Influence of Artificial Aging of ZnAlCu Alloys on Microstructure and Compressive Yield Strength
Abstract
1. Introduction
2. Materials and Methods
3. Results
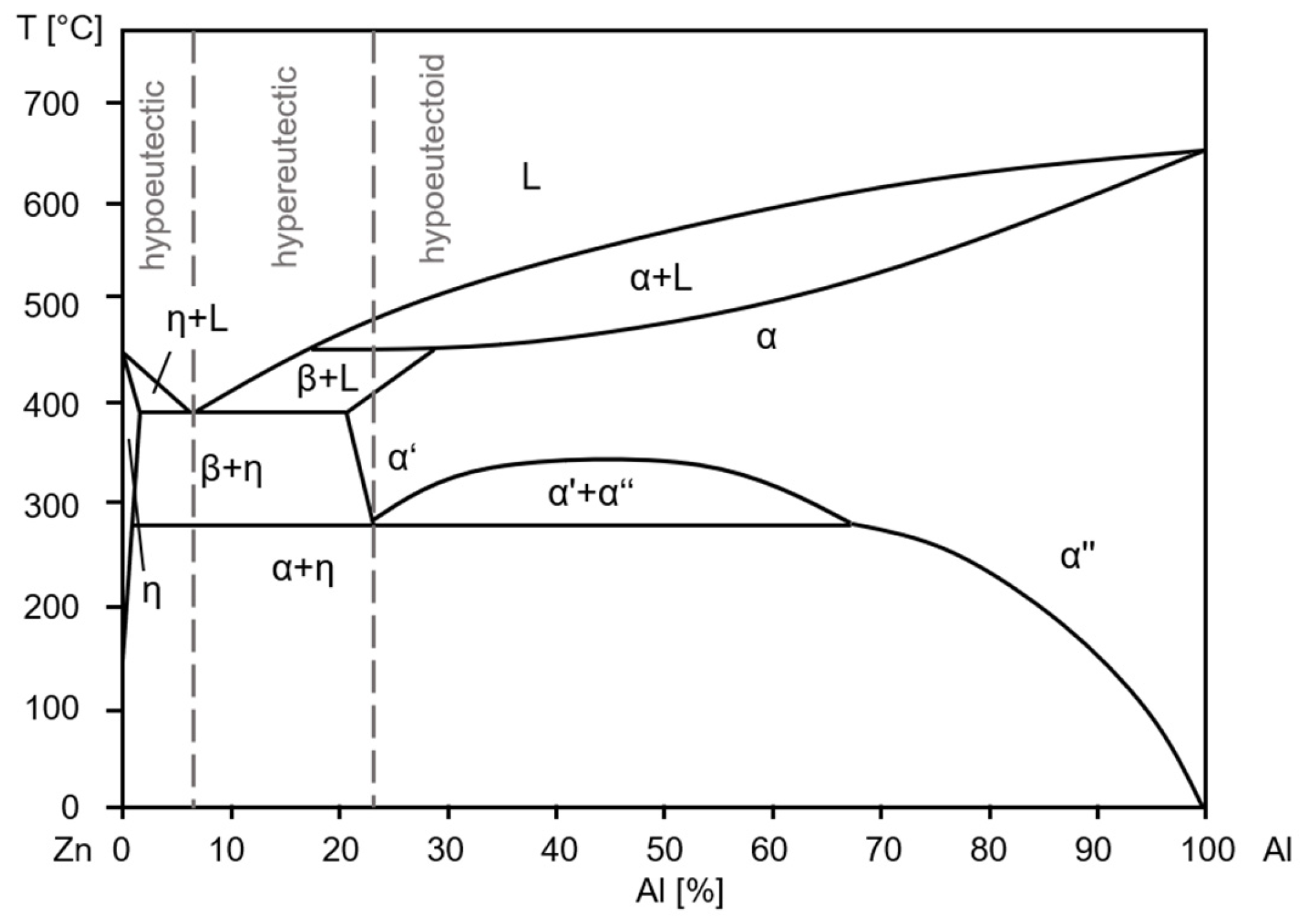
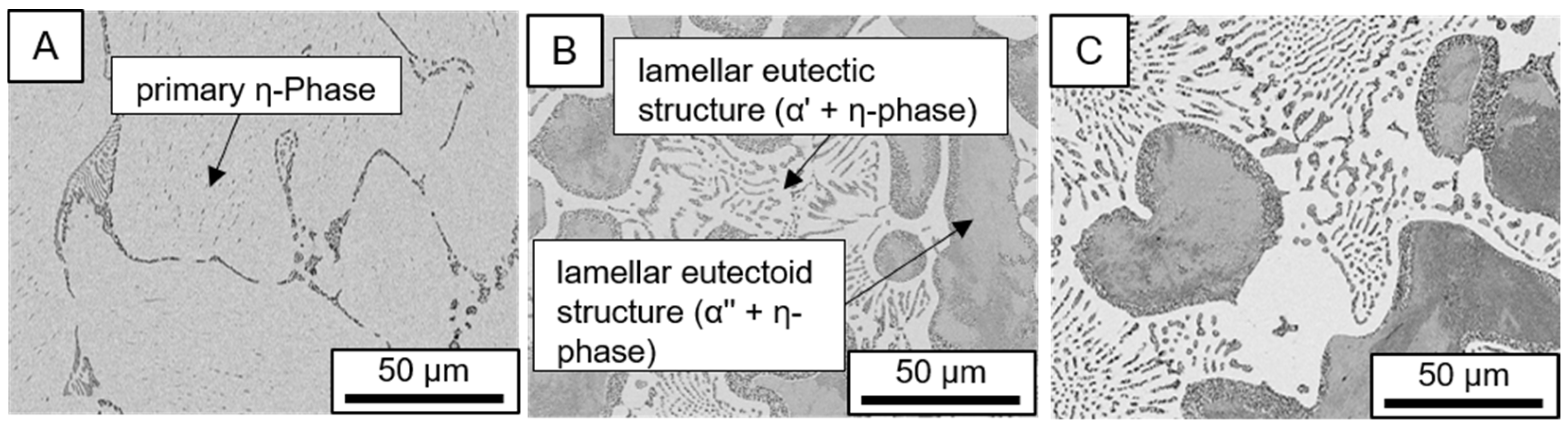
3.1. Microstructure
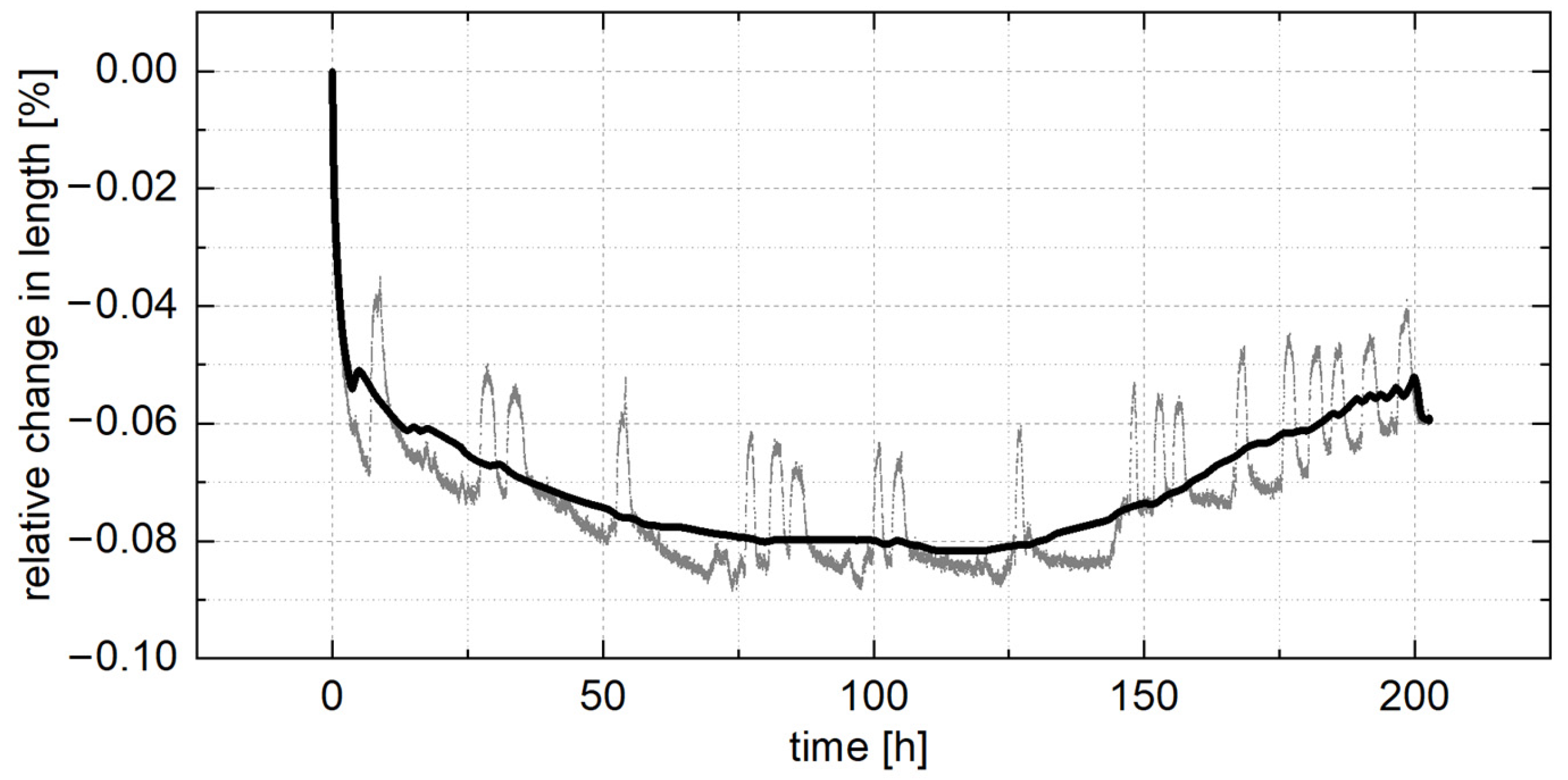
3.2. Dimensional Changes
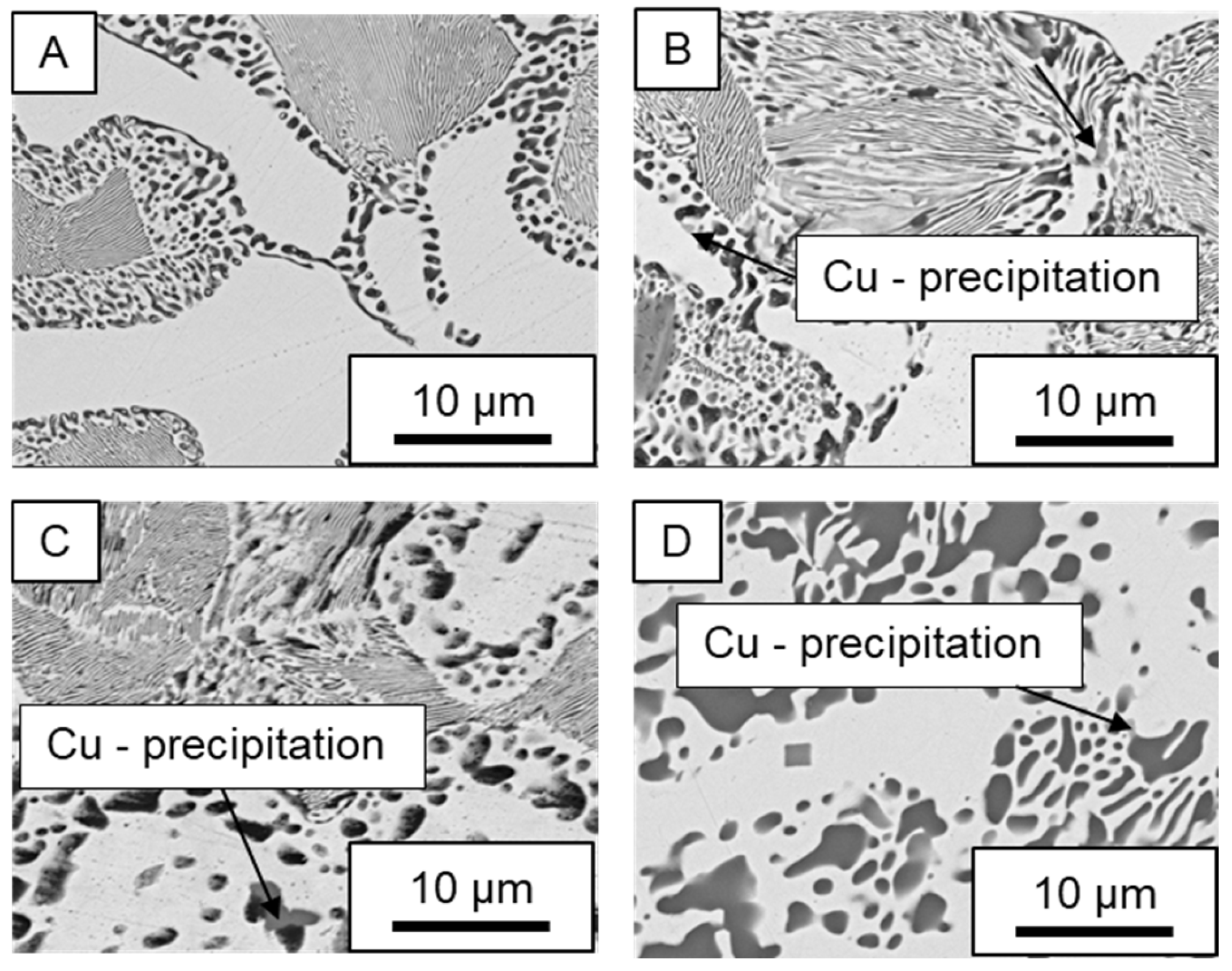
3.3. Microstructural Changes During Aging
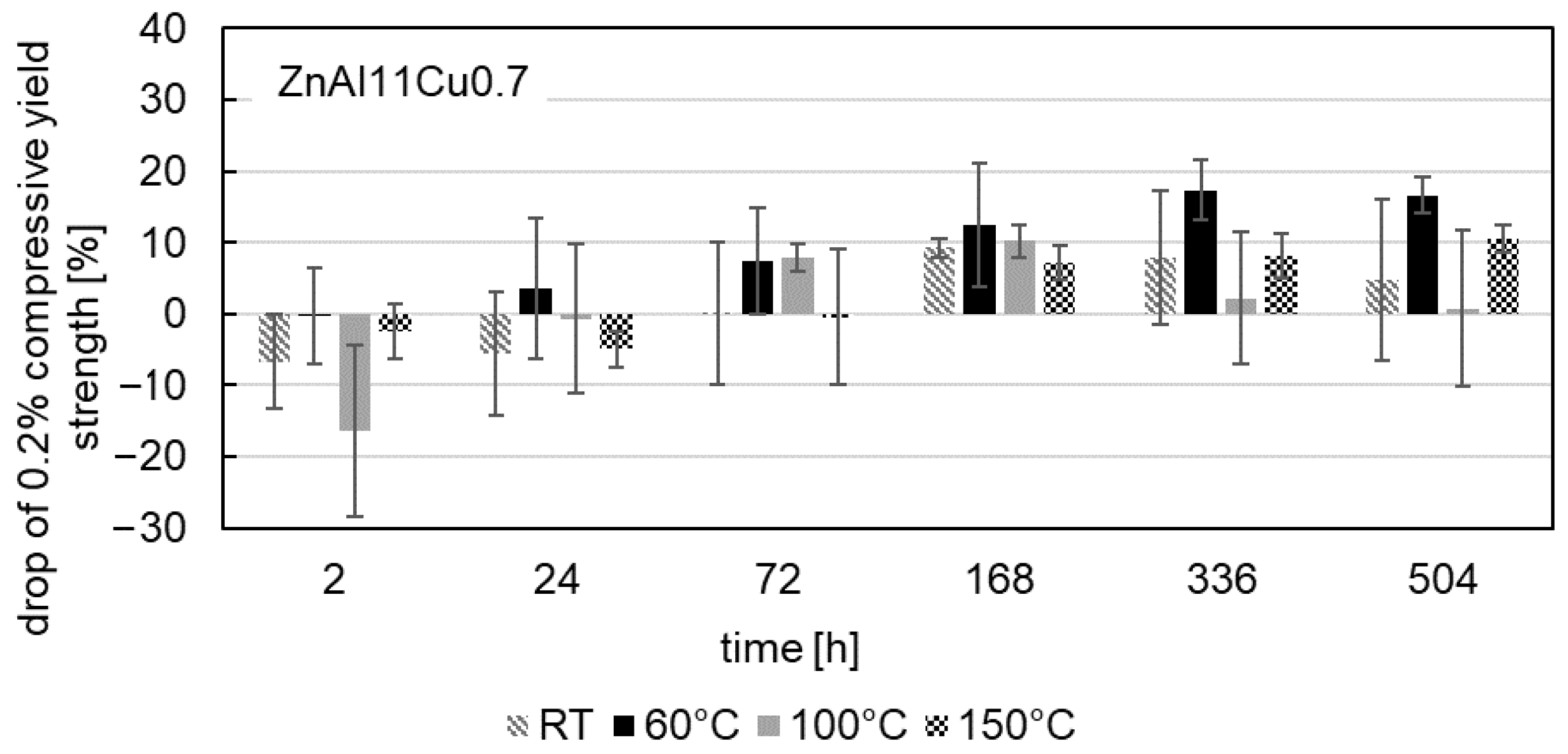
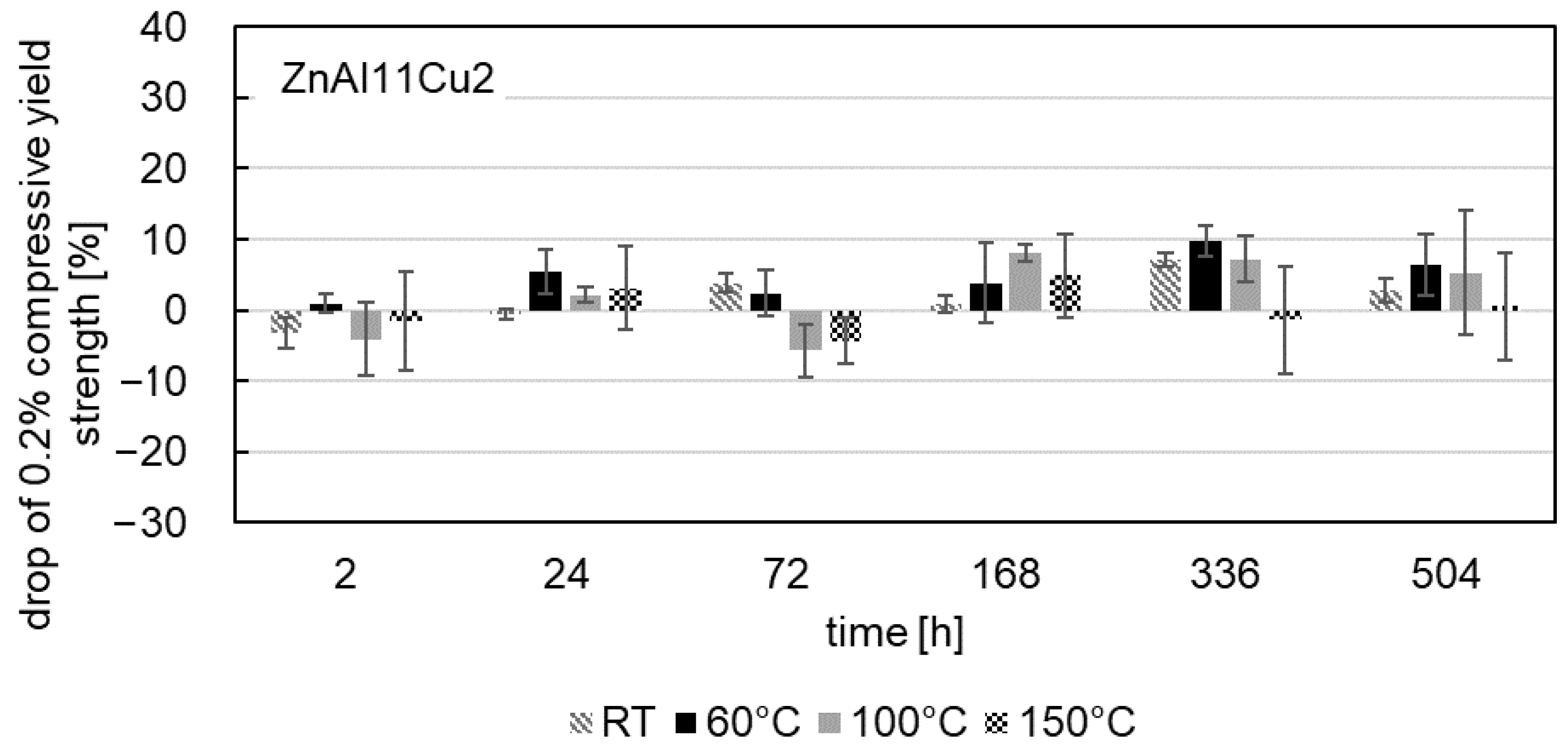
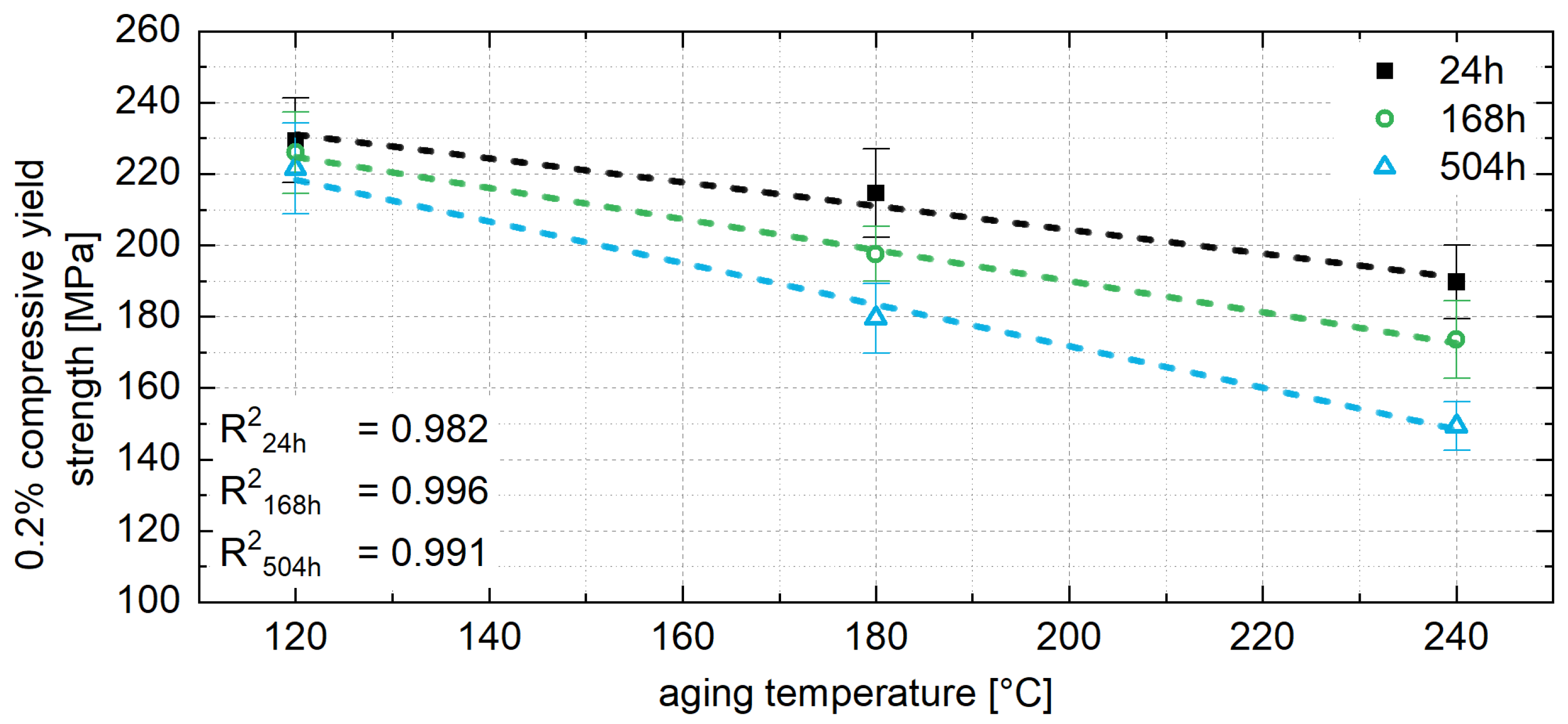
3.4. The 0.2% Compressive Yield Strength
4. Discussion
4.1. Correlation Between Microstructure and 0.2% Compressive Yield Strength During Aging
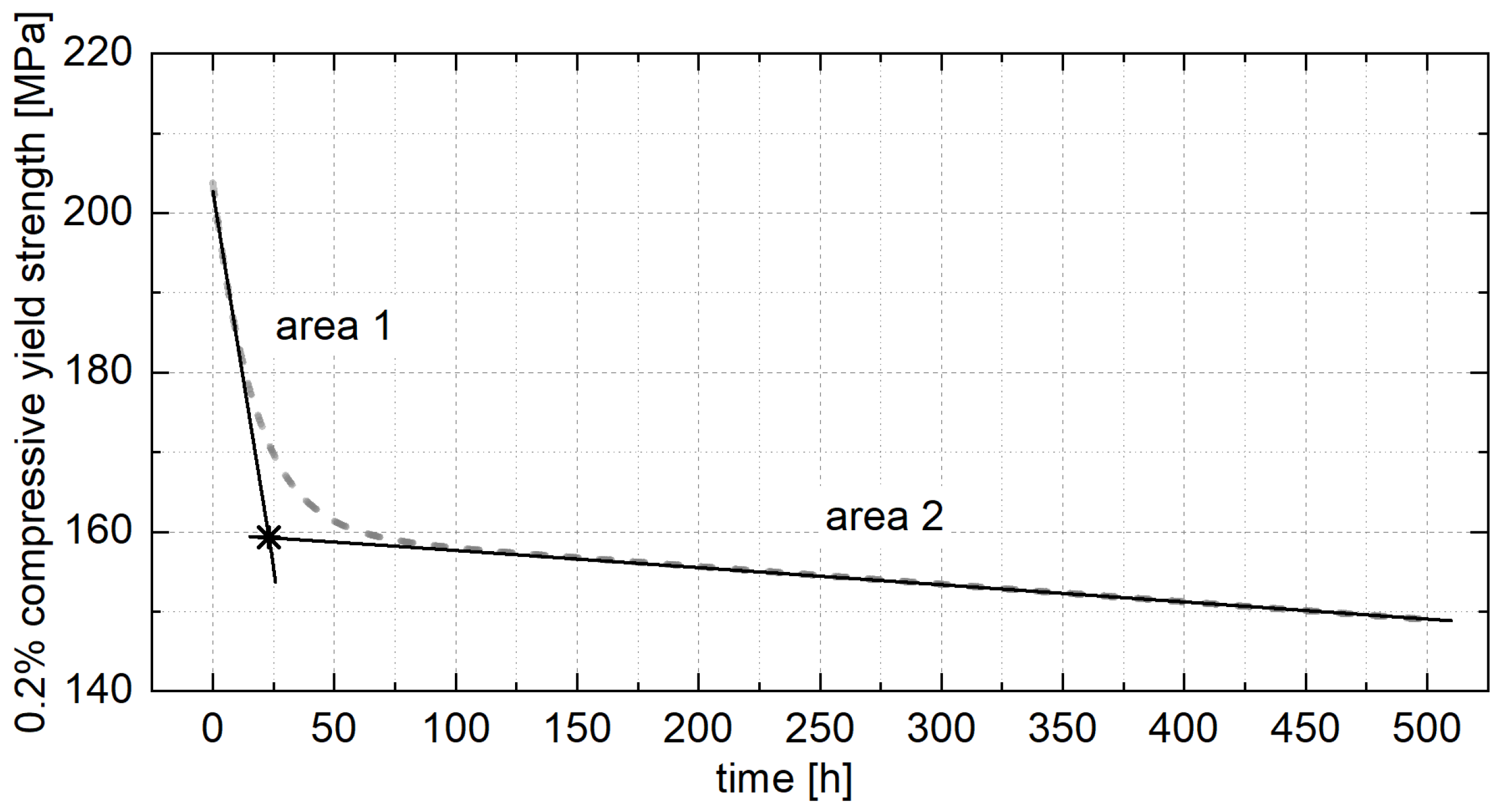
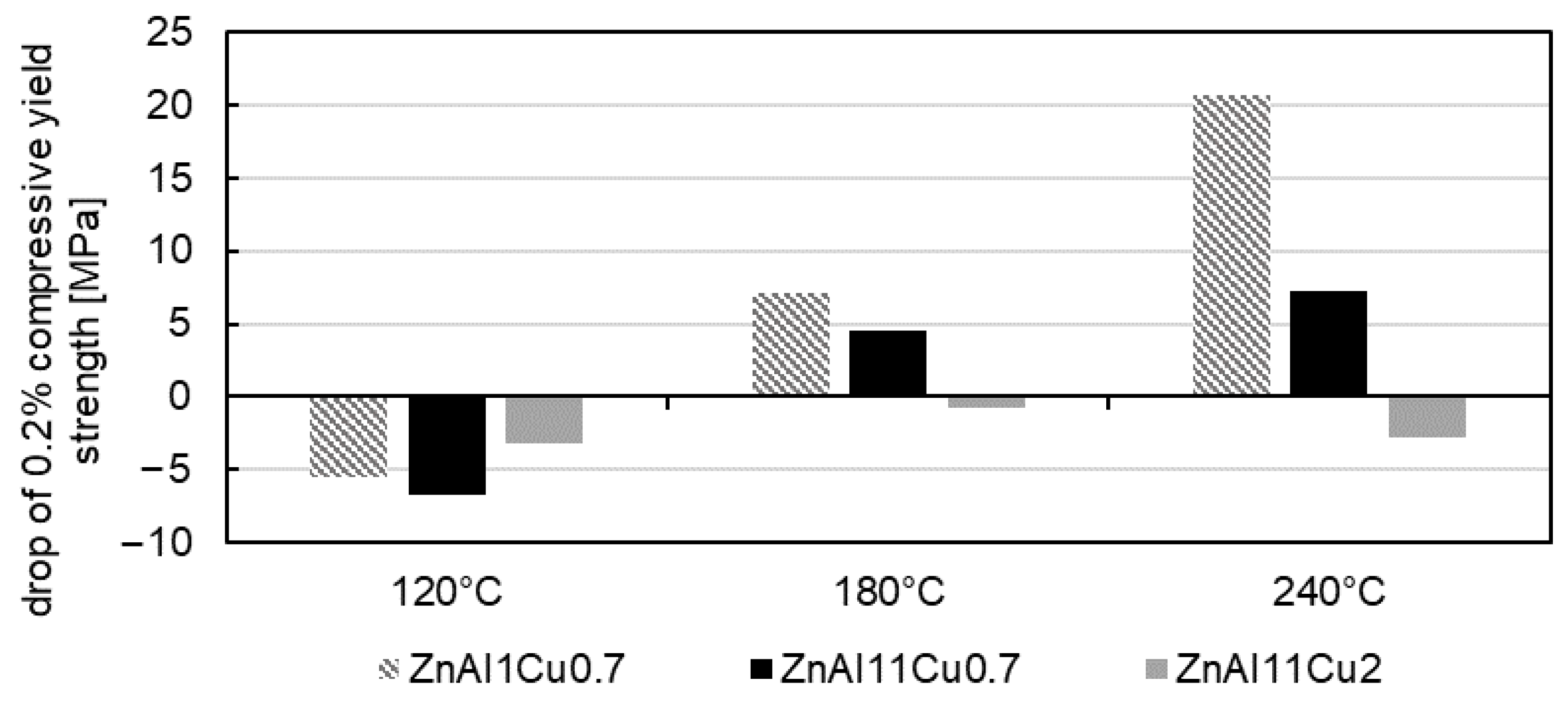
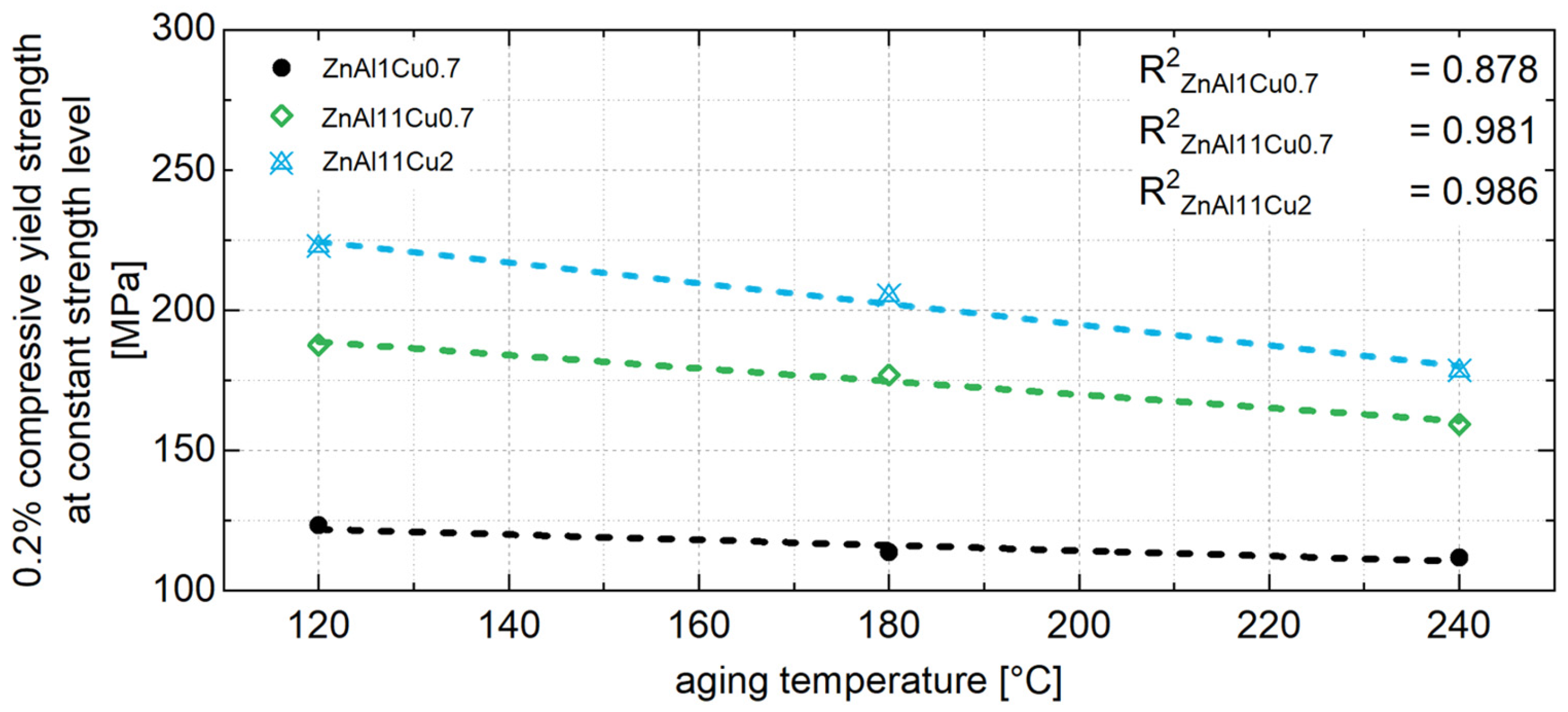
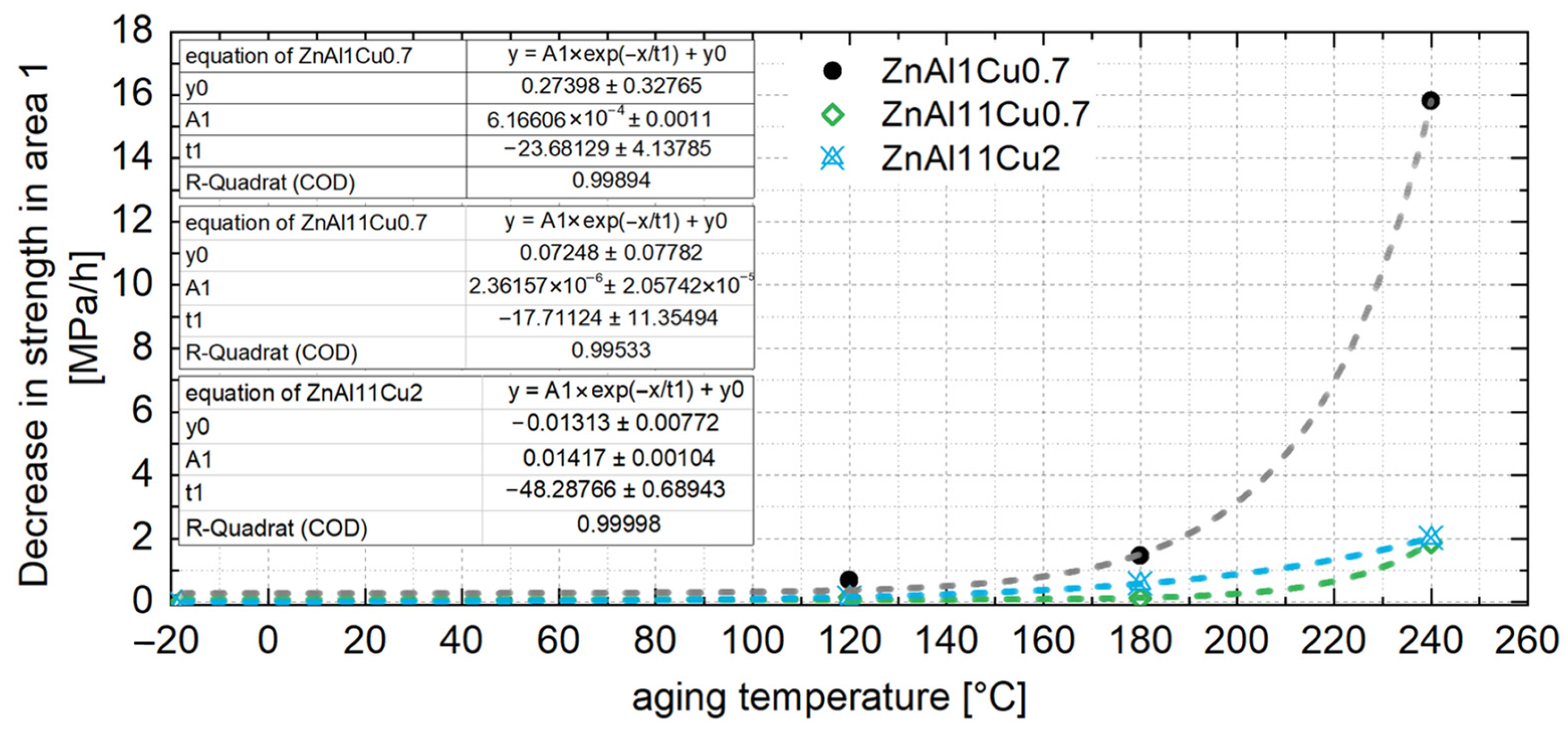
4.2. Constant Strength Level and Aging Time for Unknown Temperatures
5. Conclusions
- The largest dimensional changes occurred in the first three hours, resulting in shrinkage of approximately 0.05%, with a maximum shrinkage value of 0.08% after 100 h, followed by length increases. The dimensional changes during aging were related to changes in the lattice parameters due to aluminum precipitation and the four-phase reaction.
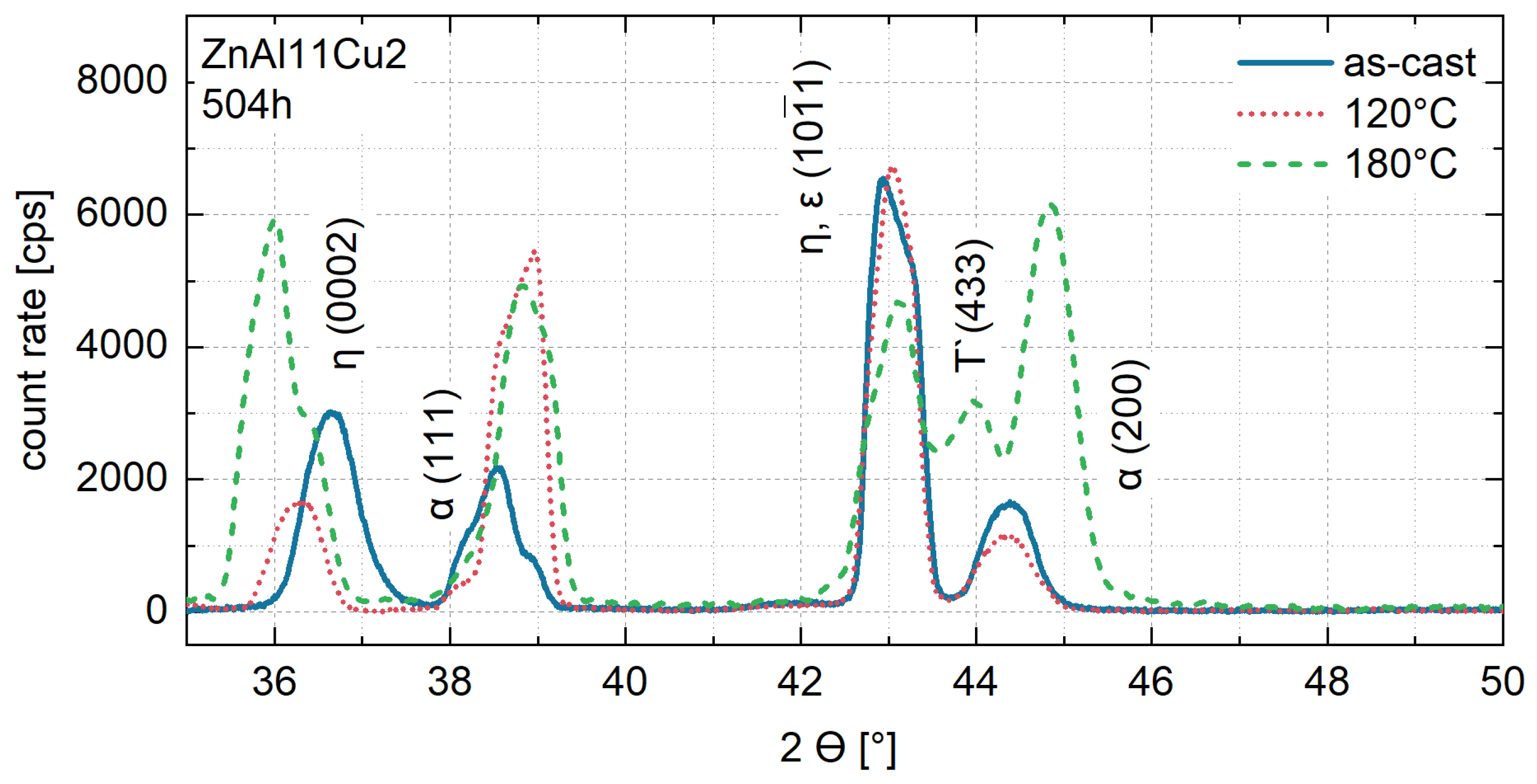
- Microstructural analysis with SEM showed no segregation at the aging temperature of 120 °C. At 240 °C, aluminum precipitations were detected after 168 h, and after 504 h, copper precipitations were observed for all three alloys. XRD measurements of ZnAl11Cu2 at 120 °C and 180 °C indicated phase transformations involving the η (0002) peak and the appearance of the τ′-phase.
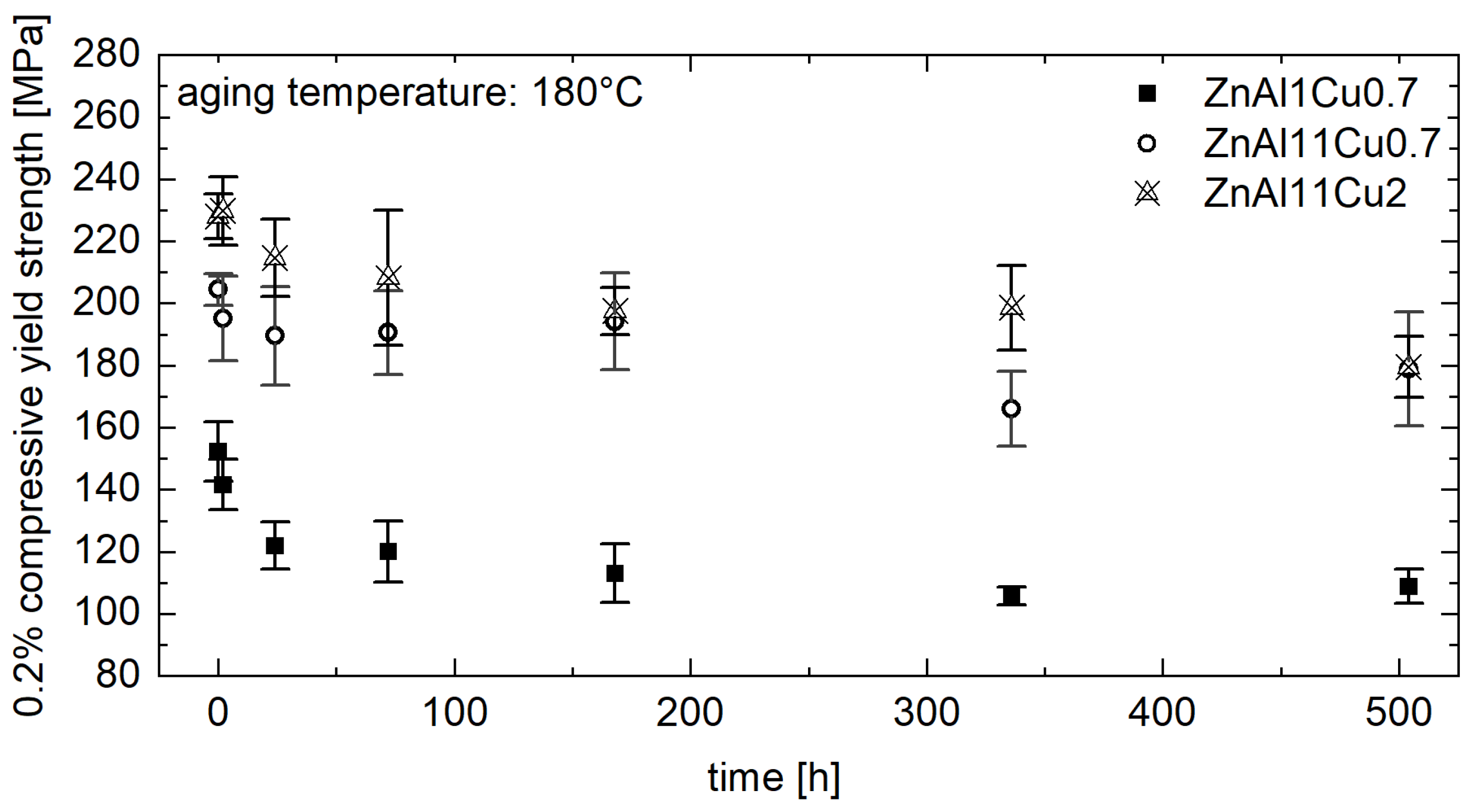
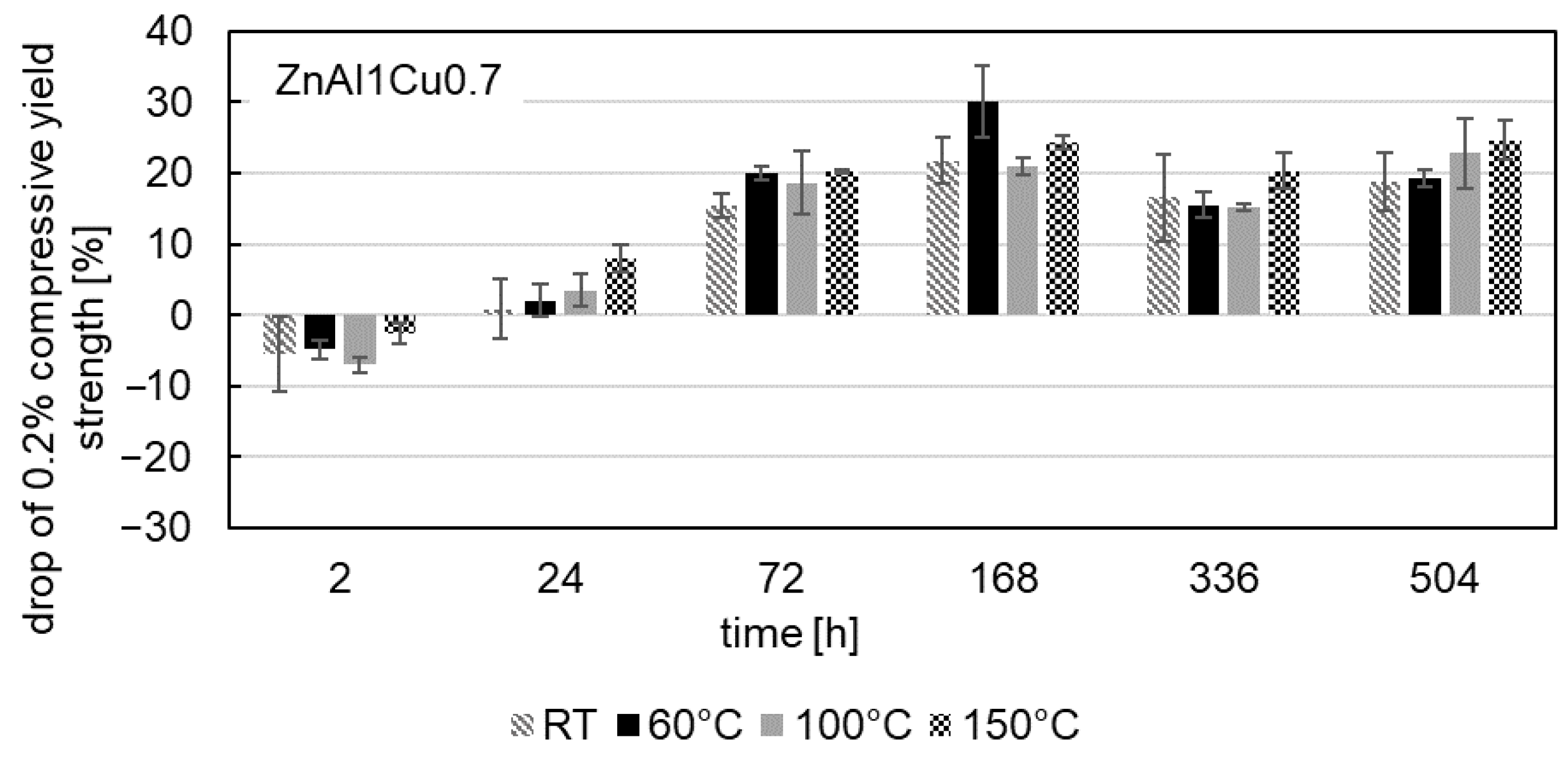
- Mechanical tests revealed a consistent decrease in the 0.2% compressive yield strength for all alloys tested over the aging time, which was most pronounced at the beginning of the aging process. With progressive aging, an approximately constant strength level was adjusted.
- In contrast to the decrease in strength, an increase was observed for ZnAl1Cu0.7 and ZnAl11Cu0.7 in the first 2 h. This increase can be attributed to nucleation followed by grain growth, which leads to the observed loss of strength. However, this increase was not observed in ZnAl11Cu2, which is due to a reduced decomposition rate of the α′-phase, resulting in a delay of the transformation process.
- A higher approximately constant strength level was achieved with higher aluminum and copper contents due to solid solution strengthening.
- Increased aging temperatures led to faster aging processes and shorter times to reach the constant stress level, which could be related to segregation but was not detected in the SEM in the present study.
- No clear correlation between microstructural changes and the decrease in 0.2% compressive yield strength could be determined.
- To ensure stable mechanical properties during operation, it is essential to age the material until the constant strength level is reached. A linear relationship between the 0.2% compressive yield strength and aging temperature was observed and used to interpolate the constant strength level for a specific operating temperature. The corresponding aging time was determined from the exponential relationship between the decrease in strength at the beginning of aging and the aging temperature.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Al | aluminum |
| Cu | copper |
| RT | room temperature |
| SEM | scanning electron microscopy |
| XRD | X-ray diffraction |
| Zn | zinc |
References
- Ünlü, B.S. Investigation of tribological and mechanical properties of metal bearings. Bull. Mater. Sci. 2009, 32, 451–457. [Google Scholar] [CrossRef]
- Choudhury, P.; Das, K.; Das, S. Evolution of as-cast and heat-treated microstructure of a commercial bearing alloy. Mater. Sci. Eng. A 2005, 398, 332–343. [Google Scholar] [CrossRef]
- Yan, S.; Xie, J.; Liu, Z.; Wang, W.; Wang, A.; Li, J. Influence of Different Al Contents on Microstructure, Tensile and Wear Properties of Zn-based Alloy. J. Mater. Sci. Technol. 2010, 26, 648–652. [Google Scholar] [CrossRef]
- Kallien, L.H.; Leis, W. Ageing of Zink Alloys. Int. Foundry Res. 2011, 2011, 64. [Google Scholar]
- Longauerová, M.; Glogovský, M.; Vojtko, M. Effect of Al Content and Heat Treatment on Microstructure of ZnAlCu Alloys. Key Eng. Mater. 2015, 647, 85–92. [Google Scholar] [CrossRef]
- Lin, G.; Zhang, R.; Wang, L.; Lei, Y.; He, J. Effects of stabilizing heat treatment on microstructures and creep behavior of Zn–10Al–2Cu–0.02Ti alloy. Trans. Nonferrous Met. Soc. China 2013, 23, 86–91. [Google Scholar] [CrossRef]
- Roberti, R.; Pola, A.; Gilles, M.; Rollez, D. Primary and steady state creep deformation in Zamak5 die-casting alloy at 80 °C. Mater. Charact. 2008, 59, 1747–1752. [Google Scholar] [CrossRef]
- Pola, A.; Tocci, M.; Goodwin, F.E. Review of Microstructures and Properties of Zinc Alloys. Metals 2020, 10, 253. [Google Scholar] [CrossRef]
- Rollez, D.; Pola, A.; Montesano, L.; Brisotto, M.; de Felicis, D.; Gelfi, M. Effect of aging on microstructure and mechanical properties of ZnAl15Cu1 alloy for wrought applications. Int. J. Mater. Res. 2017, 108, 447–454. [Google Scholar] [CrossRef]
- Wu, Z.; Sandlöbes, S.; Wang, Y.; Gibson, J.S.-L.; Korte-Kerzel, S. Creep behaviour of eutectic Zn-Al-Cu-Mg alloys. Mater. Sci. Eng. A 2018, 724, 80–94. [Google Scholar] [CrossRef]
- Zhu, Y.H. Creep induced phase transformation in cast Zn-Al alloy. J. Mater. Sci. Lett. 1996, 15, 1358–1360. [Google Scholar] [CrossRef]
- Goodwin, F.E.; Kallien, L.H.; Leis, W. New Mechanical Properties Data for Zinc Casting Alloys. In Proceedings of the North American Die Casting Association (Hrsg.), Die Casting Congress & Tabletop, Milwaukee, WI, USA, 22–24 September 2014. [Google Scholar]
- Goodwin, F.; Leis, W.; Kallien, L.H. Ageing Properties of Zinc Alloys. In Proceedings of the North American Die Casting Association (Hrsg.), Die Casting Congress & Tabletop, Columbus, OH, USA, 19–21 September 2011. [Google Scholar]
- Goodwin, F.E.; Kallien, L.H.; Leis, W. The High Fluidity (HF) Zinc Alloy: Process-Property and Ageing Characteristics. In Proceedings of the North American Die Casting Association (Hrsg.), Die Casting Congress & Exposition, Indianapolis, IN, USA, 5–7 October 2015. [Google Scholar]
- Goodwin, F.E.; Kallien, L.H.; Leis, W. Thermally-Activated Processes in Zinc Die Castings and Their Effects on Properties. In Proceedings of the North American Die Casting Association (Hrsg.), Die Casting Congress & Exposition, Indianapolis, IN, USA, 15–17 October 2018. [Google Scholar]
- Goodwin, F.E.; Kallien, L. Improving the Relationship between Processing and Properties of Zinc Die Casting: Developments in Creep and Ageing Correlations. SAE Int. J. Mater. Manuf. 2011, 4, 1188–1197. [Google Scholar] [CrossRef]
- APola, M.; Gelfi, G.M.; La Vecchia, L. Montesano: On the aging of a hyper-eutectic Zn-Al alloy. La Metall. Ital. 2015, 4, 37–41. [Google Scholar]
- Krajewski, W.K.; Greer, A.L.; Orava, J.; Krajewski, P.K.; Tyrala, E. Dimensional stability of the high-aluminium zinc alloy modified with Ti addition. Int. Foundry Res. 2013, 65, 26–29. [Google Scholar]
- Çamurlu, H.E.; Aktuna, I. Effect of aging on Zamak 3. Mach. Technol. Mater. 2019, 13, 367–369. [Google Scholar]
- Montesano, L.; Pola, A.; Gelfi, M.; La Vecchia, G.M. Wear Behavior of Zn-15Al-1Cu-Mg Alloy after Aging. Procedia Eng. 2015, 109, 228–233. [Google Scholar] [CrossRef]
- Savaşkan, T.; Hekimoğlu, A.P. Microstructure and mechanical properties of Zn–15Al-based ternary and quaternary alloys. Mater. Sci. Eng. A 2014, 603, 52–57. [Google Scholar] [CrossRef]
- Hekimoglu, A.P.; Savaskan, T. Structure and mechanical properties of Zn-(5-25) Al alloys. Int. J. Mater. Res. 2014, 105, 1084–1089. [Google Scholar] [CrossRef]
- Agapie, M.; Peter, I.; Varga, B. Structure of cooled Zn-Al eutectoid based alloys in biphasic domain. J. Optoelectron. Adv. Mater. 2015, 17, 1842–1848. [Google Scholar]
- Wu, Z. Mechanical Behaviour and Deformation Mechanisms of Zn-Al-Cu-Mg Alloys. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2018. [Google Scholar]
- Wu, Z.; Sandlöbes, S.; Wu, L.; Hu, W.; Gottstein, G.; Korte-Kerzel, S. Mechanical behaviour of Zn–Al–Cu–Mg alloys: Deformation mechanisms of as-cast microstructures. Mater. Sci. Eng. A 2016, 651, 675–687. [Google Scholar] [CrossRef]
- Kiefel, A. Untereutektoide Zink-Aluminium-Kupfer-Legierungen für Gleitlageranwendungen. Ph.D. Thesis, RWTH, Aachen, Germany, 2025. [Google Scholar]
- Burkhardt, A. Technologie der Zinklegierungen; Springer: Berlin, Germany, 1937; ISBN 978-3-662-30701-4. [Google Scholar]
- Apelian, D.; Paliwal, M.; Herrschaft, D.C. Casting with Zinc Alloys. JOM 1981, 33, 12–20. [Google Scholar] [CrossRef]
- Savaşkan, T.; Hekimoğlu, A.P. Effect of quench–ageing treatment on the microstructure and properties of Zn-15Al-3Cu alloy. Int. J. Mater. Res. 2015, 106, 481–487. [Google Scholar] [CrossRef]
- Villegas-Cardenas, J.D.; Saucedo-Muñoz, M.L.; Lopez-Hirata, V.M.; Dorantes-Rosales, H.J.; Gonzalez-Velazquez, J.L. Effect of phase transformations on hardness in Zn-Al-Cu Alloys. Mater. Res. 2014, 17, 1137–1144. [Google Scholar] [CrossRef]
- Seenappa, K.V. Sharma: Influence of Heat Treatment on Microstructure and Mechanical Properties of Some Zinc Based Alloys. Int. J. Mater. Sci. 2011, 6, 57–64. [Google Scholar]
- Martinez Page, M.A.; Weidenfeller, B.; Hartmann, S. Influence of temperature and aging on the thermal diffusivity, thermal conductivity and heat capacity of a zinc die casting alloy. J. Alloys Compd. 2019, 786, 1060–1067. [Google Scholar] [CrossRef]
- Babić, M.; Ninković, R.; Mitrović, S.; Bobić, I. Influence of Heat Treatment on Tribological Behavior of Zn-Al Alloys. Tribol. Ind. 2007, 29. [Google Scholar]
- Babić, M.; Vencl, A.; Mitrović, S.; Bobić, I. Influence of T4 Heat Treatment on Tribological Behavior of Za27 Alloy Under Lubricated Sliding Condition. Tribol. Lett. 2009, 36, 125–134. [Google Scholar] [CrossRef]
- Savaşkan, T.; Murphy, S. Decomposition of Zn–Al alloys on quench–aging. Mater. Sci. Technol. 1990, 6, 695–704. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Man, H.C.; Dorantes-Rosales, H.J.; Lee, W.B. Ageing characteristics of furnace cooled eutectoid Zn-Al based alloy. J. Mater. Sci. 2003, 38, 2925–2934. [Google Scholar] [CrossRef]
- Savaşkan, T.; Bican, O.; Alemdağ, Y. Developing aluminium–zinc-based a new alloy for tribological applications. J. Mater. Sci. 2009, 44, 1969–1976. [Google Scholar] [CrossRef]
- Kovacheva, R.; Dobrev, R.; Zadgorski, S.; Lilova, A. Phase transformations in Zn-Al-Cu alloys. Mater. Charact. 1993, 31, 217–224. [Google Scholar] [CrossRef]
- Da Costa, E.M.; Da Costa, C.E.; Vecchia, F.D.; Rick, C.; Scherer, M.; dos Santos, C.A.; Dedavid, B.A. Study of the influence of copper and magnesium additions on the microstructure formation of Zn–Al hypoeutectic alloys. J. Alloys Compd. 2009, 488, 89–99. [Google Scholar] [CrossRef]
- Savaşkan, T.; Pürçek, G.; Hekimoğlu, A.P. Effect of Copper Content on the Mechanical and Tribological Properties of ZnAl27-Based Alloys. Tribol. Lett. 2003, 15, 257–263. [Google Scholar] [CrossRef]
- Dorantes-Rosales, H.J.; López-Hirata, V.M.; Hernández-Santiago, F.; Saucedo-Muñoz, M.L.; Paniagua-Mercado, A.M. Effect of Ag Addition to Zn22 mass%Al–2 mass%Cu Alloy on the Four-Phase Reaction η + ε → α + τ′. Mater. Trans. 2018, 59, 717–723. [Google Scholar] [CrossRef]
- Murphy, S. The Structure of the T′ Phase in the System AI-Cu-Zn. Met. Sci. 1975, 9, 163–168. [Google Scholar] [CrossRef]
- Zobac, O.; Kroupa, A.; Richter, K.W. Experimental study of the Al-Cu-Zn ternary phase diagram. J. Mater. Sci. 2020, 55, 10796–10810. [Google Scholar] [CrossRef]
- Krajewski, W.K.; Greer, A.L.; Piwowarski, G.; Krajewski, P.K. Property enhancement by grain refinement of zinc-aluminium foundry alloys. IOP Conf. Ser. Mater. Sci. Eng. 2016, 117, 12004. [Google Scholar] [CrossRef]
- Löhberg, K. Die Volumenänderungen der Zink-Aluminium-Kupfer-Legierungen bei dem Übergangsgleichgewicht ε + a 1 ⇆ n + T‘. Int. J. Mater. Res. 1983, 74, 456–457. [Google Scholar] [CrossRef]
- Zhu, Y.H.; To, S.; Liu, X.M.; Lee, W.B. Microstructural changes inside the lamellar structures of alloy ZA27. Mater. Charact. 2006, 57, 326–332. [Google Scholar] [CrossRef]
- Zhu, Y.H. Phase transformations of eutectoid Zn-Al alloys. J. Mater. Sci. 2001, 36, 3973–3980. [Google Scholar] [CrossRef]
- Gervais, E.; Barnhurst, R.J.; Loong, C.A. An Analysis of Selected Properties of ZA Alloys. JOM 1985, 37, 43–47. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Jiang, H.; Lu, X. Effects of heat treatment on microstructure and mechanical properties of ZA27 alloy. Trans. Nonferrous Met. Soc. China 2013, 23, 642–649. [Google Scholar] [CrossRef]
- Koring, R. Gleitlagertechnik im Wandel—Praxis Innovativer Hydrodynamischer Gleitlager; Expert Verlag: Renningen, Germany, 2010; ISBN 978-3-8169-3151-5. [Google Scholar]
- Zhu, Y.H. Ageing characteristics of cast Zn-Al based alloy (ZnAl7Cu3). J. Mater. Sci. 2003, 38, 1945–1952. [Google Scholar] [CrossRef]
- Dorantes-Rosales, H.J.; López-Hirata, V.M.; Zhu, Y.H. Decomposition process in a Zn-22wt.%Al-2wt.%Cu alloy. Mater. Sci. Eng. A 1999, 271, 366–370. [Google Scholar] [CrossRef]
- Löhberg, K. Alterungsvorgänge in Zinklegierungen. In Zink-Taschenbuch; Zinkberatungsstelle, G.M.B.H., Ed.; Verlag Wilhelm Knapp: Halle, Germany, 1942; pp. 55–60. [Google Scholar]
- Durman, M.; Murphy, S. Precipitation of metastable ϵ-phase in a hypereutectic zinc-aluminium alloy containing copper. Acta Metall. Mater. 1991, 39, 2235–2242. [Google Scholar] [CrossRef]
- Mykura, N.; Zhu, Y.H.; Murphy, S. Solid-State Reactions in Zn-Al Based Alloys. Can. Metall. Q. 1986, 25, 151–159. [Google Scholar] [CrossRef]
- LeHuy, H.; L’esprance, G. Ageing characteristics of dendritic and non-dendritic (stir-cast) Zn-Al alloy (ZA-27). J. Mater. Sci. 1991, 26, 559–568. [Google Scholar] [CrossRef]
- Prasad, B.K. Influence of heat treatment parameters on the lubricated sliding wear behaviour of a zinc-based alloy. Wear 2004, 257, 1137–1144. [Google Scholar] [CrossRef]
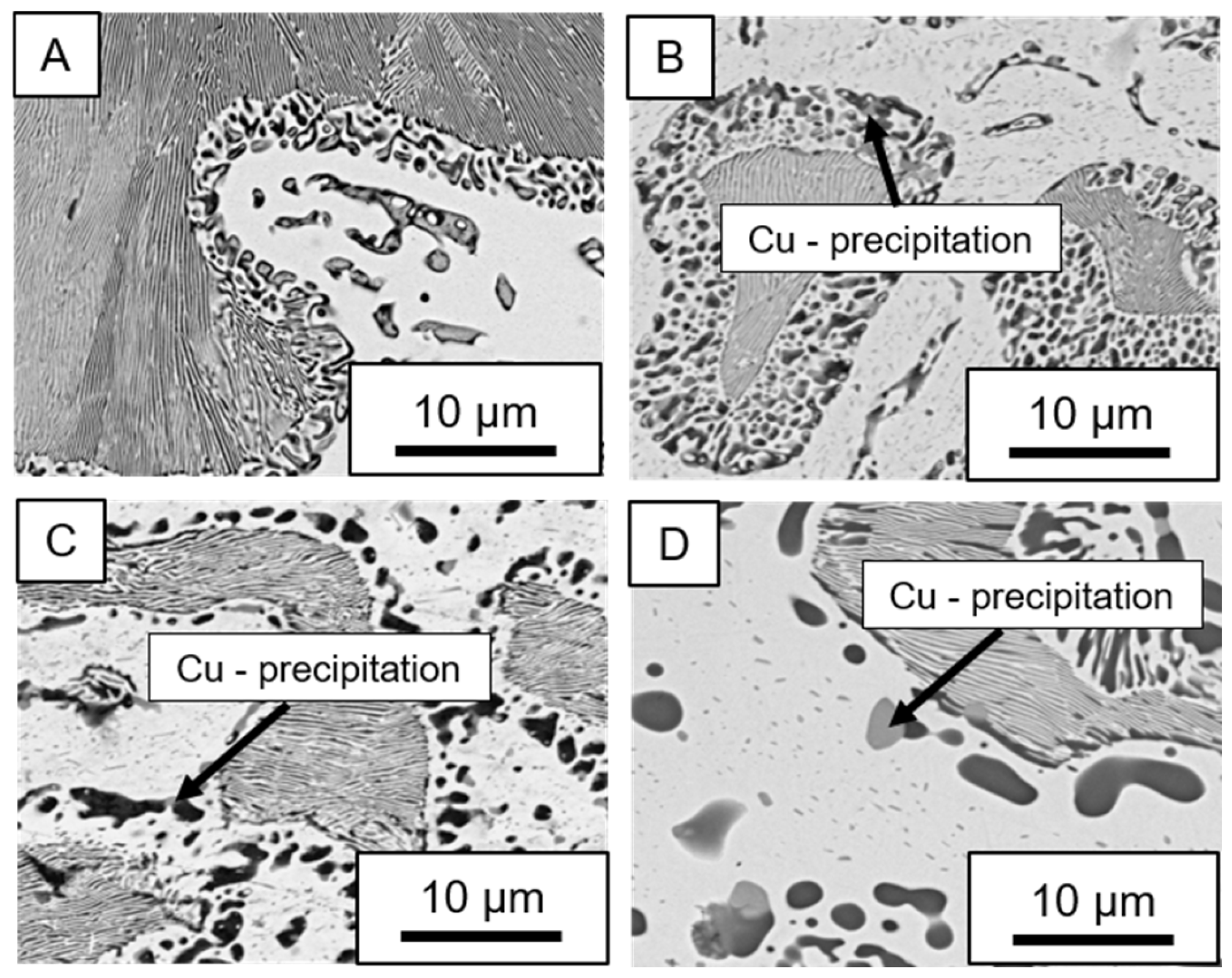

| 2 h | 24 h | 72 h | 168 h | 336 h | 504 h | 840 h | |
|---|---|---|---|---|---|---|---|
| 120 °C | x | x | x | x | x | x | x |
| 180 °C | x | x | x | x | x | x | |
| 240 °C | x | x | x | x | x | x |
| 24 h | 168 h | 504 h | |
|---|---|---|---|
| 120 °C |  |  |  |
| 180 °C |  |  |  |
| 240 °C |  |  |  |
| 24 h | 168 h | 504 h | |
|---|---|---|---|
| 120 °C |  | 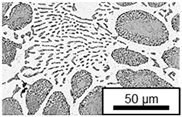 | 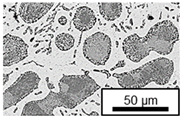 |
| 180 °C | 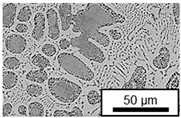 | 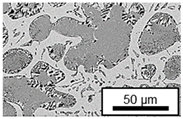 |  |
| 240 °C | 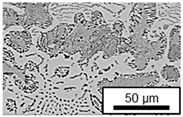 |  |  |
| 24 h | 168 h | 504 h | |
|---|---|---|---|
| 120 °C |  |  |  |
| 180 °C |  | 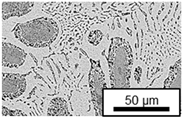 |  |
| 240 °C | 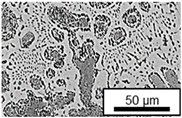 |  |  |
| Alloy | Aging Temperature [°C] | Drop in Strength in Area 1 [%] | Decrease in Strength in Area 1 [MPa/h] | Decrease in Strength in Area 2 [MPa/h] | Aging Time Until Constant Stress Level is Reached [h] | 0.2% Compressive Yield Strength at Constant Stress Level [MPa] |
|---|---|---|---|---|---|---|
| ZnAl1Cu0.7 | 120 | 19 | 0.42 | 9.23 × 10−3 | 87 | 123 |
| 180 | 25 | 1.08 | 1.87 × 10−2 | 32 | 114 | |
| 240 | 26 | 15.81 | 8.16 × 10−3 | 3 | 112 | |
| ZnAl11Cu0.7 | 120 | 9 | 0.12 | 3.80 × 10−2 | 168 | 186 |
| 180 | 13 | 0.18 | 1.63 × 10−2 | 139 | 178 | |
| 240 | 22 | 1.33 | 2.15 × 10−2 | 33 | 159 | |
| ZnAl11Cu2 | 120 | 3 | 0.15 | 2.28 × 10−2 | 58 | 222 |
| 180 | 10 | 0.58 | 5.36 × 10−2 | 39 | 206 | |
| 240 | 21 | 2.03 | 6.17 × 10−2 | 25 | 179 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiefel, A.; Bezold, A.; Broeckmann, C. Influence of Artificial Aging of ZnAlCu Alloys on Microstructure and Compressive Yield Strength. Materials 2025, 18, 4823. https://doi.org/10.3390/ma18214823
Kiefel A, Bezold A, Broeckmann C. Influence of Artificial Aging of ZnAlCu Alloys on Microstructure and Compressive Yield Strength. Materials. 2025; 18(21):4823. https://doi.org/10.3390/ma18214823
Chicago/Turabian StyleKiefel, Angelika, Alexander Bezold, and Christoph Broeckmann. 2025. "Influence of Artificial Aging of ZnAlCu Alloys on Microstructure and Compressive Yield Strength" Materials 18, no. 21: 4823. https://doi.org/10.3390/ma18214823
APA StyleKiefel, A., Bezold, A., & Broeckmann, C. (2025). Influence of Artificial Aging of ZnAlCu Alloys on Microstructure and Compressive Yield Strength. Materials, 18(21), 4823. https://doi.org/10.3390/ma18214823






