Highlights
What are the main findings?
- Proper steel slag and EDTA improved cemented paste backfill strength and CO2 uptake.
- Strength and CO2 uptake rose then fell with slag/EDTA, optimum at 10% slag, 0.5 g/L EDTA.
- EDTA chelates Ca2+/Mg2+ in steel slag, enhancing ion release and catalyzing carbonation.
- CO2 sequestration consumes hydration products, reducing compressive strength of backfill.
What are the implications of the main findings?
- Synergistic steel slag and EDTA optimize backfill by balancing strength and CO2 uptake.
- The results provide a feasible pathway for the co-utilization of coal-based wastes and steel slag.
- This approach advances green mining and high-value steel slag use.
Abstract
To enhance the support capacity of cemented paste backfill (CPB) in goaf areas and its ability to sequester CO2, steel slag and ethylenediaminetetraacetic acid (EDTA) were incorporated into gangue-based cemented backfill materials. A stress–carbonation-coupled reaction system was employed to carbonate the CPB, and the effects of steel slag and EDTA on compressive strength, CO2 uptake, and microstructure were studied. The findings indicate that steel slag remarkably enhanced the performance of the CPB, with both strength and CO2 uptake initially increasing before declining as steel slag content increased. The optimum performance was achieved at a steel slag content of 10%. The incorporation of EDTA further enhanced the compressive strength and CO2 uptake, with the best results at 0.5 g/L. Microstructural analyses demonstrated that steel slag increased the availability of Ca2+ and Mg2+ in the cement paste system, while EDTA accelerated their leaching, promoted hydration products, and catalyzed carbonation via chelation. However, excessive steel slag or EDTA reduced hydration products and deteriorated material performance. This work may provide a reference for enhancing the properties of CPB and promoting the efficient utilization of coal-based solid wastes.
1. Introduction
Coal has long served as a cornerstone energy source, underpinning national economic growth and social development. However, vast underground goaf areas have been generated by extensive coal mining. In 2024, global raw coal production amounted to approximately 9.24 billion tons, with China’s output reaching 4.78 billion tons, resulting in more than 3 billion m3 of underground void space [1,2,3,4]. These goafs pose serious environmental and safety risks, including spontaneous combustion of residual coal, water accumulation, ground subsidence, and damage to the aquifer. Cemented paste backfill (CPB) mining technology has emerged as an effective approach to mitigate these issues. By utilizing waste materials derived from coal, including fly ash and coal gangue, in combination with a cementitious binder, CPB is injected into the goafs to stabilize the overlying strata while achieving solid waste recycling [5,6,7,8]. At the same time, the substantial CO2 emissions associated with coal production and utilization remain a critical environmental concern [9,10,11]. Carbon capture, utilization, and storage (CCUS) technologies provide a promising pathway for reducing these emissions. Within this framework, the carbonation pathway—using CPB as a CO2 absorption medium—offers a promising approach for permanent geological storage of CO2, thereby enabling long-term geological storage of CO2 [12,13].
Furthermore, the massive discharge of steel slag has led to severe soil and air pollution [14,15]. Notably, mineral phases identified in steel slag include dicalcium silicate (C2S), tricalcium silicate (C3S), and dicalcium ferrite (C2F) [16,17,18], which are similar to those found in cement clinker and possess potential cementitious activity [19,20,21]. Consequently, steel slag substituting for cement in backfill materials both facilitates the utilization of steel slag and reduces the environmental burden of direct disposal [22,23,24]. In recent years, considerable research efforts have focused on this topic. Li et al. [25] prepared high-carbonation precast concrete by partially replacing cement with steel slag. Their study found that under the optimal mix proportion—with a water-to-binder ratio of 0.18, 8% silica fume, and 40% sand content—replacing 80% of cement with steel slag resulted in a concrete compressive strength of 104.9 MPa, significantly enhancing its mechanical performance. Rosales et al. [26] systematically investigated the effects of different carbonation conditions on products using steel slag as raw material. The study found that the degree of carbonation significantly increased with rising temperature and CO2 concentration, resulting in well-crystallized carbonation products and a denser pore structure. This effectively achieved CO2 sequestration and improved the reactivity of steel slag, providing a basis for its resource utilization in re-cemented materials. Herki et al. [27] experimentally analyzed the influence of different steel slag incorporation rates on the microstructure and hydration products of cement paste. The results showed that an appropriate amount of steel slag promoted the formation of C-S-H gel and Ca(OH)2, thereby improving structural density and compressive strength. When steel slag replaced 30% of cement, the compressive strength increased by approximately 45% compared to the reference group. However, excessive incorporation led to an increase in free CaO and porosity, resulting in a decline in material performance. Similarly, Gu et al. [28] studied the effects of composite activators in a steel slag–slag cementitious system. reporting that the system achieved the highest compressive strength at a 20% steel slag content, whereas higher contents (>40%) led to strength reduction.
However, previous studies have revealed that steel slag exhibits significant volumetric instability [29,30]. Excessive incorporation of steel slag might cause material expansion, thereby restricting its large-scale utilization in engineering practice [31,32]. To address this limitation, researchers have investigated the use of various chemical activators to enhance the reactivity and dimensional stability of steel slag, achieving notable progress. Ethylenediaminetetraacetic acid (EDTA) is a strong chelating agent commonly used for titrating free lime [33], periclase [34], and metal ions such as Ca2+ and Mg2+ [35]. It also serves as a wastewater treatment agent for removing heavy metal ions from water. Due to its chelating capacity, EDTA can promote the release of metal ions from steel slag [36], enhance carbonation efficiency, and improve its volume stability [37,38,39]. Numerous scholars have conducted research on this topic.
Ababneh et al. [40] employed EDTA to pretreat medical waste fly ash and investigated its feasibility for application in mortar. Experimental results demonstrated that EDTA effectively removed heavy metal ions from the fly ash. The treated fly ash could replace up to 20% of cement in mortar preparation, achieving a material strength of 20 MPa after 28 days of curing. Chen et al. [41] incorporated EDTA into the wet carbonation process of steel slag and investigated its impact on mineralization efficiency and the properties of cementitious materials. Their results demonstrated that EDTA effectively improved both the volume stability and the mineralization efficiency of steel slag, enabling the cementitious materials to achieve a uniaxial compressive strength of 48 MPa after 28 days of curing. Katre et al. [42] studied the CO2 mineralization behavior and mineral transformation patterns of gabbro-peridotite and picritic basalt in solutions containing NaHCO3 and Na2H2EDTA·2H2O. The results indicated that EDTA enhanced carbonate formation by promoting mineral dissolution and the release of Ca2+ and Mg2+, significantly accelerating the mineralization rate. Thumm et al. [43] investigated the influence of EDTA on the adsorption behavior of Eu (III) and Cm (III) on calcium silicate hydrate under high-salinity conditions and varying calcium-to-silicon ratios. The findings revealed that under high-calcium conditions, stable ternary Ca–An (III)/Ln (III)–EDTA complexes formed, effectively stabilizing An3+ and Ln3+ in the liquid phase and inhibiting their incorporation into the C-S-H structure. This significantly enhanced their mobility, confirming the catalytic role of EDTA in the leaching of metal ions from steel slag. Similarly, Yichao Zhang et al. [44] examined the EDTA-assisted semi-dry carbonation process and found that EDTA markedly enhanced CO2 sequestration performance by chelating Ca2+, promoted cement hydration, and increased the strength of mortars incorporating carbonated steel slag. Nevertheless, existing research has primarily focused on the effects of EDTA on steel slag, with relatively few studies investigating the combination of steel slag and EDTA in cemented paste backfill (CPB) systems.
Based on the above research, this study integrates steel slag into CPB to systematically evaluate the effect of slag content on the mechanical performance and CO2 sequestration behavior of the backfill. Following the identification of the optimal steel slag content, EDTA was introduced as a chelating regulator to further enhance the performance of the CPB. The synergistic influences of steel slag and EDTA on the compressive strength and CO2 sequestration performance of CPB were comprehensively investigated. Furthermore, X-ray diffraction (XRD), scanning electron microscopy, and energy-dispersive X-ray spectroscopy (SEM–EDS), and other microstructural characterization techniques were employed to reveal the underlying mechanisms governing the effects of steel slag and EDTA on the microstructure and performance of the CPB.
2. Materials and Methods
2.1. Raw Materials
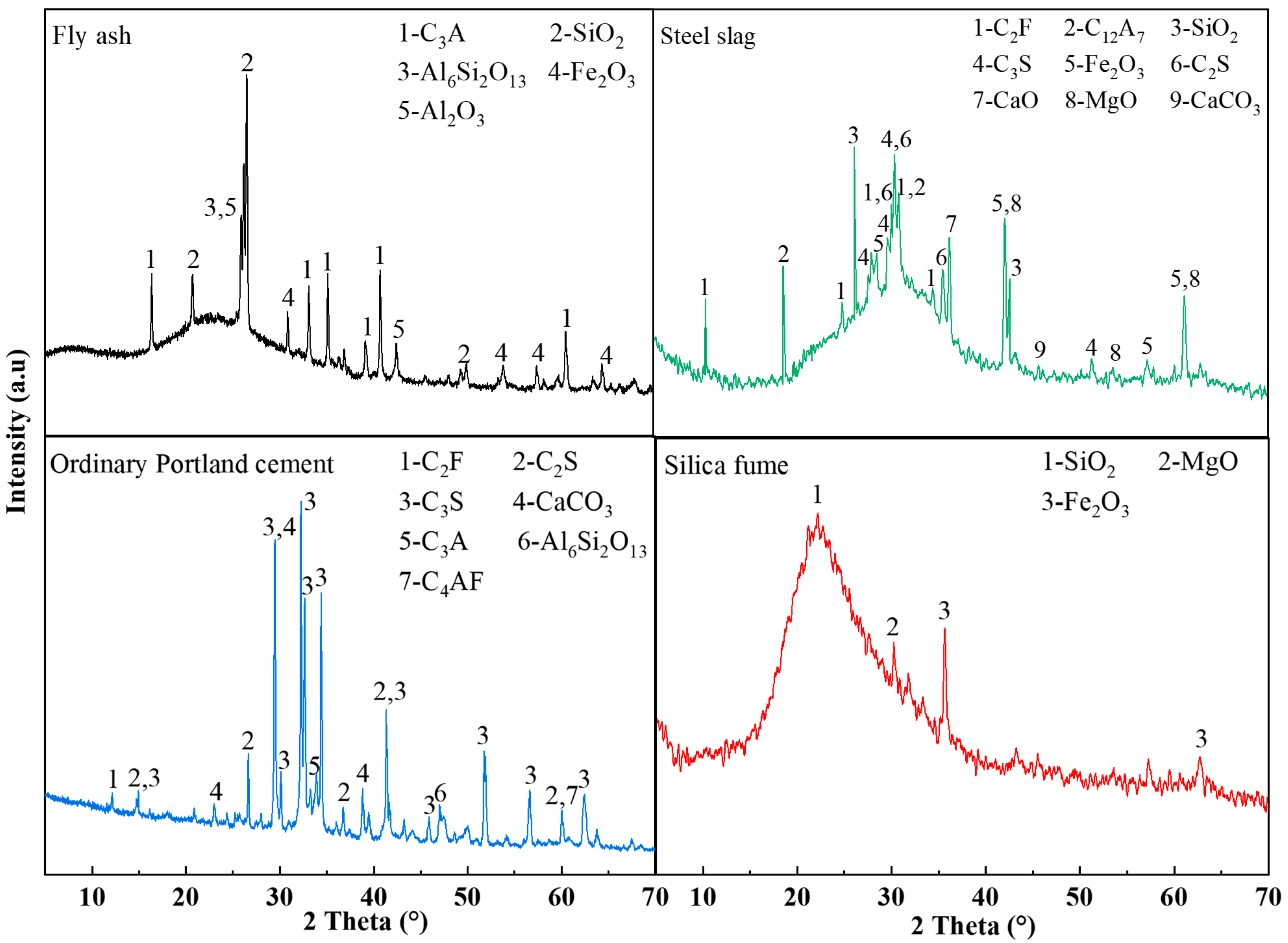
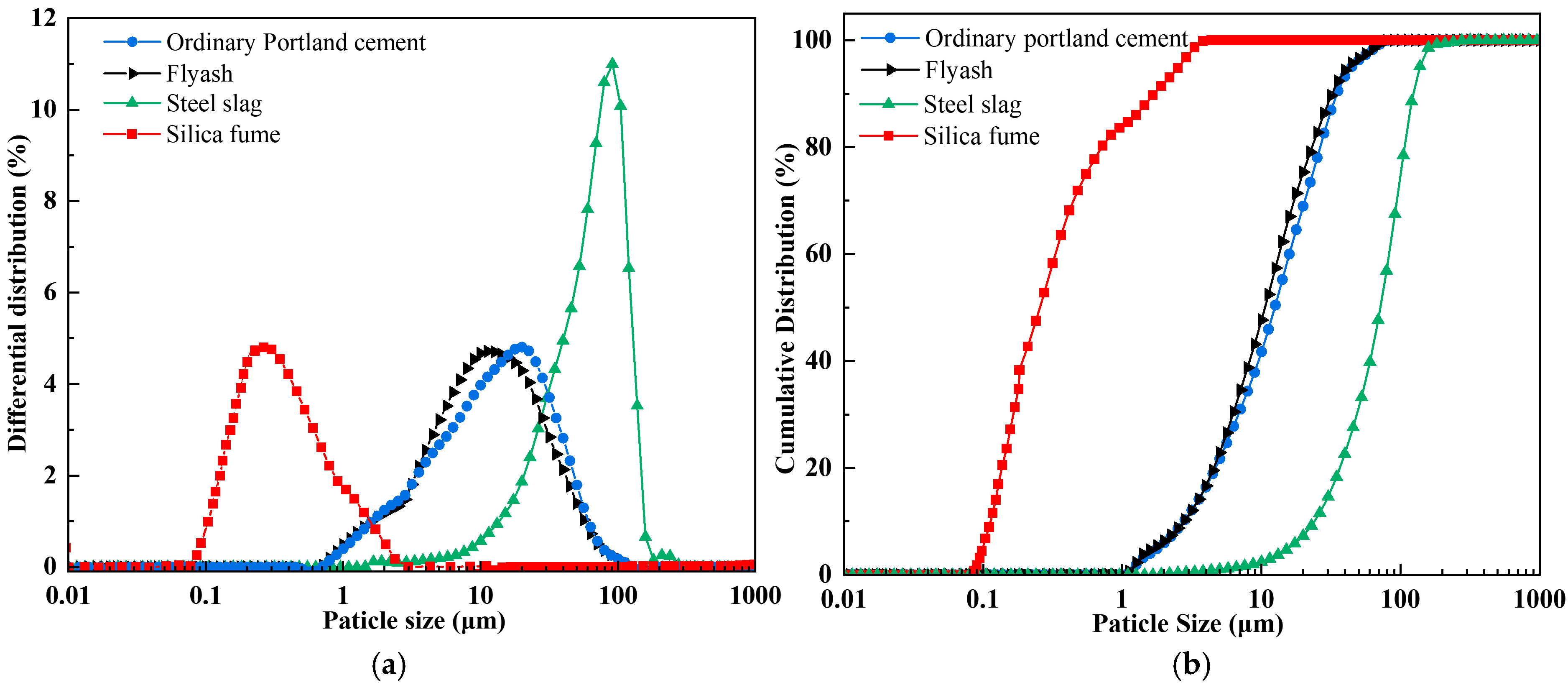
The raw materials utilized in this investigation encompassed coal gangue (CG), fly ash (FA), ordinary Portland cement (OPC), steel slag (SS), silica fume (SF), and ethylenediaminetetraacetic acid (EDTA). Chemical compositions of these materials were characterized using X-ray fluorescence (XRF); the detailed analytical data are listed in Table 1. The coal gangue was sourced from the Ordos coal-producing region of Inner Mongolia, with particle sizes of 0–2 mm: 2–5 mm: 5–10 mm in a mass ratio of 1:1:1. Both the fly ash and steel slag were supplied by Henan Borun Foundry Materials Co., Ltd. The mineral phase of the materials was characterized by XRD, and the principal mineral phase identified as corundum (Al2O3), while the main mineral phase of the steel slag was calcium oxide (CaO). The OPC used was P.O.42.5 ordinary Portland cement from China Huai’an Conch Cement Co., Ltd., with dominant mineral phases being CaO and quartz (SiO2). The main mineral phase of the silica fume was SiO2. The EDTA used was of analytical grade and obtained from China Tianjin Zhonglian Chemical Reagent Co., Ltd. The mineralogical compositions of the FA, SS, OPC, and SF, characterized by XRD, are presented in Figure 1. The median particle sizes (D50) of SF, SS, FA, and OPC were 0.24 , 71.82 , 10.60 , and 12.37 , respectively. The particle size distributions of FA, SS, OPC, and SF are presented in Figure 2.

Table 1.
Chemical composition of raw materials.
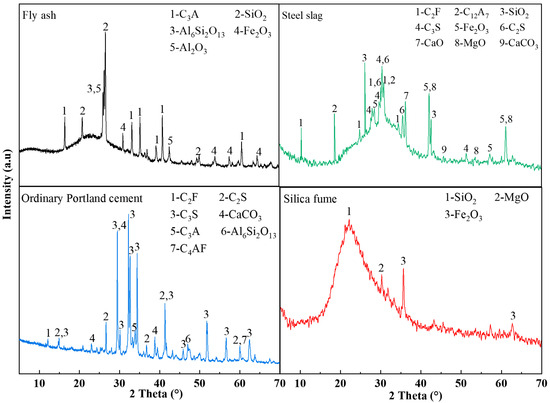
Figure 1.
XRD patterns of raw materials.
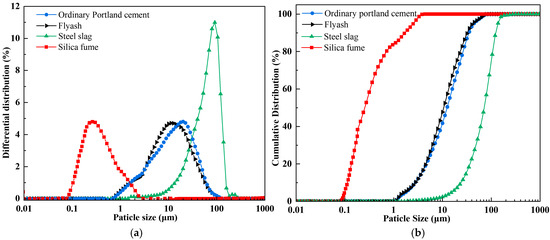
Figure 2.
Particle size distribution of raw materials: (a) differential distribution; (b) cumulative distribution.
2.2. Specimen Preparation
Test specimens were prepared with varying steel slag contents and EDTA concentrations. Based on preliminary experiments considering both compressive strength and CO2 sequestration performance, the mass ratio of gangue, fly ash, and cement was set at 6:3:1, with 2.5% of the cement replaced by silica fume. A constant water–to–binder ratio of 0.50 was adopted. On this basis, different variable levels were designed. First, the influence of steel slag content on the performance of the backfill was investigated. The steel slag content levels were set at 0%, 5%, 10%, and 15%, denoted as SS0, SS1, SS2, and SS3, respectively. Among these, the 10% steel slag content demonstrated the best compressive strength and carbon sequestration performance. Therefore, in the experiments involving the addition of EDTA, the steel slag content was fixed at 10% to ensure that the effects of EDTA were evaluated under the optimal steel slag conditions. And EDTA concentrations of 0 g/L, 0.1 g/L, 0.5 g/L, and 1.0 g/L, designated as E0, E1, E2, and E3, respectively. The experimental mix proportions are presented in Table 2. The specimen codes followed the format “ExSSx,” where “ExSSx-C” denotes samples after carbonation. For instance, E1SS1–C represents the sample containing 5% steel slag and 0.1 g/L EDTA after carbonation, while E0SS0 corresponds to the control group.

Table 2.
Mix proportions of samples.
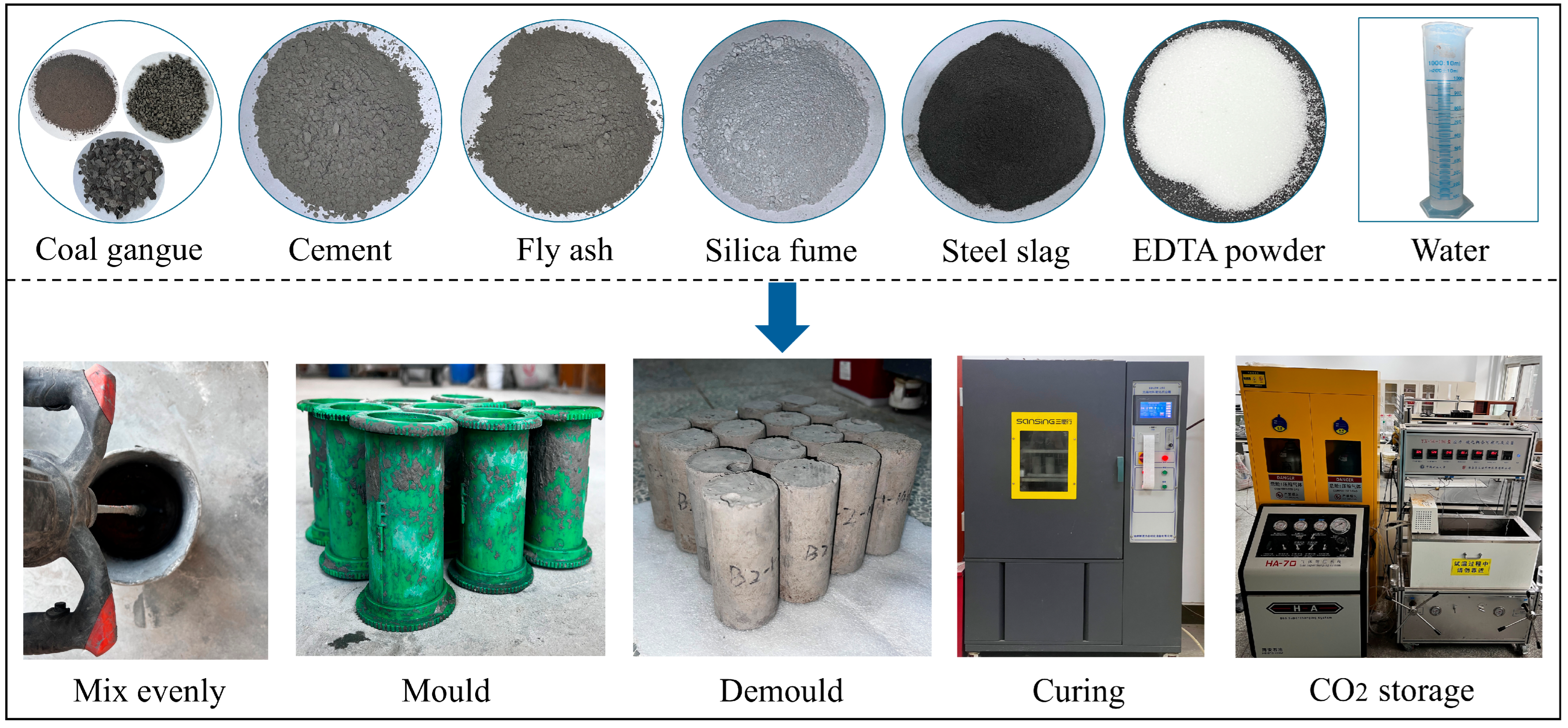
Based on the mix proportions detailed in Table 2, the solid raw materials were precisely weighed and homogenized through thorough mixing, followed by the addition of either pure water or EDTA solutions at the designated concentrations. After 5 min of uniform mixing, the prepared mixture was put into lubricated cylindrical molds (50 × 100 mm) for easy demolding. After 24 h of natural curing at ambient conditions, the samples were demolded and then transferred to a curing chamber that was maintained at 20 ± 2 °C and 95% relative humidity for curing periods of 3, 7, 14, and 28 d. The schematic diagram of the specimen preparation procedure is illustrated schematically in Figure 3.

Figure 3.
Specimen preparation procedure.
2.3. Testing Methods
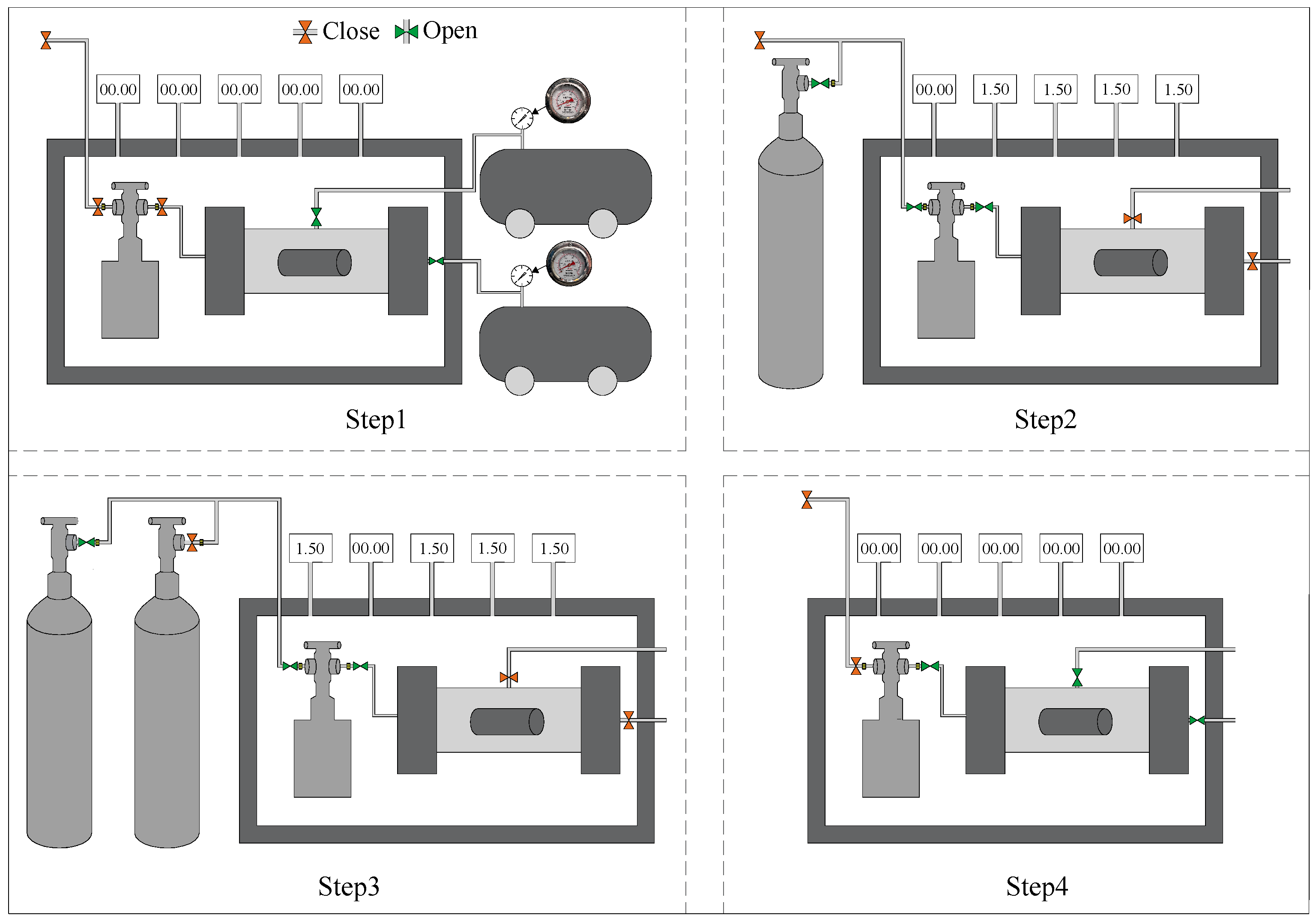
In this study, CO2 sequestration experiments were carried out using a self-developed stress–carbonation-coupled reaction vessel. The apparatus integrates triaxial stress loading, temperature regulation, and high-pressure gas injection systems, enabling simulation of the CO2 sequestration process of backfill materials under underground triaxial stress conditions. During testing, a confining pressure and axial pressure of 1.5 MPa were applied to the specimens, with CO2 continuously injected at 30 °C for a duration of 2 h. The experimental procedure for CO2 sequestration is illustrated in Figure 4. Following the carbonation treatment, the specimens were weighed and subsequently subjected to compressive strength testing, thermogravimetric (TG) analysis, and microstructural characterization.
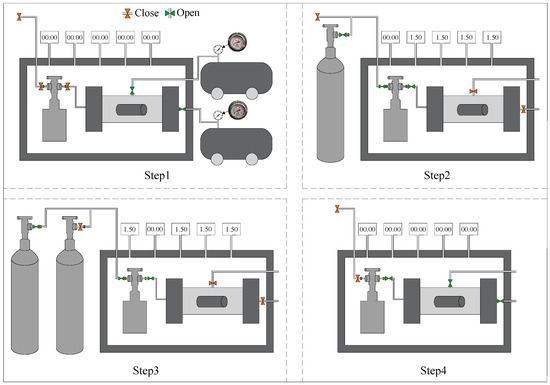
Figure 4.
CO2 sequestration steps.
2.3.1. Compressive Strength
The Chinese standard GB/T 17671–2021, Methods of Testing Cements–Determination of Strength [45] was used as the basis for conducting compressive strength tests. Measurements were performed on samples at 3, 7, 14, and 28 d, as well as on specimens subjected to CO2 sequestration. The tests were carried out using a YAW-1000H universal testing system under displacement control at 0.5 mm/min. Test data are presented as the average of three replicates per experimental group [46].
2.3.2. Carbon Sequestration Test
The CO2 uptake was employed to evaluate the ability of the CPB to store CO2. Thermogravimetric-differential thermogravimetric (TG-DTG) analysis was employed for this determination. Following CO2 sequestration, the specimens were ground to pass a 250-mesh sieve, and approximately 10 mg of the homogenized powder was analyzed using a simultaneous thermal analyzer METTLER TOLEDO TGA2 (Switzerland). High-purity nitrogen was used as the protective gas. The temperature was raised from ambient state to a maximum of 1000 °C, with a constant ramp rate of 10 °C/min maintained throughout the process. The carbonated CO2 content was quantified from the CO2 weight loss peaks in the TG curves. Equations (1) and (3) were applied to calculate the CO2 uptake and the CaCO3 content, respectively.
where denotes CO2 mass percentage of the carbonated sample (%); denotes initial CO2 mass percentage of uncarbonated sample (%); , ,and are residual mass percentage of samples at 550 °C, 850 °C and 60 °C, respectively (%).
In the equation, denotes the molar mass of CaCO3, and denotes the molar mass of CO2 [47].
2.3.3. Microstructural Analysis
XRF (Zeiss Gemini 300, Oberkochen, Germany) was used to determine the raw materials’ chemical composition. The mineralogical phases of the specimens with different mix ratios, both before and after carbonation, were identified by XRD (Ultima IV, Kyoto, Japan) equipped with Cu Kα radiation (λ = 1.541874 A, 30 kV, 40 mA). XRD analysis was performed, its scanning range was 5–70° (2θ) using a step width of 0.02° and at 4°/min. The microstructure of the samples was characterized by SEM (TESCAN MIRA4, Brno, Czech Republic) at magnifications of 500–10,000, with a resolution of 1. EDS was used to further characterize the elemental composition and content of the hydration products.
3. Results
3.1. Compressive Strength
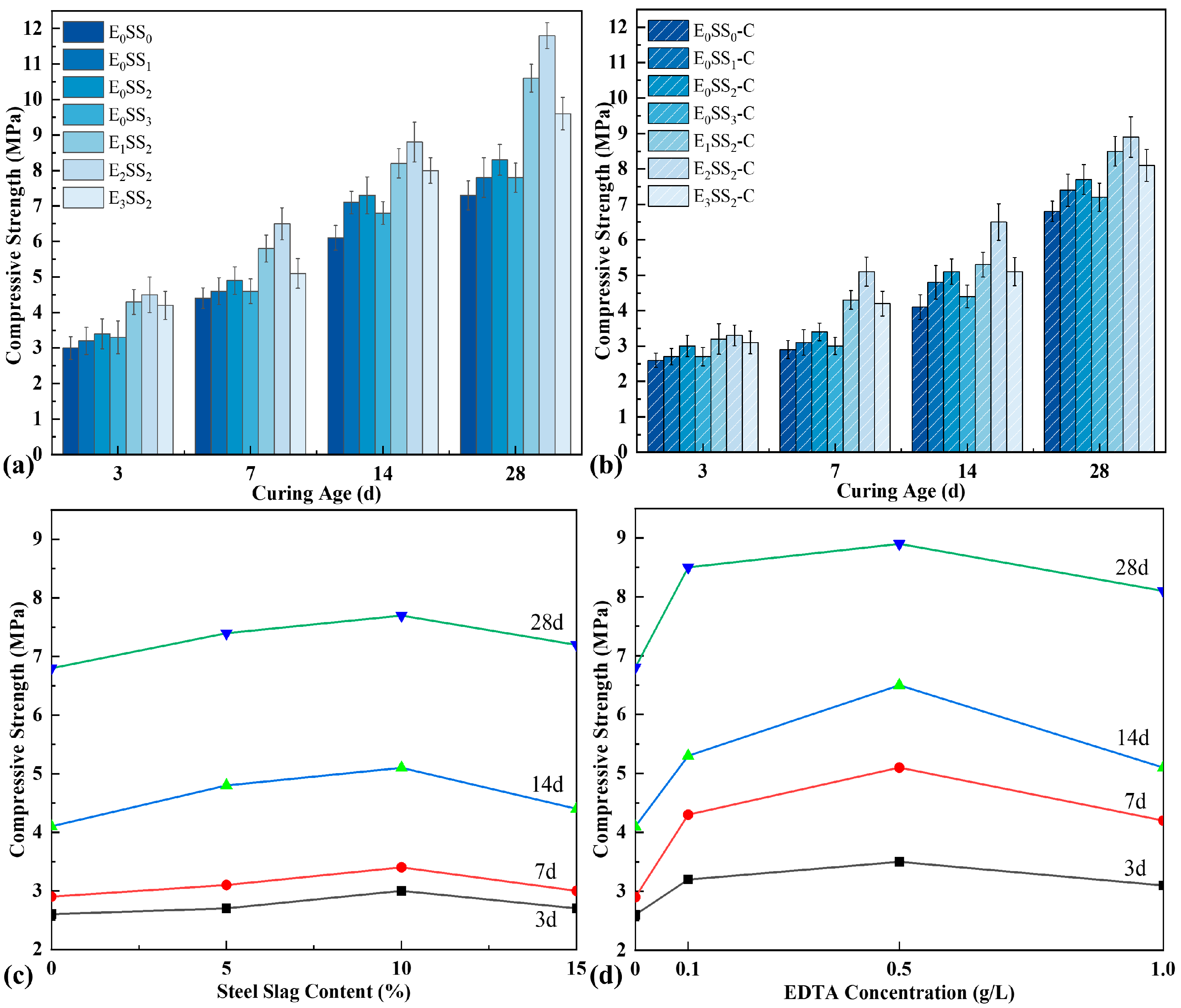
As depicted in Figure 5, the uniaxial compressive strength of CPB at different curing ages was examined both before and after carbonation. And each data point represents the average value of three specimens. The results demonstrate that the strength of uncarbonated samples exhibited an incremental rise during the 3–7 d and 7–14 d periods, followed by a pronounced growth at 14–28 d. For the control group, the strengths were 3.0 MPa, 4.4 MPa, 6.1 MPa, and 7.3 MPa at 3, 7, 14, and 28 d, respectively. With increasing steel slag content, the compressive strength of CPB initially increased and then declined, peaking at 10% steel slag, corresponding to an improvement of 11.36–19.67% compared with the control. It is noteworthy that the early-age strength differences among specimens with varying steel slag contents were relatively minor. This may be attributed to the low early reactivity of steel slag, as the hydration of minerals such as C2S proceeds slowly, while the presence of a dense glassy layer on particle surfaces further restricts Ca2+ leaching, thereby limiting early strength development [48]. The incorporation of EDTA markedly enhanced the compressive strength. A discernible trend was observed, whereby the compressive strength initially increased and subsequently decreased with increasing EDTA concentration. Under an EDTA concentration of 0.5 g/L, the strength improvement was most pronounced, with strength increases of 44.26–61.64% over the control and 20.55–42.17% over the specimens without EDTA. When the EDTA concentration reached 1.0 g/L, the strength decreased slightly but remained higher than that of specimens without EDTA. The significant enhancement in compressive strength with EDTA addition is closely linked to its catalytic effects on hydration and carbonation reactions, which facilitate the formation of a denser and more cohesive microstructure [49].
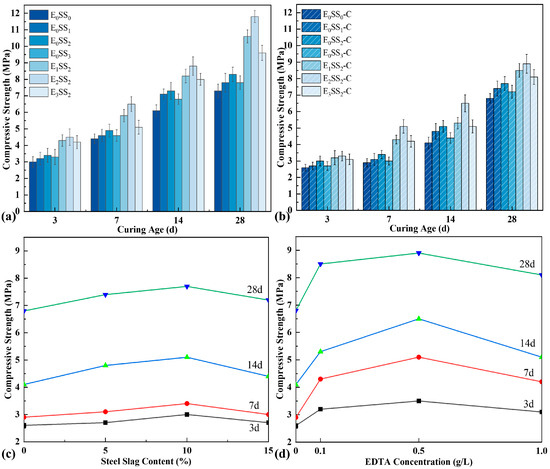
Figure 5.
Compressive strength of specimens (a) before carbonation and (b) after carbonation; (c) effect of steel slag content on carbonated sample strength; (d) effect of EDTA concentration on carbonated sample strength.
After carbonation, the compressive strength of all specimen groups decreased to varying degrees; however, the overall trends with respect to steel slag content and EDTA concentration remained consistent with those observed before carbonation. In particular, the CPB incorporating 10% steel slag and 0.5 g/L EDTA exhibited the highest strength. At curing ages of 1–28 d, the strengths of this optimal mixture were 3.3 MPa, 5.1 MPa, 6.5 MPa, and 8.9 MPa, corresponding to an improvement of 26.92–75.86% compared with the carbonated control specimens. The reduction in strength relative to uncarbonated specimens resulted from hydration products being consumed during the carbonation process. Specifically, when CO2 was introduced, part of the previously formed hydration products reacted with CO2 to generate carbonates, thereby weakening the cementitious bonding within the matrix and resulting in a decline in compressive strength.
To evaluate the significance of the effects of steel slag content and EDTA concentration on the mechanical properties of CPB, a one-way analysis of variance (ANOVA) was performed on the compressive strength data of each group. Taking the 28 d cured specimens before carbonation as an example, the analysis results are shown in Table 3. Under the condition of only adding steel slag, a p-value of 0.002 was obtained, while under the condition of fixed steel slag content with the addition of EDTA, a p-value of <0.001 was obtained. These results indicate that both steel slag content and EDTA concentration have a significant influence on compressive strength and possess statistical significance.

Table 3.
ANOVA analysis of 28 d compressive strength before carbonation.
3.2. TG-DTG
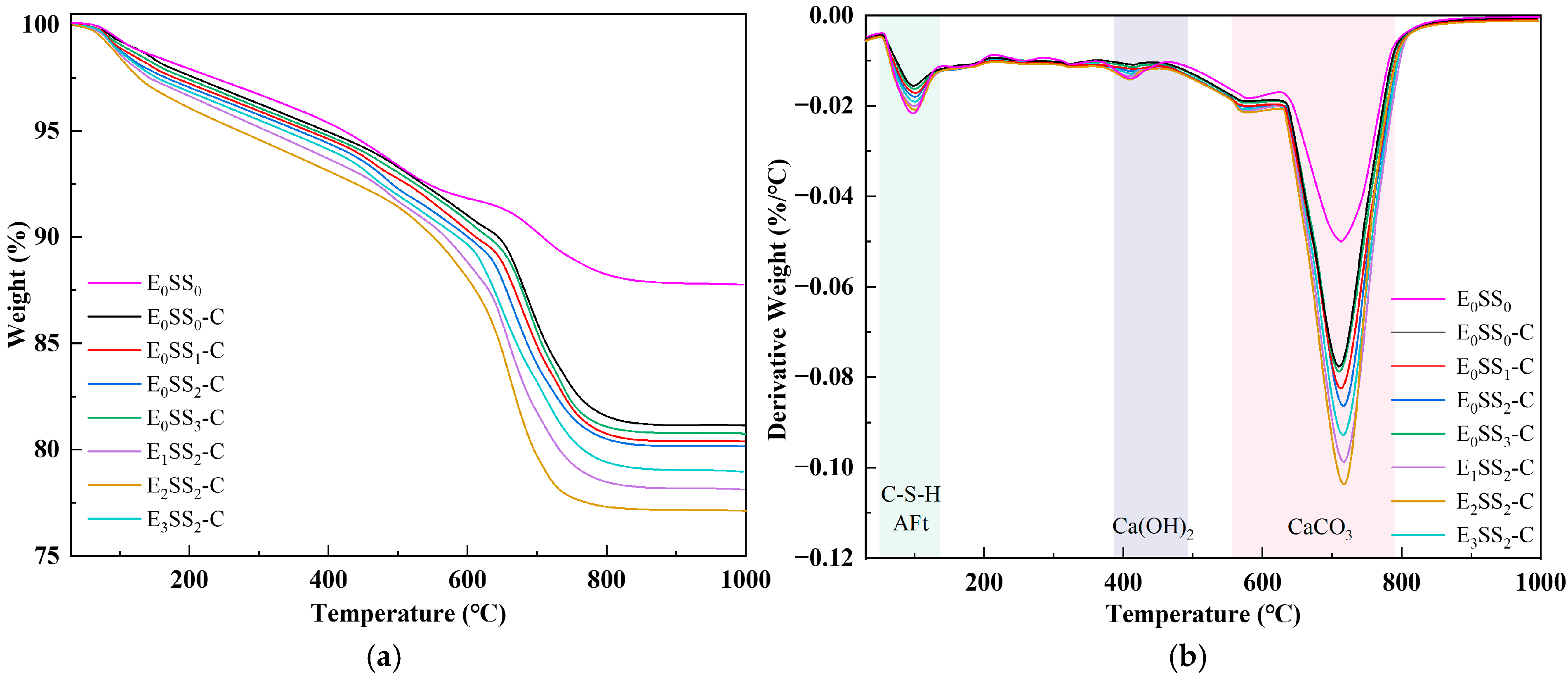
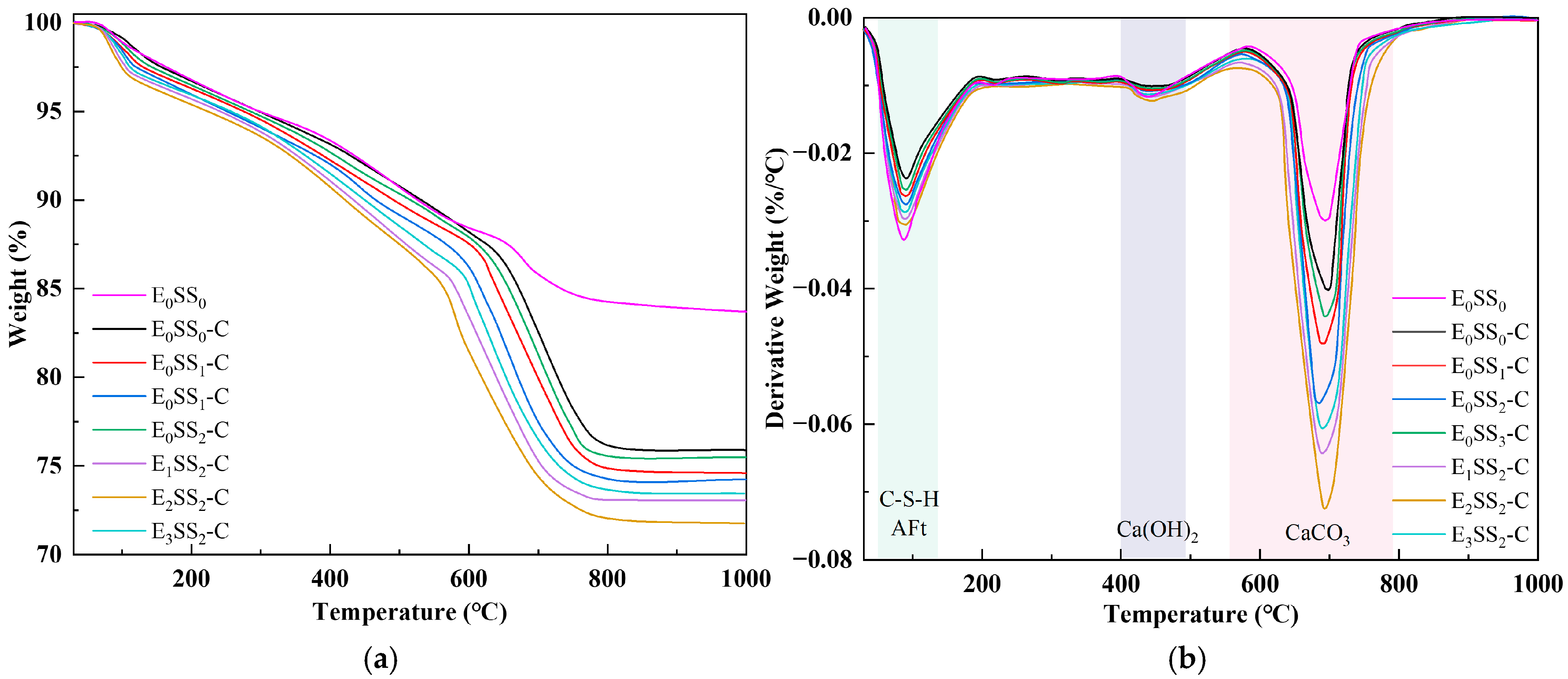
To quantify CO2 sequestration at different hydration and carbonation stages, TG-DTG analysis was performed on specimens cured for 7 d and 28 d. To ensure sample homogeneity and minimize experimental error, this test was performed on phenolphthalein-tested sections that remained colorless, indicating complete carbonation. Figure 6 and Figure 7 present the TG-DTG curves of the carbonated CPB after 7 d and 28 d of curing, respectively. As shown in Figure 6b and Figure 7b, three distinct weight loss peaks three distinct weight loss peaks were observed, corresponding to the dehydration of C-S-H, AFt (70–120 °C), the decomposition of Ca(OH)2 (400–480 °C), and CaCO3 (550–850 °C) were identified [50]. The TG-DTG curves indicate that, compared to the carbonated samples, the control group before carbonation exhibits larger weight loss peaks and higher weight loss rates in the temperature ranges of 0–200 °C and 400–500 °C. This suggests that the cemented backfill system before carbonation is predominantly composed of hydration products. However, in the range of 550–850 °C, the curve shows a gradual change with a slight weight loss peak, corresponding to the decomposition of a small amount of CaCO3. This demonstrates that the carbonate content in the samples before carbonation is relatively low, and no significant carbonation reaction has occurred in the system. Compared with the control group, the specimens containing steel slag and EDTA exhibited significantly greater weight losses, confirming the pronounced influence of steel slag content and EDTA concentration on CO2 uptake in the CPB. Among all specimens, those incorporating 10% steel slag and 0.5 g/L EDTA exhibited the most pronounced weight loss peaks in the 550–850 °C range at both curing ages. After 28 d of curing, the TG-DTG profiles displayed trends similar to those observed at 7 d but with lower overall weight loss. This reduction may result from the evolution of the pore structure, as continuous hydration leads to the generation of C-S-H, AFt, Ca(OH)2, and other products that progressively fill the pores, thereby reducing CO2 ingress and ultimately lowering CO2 sequestration capacity. These findings are consistent with the results of the compressive strength analysis.
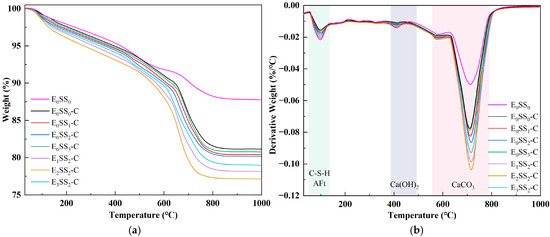
Figure 6.
TG-DTG curves of carbonated specimens at 7 d; (a) TG curves; (b) DTG curves.
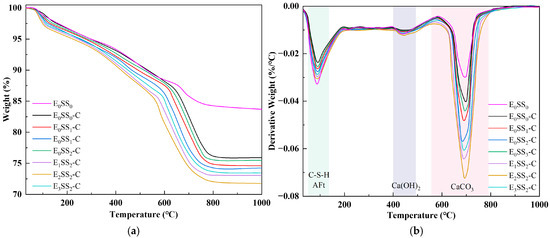
Figure 7.
TG-DTG curves of carbonated specimens at 28 d; (a) TG curves; (b) DTG curves.
Based on Equations (1) and (3), the CO2 uptake and CaCO3 content were calculated, and the results are summarized in Table 4. For the control group, CO2 uptake was 7.51% and 6.01% at 7 and 28 d, respectively, with corresponding CaCO3 contents of 17.15% and 13.65%. With the incorporation of steel slag and EDTA, both CO2 uptake and CaCO3 content increased. When the steel slag content increased from 0% to 15%, the CO2 uptake of samples at 7 d and 28 d curing ages increased by 3.14–6.79% and 1.16–3.00%, respectively, compared to the control group, while the CaCO3 content rose significantly. This indicates that steel slag provides additional Ca2+ for carbonate reaction. The maximum CO2 uptake of 8.02% and 6.19% was achieved at a 10% steel slag content. Building upon this optimal steel slag content, the introduction of EDTA further enhanced both CO2 uptake and CaCO3 content. Compared to the control group, the addition of 0.1 g/L to 1.0 g/L EDTA increased CO2 uptake by 7.46–11.05% at 7 days and 7.82–17.80% at 28 days. The sample with 0.5 g/L EDTA exhibited the highest CO2 uptake, reaching 8.34% and 7.09%, respectively. However, further increasing the EDTA concentration led to a decline in both CO2 uptake and CaCO3 content, which is hypothesized to result from excessive chelation of ions by EDTA, interfering with the carbonation reaction. The specific mechanism will be substantiated in the Discussion section. Additionally, the lower CO2 uptake at 28 days compared to 7 days is primarily attributed to the formation of more hydration products over the extended curing period, which filled pore spaces, reduced porosity, and consequently limited CO2 ingress into the samples. Thus, longer curing ages diminish the CO2 sequestration capacity of the material, a finding that will be corroborated by SEM and XRD analysis.

Table 4.
CO2 uptake and CaCO3 content of carbonated samples.
3.3. XRD
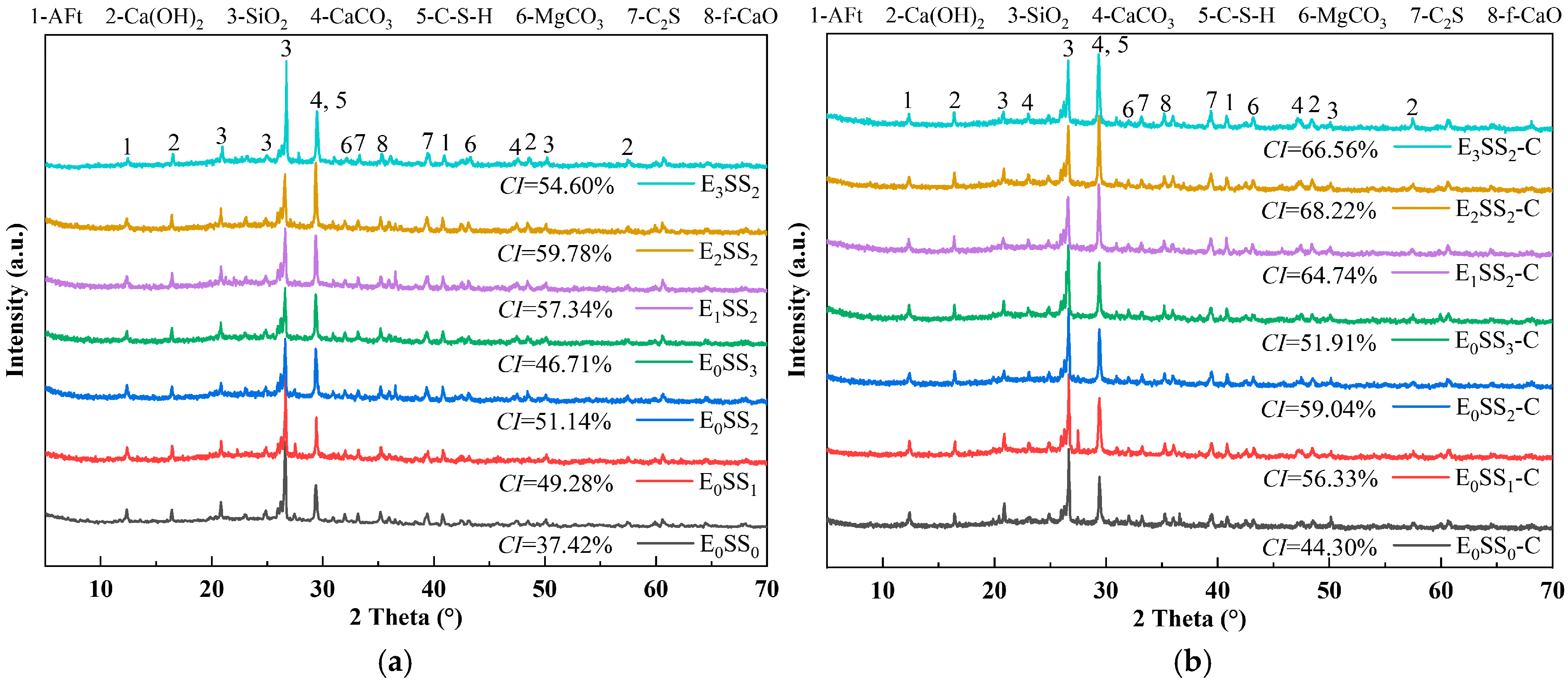
Figure 8 presents the XRD patterns of CPB specimens with varying steel slag contents and EDTA concentrations before and after carbonation at 28 d of curing. The major diffraction peaks were identified as calcium silicate hydrate (C-S-H), ettringite (AFt), SiO2, and calcite (CaCO3). Before carbonation, the hydration products were primarily composed of C-S-H gel, accompanied by residual unhydrated C3S, which served as the principal contributor to the mechanical strength of the CPB [51]. C-S-H is typically amorphous or weakly crystalline, producing a broad peak near 2θ = 29.35°, while calcium hydroxide (Ca(OH)2) displayed characteristic peaks at 2θ = 16.47°, 48.59°, and 57.49°. In the control group, the weaker C-S-H and Ca(OH)2 diffraction peaks indicated a lower Ca2+ content in the hydration products of specimens without steel slag and EDTA [52]. After carbonation, CaCO3 emerged as the predominant product, with characteristic calcite peaks observed at 2θ = 29.35°, 43.18°, and 47.14°. A marked increase in the intensity of CaCO3 peaks was accompanied by a notable reduction in Ca(OH)2 and AFt. These results indicate that CaCO3 was primarily formed through the carbonation reaction of C-S-H gel and Ca(OH)2 [53]. This transformation further corroborates that the reduction in hydration products after carbonation led to a decline in the compressive strength of the CPB.
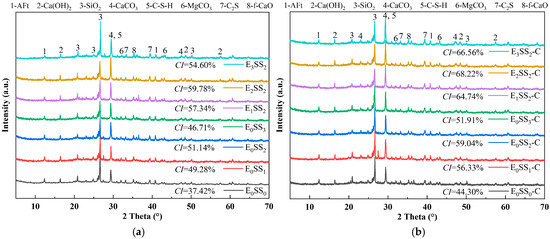
Figure 8.
XRD patterns of samples at 28 d (a) before carbonation; (b) after carbonation.
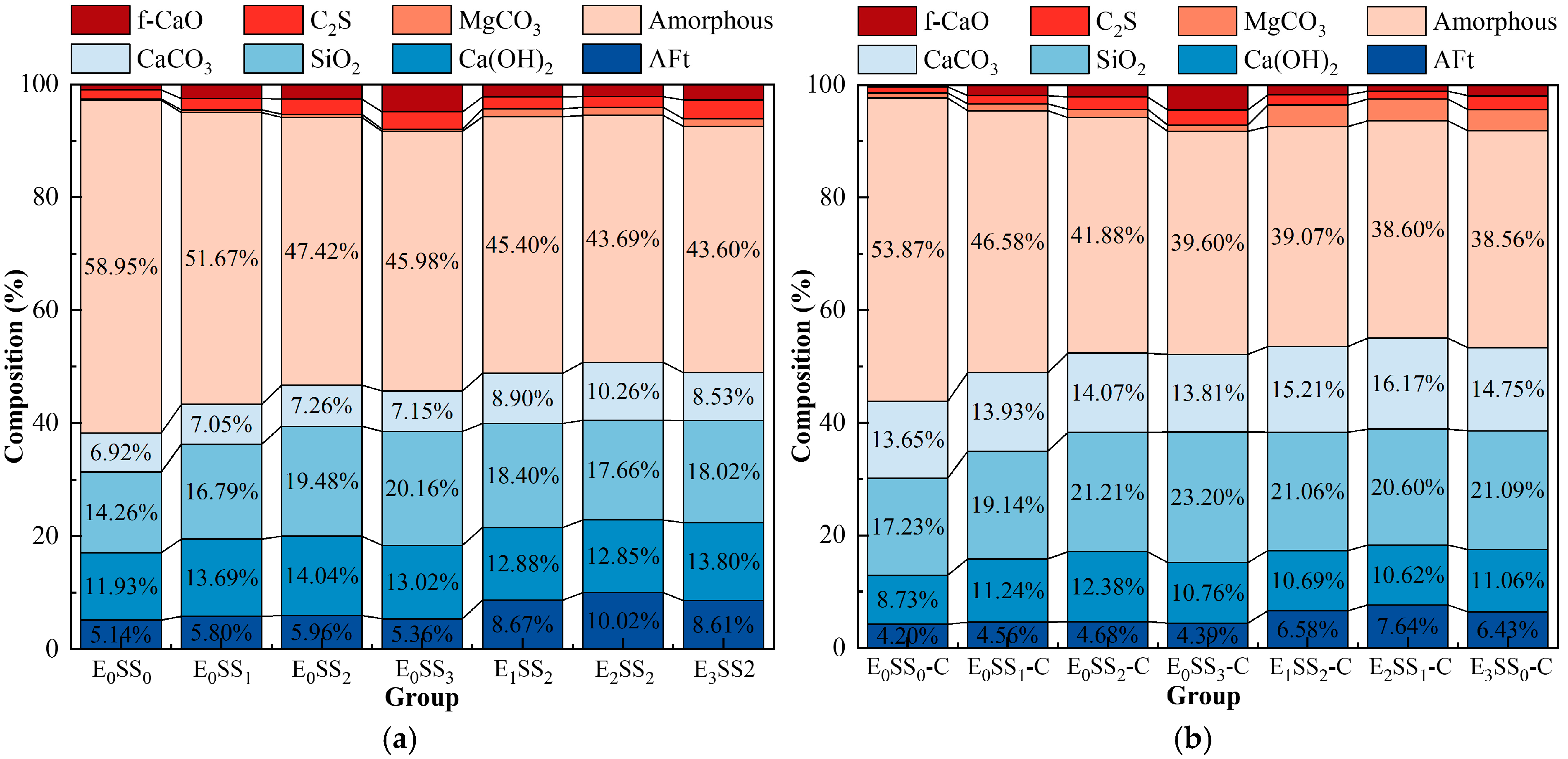
The quantitative analysis of the main mineral phases in the samples was conducted using XRD, and the Quantitative X-ray Diffraction (QXRD) results are shown in Figure 9. The incorporation of steel slag significantly altered the phase composition and reaction pathways of the system. As the steel slag content increased, the SiO2 content rose from 14.26% to 20.16%, and the Ca(OH)2 content increased from 11.93% to 14.04%. After carbonation, the Ca(OH)2 content decreased, while the contents of CaCO3 and magnesite (MgCO3) increased, indicating that CaO and MgO from the steel slag participated in the carbonation reaction to form CaCO3 and MgCO3. Upon the addition of EDTA, the contents of AFt and CaCO3 increased significantly. The highest CaCO3 content was observed in the sample with 10% steel slag and 0.5 g/L EDTA, which was 18.46% higher than that of the control group, demonstrating the optimal carbonation performance of this mixture and confirming that EDTA catalyzed both the hydration and carbonation reactions. It is noteworthy that the CaCO3 peak intensity decreased in the 15% steel slag group compared with the 10% group, and in the 1.0 g/L EDTA group compared with the 0.5 g/L group. These XRD results are consistent with the observed variations in compressive strength and CO2 sequestration performance of the CPB with different mix proportions.
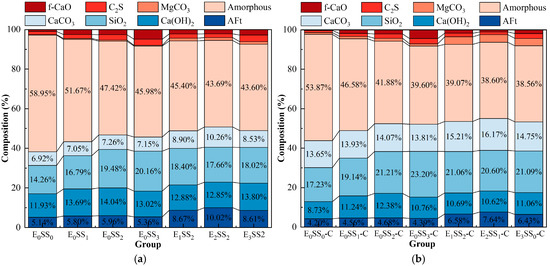
Figure 9.
QXRD results of samples at 28 d (a) before carbonation; (b) after carbonation.
The crystallinity index (CI) of each sample group was calculated from the XRD data using the peak area method [54], as shown in Figure 8. The results indicate that the crystallinity of the samples before carbonation ranged from 37.42% to 59.78%, which increased to 44.30–68.22% after carbonation. The E2SS2 group exhibited the highest crystallinity index, with a post-carbonation CI of 68.22%. This demonstrates that the synergistic effect of an appropriate amount of steel slag and EDTA promotes the formation of carbonate crystals, resulting in a more ordered crystal arrangement, improved structural stability, and a significant increase in crystallinity. In contrast, excessive incorporation led to a decline in crystallinity. Overall, the trend in crystallinity changes aligns with the patterns observed in compressive strength and CO2 sequestration: samples with higher crystallinity demonstrate superior mechanical properties and carbon sequestration capacity.
3.4. SEM-EDS
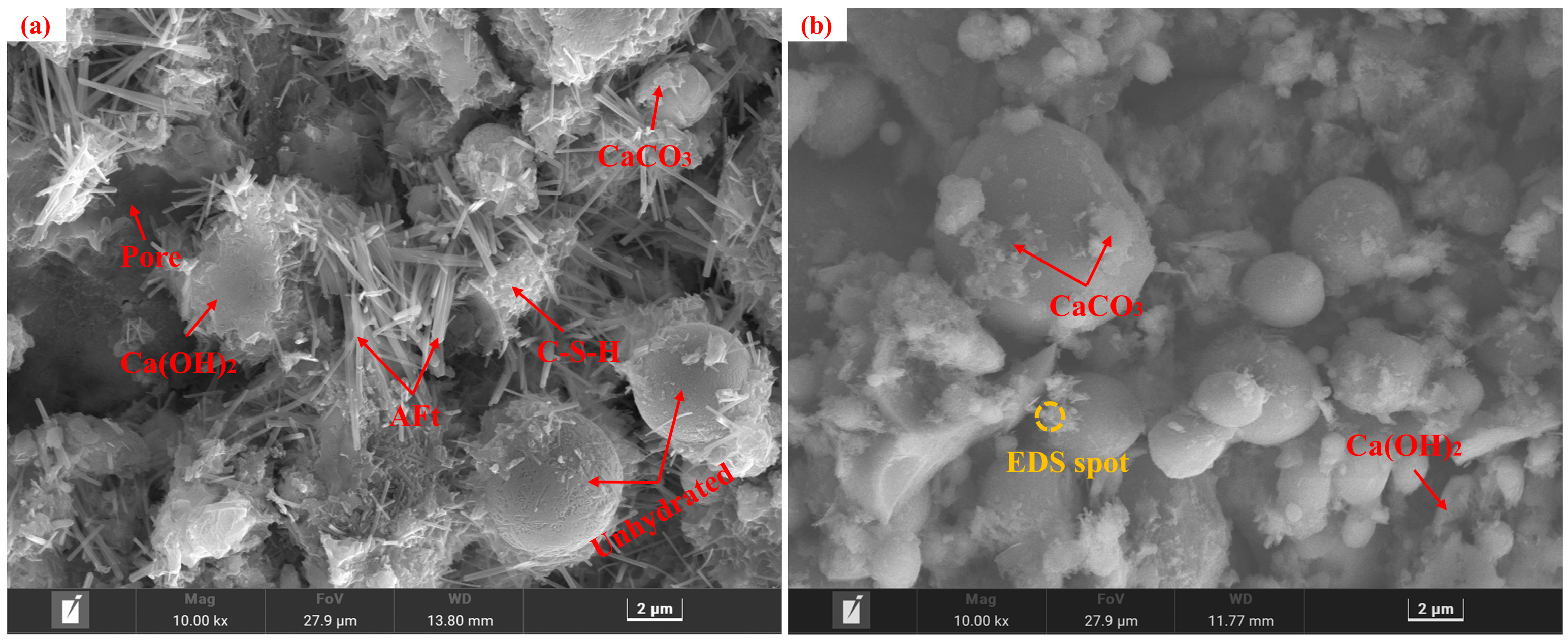
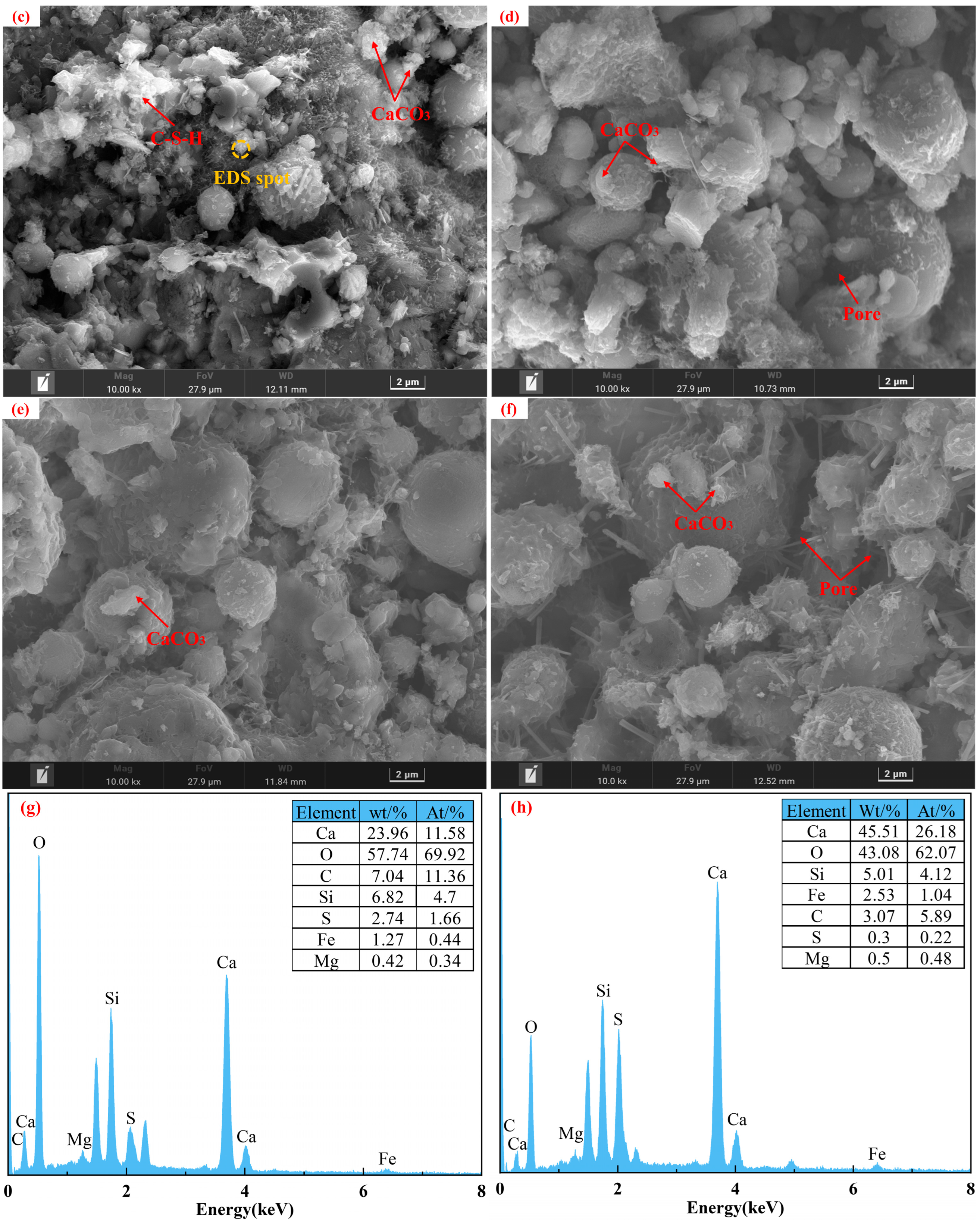
Specimens from the control group before and after carbonation at 28 d of curing, along with the carbonated E0SS2, E0SS3, E2SS2, and E3SS2 groups, were selected for SEM-EDS analysis, as shown in Figure 10. In the uncarbonated control sample, Ca(OH)2 crystals, unhydrated particles, and flocculent C-S-H gel were identified, exhibiting a relatively loose and porous structure [51]. After carbonation, the formation of CaCO3 crystals was evident, while residual Ca(OH)2 and C-S-H gel remained detectable. EDS analysis indicates that the primary elements on the sample surface are Ca, Si, C, O, and Mg. With the incorporation of steel slag, Mg was introduced. SEM examination revealed that the crystalline morphology of CaCO3 gradually evolved from regular forms to cauliflower-like forms. This transformation is attributed to Mg2+ promoting the growth of calcite crystals in asymmetrical directions, thereby inducing morphological changes in the crystals. This observation was corroborated by XRD mineral composition analysis [55,56]. With the incorporation of appropriate amounts of steel slag and EDTA, the generation of CaCO3 was further enhanced in the carbonation reaction, effectively filling pore spaces and resulting in a denser microstructure [57,58,59,60]. Furthermore, the synergy between steel slag and EDTA facilitated the formation of additional hydration products, providing a microstructural explanation for the observed improvements in the macroscopic strength of the CPB.
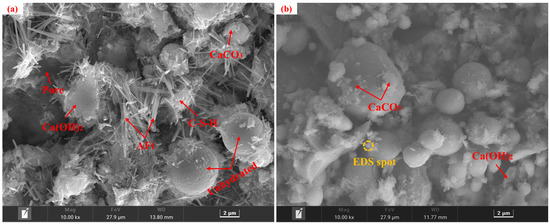
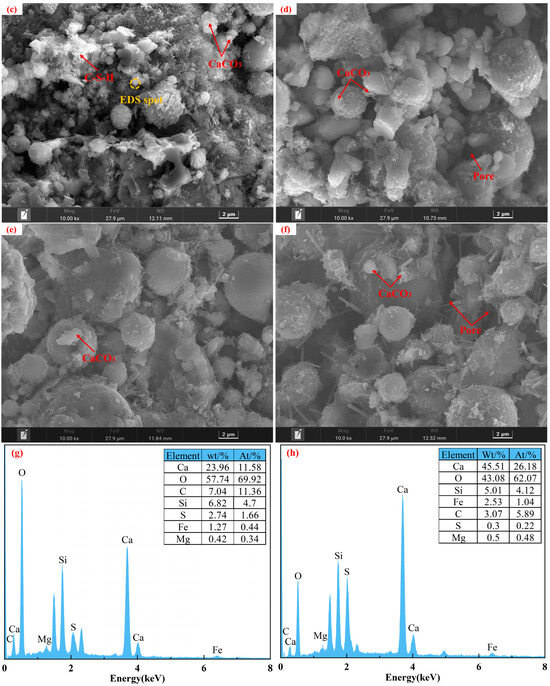
Figure 10.
Microstructural morphology of different specimens: (a) E0SS0 group; (b) E0SS0-C group; (c) E0SS2-C group; (d) E0SS3-C group; (e) E2SS2-C group; (f) E2SS3-C group; (g) EDS of E0SS0-C; (h) EDS of E2SS2-C.
Excessive incorporation of steel slag or EDTA was found to affect the microstructural development of the CPB negatively. Hydration products were markedly reduced in the 15% steel slag group and the 1.0 g/L EDTA group, with visible pores within the matrix. With increasing steel slag content, the proportion of free CaO and MgO in the system increased, promoting the rapid generation of loose hydration products. In addition, the newly formed carbonates tended to envelop C-S-H gel and other hydration products, thereby impeding further carbonation reactions and gradually deteriorating the matrix structure. Similarly, at excessively high EDTA concentrations, the resulting carbonate crystals exhibited irregular sizes and indistinct morphologies. These observations were consistent with the CO2 sequestration results derived from TG analysis, further verifying the existence of an optimal dosage range for both steel slag and EDTA.
4. Discussion
4.1. Mechanism of Steel Slag Affecting CPB Properties
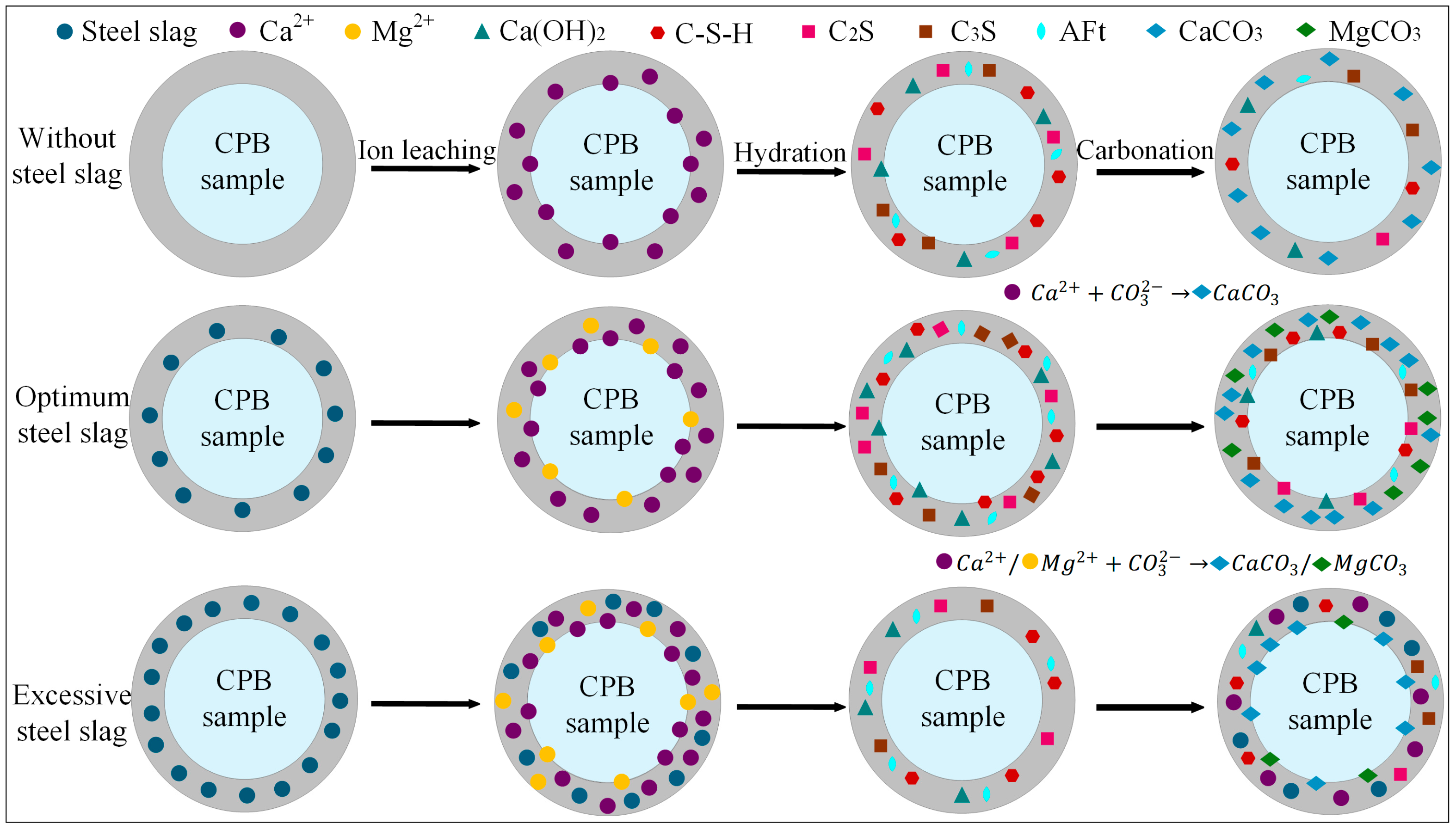
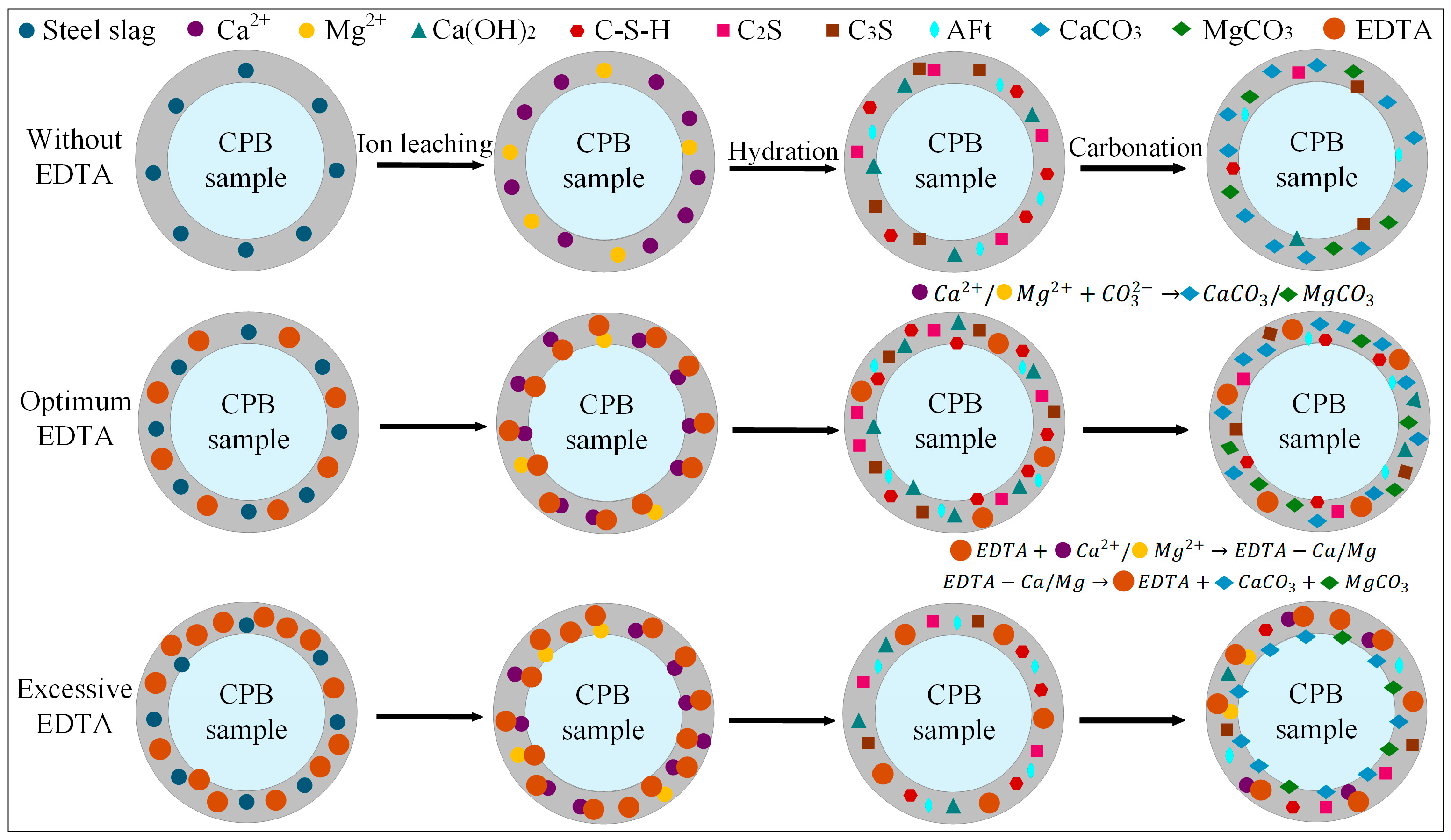
The results demonstrate that the incorporation of steel slag into the CPB significantly influences both its compressive strength and CO2 uptake. Figure 11 depicts the reaction mechanism of steel slag within the cemented backfill matrix, and the process can be broadly divided into three stages:
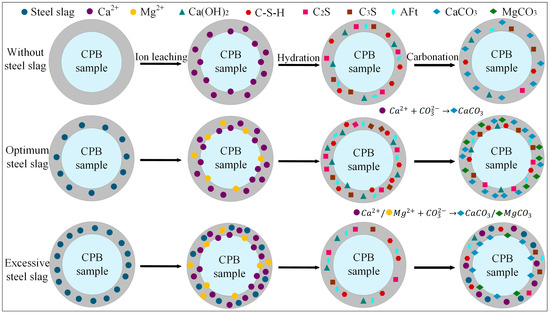
Figure 11.
The effect mechanism of steel slag in CPB.
(1) Ion Leaching
Based on the QXRD analysis (Figure 9), it is evident that steel slag is rich in free CaO and MgO. The incorporation of 10% steel slag increased the f-CaO content in the system of the pre-carbonated samples by nearly threefold, and raised the Ca(OH)2 content to 14.04%. During the ion leaching stage, the hydrolysis of steel slag releases substantial amounts of Ca2+ and Mg2+, markedly increasing these ions within the matrix. Simultaneously, the calcium and magnesium oxides in steel slag react with water to form Ca(OH)2 and Mg(OH)2, and the resulting alkaline environment further promotes the continuous release of ions [19].
(2) Hydration Reaction
The incorporation of steel slag creates a highly alkaline environment, promoting the release of ions such as Ca2+, Mg2+, H2SiO42−, and HSiO3−. The increased intensity of the C-S-H diffraction peak in the XRD pattern (Figure 8) indicates that mineral phases like C2S and C3S in steel slag undergo hydration reactions, facilitating the extensive formation of C-S-H gel. This gel effectively binds unhydrated steel slag particles, fly ash glassy phases, and gangue, forming a skeletal structure that enhances the compressive strength of the steel slag-incorporated groups. Specifically, the addition of 10% steel slag resulted in compressive strengths of 3.4 MPa, 4.9 MPa, 7.3 MPa, and 8.3 MPa for pre-carbonated samples at different curing ages, respectively. However, when the steel slag content is excessively high, surplus Ca2+ competes with silicate polymers ([SiO4]4−) for binding sites, leading to a reduction in C-S-H peak intensity and diminished formation of hydration products. This weakens the cementing effect within the system, a finding consistent with the research of Hao et al. [61].
(3) Carbonation Reaction
Based on the CO2 sequestration capacity (Table 4) and QXRD phase content results (Figure 9), it was observed that at 7 d curing age, the CO2 uptake could be increased by up to 6.79% compared to the control group. Under the three different steel slag incorporation rates, the CaCO3 content after carbonation reached 13.93%, 14.07%, and 13.81%, respectively, while the content of hydration products such as C2S and AFt decreased to varying degrees. Additionally, the compressive strength of the samples after carbonation was reduced by up to 54.5% compared to that before carbonation (Figure 5). CO2 diffuses through the pore structure and dissolves in the water, forming CO32−, which subsequently reacts with Ca2+ and Mg2+ to generate stable carbonate minerals such as CaCO3 and MgCO3. This process enables long-term CO2 sequestration. However, carbonation also consumes part of the hydration products, resulting in a decrease in compressive strength after CO2 treatment. At higher steel slag contents, the increased concentrations of Ca2+ and Mg2+ accelerate the precipitation of carbonate crystals. These newly formed CaCO3 and MgCO3 crystals encapsulate unreacted particles, obstructing the further release of ions, impeding subsequent mineralization reactions, and ultimately diminishing the CO2 sequestration efficiency of the material. The mechanism is consistent with the conclusions drawn by Ababneh et al. [40].
4.2. Mechanism of the Synergistic Effect of Steel Slag and EDTA on CPB Properties
Based on the optimal steel slag content, the incorporation of an appropriate amount of EDTA significantly enhanced both the compressive strength and carbon sequestration capacity of the CPB. The E2SS2-C group achieved the peak compressive strength, reaching 8.9 MPa at 28 d of curing (Figure 5). This improvement is primarily attributed to the chelating effect of EDTA, with the reaction mechanism illustrated in Figure 12. Upon introduction into the system, EDTA dissolves in water and releases H+, promoting the leaching of calcium and magnesium ions from the steel slag [62,63]. Subsequently, EDTA complexes with Ca2+ and Mg2+ to form EDTA-Ca and EDTA-Mg, which facilitates the formation of hydration products and enhances the cementitious action of the system, thereby improving the material’s strength. As a result, the 28-day compressive strength of the samples increased by 23.08–51.28% compared to the group without EDTA. However, when the EDTA concentration was excessively high, the AFt content decreased from 7.64–6.43%, and the 28 d compressive strength dropped from 8.9 MPa to 8.1 MPa. This is because the excessively chelated Ca2+ competed with silicate polymers ([SiO4]4−) for binding sites, inhibiting the formation of hydration products and weakening the cementing effect of the system. After carbonation, the contents of CaCO3 and MgCO3 in the system increased by 6.81–17.09% and 235.71–247.32%, respectively, compared to the group without EDTA (Figure 9), leading to a corresponding improvement in CO2 sequestration capacity. During the carbonation process, the decomposition of EDTA-Ca and EDTA-Mg complexes promotes the precipitation of carbonate compounds, primarily CaCO3 and MgCO3, thereby enhancing CO2 uptake and improving carbonation efficiency. However, at high EDTA concentrations, the accelerated precipitation of CaCO3 and MgCO3 results in carbonate layers that coat unreacted particles, hindering further ion leaching. This reduces the availability of Ca2+ and Mg2+ for subsequent mineralization [24], ultimately limiting the carbonation efficiency—an effect similar to that observed with excessive steel slag incorporation. Therefore, appropriate dosages of both EDTA and steel slag are crucial for maximizing the strength and CO2 sequestration performance of CPB, whereas overdosing diminishes these benefits. This finding aligns with the results reported by Chen et al. [41].
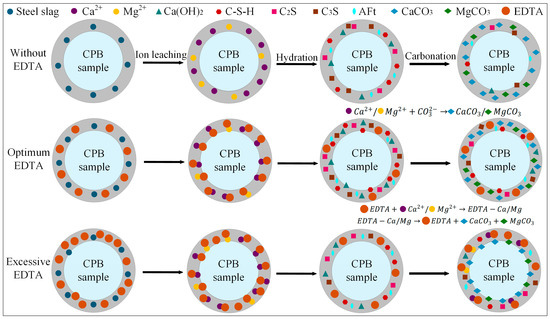
Figure 12.
The effect mechanism of EDTA in CPB.
5. Conclusions
This study investigated the effects of steel slag content and EDTA concentration on compressive strength and CO2 sequestration performance of CPB. It further analyzes the mechanisms by which steel slag, and the synergistic interaction between steel slag and EDTA, influence material performance. The main conclusions can be drawn:
- (1)
- Compared with the control group, the incorporation of appropriate incorporation of steel slag and EDTA significantly enhanced the compressive strength of the CPB. With increasing steel slag content and EDTA concentration, the compressive strength first increased and then decreased, with the optimal mixture containing 10% steel slag and 0.5 g/L EDTA. Under these conditions, the specimen achieved a compressive strength of 6.4 MPa after 28 d of curing.
- (2)
- The incorporation of steel slag and EDTA also significantly improved the CO2 sequestration performance of the CPB, exhibiting a similar trend to that of compressive strength with an optimal dosage. Under the optimal conditions of 10% steel slag and 0.5 g/L EDTA, the specimen cured for 7 d achieved a CO2 uptake of 7.94%. Moreover, extending the curing age reduced the CO2 uptake, as the value after 28 d of curing was lower than that after 7 d.
- (3)
- The incorporation of steel slag and EDTA affects the performance of cemented backfill materials across the three crucial stages of ion leaching, hydration, and carbonation. Steel slag serves as a source for additional Ca2+ and Mg2+ ions, while the presence of EDTA significantly enhances their leaching and dissolution into the aqueous phase. This synergistic action accelerates the formation of both hydration products and carbonates within the cemented matrix, leading to a stable skeletal structure, improved compressive strength, and enabling long-term CO2 sequestration.
- (4)
- Conversely, when the content of steel slag or the concentration of EDTA is excessively high, a detrimental effect is observed. The resulting surplus of Ca2+ and Mg2+ ions leads to competitive binding with silicate polymers ([SiO4]4−), thereby reducing the formation of hydration products. Furthermore, excessive carbonate precipitates may rapidly form and fully envelop the unreacted particles and existing hydration products, restricting further reaction processes. This ultimately creates porosity and matrix deterioration, leading to a decline in both the material’s compressive strength and carbonation performance.
Author Contributions
Conceptualization, N.Z.; Methodology, D.K., N.Z. and Z.L.; Data curation, X.L. and Z.L.; Software, X.L.; Formal analysis, X.L., Z.L. and Q.C.; Investigation, X.L., D.K., Z.L. and Q.C.; Resources, Z.L. and Q.C.; Writing—original draft, X.L.; Writing—review & editing, N.Z. and Z.L.; Supervision, Z.L.; Validation, N.Z.; Visualization, X.L.; Project administration, N.Z. and D.K.; Funding acquisition, N.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R & D Program of China (2023YFC3904304), the Key R&D Program of Xinjiang Uygur Autonomous Region (2023B01009), the Research Project of Jiangsu Key Laboratory for Clean Utilization of Carbon Resources (BM2024007).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
Author Dan Kang was employed by the company Yankuang Energy Group Company Limited, but he declares that he has no known personal relationships or competing financial interests that could have appeared to influence the work reported in this paper. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EDTA | Ethylenediaminetetraacetic Acid |
| CPB | Cemented paste backfill |
| CCUS | Carbon capture, utilization, and storage |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscopy |
| CG | Coal gangue |
| FA | Fly ash |
| OPC | Ordinary Portland cement |
| SS | Steel slag |
| SF | Silica fume |
| XRF | X-ray fluorescence spectroscopy |
| TG | Thermogravimetric |
| DTG | Differential thermogravimetric |
| QXRD | Quantitative X-ray Diffraction |
| CI | crystallinity index |
References
- Zhu, M.; Liu, L.; Wang, S.; Wei, B.; Rong, R.; Zhang, W.; Zhuang, D.; Jia, Q. Backfill-strip mining and CO2 mineralization sequestration in coal mine goaves: A synergetic method and its key technologies. Coal Geol. Explor. 2025, 53, 143–155. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, N.; Liu, H.; Xie, Y.; Sun, Z.; Ouyang, S. Research progress and prospect of underground utilization of coal-based solid waste functional materials. Coal Sci. Technol. 2025, 53, 1–28. [Google Scholar] [CrossRef]
- Jiao, Y.; Lv, B.; Fan, W.; Li, L.; Wang, M.; Xing, B. Preparation and application prospects of coal-based carbon materials: A comprehensive review. J. Alloys Compd. 2025, 1016, 178951. [Google Scholar] [CrossRef]
- Keskin, T.; Yilmaz, E.; Kasap, T.; Sari, M.; Cao, S. Toward viable industrial solid residual waste recycling: A review of its innovative applications and future perspectives. Minerals 2024, 14, 943. [Google Scholar] [CrossRef]
- Zhang, F. Study on mechanical properties and load-bearing failure characteristics of coal-based solid waste cemented backfill. Coal 2025, 34, 100–104. [Google Scholar] [CrossRef]
- Zhao, H. Experimental Study on Carbon Sequestration Slurry Materials for Goaf Backfilling and Reclamation. Master’s Thesis, China Coal Research Institute, Beijing, China, 2025. [Google Scholar]
- Bordoloi, S.; Afolayan, O.D.; Ng, C.W.W. Feasibility of construction demolition waste for unexplored geotechnical and geo-environmental applications—A review. Constr. Build. Mater. 2022, 356, 129230. [Google Scholar] [CrossRef]
- Krishna, R.S.; Shaikh, F.; Mishra, J.; Lazorenko, G.; Kasprzhitskii, A. Mine tailings-based geopolymers: Properties, applications and industrial prospects. Ceram. Int. 2021, 47, 17826–17843. [Google Scholar] [CrossRef]
- Zajac, M.; Bremeier, R.; Deja, J.; Król, M.; Ben Haha, M. Carbonation hardening of portland cement with recycled supplementary cementitious materials. Cem. Concr. Compos. 2025, 157, 105904. [Google Scholar] [CrossRef]
- Alves, M.O.; Nelson de Goes, L.M.; Simonelli, G. Impact on greenhouse gas emissions in the ammonia production process by reusing heat in the CO2 absorption stage. Energy Convers. Manag. 2026, 347, 120534. [Google Scholar] [CrossRef]
- Saletnik, A.; Saletnik, B.; Puchalski, C. Coal as the world’s dominant energy source and its role in the energy transformation and regulations of european green deal. J. Environ. Manag. 2025, 392, 126815. [Google Scholar] [CrossRef]
- Liu, L.; Fang, Z.; Wang, S.; Gao, G.; Zhang, B.; Zhao, Y.; Zhu, M.; Liu, Z.; Wang, J.; Zhou, J.; et al. Theoretical basis and technical conception of backfill carbon fixation in coal mine. Coal Sci. Technol. 2024, 52, 292–308. [Google Scholar] [CrossRef]
- Kusin, F.M.; Syed Hasan, S.N.M.; Molahid, V.L.M.; Soomro, M.H. Dual adoption opportunities and prospects for mining and industrial waste recovery through an integrated carbon capture, utilization and storage. Sustain. Prod. Consum. 2024, 48, 181–204. [Google Scholar] [CrossRef]
- Zhang, S.; Niu, D.; Luo, D.; Wang, Y. Activation of activator on steel slag-cement and its hydration mechanism. J. Harbin Inst. Tech. 2024, 56, 165–172. [Google Scholar] [CrossRef]
- Biava, G.; Zacco, A.; Zanoletti, A.; Sorrentino, G.P.; Capone, C.; Princigallo, A.; Depero, L.E.; Bontempi, E. Accelerated direct carbonation of steel slag and cement kiln dust: An industrial symbiosis strategy applied in the Bergamo–Brescia area. Materials 2023, 16, 4055. [Google Scholar] [CrossRef]
- Montoya, D.; Pistofidis, N.; Giannakopoulos, G.; Iacobescu, R.; Katsiotis, M.; Pontikes, Y. Revisiting the iron-rich “ordinary portland cement” towards valorisation of wastes: Study of Fe-to-Al ratio on the clinker production and the hydration reaction. Mater. Struct. 2021, 54, 30. [Google Scholar] [CrossRef]
- Wang, X.; Guo, M.; Ling, T. Review on CO2 curing of non-hydraulic calcium silicates cements: Mechanism, carbonation and performance. Cem. Concr. Compos. 2022, 133, 104641. [Google Scholar] [CrossRef]
- Na, H.; Wang, Y.; Zhang, X.; Li, J.; Zeng, Y.; Liu, P. Hydration activity and carbonation characteristics of dicalcium silicate in steel slag: A review. Metals 2021, 11, 1580. [Google Scholar] [CrossRef]
- Song, X.; Zhao, C.; Song, Y.; Guo, Y.; Kong, X. Mineralization curing of steel slag-fly ash solid waste composite alkali-activated cementitious material. Clean Coal Tech. 2024, 30, 88–98. [Google Scholar] [CrossRef]
- Nielsen, P.; Boone, M.A.; Horckmans, L.; Snellings, R.; Quaghebeur, M. Accelerated carbonation of steel slag monoliths at low CO2 pressure—Microstructure and strength development. J. CO2 Util. 2020, 36, 124–134. [Google Scholar] [CrossRef]
- Jhatial, A.A.; Nováková, I.; Gjerløw, E. A review on emerging cementitious materials, reactivity evaluation and treatment methods. Buildings 2023, 13, 526. [Google Scholar] [CrossRef]
- Nunes, V.A.; Borges, P.H.R. Recent advances in the reuse of steel slags and future perspectives as binder and aggregate for alkali-activated materials. Constr. Build. Mater. 2021, 281, 122605. [Google Scholar] [CrossRef]
- Jahami, A.; Issa, C.A. Exploring the use of mixed waste materials (MWM) in concrete for sustainable construction: A review. Constr. Build. Mater. 2023, 398, 132476. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, W.; Zheng, Y.; Hu, S. Effect of recycled aggregate and slag as substitutes for natural aggregate and cement on the properties of concrete: A review. J. Renew. Mater. 2023, 11, 1853–1879. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Liu, Z.; Li, N.; Zhao, S.; Hu, S. Steel slag accelerated carbonation curing for high-carbonation precast concrete development. Materials 2024, 17, 2968. [Google Scholar] [CrossRef]
- Rosales, J.; Agrela, F.; Entrenas, J.A.; Cabrera, M. Potential of stainless steel slag waste in manufacturing self-compacting concrete. Materials 2020, 13, 2049. [Google Scholar] [CrossRef]
- Herki, B.M.A.; Ali, A.I.; Smail, Y.S.; Omer, K.M. An innovative approach to enhancing concrete sustainability: Utilising unprocessed steel slag with low CaO and high SiO2 content. Buildings 2025, 15, 1514. [Google Scholar] [CrossRef]
- Gu, X.; Wang, Y.; Sun, D.; Sun, R. Macroscopic and microscopic characteristics of slag-steel slag ce-mentitious system under the action of composite activator. Coal Sci. Technol. 2025, 53, 375–386. [Google Scholar]
- Zhang, X.; Yang, X.; Yang, J.; Yin, S.; Bian, W.; Qi, Y. Development of magnesium slag–steel slag-based composite cementitious material and its backfilling application. Arch. Civ. Mech. Eng. 2025, 25, 194. [Google Scholar] [CrossRef]
- Sunkara, Y.; Vinod, J.S.; Kumari, W.G.P.; Saride, S. Shear behaviour of basic oxygen furnace slag-granulated blast furnace slag mixtures: A laboratory investigation. Constr. Build. Mater. 2025, 494, 143443. [Google Scholar] [CrossRef]
- Yi, G.; Tian, W.; Shi, J.; Guo, J.; Cheng, X. CO2 mineralization curing steel slag-fly ash-portland cement ternary paste: Mechanical properties, microstructure and life cycle assessment. J. Cent. South Univ. 2025, 32, 2487–2510. [Google Scholar] [CrossRef]
- Li, N.; Unluer, C. A comparative study of ethylenediamine tetraacetic acid induced gas-solid and liquid-solid accelerated carbonation for enhancement of steel slag aggregates. Constr. Build. Mater. 2023, 400, 132539. [Google Scholar] [CrossRef]
- Leão, A.; Collin, M.; Ghodkhande, S.; Bouissonnié, A.; Chen, X.; Malin, B.; Liu, Y.; Hovey, G.; Govindhakannan, J.; Plante, E.L.; et al. ZeroCAL: Eliminating carbon dioxide emissions from limestone’s decomposition to decarbonize cement production. Acs Sustain. Chem. Eng. 2024, 12, 15762–15787. [Google Scholar] [CrossRef]
- Jose, V.; Jose, V.; Kuruvilla, E.; Nesaraj, A.S. Fabrication, phytotoxicity, and electrochemical performance of rare-earth metal-based mixed la–ce cobaltite nanospheres: Applications in energy storage and environmental remediation. J. Phys. Chem. Solids 2025, 207, 112935. [Google Scholar] [CrossRef]
- Parhizgar Keradeh, M.; Tabatabaei-Nezhad, S.A. Investigation of the effect of diethylene triamine pentaacetic acid chelating agent as an enhanced oil recovery fluid on wettability alteration of sandstone rocks. Petrol. Explor. Dev. 2023, 50, 675–687. [Google Scholar] [CrossRef]
- Gu, L.; Deng, J.; Yuan, H.; Zhang, X. Effect of ethylenediaminetetraacetic acid and its sodium salt on carbonation performance of steel slag block. Green Bulid. Mater. 2024, 11, 140–145. [Google Scholar]
- Liu, S.; Su, Y.; Yang, M.; Xu, P.; Yi, C.; Nie, Y.; Wang, L. Preparation and activation of slag-blended cementitious materials: A study on the activation of cementitious activity. Met. Mine 2022, 57, 252–258. [Google Scholar] [CrossRef]
- Krevor, S.; de Coninck, H.; Gasda, S.E.; Ghaleigh, N.S.; de Gooyert, V.; Hajibeygi, H.; Juanes, R.; Neufeld, J.; Roberts, J.J.; Swennenhuis, F. Subsurface carbon dioxide and hydrogen storage for a sustainable energy future. Nat. Rev. Earth Environ. 2023, 4, 102–118. [Google Scholar] [CrossRef]
- Librandi, P.; Nielsen, P.; Costa, G.; Snellings, R.; Quaghebeur, M.; Baciocchi, R. Mechanical and environmental properties of carbonated steel slag compacts as a function of mineralogy and CO2 uptake. J CO2 Util. 2019, 33, 201–214. [Google Scholar] [CrossRef]
- Ababneh, A.; Al-Rousan, R.; Gharaibeh, W.; Abu-Dalo, M. Recycling of pre-treated medical waste fly ash in mortar mixtures. J. Mater. Cycles Waste Manag. 2020, 22, 207–220. [Google Scholar] [CrossRef]
- Chen, T.; Xue, Y.; Zhao, X.; Liu, J. Effects of EDTA on the accelerated carbonation behavior of steel slag used as cementitious materials. J. Mater. Cycles Waste Manag. 2023, 25, 1498–1508. [Google Scholar] [CrossRef]
- Katre, S.; Ochonma, P.; Mamidala, A.; Sahu, S.; Nair, A.M.; Ravi, K.; Gadikota, G. Organic ligands and CO2 unlock the potential for energy relevant metals recovery and carbon mineralization from mafic rocks. Sci. Rep. 2025, 15, 10882. [Google Scholar] [CrossRef]
- Thumm, A.K.; Skerencak-Frech, A.; Gaona, X.; Altmaier, M.; Geckeis, H. Uptake of cm (III) and eu (III) by c–s–h phases under saline conditions in presence of EDTA: A batch sorption and TRLFS study. Appl. Geochem. 2024, 170, 106087. [Google Scholar] [CrossRef]
- Zhang, Y.; Shan, J.; Ma, Z.; Liu, Z.; Huang, J.; Fang, Y. Effects of ethylenediaminetetraacetic acid on the properties of accelerated carbonated steel slag as supplementary cementitious materials. Constr. Build. Mater. 2025, 487, 142093. [Google Scholar] [CrossRef]
- GB-T 17671-2021; Test Method of Cement Mortar Strength (ISO Method). National Cement Standardization Technical Committee: Beijing, China, 2021.
- Liu, X.; Wu, P.; Liu, X.; Zhang, Z.; Ai, X. The utilization of carbonated steel slag as a supplementary cementitious material in cement. Materials 2024, 17, 4574. [Google Scholar] [CrossRef]
- Cheng, X.; Tian, W.; Yuan, Q.; Chen, W.; Wan, J.; Guo, J.; Cai, J. Effect of carbon dioxide mineralization curing on mechanical properties and microstructure of portland cement–steel slag–granulated blast furnace slag ternary paste. Constr. Build. Mater. 2024, 431, 136553. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, B.; Li, T.; Wang, Y.; Li, N. High volume steel slag powder-cement alkali excitation characteristics. China Powder Technol. 2023, 29, 55–65. [Google Scholar]
- Wang, X.; Ni, W.; Li, J.; Zhang, S.; Hitch, M.; Pascual, R. Carbonation of steel slag and gypsum for building materials and associated reaction mechanisms. Cem. Concr. Res. 2019, 125, 105893. [Google Scholar] [CrossRef]
- Huo, B.; Zhang, J.; Zhou, N.; Li, M.; Guo, Q. Effect of sodium dodecyl sulfate on CO2 storage and rheological properties of alkali-activated coal gasification slag filling slurry. J. Min. Saf. Eng. 2024, 41, 1279–1288. [Google Scholar]
- Rui, Y.; Qian, C.; Zhang, X.; Ma, Z. Different carbon treatments for steel slag powder and their subsequent effects on properties of cement-based materials. J. Clean. Prod. 2022, 362, 132407. [Google Scholar] [CrossRef]
- Tian, W.; Wan, J.; Cheng, X.; Zhang, J.; Guo, J. Synergistic carbon sequestration performance and microstructure of steel slag-base cementitious materials. J. Build. Mater. 2025, 28, 434–441. [Google Scholar]
- Zhao, J.; Li, Y.; Yang, J.; Cao, X.; Wang, J.; Wang, G.; Sun, L.; Huang, Q.; Cheng, W.; Lyu, Z. Preparation of slag-based foam concrete and its carbon dioxide sequestration performance. Int. J. Greenh. Gas Control. 2024, 135, 104156. [Google Scholar] [CrossRef]
- Ngiwngam, K.; Chinvorarat, S.; Rachtanapun, P.; Auras, R.; Wittaya, T.; Tongdeesoontorn, W. Effect of chemical and steam explosion pulping on the physical and mechanical properties of sugarcane straw pulp trays. Polymers 2023, 15, 3132. [Google Scholar] [CrossRef]
- Saillio, M.; Baroghel-Bouny, V.; Pradelle, S.; Bertin, M.; Vincent, J.; d’Espinose de Lacaillerie, J. Effect of supplementary cementitious materials on carbonation of cement pastes. Cem. Concr. Res. 2021, 142, 106358. [Google Scholar] [CrossRef]
- Han, Y.; Aizenberg, J. Effect of magnesium ions on oriented growth of calcite on carboxylic acid functionalized self-assembled monolayer. J. Am. Chem. Soc. 2003, 125, 4032–4033. [Google Scholar] [CrossRef]
- Li, X.; Mehdizadeh, H.; Ling, T. Environmental, economic and engineering performances of aqueous carbonated steel slag powders as alternative material in cement pastes: Influence of particle size. Sci. Total Environ. 2023, 903, 166210. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, K.; Wang, B.; Wu, Z.; Zhou, M. Design and research on the preparation of pervious concrete using carbonized steel slag as a full component. Buildings 2025, 15, 1526. [Google Scholar] [CrossRef]
- Li, K.; Zhu, L.; Wu, Z.; Wang, X. Properties of cemented filling materials prepared from phosphogypsum-steel slag–blast-furnace slag and its environmental effect. Materials 2024, 17, 3618. [Google Scholar] [CrossRef]
- Wang, J.; Ma, X.; Guo, H.; Wang, H.; Qu, Y.; Jiao, W.; Ma, Z. The influence mechanism of non-free calcium on the dissolution-polymerization reaction of coal gasification ash in alkaline environment. J. Fuel Chemis Tech. 2025, 53, 1416–1426. [Google Scholar] [CrossRef]
- Hao, J.; Zhou, Z.; Chen, Z.; Zhou, Y.; Wang, J. Damage characterization and microscopic mechanism of steel slag-cemented paste backfill under uniaxial compression. Constr. Build. Mater. 2023, 409, 134175. [Google Scholar] [CrossRef]
- Han, J.; Kim, S.; Lee, Y.; Hur, D. Chemical cleaning of magnetite deposits on the flow mini-channels of a printed circuit heat exchanger in an EDTA-based solution. Materials 2022, 15, 1471. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, D.; Gu, L.; Yuan, H.; Zhang, B. Enhanced carbonation of steel slag blocks using various chemical additives. J. Build. Eng. 2025, 105, 112518. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).