Synthesis of DHTA-Modified Poly(Epoxysuccinic Acid) and Scale Inhibition of Fluoride Scale
Abstract
1. Introduction
2. Materials and Methods
2.1. Main Reagents and Instruments
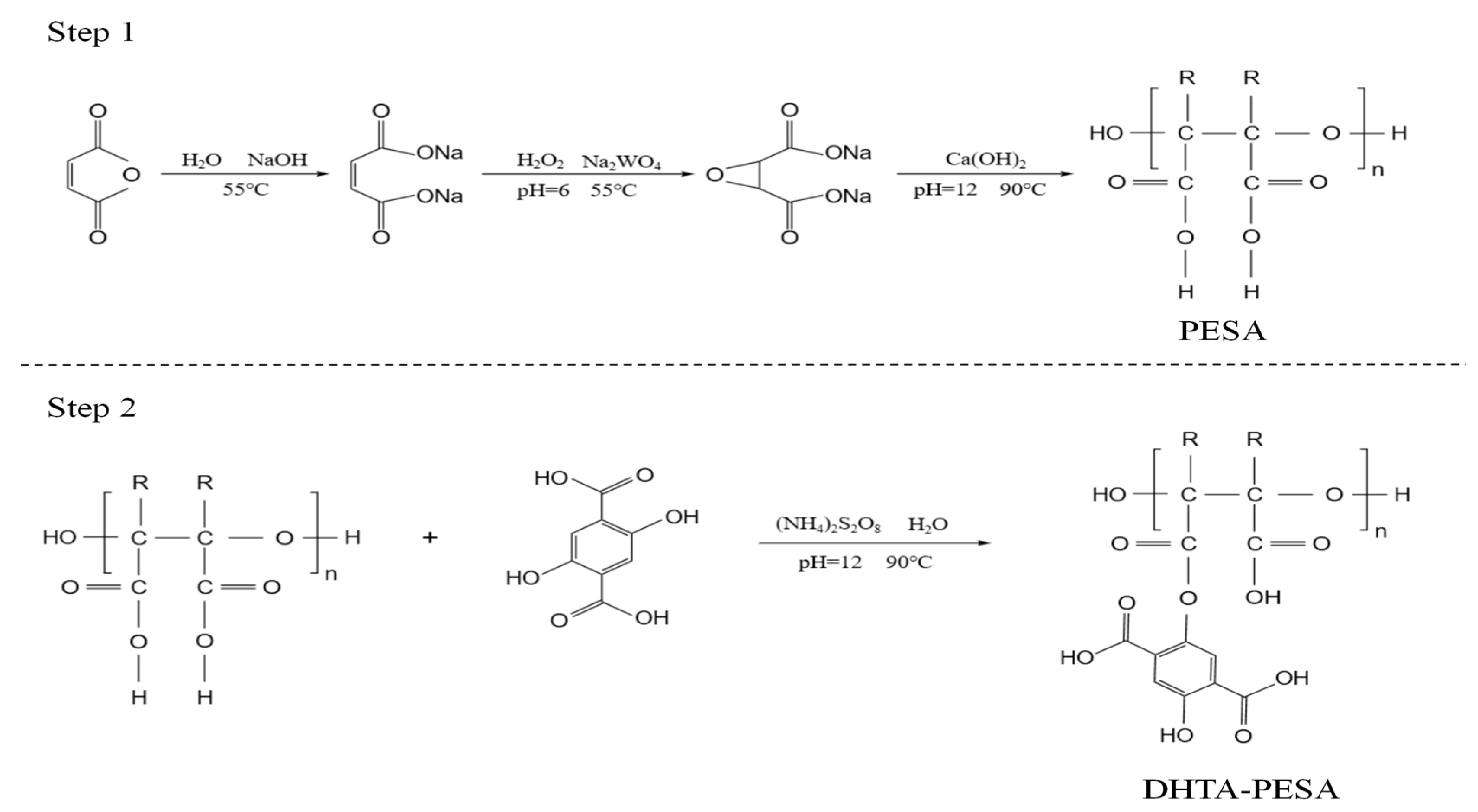
2.2. Synthesis of Scale Inhibitor
2.3. Characterization of Scale Inhibitors and Scale Samples
2.4. Viscosity-Average Molecular Weight Measurement
2.5. Scale Inhibition Performance Evaluation
2.5.1. Static Scale Inhibition Experiment
2.5.2. Conductivity Measurement
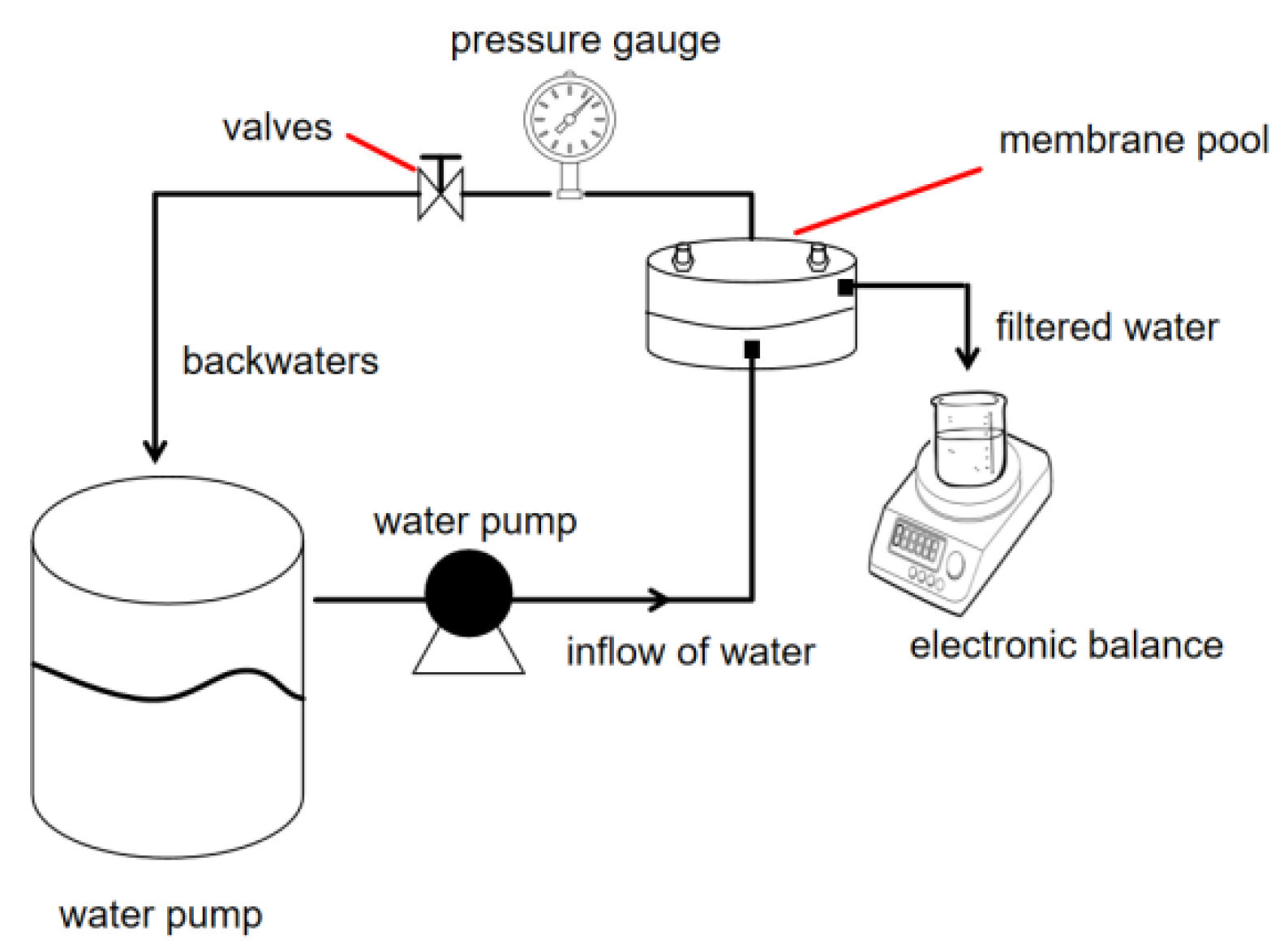
2.5.3. Dynamic Experiment
3. Results and Discussion
3.1. Optimization of Synthesis Conditions
3.2. Performance Characterization of Synthesized Products
3.2.1. Infrared Spectroscopy
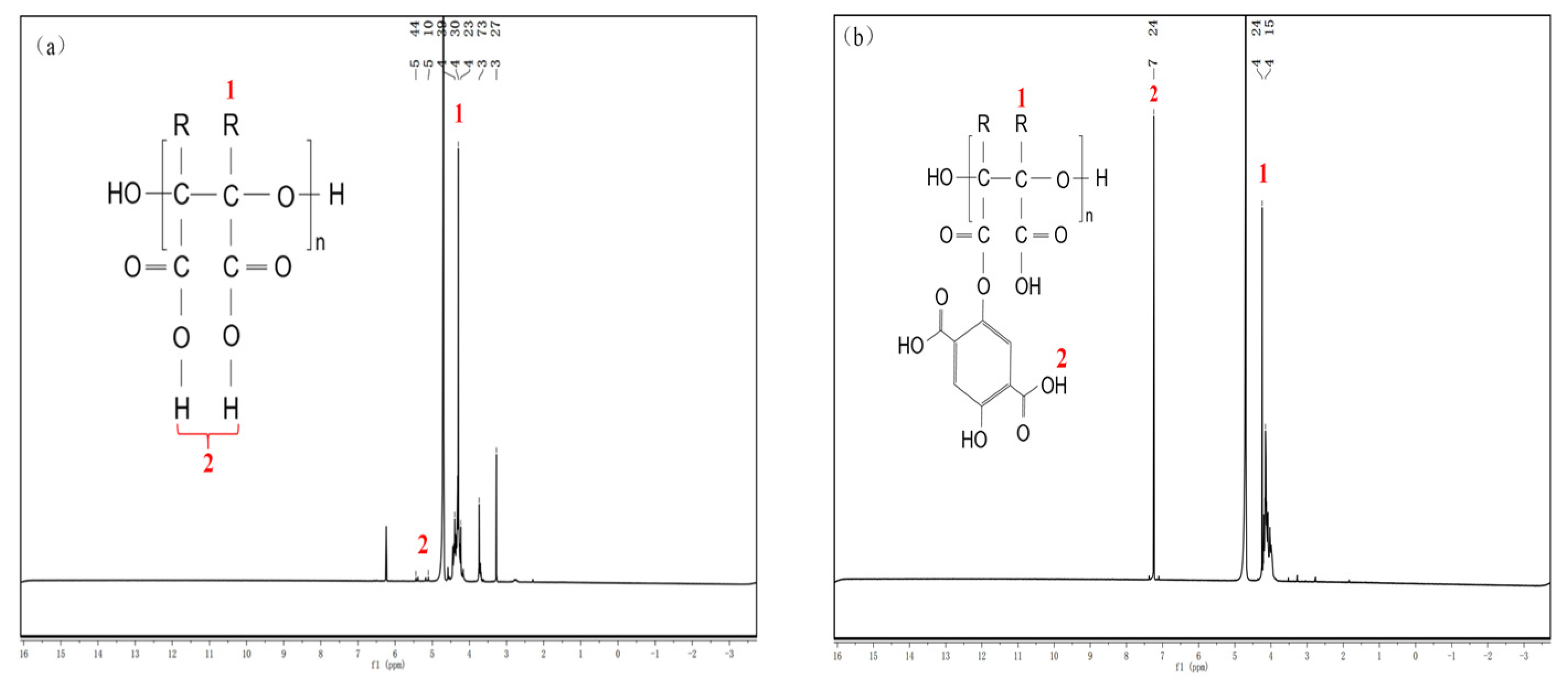
3.2.2. Nuclear Magnetic Hydrogen Spectroscopy
3.2.3. Molecular Weight Determination
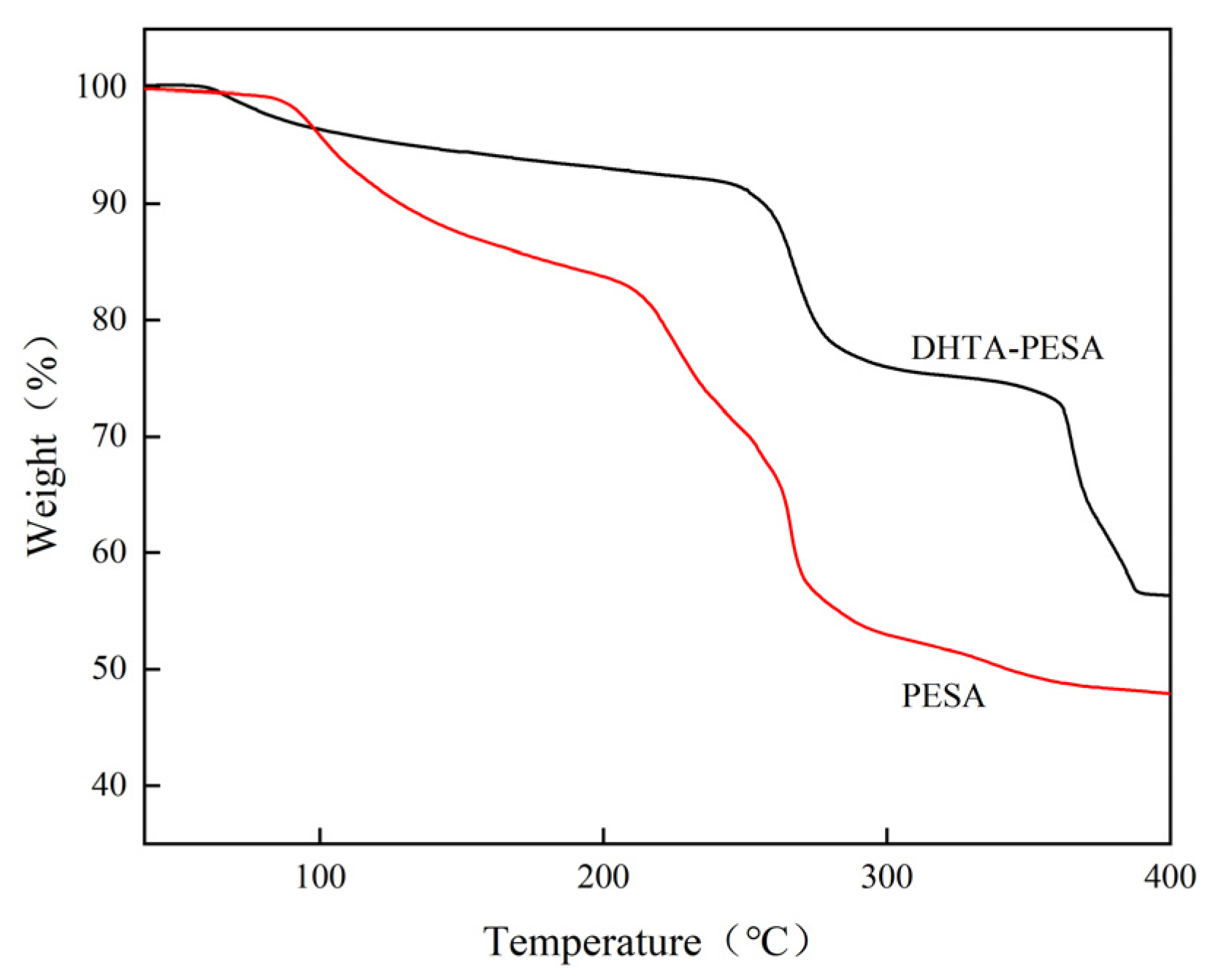
3.2.4. Thermal Stability Analysis
3.3. Scale Inhibition Performance
3.3.1. Static Scale Inhibition
- (1)
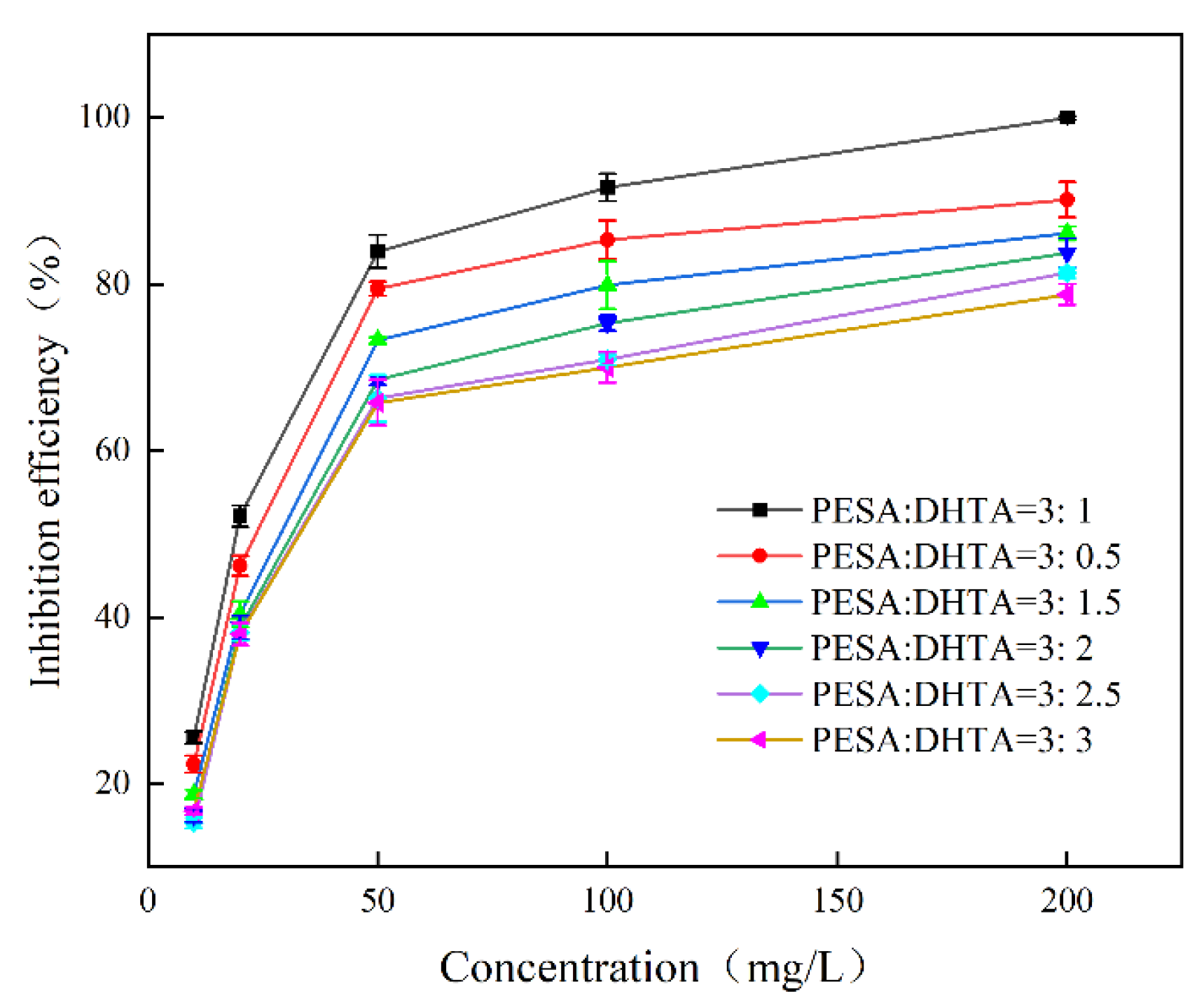
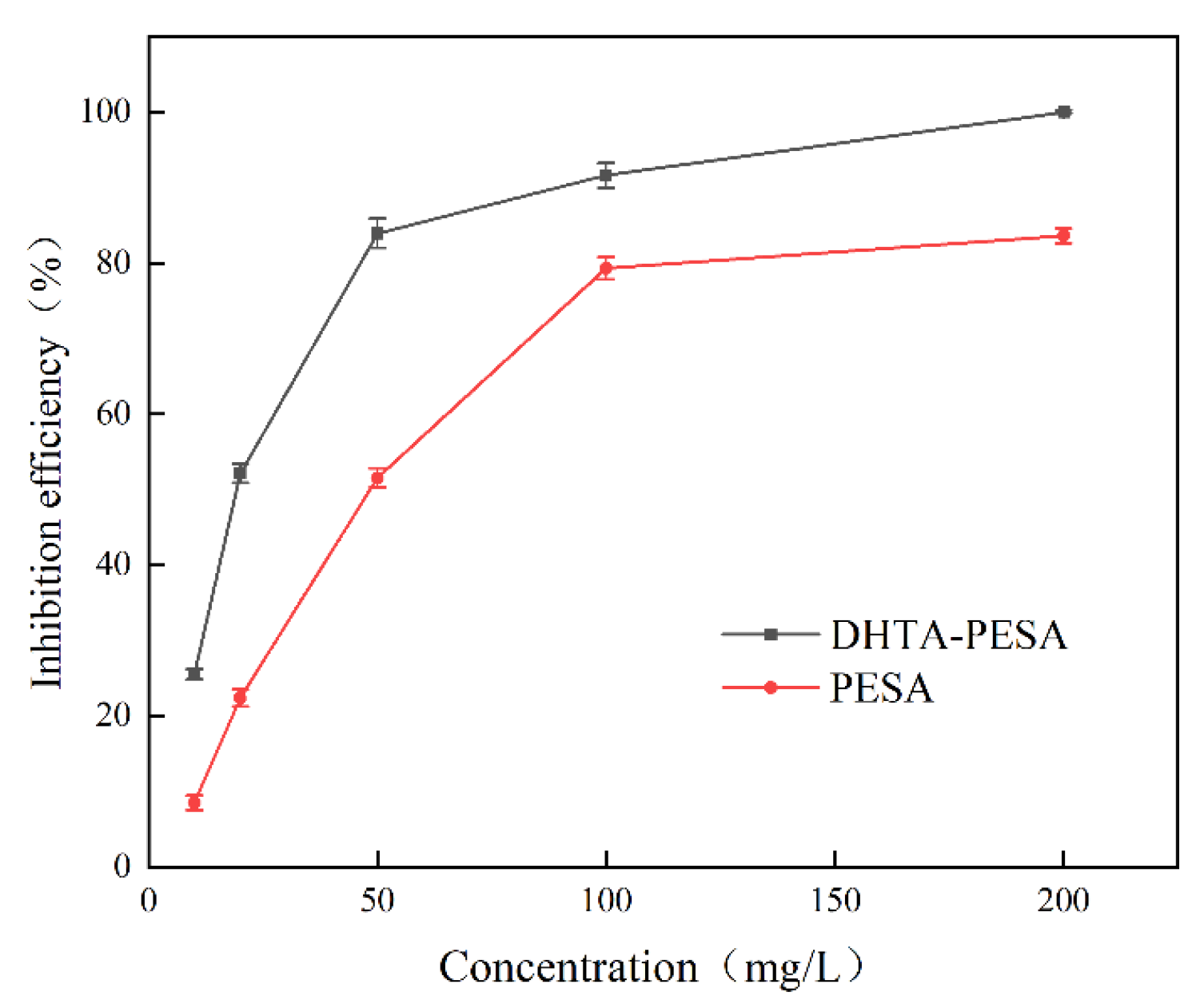
- The influence of concentration on scale inhibition rate
- (2)
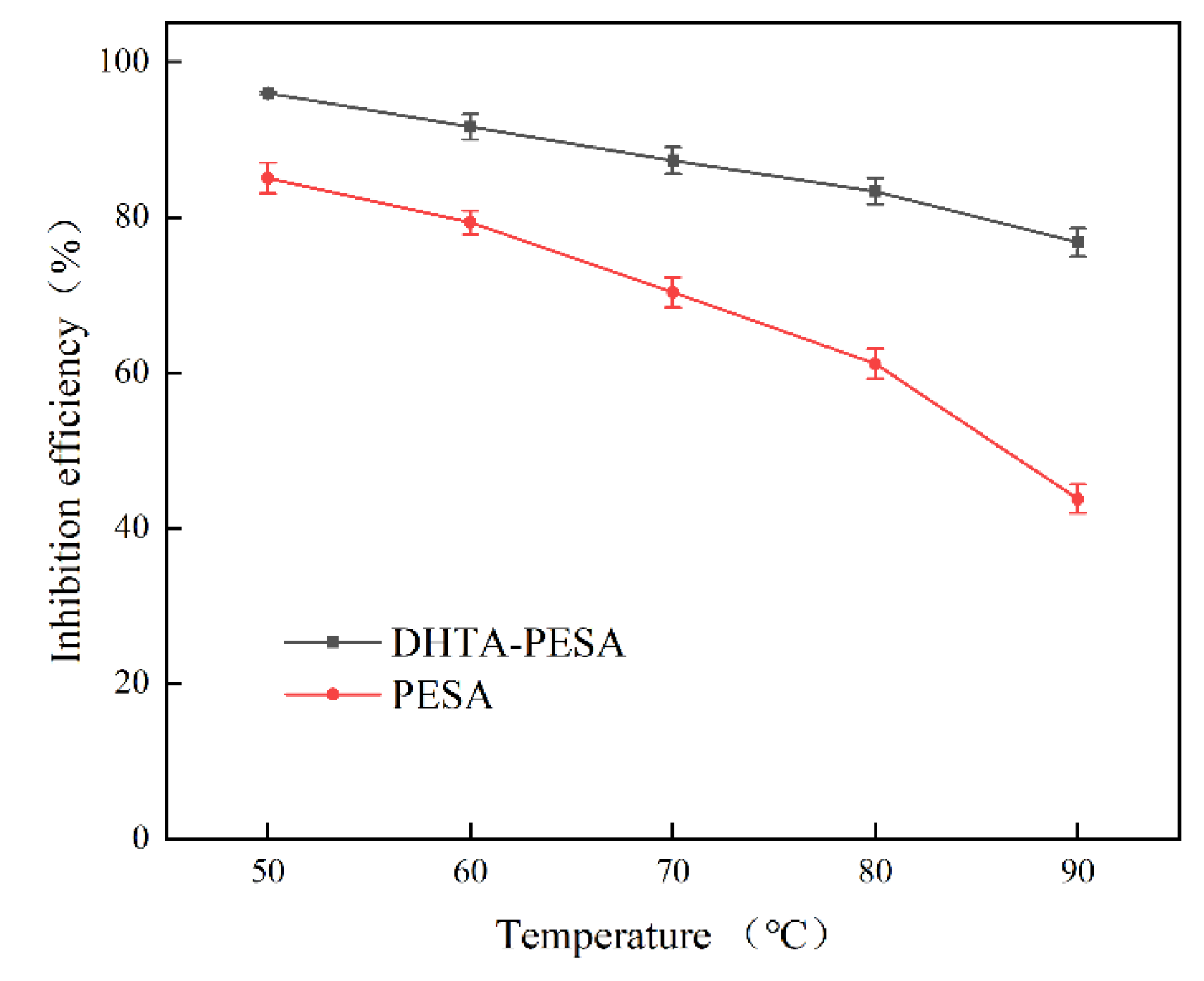
- Influence of Temperature on Scale Inhibition Rate
- (3)
- Influence of Calcium Ion Concentration on Scale Inhibition Rate
- (4)
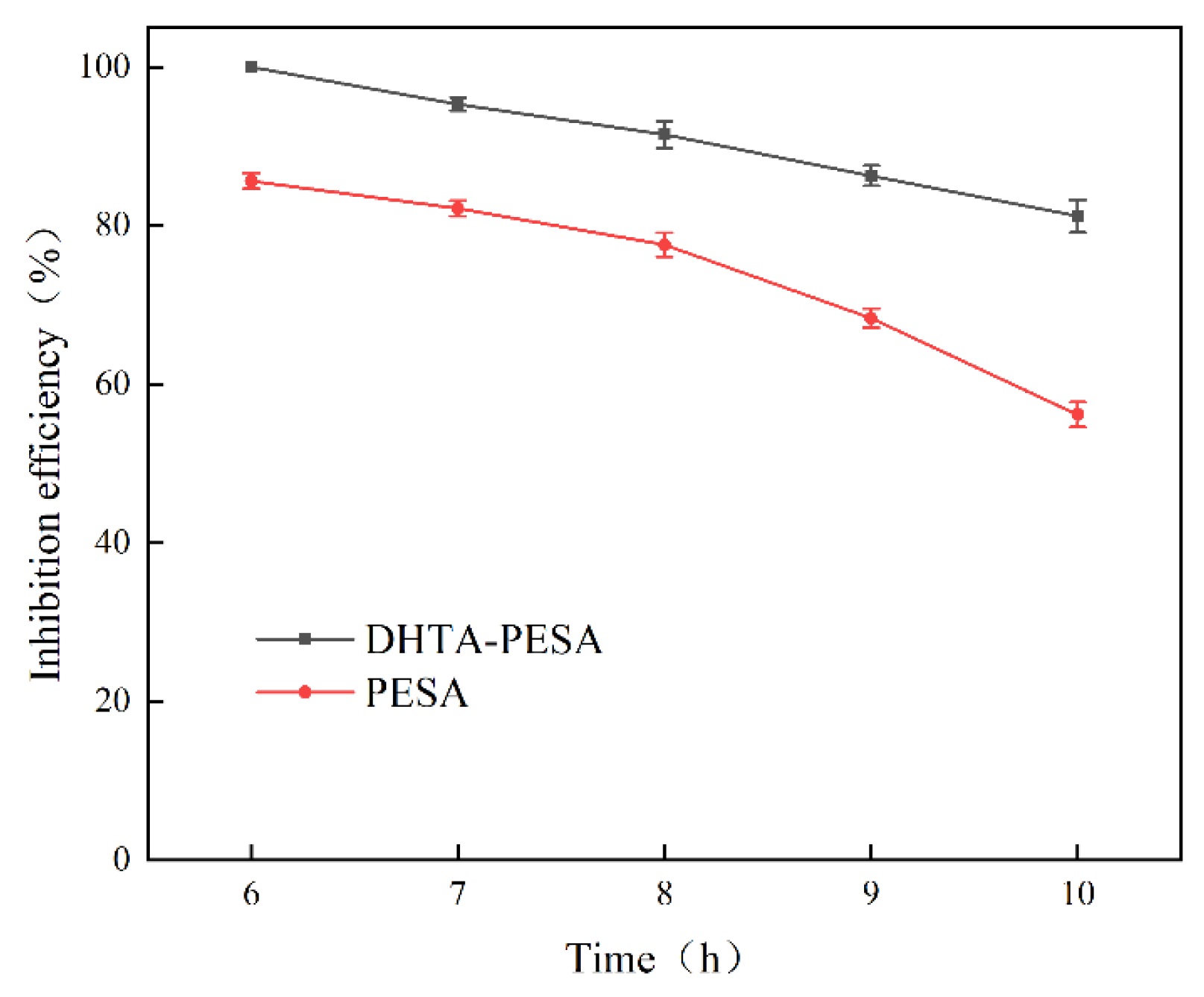
- Effect of Heating Time on Scale Inhibition Rate
- (5)
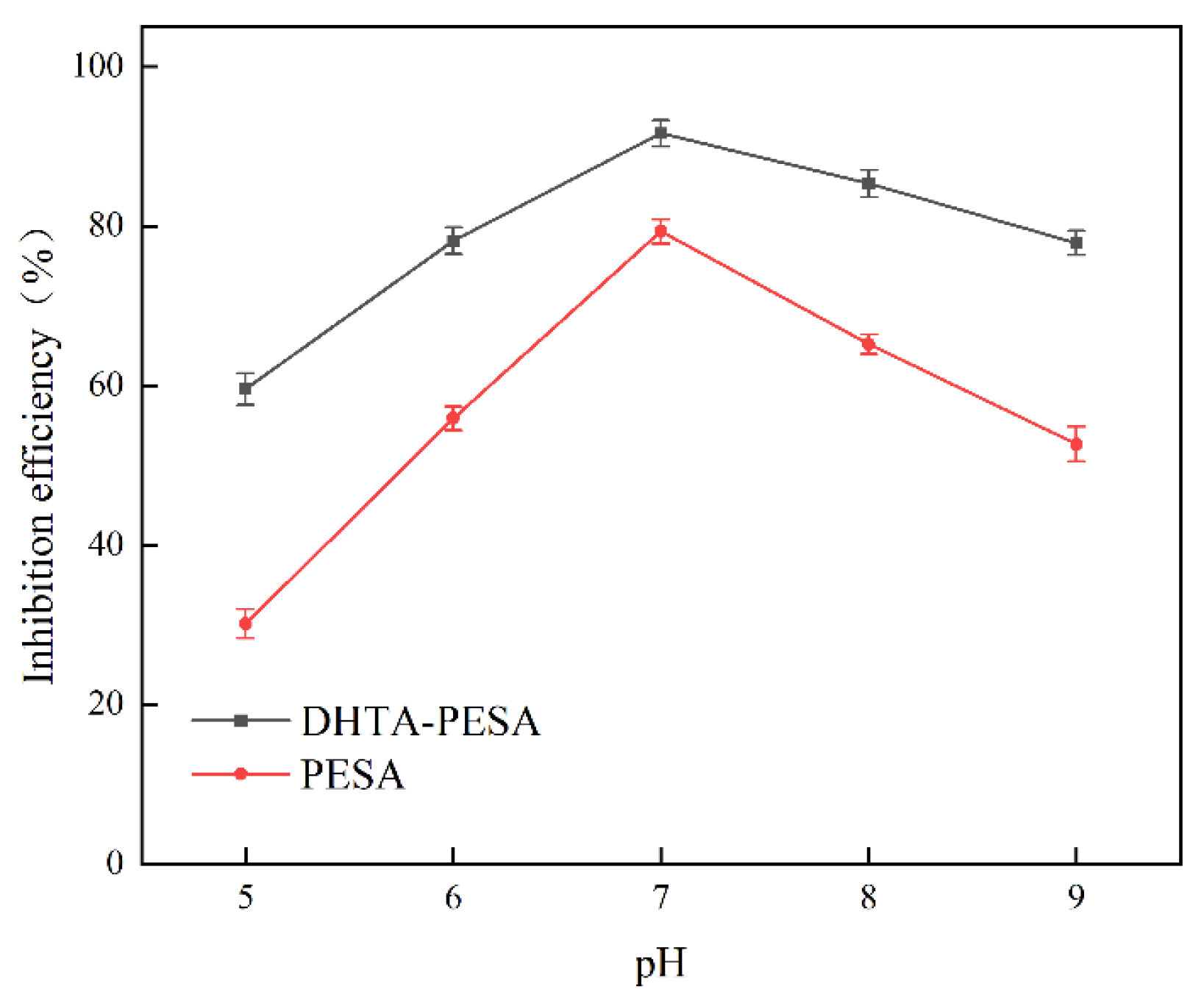
- Influence of pH on Scale Inhibition Rate
- (6)
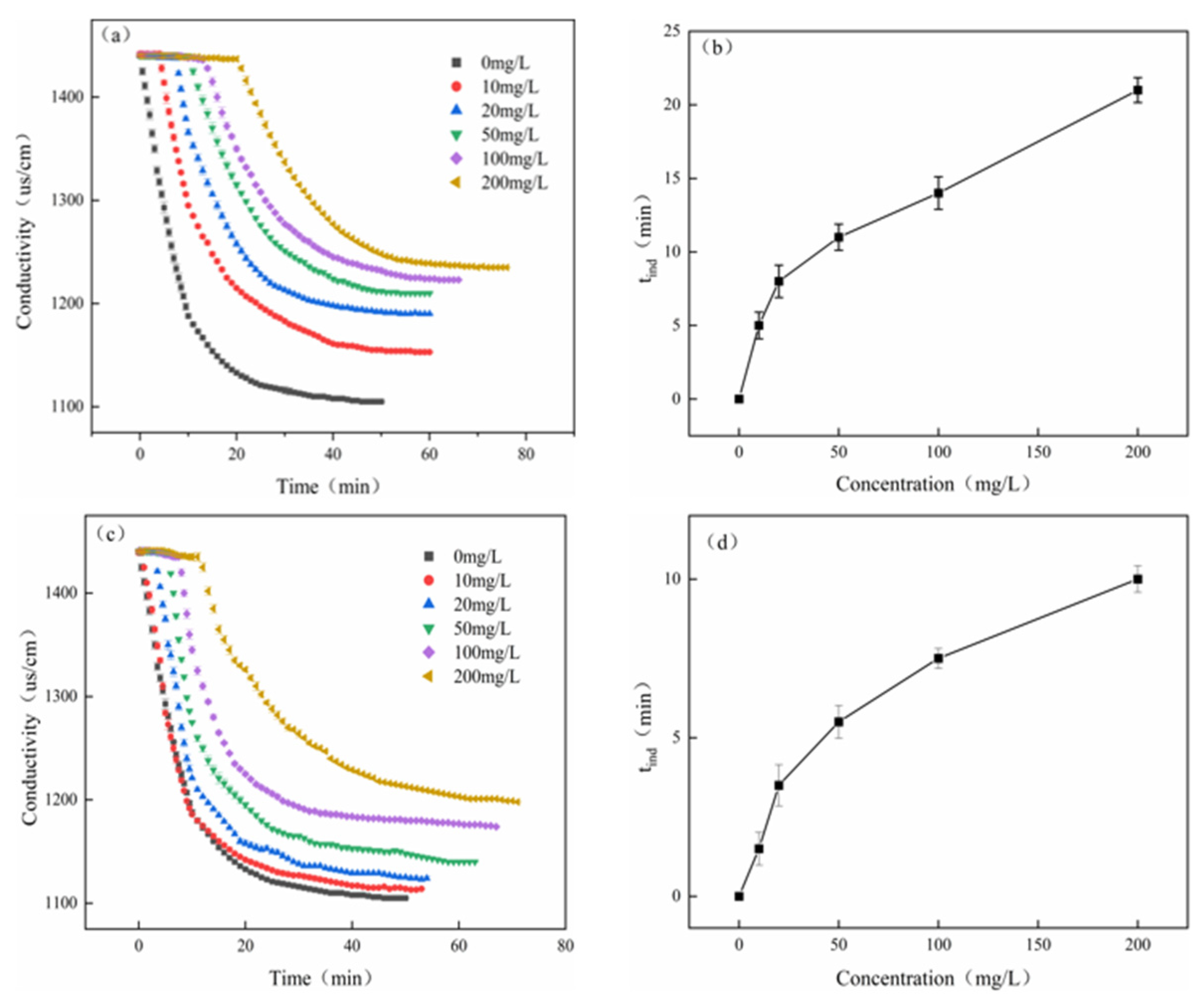
- Static conductance experiment
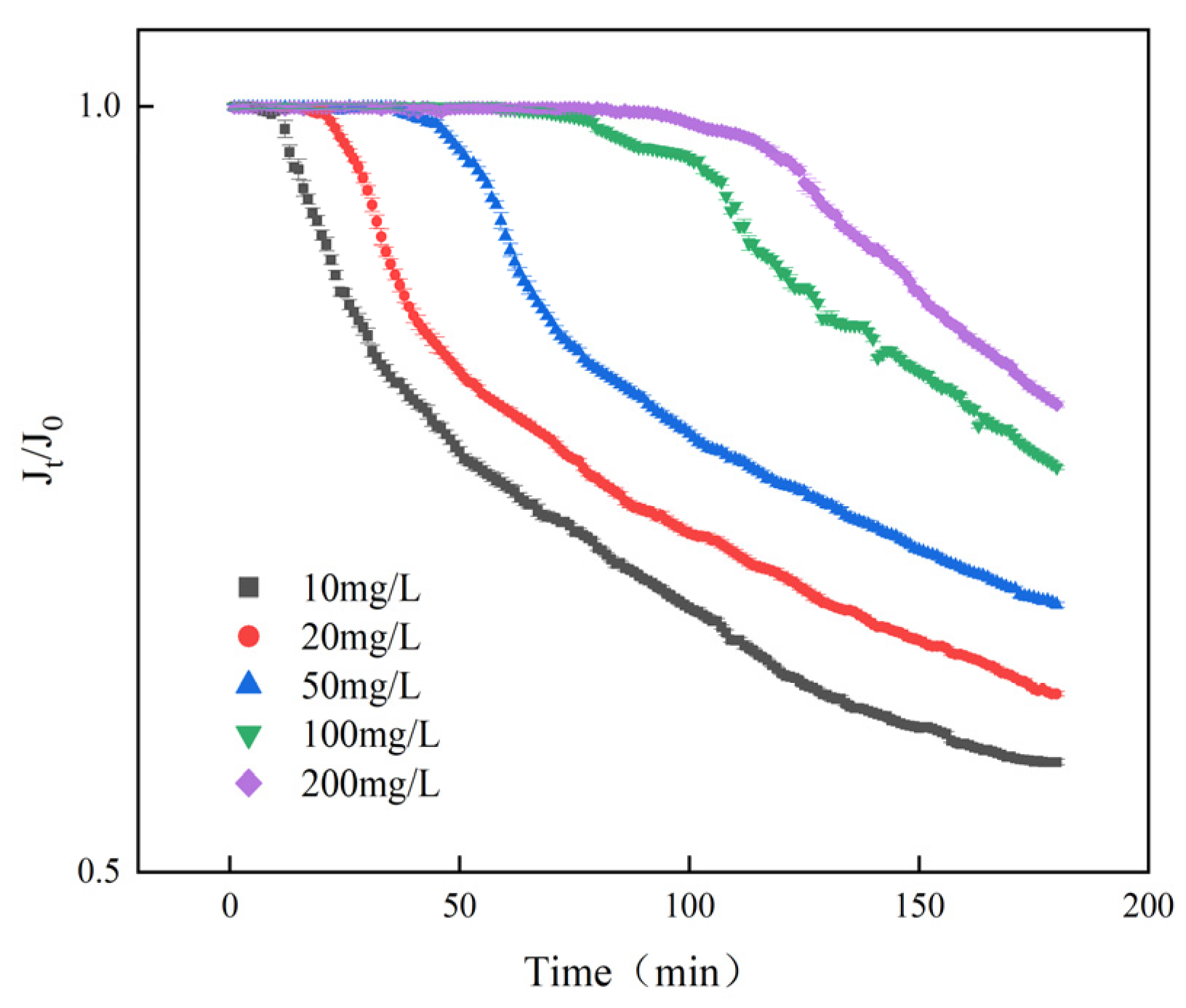
3.3.2. Dynamic Scale Inhibition
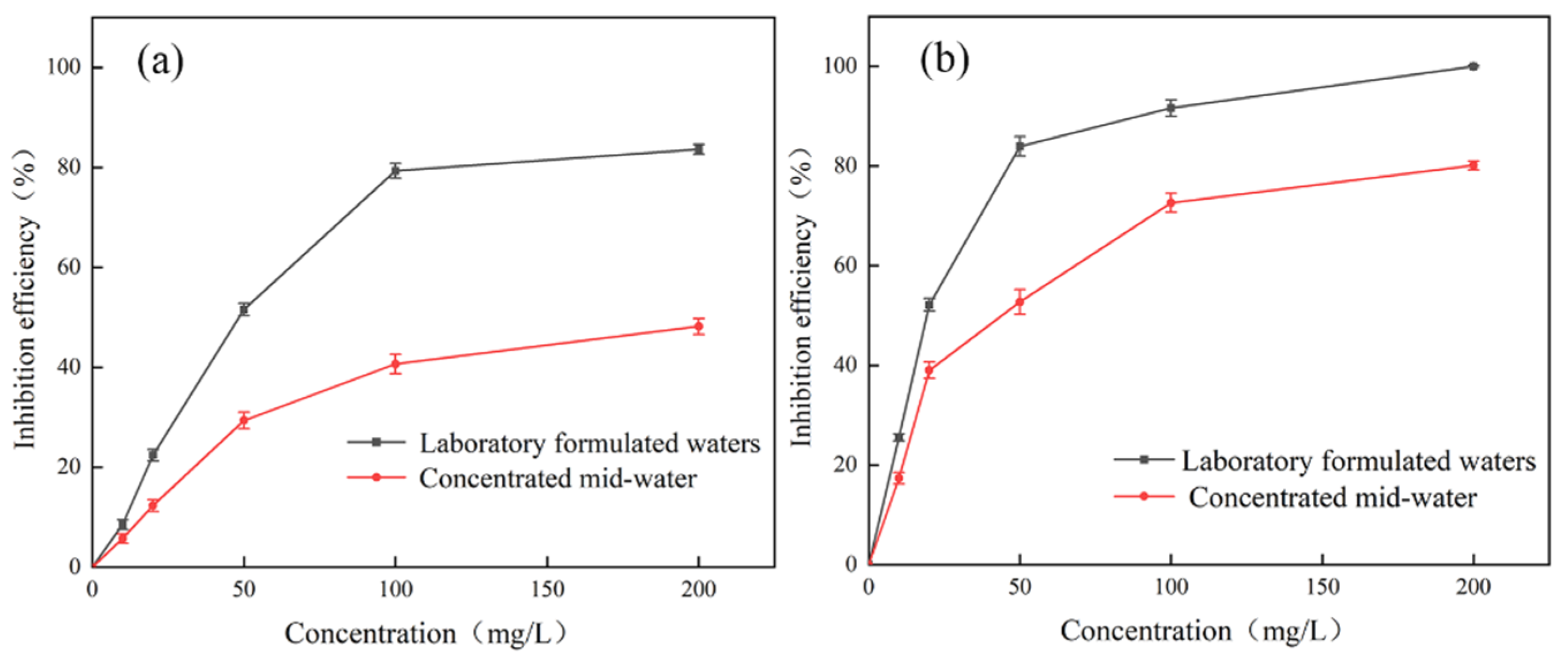
3.3.3. Water Trial Experiment
3.4. Analysis of Scale Inhibition Mechanism
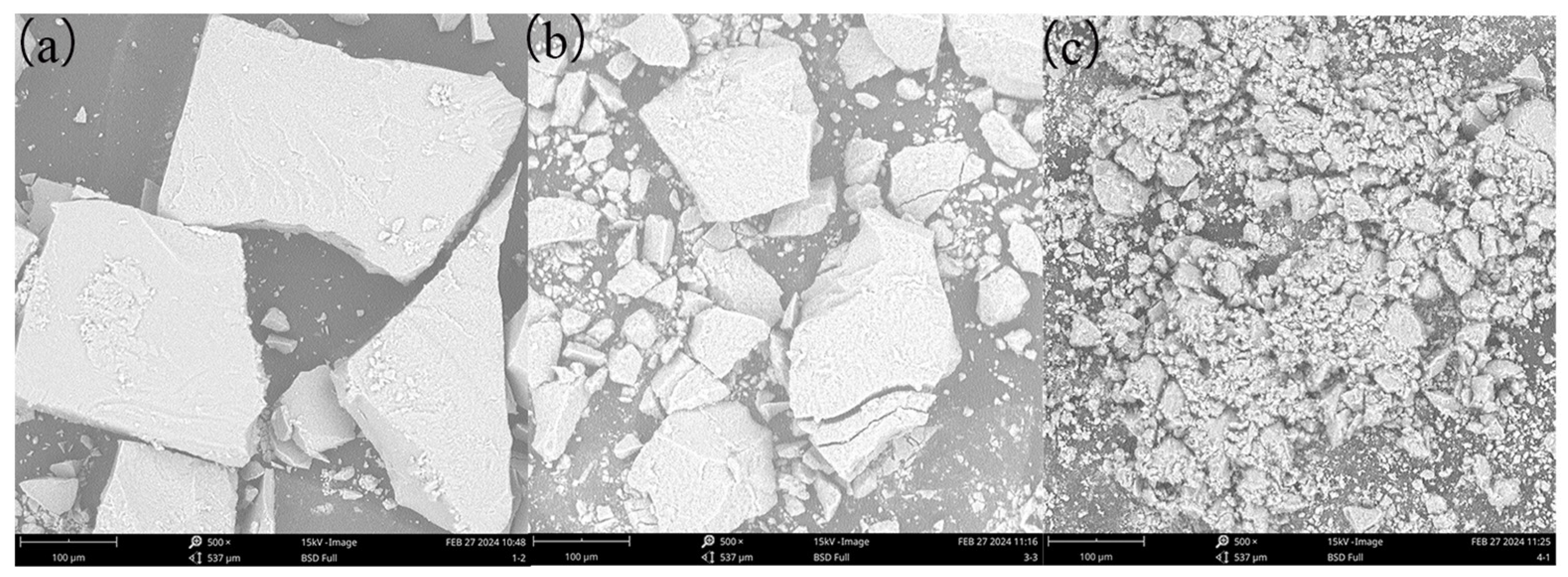
3.4.1. SEM Analysis
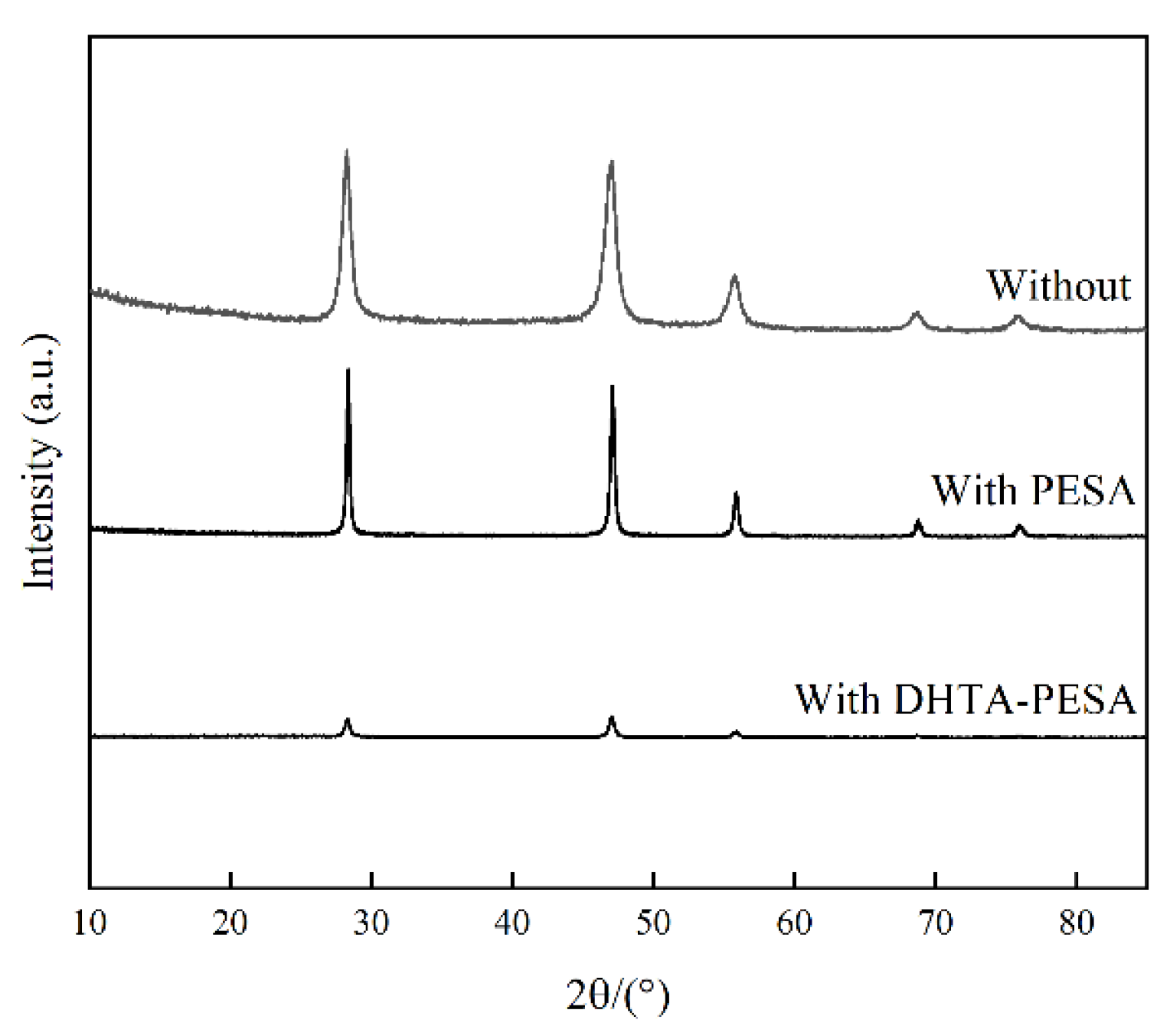
3.4.2. XRD Analysis
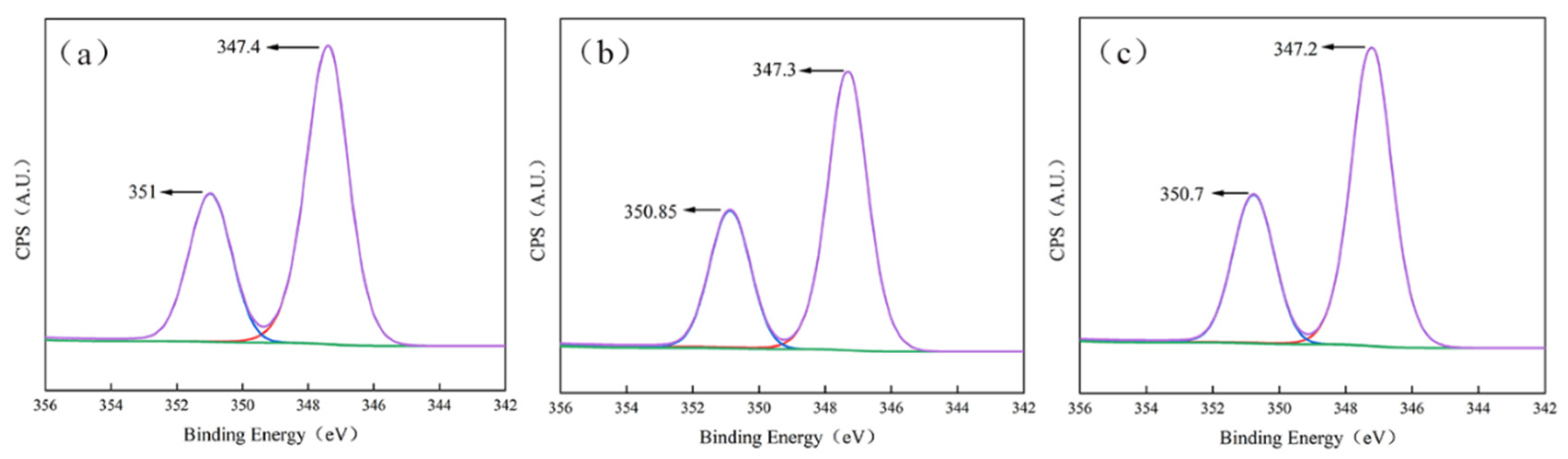
3.4.3. XPS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Long, T.; Wang, Z.; Luukkanen, S.; Yang, W.; Yang, C.; Deng, S.; Gu, T. Effect of environmentally friendly reverse osmosis scale inhibitors on inorganic calcium carbonate scale. Colloids Surf. A Physicochem. Eng. Asp. 2024, 702, 134883. [Google Scholar] [CrossRef]
- Zelner, M.; Jahn, P.; Ulbricht, M.; Freger, V. A mixed-charge polyelectrolyte complex nanofiltration membrane: Preparation, performance and stability. J. Membr. Sci. 2021, 636, 119579. [Google Scholar] [CrossRef]
- Tong, T.; Wallace, A.F.; Zhao, S.; Wang, Z. Mineral scaling in membrane desalination: Mechanisms, mitigation strategies, and feasibility of scaling-resistant membranes. J. Membr. Sci. 2019, 579, 52–69. [Google Scholar] [CrossRef]
- Can, H.K.; Üner, G. Water-soluble anhydride containing alternating copolymers as scale inhibitors. Desalination 2015, 355, 225–232. [Google Scholar] [CrossRef]
- Wang, C.; Shen, T.; Li, S.; Wang, X. Investigation of influence of low phosphorous co-polymer antiscalant on calcium sulfate dihydrate crystal morphologies. Desalination 2014, 348, 89–93. [Google Scholar] [CrossRef]
- Wu, S.; Kaiser, J.; Guo, X.; Li, L.; Lu, Y.; Ballauff, M. Recoverable platinum nanocatalysts immobilized on magnetic spherical polyelectrolyte brushes. Ind. Eng. Chem. Res. 2012, 51, 5608–5614. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, F.; Han, J.; Tian, T.; Jin, Y.; Zhang, Z.; Chen, J. Evaluation of arginine-modified polyepoxysuccinic acid as anti-scaling and anti-corrosion agent. Chem. Eng. Technol. 2021, 44, 1131–1140. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, Y.; Miao, C.; Huang, W.; Wang, S.; Wang, Y. Synthesis and evaluation of amino acid modified polyepoxysuccinic acid as inhibitor of calcium carbonate scale. Water Supply 2022, 22, 8923–8941. [Google Scholar] [CrossRef]
- Jia, J.; Liu, X.; Wang, L.; Fu, Z.; Wei, J.; Wang, P.; Wang, L.; Zhao, T. Study on Scale and Corrosion Inhibition Properties of Polyepoxy-succinic Acid Derivatives and Composite Formulas in Natural Seawater. Surf. Technol. 2021, 50, 390–399. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, Y.; Cui, J.; Liu, X. Study on calcium scale inhibition and biodegradation of polyepoxysuccinic acid derivatives. Ind. Water Treat. 2019, 39, 41–43+110. [Google Scholar]
- Htet, T.T.; Zeng, D. Study on the corrosion and scale inhibition mechanism of the thiourea-modified polyepoxysuccinic acid (CNS-PESA). J. Chem. 2022, 2022, 1–15. [Google Scholar] [CrossRef]
- Huang, H.; Yao, Q.; Jiao, Q.; Liu, B.; Chen, H. Polyepoxysuccinic acid with hyper-branched structure as an environmentally friendly scale inhibitor and its scale inhibition mechanism. J. Saudi Chem. Soc. 2019, 23, 61–74. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, Z.; Bai, X.; Ma, T.; Zhao, C.; Lu, D. Synergistic Modification of Poly(epoxysuccinic acid) with PBTCA-Tyr and Scale Inhibition Properties Against Fluoride Scale. Polym. Mater. Sci. Eng. 2024, 40, 127–136. [Google Scholar] [CrossRef]
- Hu, L.; Hu, X.; Tan, Z.; Guo, L.; Wu, J.; Wei, J.; Qi, J.; Zou, C. A combination of citric acid-dopamine-epichlorohydrin polymer and linear carboxymethyl β-cyclodextrin-epichlorohydrin polymer as an eco-friendly scale inhibitor in artificial seawater. J. Mol. Liq. 2022, 366, 120263. [Google Scholar] [CrossRef]
- Song, D.; Chen, W.; Yu, W.; Yang, H. Study on the scale and corrosion inhibition performance of carboxymethyl chitosan-graft-poly(acrylic acid). Acta Sci. Circumstantiae 2022, 42, 81–95. [Google Scholar] [CrossRef]
- Leng, M.; Lu, X.; Feng, W.; Wang, X.; Fu, W.; Yang, Z.; Wang, H. Study on synthesis and modification of polyepoxysuccinic acid. Front. Educ. Res. 2020, 3, 30–33. [Google Scholar] [CrossRef]
- Han, Y.; Lv, X.; Zhang, Y.; Wang, Y.; Cai, Y.; Zhao, X.; Zhao, L.; Xu, Y. Synthesis and scale inhibition mechanism of poly(aspartic acid)/ethylenediamine/bromoacetic acid with different grafting ratios. J. Mol. Struct. 2025, 1348, 143266. [Google Scholar] [CrossRef]
- Luo, H.; Chen, D.; Yang, X.; Zhao, X.; Feng, H.; Li, M.; Wang, J. Synthesis and performance of a polymeric scale inhibitor for oilfield application. J. Pet. Explor. Prod. Technol. 2015, 5, 177–187. [Google Scholar] [CrossRef]
- Wang, Y.; Li, A.; Yang, H. Effects of substitution degree and molecular weight of carboxymethyl starch on its scale inhibition. Desalination 2017, 408, 60–69. [Google Scholar] [CrossRef]
- Yu, R.; Liu, Y.; Wang, Z.; Lian, Z.; Lv, F. Synergistic Scale Inhibition of Calcium Carbonate by Functional Groups in SAS/AMPS/IA Copolymers. Mater. Rep. 2021, 35, 2207–2212. [Google Scholar]
- Yang, Q.; Liu, Y.; Li, Y. Humic acid fouling mitigation by antiscalant in reverse osmosis system. Environ. Sci. Technol. 2010, 44, 5153–5158. [Google Scholar] [CrossRef]
- Chen, M.; Sun, W.; Zhang, Y.; Lu, W.; Han, F. Preparation of Polymaleic Anhydride-Based Green Scale Inhibitor and Its Application in Cement Production Lines. Cem. Guide New Epoch 2024, 30, 53–56. [Google Scholar] [CrossRef]
- Yao, Q.; Zhan, R.; Ren, H.; Yang, B.; Yang, Y. Carboxyl or phosphate functionalization polyamidoamine dendrimer efficient scale inhibitor: Preparation and properties. J. Mol. Struct. 2022, 1252, 132130. [Google Scholar] [CrossRef]
- Huang, H.; Yao, Q.; Chen, H.; Liu, B. Scale inhibitors with a hyper-branched structure: Preparation, characterization and scale inhibition mechanism. RSC Adv. 2016, 6, 92943–92952. [Google Scholar] [CrossRef]
- Xia, C.; Xia, H.; Ding, R.; Ding, Y.; Chen, Z.; Xu, H.; Yang, W. Synthesis, characterization, and performance evaluation of AA/AMPS copolymers with different molecular weights and explanation of the inhibition mechanism of calcium carbonate and calcium sulfate. Colloids Surf. A Physicochem. Eng. Asp. 2024, 698, 134558. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Zhang, M.; Jaspe, C.J.S.; Cheng, Y.; Cao, Z.; Wu, Y.; Xu, Y. Synthesis, scale inhibition performance evaluation and mechanism study of 3-amino-1-propane sulfonic acid modified polyaspartic acid copolymer. J. Mol. Struct. 2023, 1272, 134141. [Google Scholar] [CrossRef]
- Yu, W.; Chen, W.; Yang, H. Application of modified cellulose-based antiscalant in reverse osmosis membrane scaling control. Sci. Sin. Technol. 2022, 52, 431–446. (In Chinese) [Google Scholar] [CrossRef]
- Ashfaq, M.Y.; Al-Ghouti, M.A.; Disi, Z.A.A.; Zouari, N. Interaction of seawater microorganisms with scalants and antiscalants in reverse osmosis systems. Desalination 2020, 487, 114480. [Google Scholar] [CrossRef]
- Hasson, D.; Drak, A.; Semiat, R. Inception of CaSO4 scaling on RO membranes at various water recovery levels. Desalination 2001, 139, 73–81. [Google Scholar] [CrossRef]
- Hasson, D.; Drak, A.; Semiat, R. Induction times induced in an RO system by antiscalants delaying CaSO4 precipitation. Desalination 2003, 157, 193–207. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W.; Benamor, A.; Hilal, N. Hybrid coagulation–NF membrane processes for brackish water treatment: Effect of pH and salt/calcium concentration. Desalination 2016, 390, 25–32. [Google Scholar] [CrossRef]
- Yuan, W.; Chen, X.; Yu, Z.; Wan, Y.; Lin, J.; Ye, W. Critical review of membrane fouling in reverse osmosis treatment: Characterizations, models, mechanisms, and controls. Sep. Purif. Technol. 2025, 363, 132119. [Google Scholar] [CrossRef]
- Yi, X.; Yang, S.; He, X.; Wang, Z.; Rui, M.; Tang, Y. Use of modified poly-epoxysuccinic acid as an efficient scale inhibitor to control CaSO4 scaling in NF processes: Performance and mechanisms. Desalination 2024, 586, 117821. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Z.; Li, Z.; Chen, S.; Yang, X.; Duan, L.; Cai, J. Synthesis and evaluation of scale inhibitor with high-temperature resistance and low corrosion capability for geothermal exploitation. J. Pet. Sci. Eng. 2022, 218, 110976. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Y.; Gao, Y.; Yang, Y.; Li, W.; Ma, N.; Zhao, J. Synthesis of polyaspartic acid-glycidyl adduct and evaluation of its scale inhibition performance and corrosion inhibition capacity for Q235 steel applications. Arab. J. Chem. 2023, 16, 104515. [Google Scholar] [CrossRef]
- Lai, C.; Wang, W.; Lv, S.; Deng, Y.; Gan, X.; Gou, W. Study on scale and corrosion inhibition performance of ethane-1,2-diaminium O,O’-dicyclohexyldithiophosphate. Int. J. Electrochem. Sci. 2020, 15, 11306–11315. [Google Scholar] [CrossRef]
- Chen, W.; Si, Y.; Wu, X.; Hu, Z.; Ren, Z.; Ma, J.; Zhang, Y. The inhibitory effect of low phosphorus and low molecular weight terpolymer scale inhibitor on calcium scale in oilfields. J. Appl. Polym. Sci. 2024, 142, e56544. [Google Scholar] [CrossRef]
- Zhao, W.; Shi, L.; Wang, X.; Han, Y.; Zhang, X.; Huang, Z.; Pan, X.; Yang, C. The isolation, compound, and application of scale inhibition biological agents for treating recirculating cooling water system. Clean. Water 2025, 4, 100088. [Google Scholar] [CrossRef]
- Yu, X.; Kan, J.; Han, J.; Liu, K.; Li, J.; Zhang, H.; Chen, J. Synthesis, scale and corrosion inhibition evaluation and mechanism of 2-aminobenzimidazole modified polyaspartic acid. J. Environ. Chem. Eng. 2024, 12, 112950. [Google Scholar] [CrossRef]



| Number of Times | t0 | t1 | ηr | ηsp | [η] | Mη |
|---|---|---|---|---|---|---|
| 1 | 83.34 | 85.61 | 1.0272 | 0.0272 | 0.0269 | 497.71 |
| 2 | 83.42 | 85.63 | 1.0264 | 0.0265 | 0.0263 | 471.07 |
| 3 | 83.38 | 85.58 | 1.0263 | 0.0264 | 0.0262 | 467.30 |
| Number of Times | t0 | t1 | ηr | ηsp | [η] | Mη |
|---|---|---|---|---|---|---|
| 1 | 83.34 | 87.35 | 1.0481 | 0.0481 | 0.0474 | 1532.33 |
| 2 | 83.42 | 87.45 | 1.0483 | 0.0483 | 0.0476 | 1544.49 |
| 3 | 83.38 | 87.44 | 1.0487 | 0.0487 | 0.0479 | 1568.69 |
| Item | Ca2+ (mg/L) | Cl− (mg/L) | K+ (mg/L) | F− (mg/L) | Mg2+ (mg/L) | Na+ (mg/L) | pH |
|---|---|---|---|---|---|---|---|
| Actual reclaimed water | 250.96 | 907.5 | 45.89 | 14.0 | 73.11 | 745.69 | 7.45~8.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, B.; Zhu, X.; Zhao, C.; Qin, Z.; Liu, Z.; Lu, D. Synthesis of DHTA-Modified Poly(Epoxysuccinic Acid) and Scale Inhibition of Fluoride Scale. Materials 2025, 18, 4701. https://doi.org/10.3390/ma18204701
Zhang Y, Liu B, Zhu X, Zhao C, Qin Z, Liu Z, Lu D. Synthesis of DHTA-Modified Poly(Epoxysuccinic Acid) and Scale Inhibition of Fluoride Scale. Materials. 2025; 18(20):4701. https://doi.org/10.3390/ma18204701
Chicago/Turabian StyleZhang, Yihao, Bo Liu, Xiaolong Zhu, Chunxia Zhao, Zhe Qin, Zixue Liu, and Da Lu. 2025. "Synthesis of DHTA-Modified Poly(Epoxysuccinic Acid) and Scale Inhibition of Fluoride Scale" Materials 18, no. 20: 4701. https://doi.org/10.3390/ma18204701
APA StyleZhang, Y., Liu, B., Zhu, X., Zhao, C., Qin, Z., Liu, Z., & Lu, D. (2025). Synthesis of DHTA-Modified Poly(Epoxysuccinic Acid) and Scale Inhibition of Fluoride Scale. Materials, 18(20), 4701. https://doi.org/10.3390/ma18204701




