.05 | La0.7Ca0.25Sr0.05MnO3 Pbnm | 100 ± 0.00 | 77.40 ± 4.26 | 4.76 × 10−4 ± 3.11 × 10−6 | 5.44 ± 6 × 10−4 | 7.76 ± 3 × 10−4 | 5.47 ± 6 × 10−4 | 20.89 | 1.18 |
| LCSM0.1 | La0.7Ca0.2Sr0.1MnO3 Pbnm | 100 ± 0.00 | 79.01 ± 2.26 | 6.36 × 10−4 ± 3.72 × 10−6 | 5.48 ± 1 × 10−4 | 7.69 ± 1 × 10−4 | 5.46 ± 9 × 10−4 | 15.80 | 1.87 |
| Composite | La0.7Ca0.25Sr0.05MnO3 Pbnm | 50.40 ± 0.65 | 93.94 ± 5.46 | 1.77 × 10−4 ± 2.46 × 10−6 | 5.48 ± 5 × 10−3 | 7.72 ± 5 × 10−3 | 5.47 ± 5 × 10−3 | 21.26 | 2.47 |
| La0.7Ca0.2Sr0.1MnO3 Pbnm | 49.60 ± 1.46 | 100 ± 5.50 | 6.09 × 10−4 ± 2.26 × 10−6 | 5.46 ± 4 × 10−3 | 7.71 ± 9 × 10−3 | 5.48 ± 7 × 10−3 | |||
| Sample | Phase | Mn-O-Mn Bond Angle (Degree) | |
|---|---|---|---|
| Mn1-O-Mn1 | Mn2-O-Mn2 | ||
| LCSM0.05 | La0.7Ca0.25Sr0.05MnO3 | 160.29 | 161.32 |
| LCSM0.1 | La0.7Ca0.2Sr0.1MnO3 | 160.15 | 161.37 |
| Composite | La0.7Ca0.25Sr0.05MnO3 | 160.19 | 161.36 |
| La0.7Ca0.2Sr0.1MnO3 | 160.24 | 161.36 | |
| Composite |
Tc
(K) | kOe |
OTW
(K) | (J·kg−1·K−1) | (J·kg−1) | Reference |
|---|---|---|---|---|---|---|
| 0.5La0.75Ca0.25Sr0.05MnO3/0.5La0.7Ca0.2Sr0.1MnO3 | 286–307 | 18 | 85 | 5.86 | 189 | This work |
| 0.5La0.7Ca0.2Sr0.1MnO3/0.5La0.7Te0.3MnO3 | 255–303 | 20 | 70 | 2.07 | 125 | [7] |
| 0.95La0.45Nd0.25Sr0.3MnO3/0.05CuO | 285 | 15 | 95 | 2.55 | 75 | [8] |
| 0.4La0.6Ca0.4MnO3/0.6La0.6Sr0.4MnO3 | 255–350 | 30 | 250 | 0.92 | 215 | [10] |
| 0.5La0.65Ca0.35MnO3/0.5Pr0.5Sr0.5MnO3 | 277–298 | 20 | 100 | 0.68 | 80 | [11] |
| 0.75La0.7Ca0.2Sr0.1MnO3 /0.25La0.7Te0.3MnO3 | 290 | 20 | 70 | 2.5 | 138 | [12] |
| 0.5La0.7Ca0.3MnO3/0.5La0.7Ag0.3MnO3 | 260–304 | 20 | 98 | 2.65 | 139 | [14] |
| 0.75La0.7Ca0.3MnO3/0.25La0.84Sr0.16MnO3 | 255 | 20 | 140 | 0.72 | 89 | [15] |
| 0.5La0.67Ca0.27Sr0.06MnO3/0.5La0.7Sr0.3Mn0.95Cu0.05O3 | 264–360 | 20 | 75 | 1.82 | 138 | [16] |
| 0.5La0.7Ca0.2Sr0.1MnO3/0.5La0.7Ca0.15Sr0.15MnO3 | 310–336 | 20 | 90 | 1.26 | 115 | [19] |
| 0.33Pr0.67Ca0.33MnO3/0.66La0.67Sr0.33MnO3 | 346 | 20 | 250 | 0.98 | 77 | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes Patricio, F.I.; Taboada Moreno, C.A.; Bolarín Miró, A.M.; Cortés Escobedo, C.A.; Reyes Valderrama, M.I.; Sánchez De Jesús, F. An Enhancement in the Magnetocaloric Effect in a Composite Powder Based on Lanthanum Manganites. Materials 2025, 18, 4869. https://doi.org/10.3390/ma18214869
Reyes Patricio FI, Taboada Moreno CA, Bolarín Miró AM, Cortés Escobedo CA, Reyes Valderrama MI, Sánchez De Jesús F. An Enhancement in the Magnetocaloric Effect in a Composite Powder Based on Lanthanum Manganites. Materials. 2025; 18(21):4869. https://doi.org/10.3390/ma18214869
Chicago/Turabian StyleReyes Patricio, Fidel Ivan, Cristhian Antonio Taboada Moreno, Ana María Bolarín Miró, Claudia Alicia Cortés Escobedo, María Isabel Reyes Valderrama, and Félix Sánchez De Jesús. 2025. "An Enhancement in the Magnetocaloric Effect in a Composite Powder Based on Lanthanum Manganites" Materials 18, no. 21: 4869. https://doi.org/10.3390/ma18214869
APA StyleReyes Patricio, F. I., Taboada Moreno, C. A., Bolarín Miró, A. M., Cortés Escobedo, C. A., Reyes Valderrama, M. I., & Sánchez De Jesús, F. (2025). An Enhancement in the Magnetocaloric Effect in a Composite Powder Based on Lanthanum Manganites. Materials, 18(21), 4869. https://doi.org/10.3390/ma18214869







