Evaluating Biochar’s Role in Dye Adsorption and Wheat Performance Under Saline Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar Preparation
2.2. Crystal Violet Removal
2.3. Batch Adsorption Experiment
2.4. Biochar Characterization
2.5. Adsorption Isotherm
2.6. Soil Amendment and Irrigation Experiment
3. Results
3.1. Biochar Characteristics
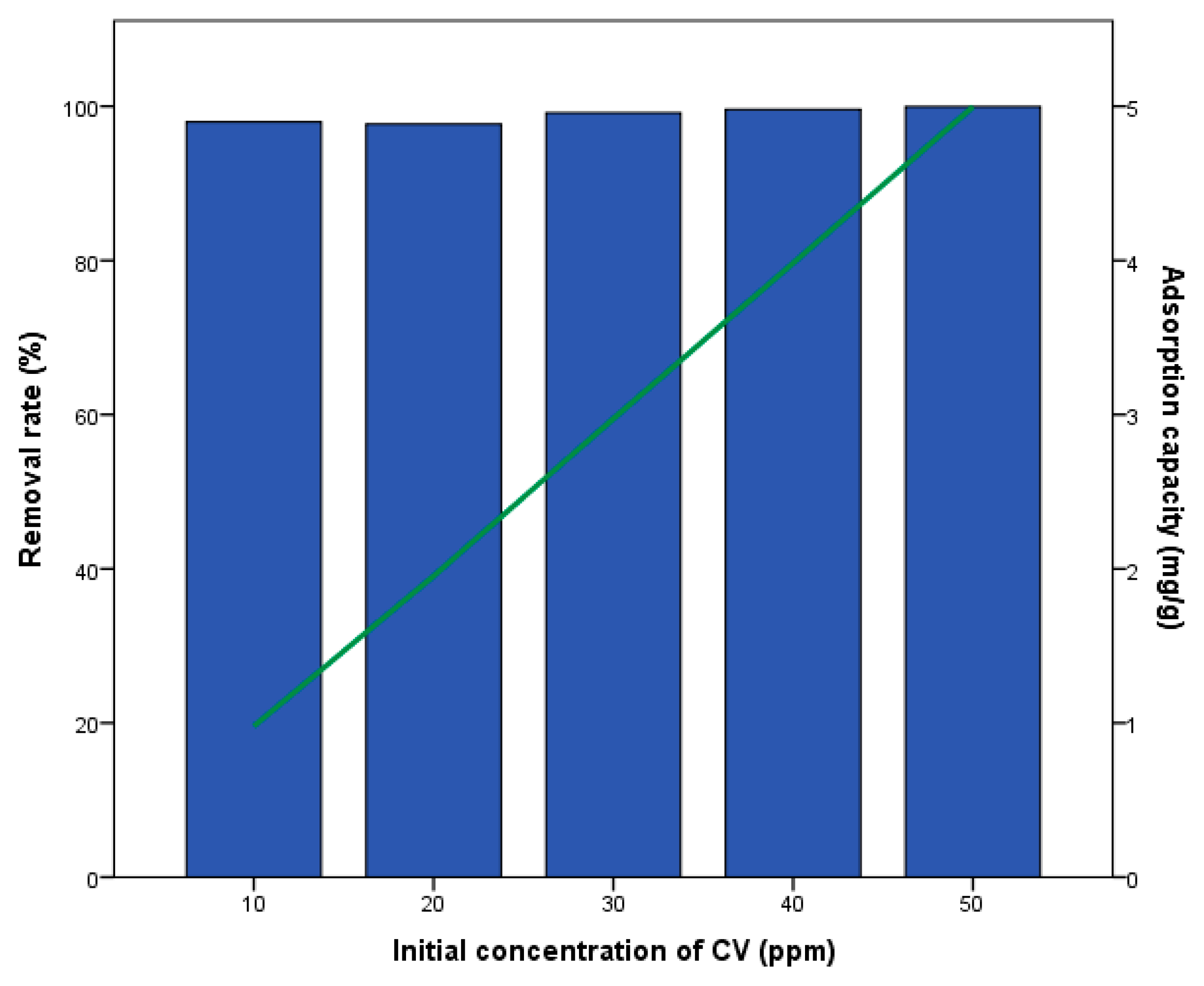
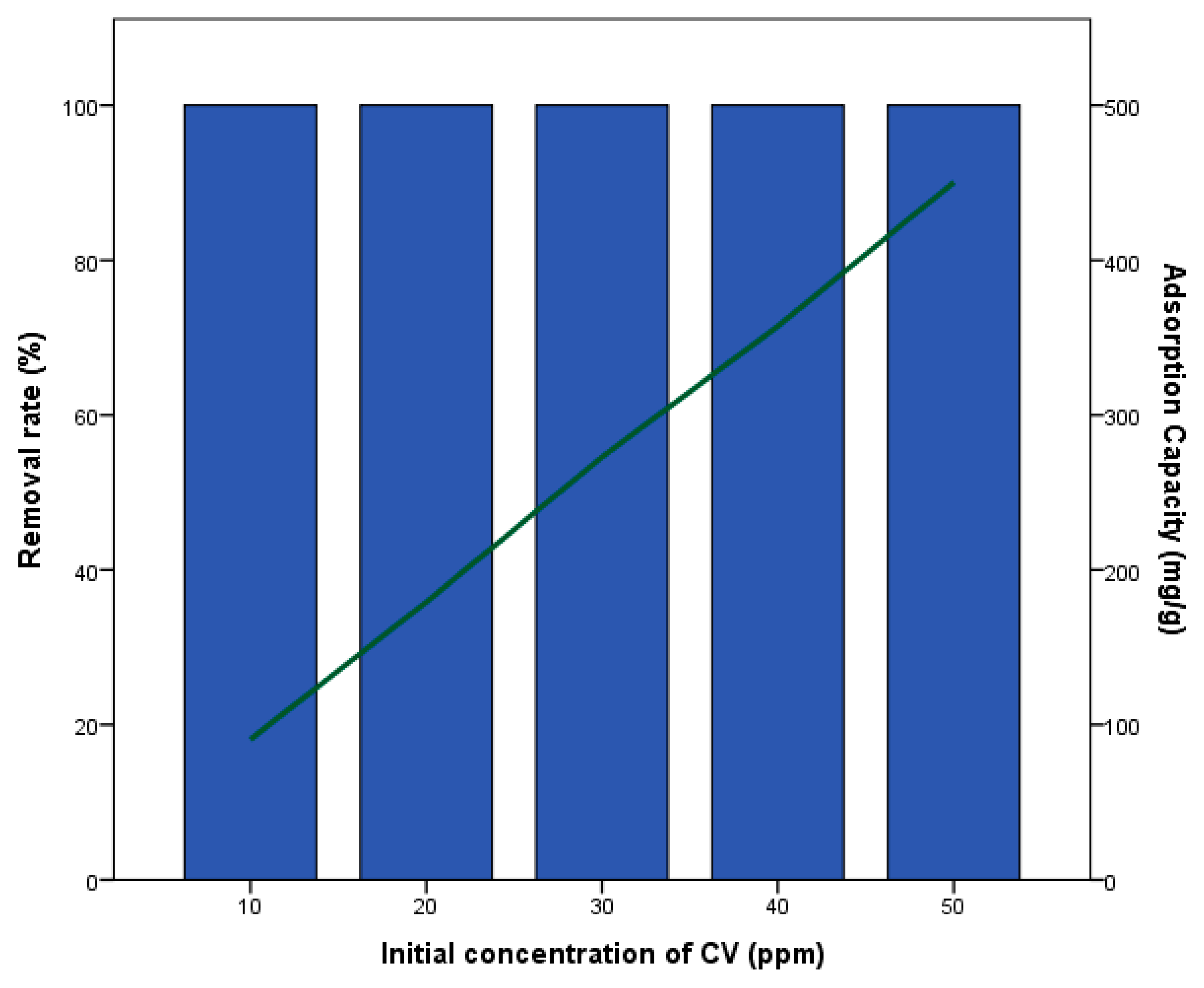
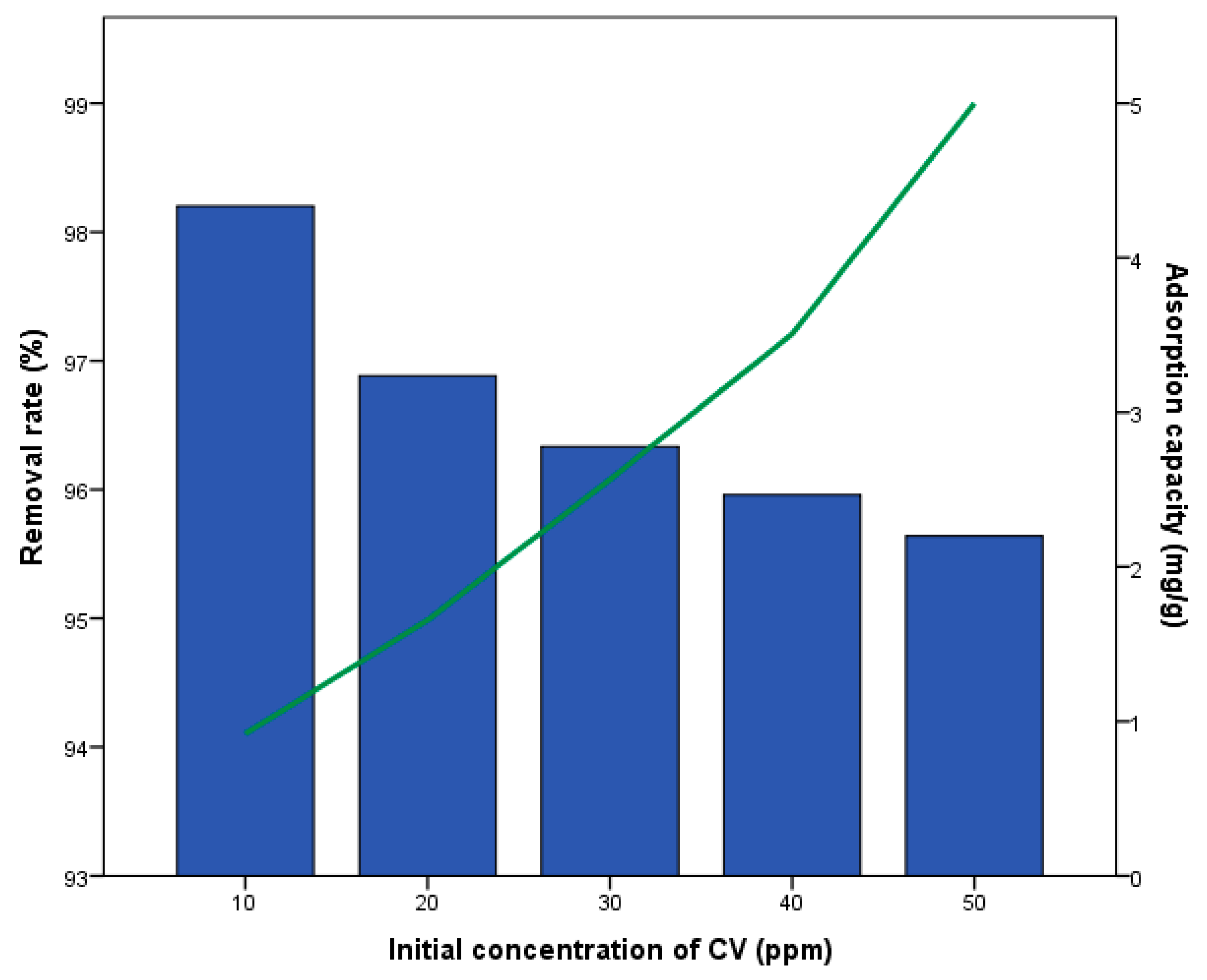
3.2. Crystal Violet Removal
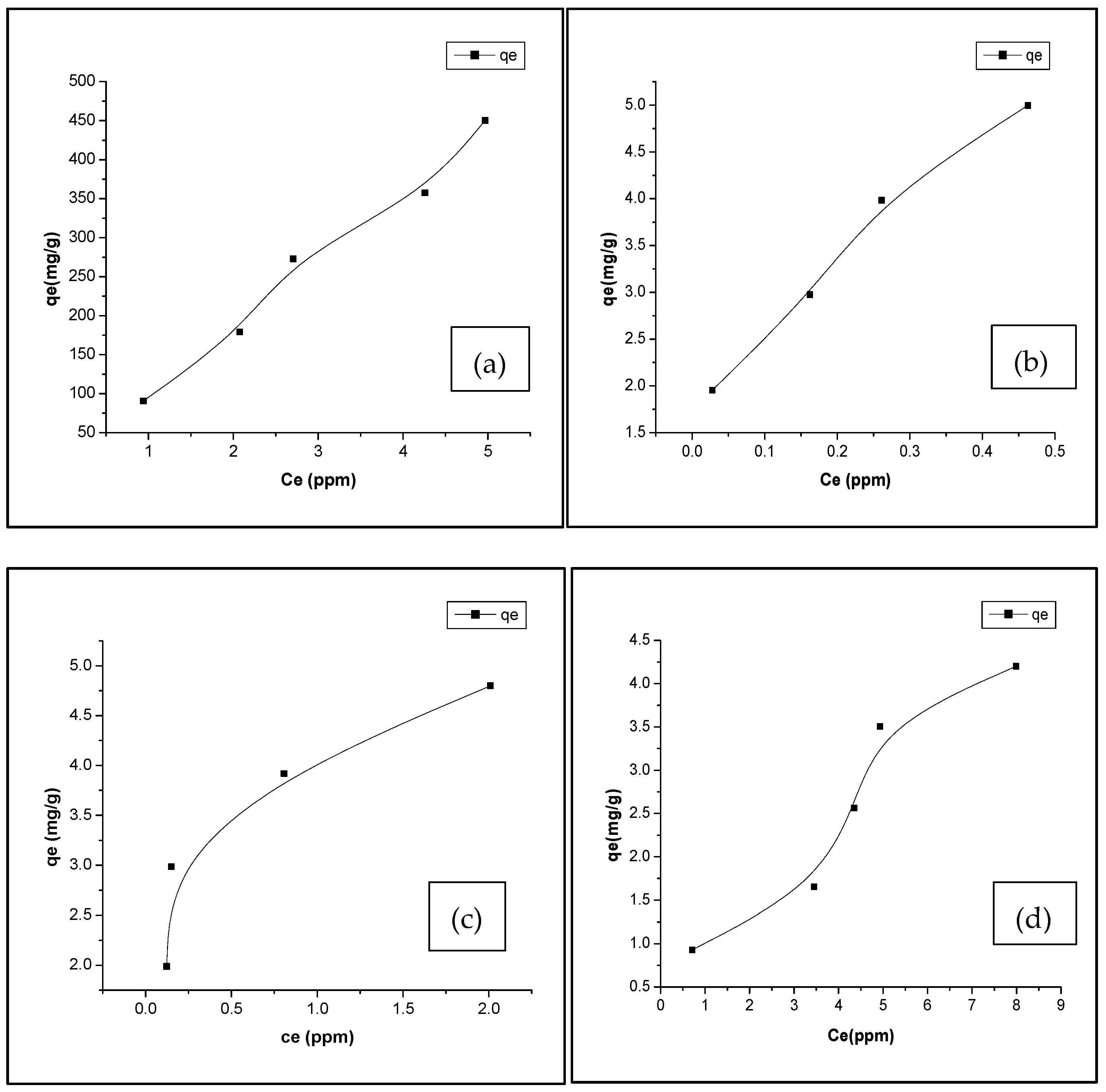
3.3. Adsorption Isotherm
3.4. Soil Amendment and Irrigation
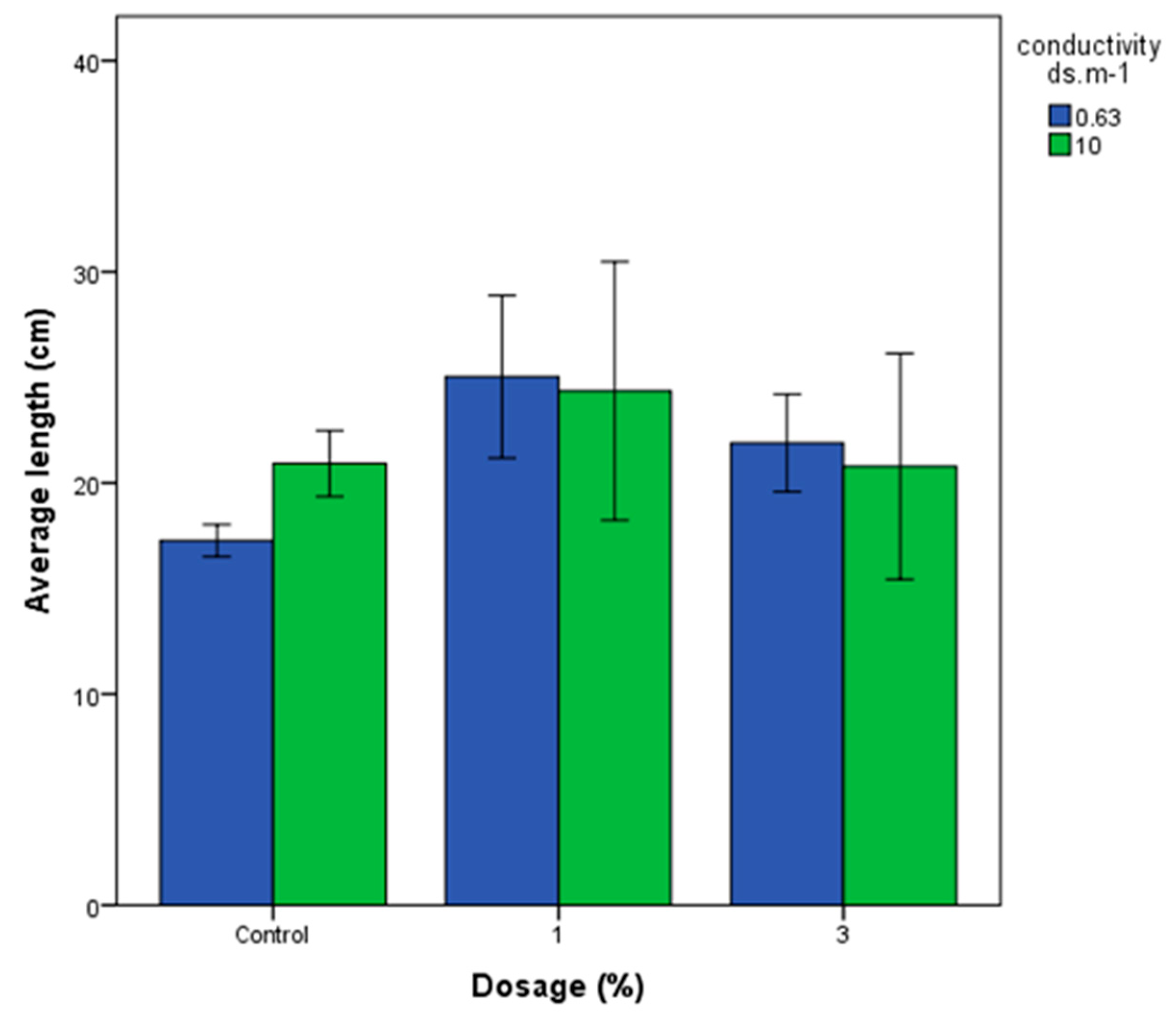
3.4.1. Wheat Growth
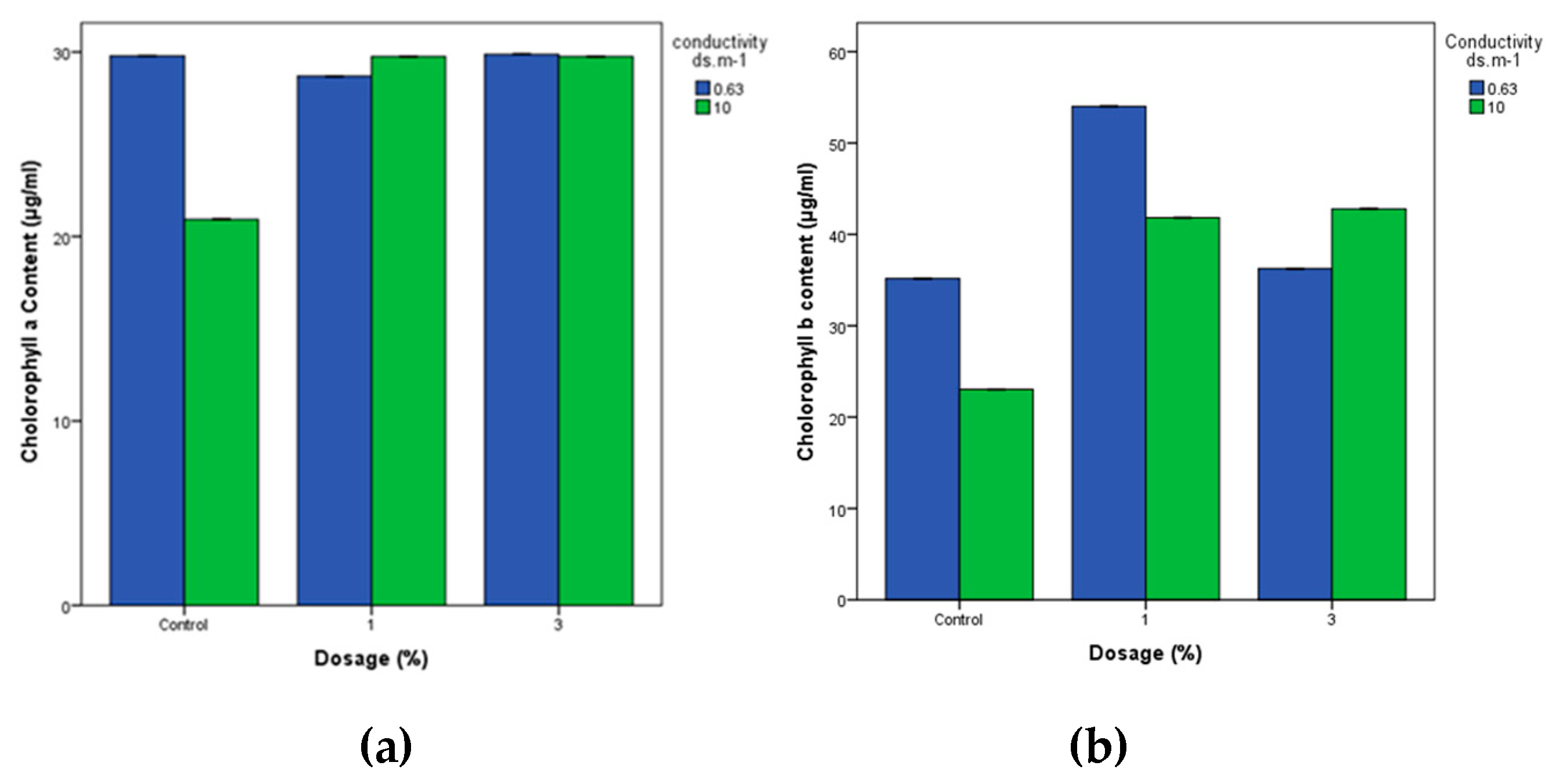
3.4.2. Chlorophyll Levels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Woodward, G.; Perkins, D.M.; Brown, L.E. Climate change and freshwater ecosystems: Impacts across multiple levels of organization. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2093–2106. [Google Scholar] [CrossRef] [PubMed]
- Duran-Encalada, J.A.; Paucar-Caceres, A.; Bandala, E.R.; Wright, G.H. The impact of global climate change on water quantity and quality: A system dynamics approach to the US–Mexican transborder region. Eur. J. Oper. Res. 2017, 256, 567–581. [Google Scholar] [CrossRef]
- Kesari, K.K.; Soni, R.; Jamal, Q.M.S. Wastewater treatment and reuse: A review of its applications and health implications. Water Air Soil Pollut. 2021, 232, 208. [Google Scholar] [CrossRef]
- Upadhyay, R.; Przystaś, W.; Dave, B. Myco-remediation of synthetic dyes: A comprehensive review on contaminant alleviation mechanism, kinetic study and toxicity analysis. Int. J. Environ. Sci. Technol. 2025, 22, 521–538. [Google Scholar] [CrossRef]
- Silva, V.C.; Barbosa Araújo, M.E.; Rodrigues, A.M.; Vitorino, B.C.; Cartaxo, J.M.; Menezes, R.R.; Neves, G.A. Adsorption behavior of crystal violet and Congo red dyes on heat-treated Brazilian palygorskite: Kinetic, isothermal and thermodynamic studies. Materials 2021, 14, 5688. [Google Scholar] [CrossRef]
- Ben Aissa, M.A.; Modwi, A.; Albadri, A.E.; Saleh, S.M. Dependency of crystal violet dye removal behaviors onto mesoporous V2O5-g-C3N4 constructed by simplistic ultrasonic method. Inorganics 2023, 11, 146. [Google Scholar] [CrossRef]
- Valli Nachiyar, C.; Rakshi, A.; Sandhya, S.; Britlin Deva Jebasta, N.; Nellore, J. Developments in treatment technologies of dye-containing effluent: A review. Case Stud. Chem. Environ. Eng. 2023, 7, 100339. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Guerrero-Barajas, C.; Ahmad, A.; Ibrahim, M.N.M.; Alshammari, M.B. Advanced technologies for wastewater treatment. In Green Chemistry for Sustainable Water Purification; Wiley: Hoboken, NJ, USA, 2023; pp. 179–202. [Google Scholar] [CrossRef]
- Nishat, A.; Yusuf, M.; Qadir, A.; Ezaier, Y.; Vambol, V.; Ijaz Khan, M.; Ben Moussa, S.; Kamyab, H.; Sehgal, S.S.; Prakash, C.; et al. Wastewater treatment: A short assessment on available techniques. Alex. Eng. J. 2023, 76, 505–516. [Google Scholar] [CrossRef]
- Satyam, S.; Patra, S. Innovations and challenges in adsorption-based wastewater remediation: A comprehensive review. Heliyon 2024, 10, e29573. [Google Scholar] [CrossRef]
- Adesanmi, B.M.; Hung, Y.-T.; Paul, H.H.; Huhnke, C.R. Comparison of dye wastewater treatment methods: A review. GSC Adv. Res. Rev. 2022, 10, 126–137. [Google Scholar] [CrossRef]
- Olugbenga, S.; Adeleye, P.G.; Oladipupo, S.B.; Adeleye, A.T.; Igenepo, J.K. Biomass-derived biochar in wastewater treatment: A circular economy approach. Waste Manag. Bull. 2024, 1, 1–14. [Google Scholar] [CrossRef]
- Jagadeesh, N.; Sundaram, B. Adsorption of pollutants from wastewater by biochar: A review. J. Hazard. Mater. Adv. 2023, 9, 100226. [Google Scholar] [CrossRef]
- Srivatsav, P.; Bhargav, B.S.; Shanmugasundaram, V.; Arun, J.; Gopinath, K.P.; Bhatnagar, A. Biochar as an eco-friendly and economical adsorbent for the removal of colorants (dyes) from aqueous environment: A review. Water 2020, 12, 3561. [Google Scholar] [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Zularisam, A.; Nasrullah, M. A comprehensive assessment of the method for producing biochar, its characterization, stability, and potential applications in regenerative economic sustainability—A review. Clean. Mater. 2022, 3, 100045. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Zhu, Y.; Fang, W.; Tan, Y.; He, Z.; Liao, H. Research status, trends, and mechanisms of biochar adsorption for wastewater treatment: A scientometric review. Environ. Sci. Eur. 2024, 36, 25. [Google Scholar] [CrossRef]
- Jha, S.; Gaur, R.; Shahabuddin, S.; Tyagi, I. Biochar as sustainable alternative and green adsorbent for the remediation of noxious pollutants: A comprehensive review. Toxics 2023, 11, 117. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Illankoon, W.A.; Milanese, C.; Calatroni, S.; Caccamo, F.M.; Girella, A.; Sorlini, S. Preparation and modification of biochar derived from agricultural waste for metal adsorption from urban wastewater. Water 2023, 16, 698. [Google Scholar] [CrossRef]
- Gupta, M.; Savla, N.; Pandit, C.; Pandit, S.; Gupta, P.K.; Pant, M.; Khilari, S.; Kumar, Y.; Agarwal, D.; Nair, R.R.; et al. Use of biomass-derived biochar in wastewater treatment and power production: A promising solution for a sustainable environment. Sci. Total Environ. 2022, 825, 153892. [Google Scholar] [CrossRef]
- Soares Dias, A.P.; Santos, F.A.; Rijo, B.; Simes, D.C.; Pereira, L.; Pereira, M.F. Seaweed-Derived Biochar for Effective Treatment of Dye-Contaminated Wastewater. Water 2024, 17, 1215. [Google Scholar] [CrossRef]
- Wang, W.; Huang, J.; Wu, T.; Ren, X.; Zhao, X. Research on the preparation of biochar from waste and its application in environmental remediation. Water 2022, 15, 3387. [Google Scholar] [CrossRef]
- Vyavahare, G.; Jadhav, P.; Jadhav, J.; Patil, R.; Aware, C.; Patil, D.; Gophane, A.; Yang, Y.; Gurav, R. Strategies for crystal violet dye sorption on biochar derived from mango leaves and evaluation of residual dye toxicity. J. Clean. Prod. 2019, 207, 296–305. [Google Scholar] [CrossRef]
- Nouioua, A.; Ben Salem, D.; Ouakouak, A.; Rouahna, N.; Baigenzhenov, O.; Hosseini-Bandegharaei, A. Production of biochar from Melia azedarach seeds for the crystal violet dye removal from water: Combining hydrothermal carbonization and pyrolysis. Bioengineered 2023, 14, 290–306. [Google Scholar] [CrossRef]
- Goswami, L.; Kushwaha, A.; Kafle, S.R.; Kim, B. Surface modification of biochar for dye removal from wastewater. Catalysts 2022, 12, 817. [Google Scholar] [CrossRef]
- de Vasconcelos, A.C.F. Biochar Effects on Amelioration of Adverse Salinity Effects in Soils; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, J.; Wang, H. Biochar addition alleviates the negative effects of drought and salinity stress on soybean productivity and water use efficiency. BMC Plant Biol. 2020, 20, 288. [Google Scholar] [CrossRef] [PubMed]
- Kul, R.; Arjumend, T.; Ekinci, M.; Yildirim, E.; Turan, M.; Argin, S. Biochar as an organic soil conditioner for mitigating salinity stress in tomato. Soil Sci. Plant Nutr. 2021, 67, 693–706. [Google Scholar] [CrossRef]
- Al-Hazmi, H.; Mohammadi, A.; Hejna, A.; Majtacz, J.; Esmaeili, A.; Habibzadeh, S.; Saeb, M.; Badawi, M.; Lima, E.; Makinia, J. Wastewater reuse in agriculture: Prospects and challenges. Environ. Res. 2023, 236, 116711. [Google Scholar] [CrossRef]
- Kataya, G.; Issa, M.; Jeguirim, M.; Hijazi, A. Characterization and environmental application potential of banana peels bio-char. Eng. Proc. 2023, 37, 105. [Google Scholar] [CrossRef]
- Peixoto, B.S.; Mota, L.S.; Oliveira, P.C.; Veloso, M.C.; Romeiro, G.A.; Moraes, M.C. Highly functionalized microporous activated biochar from Syagrus coronata waste: Production, characterization, and application in adsorption studies. Water 2021, 14, 3525. [Google Scholar] [CrossRef]
- Chen, Y.; He, Q.; Liu, B.S.; Chang, L.; Fang, J.; Li, G. Bactericidal Biochar Loaded with Silver Particles and Preparation Method Thereof. CN110550709B, 11 February 2019. [Google Scholar]
- Mohan, D.; Kumar, H.; Sarswat, A.; Alexandre-Franco, M.; Pittman, C.U. Cadmium and lead remediation using magnetic oak wood and oak bark fast pyrolysis bio-chars. Chem. Eng. J. 2014, 236, 513–528. [Google Scholar] [CrossRef]
- Sun, X.; Fu, H.; Bao, M. Development of a new hydrophobic magnetic biochar for removing oil spills on the water surface. Biochar 2022, 4, 60. [Google Scholar] [CrossRef]
- Yerli, C. Examining the effects of biochar in spinach cultivation with increased irrigation water salinity on some physical and physiological properties of the plant. In Proceedings of the International Conference on Applied Engineering and Natural Sciences, Konya, Turkey, 10–12 July 2023. [Google Scholar] [CrossRef]
- Shaikh, W.; Kumar, A.; Chakraborty, S.; Islam, R.; Bhattacharya, T.; Biswas, J. Biochar-based nanocomposite from waste tea leaf for toxic dye removal: From facile fabrication to functional fitness. Chemosphere 2021, 282, 132788. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.D.N.; Bui, T.H.; Nguyen, A.P.; Nguyen, T.T.; Nguyen, V.M.; Duong, N.L.; Nguyen, T. The ability of silver-biochar green-synthesized from Citrus maxima peel to adsorb pollutant organic compounds and antibacterial activity. Green Chem. Lett. Rev. 2021, 15, 18–27. [Google Scholar] [CrossRef]
- Khan, M.N.; Siddique, M.; Mirza, N.; Khan, R.; Bilal, M.; Riaz, N.; Waheed, U.; Shahzadi, I.; Ali, A.; Abdellattif, M.H.; et al. Synthesis, characterization, and application of Ag-biochar composite for sono-adsorption of phenol. Front. Environ. Sci. 2022, 10, 823656. [Google Scholar] [CrossRef]
- Sankar Sana, S.; Haldhar, R.; Parameswaranpillai, J.; Chavali, M.; Kim, S. Silver nanoparticles-based composite for dye removal: A comprehensive review. Clean. Mater. 2022, 6, 100161. [Google Scholar] [CrossRef]
- Baharim, N.H.; Sjahrir, F.; Taib, R.M.; Idris, N.; Daud, T.A.T. Removal of crystal violet from aqueous solution using post-treated activation biochar derived from banana pseudo stem. Chem. Eng. Trans. 2023, 98, 45–50. [Google Scholar] [CrossRef]
- Mohanty, K.; Naidu, J.T.; Meikap, B.C.; Biswas, M.N. Removal of crystal violet from wastewater by activated carbons prepared from rice husk. Ind. Eng. Chem. Res. 2006, 45, 5165–5171. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Zhang, C.; Yue, Q.; Li, Y.; Li, C. Equilibrium and kinetic studies of methyl orange and methyl violet adsorption on activated carbon derived from Phragmites australis. Desalination 2010, 252, 149–156. [Google Scholar] [CrossRef]
- Sun, S.; Zhu, Y.; Gu, Z.; Chu, H.; Hu, C.; Gao, L.; Zhao, X. Adsorption of crystal violet on activated bamboo fiber powder from water: Preparation, characterization, kinetics and isotherms. RSC Adv. 2023, 13, 6108–6123. [Google Scholar] [CrossRef]
- Kumbhar, P.; Narale, D.; Bhosale, R.; Jambhale, C.; Kim, J.; Kolekar, S. Synthesis of tea waste/Fe3O4 magnetic composite (TWMC) for efficient adsorption of crystal violet dye: Isotherm, kinetic and thermodynamic studies. J. Environ. Chem. Eng. 2022, 10, 107893. [Google Scholar] [CrossRef]
- Luyen, N.; Linh, H.; Huy, T. Preparation of rice husk biochar-based magnetic nanocomposite for effective removal of crystal violet. J. Electron. Mater. 2019, 49, 10. [Google Scholar] [CrossRef]
- Malika, M.G.; Hema Kumar, C.; Bhavani, A.D.; Surya, D. Fe-biochar composite for the removal of dye in wastewater using adsorption method. Int. Res. J. Eng. Technol. (IRJET) 2023, 10, 70–74. [Google Scholar]
- Anastasiou, M.; Sakkas, V.; Sleiman, M. Activated Biochar from Sewage Sludge: A Sustainable Solution for Effective Removal of Emerging Water Contaminants. Molecules 2024, 30, 3514. [Google Scholar] [CrossRef]
- Shafiq, M.; Alazba, A.A.; Amin, M.T. Kinetic and Isotherm Studies of Ni2+ and Pb2+ Adsorption from Synthetic Wastewater Using Eucalyptus camdulensis—Derived Biochar. Sustainability 2020, 13, 3785. [Google Scholar] [CrossRef]
- Mojid, M.A.; Ibn Murad, K.F.; Tabriz, S.; Wyseure, G. An advantageous level of irrigation water salinity for wheat cultivation. J. Bangladesh Agric. Univ. 2014, 11, 141–146. [Google Scholar] [CrossRef]
- Tekin, S.; Sezen, S.M.; Arslan, S.; Boyaci, S.; Yildiz, M. Water production functions of wheat irrigated with saline water using line source sprinkler system under the Mediterranean type climate. Türk Tarım Ve Doğa Bilim. Derg. 2014, 1, 1017–1024. [Google Scholar]
- Wang, H.; Zheng, C.; Ning, S.; Cao, C.; Li, K.; Dang, H.; Wu, Y.; Zhang, J. Impacts of long-term saline water irrigation on soil properties and crop yields under maize-wheat crop rotation. Agric. Water Manag. 2023, 286, 108383. [Google Scholar] [CrossRef]
- El Sayed, M.; Hazman, M.; Gamal, A.; Almas, L.; McFarland, M.; Burian, S. Biochar reduces the adverse effect of saline water on soil properties and wheat production profitability. Agriculture 2021, 11, 1112. [Google Scholar] [CrossRef]
- Helaoui, S.; Boughattas, I.; Mkhinini, M.; Ghazouani, H.; Jabnouni, H.; Kribi-Boukhris, S.E.; Marai, B.; Slimani, D.; Arfaoui, Z.; Banni, M. Biochar application mitigates salt stress on maize plant: Study of the agronomic parameters, photosynthetic activities and biochemical attributes. Plant Stress 2023, 9, 100182. [Google Scholar] [CrossRef]
- Vu, N.; Bui, T.; Vu, T.; Nguyen, T.; Le, T.; Tran, A.; Vu, N.; Tran, V.; Tong, V.; Nguyen, X.; et al. Biochar improved sugarcane growth and physiology under salinity stress. Appl. Sci. 2023, 13, 7708. [Google Scholar] [CrossRef]
- Kanwal, S.; Ilyas, N.; Shabir, S.; Saeed, M.; Gul, R.; Zahoor, M.; Batool, N.; Mazhar, R. Application of biochar in mitigation of negative effects of salinity stress in wheat (Triticum aestivum L.). J. Plant Nutr. 2017, 40, 1923–1930. [Google Scholar] [CrossRef]

| Peak BE | FWHM eV | Area (P) CPS.eV | Atomic Percentage (%) |
| C1s | 284.84 | 1.63 | 4514.22 | 35.0 |
| N1s | 400.27 | 2.13 | 337.44 | 1.6 |
| O1s | 530.27 | 1.87 | 13,606.26 | 39.1 |
| Na1s | 1071.83 | 1.97 | 1824.19 | 1.9 |
| Si2p | 101.35 | 3.49 | 298.37 | 2.5 |
| Fe2p | 710.93 | 3.79 | 29,340.45 | 18.1 |
| Cl2p | 198.63 | 1.33 | 237.00 | 0.7 |
| S2p | 168.93 | 2.43 | 312.27 | 1.3 |
| Peak BE | FWHM eV | Area (P) CPS.eV | Atomic Percentage (%) |
| C1s | 284.84 | 1.73 | 7366.98 | 64.4 |
| K2p | 293.03 | 1.78 | 3863.72 | 7.2 |
| Cl2p | 198.68 | 2.78 | 395.72 | 1.2 |
| P2s | 190.16 | 2.18 | 96.76 | 0.7 |
| O1s | 531.47 | 2.56 | 6727.45 | 21.8 |
| Na1s | 1071.43 | 1.63 | 614.24 | 0.7 |
| N1s | 399.64 | 3.35 | 456.59 | 2.4 |
| Ca2p | 346.95 | 1.71 | 820.52 | 1.2 |
| Ag3d5 | 367.82 | 1.23 | 227.36 | 0.2 |
| Peak BE | FWHM eV | Area (P) CPS.eV | Atomic Percentage (%) |
| C1s | 284.82 | 1.61 | 7941.62 | 69.6 |
| S2p | 168.77 | 2.35 | 655.13 | 3.0 |
| N1s | 401.04 | 2.60 | 589.25 | 3.2 |
| O1s | 532.12 | 3.04 | 7453.56 | 24.2 |
| Sample | Surface Area (m2/g) |
|---|---|
| Biochar-H2SO4 | 33.0 |
| Biochar-Ag | 3.4 |
| Biochar | 4.8 |
| Biochar-Fe | 4.76 |
| Biochar | Model | qm (mg/g) | KL | RL | R2 | KF | n |
|---|---|---|---|---|---|---|---|
| Non-activated | Langmuir | 7.72 | 0.12 | 0.13 | 0.44 | – | – |
| Freundlich | – | – | – | 0.89 | 1.05 | 1.59 | |
| H2SO4-biochar | Langmuir | 488.20 | 0.02 | 0.50 | 0.982 | – | – |
| Freundlich | – | – | – | 0.97 | 97.07 | 1.06 | |
| Ag-biochar | Langmuir | 5.56 | 17.99 | 0.001 | 0.44 | – | – |
| Freundlich | – | – | – | 0.998 | 5.5 | 3.57 | |
| Fe-biochar | Langmuir | 5.26 | 7.20 | 0.028 | 0.909 | – | – |
| Freundlich | – | – | – | 0.912 | 4.078 | 4.06 |
| Source | Type III Sum of Squares | df | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Corrected Model | 97.1601 | 3 | 32.387 | 9.121 | 0.001 |
| Intercept | 8482.748 | 1 | 8482.748 | 2388.904 | 0.000 |
| Dosage | 95.420 | 2 | 47.710 | 13.436 | 0.001 |
| conductivity | 1.739 | 1 | 1.739 | 0.490 | 0.495 |
| Error | 49.713 | 14 | 3.551 | ||
| Total | 8629.620 | 18 | |||
| Corrected Total | 146.872 | 17 |
| Dosage (I) | Dosage (J) | Mean Difference (I-J) | Std. Error | Sig. | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| 0 | 1% | −5.603 * | 1.069 | 0.000 | −8.48 | −2.72 |
| 3% | −2.248 | 1.069 | 0.159 | −5.13 | 0.63 | |
| 1% | 0 | 5.603 * | 1.069 | 0.000 | 2.72 | 8.48 |
| 3% | 3.356 * | 1.069 | 0.020 | 0.48 | 6.24 | |
| 3% | 0 | 2.248 | 1.069 | 0.159 | −0.63 | 5.13 |
| 1% | −3.356 * | 1.069 | 0.020 | −6.24 | −0.48 | |
| Source | Type III Sum of Squares | df | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Corrected Model | 101.555 a | 3 | 33.852 | 5.379 | 0.011 |
| Intercept | 14,243.979 | 1 | 14,243.979 | 2263.266 | 0.000 |
| dosage | 70.240 | 2 | 35.120 | 5.580 | 0.017 |
| conductivity | 31.314 | 1 | 31.314 | 4.976 | 0.043 |
| Error | 88.110 | 14 | 6.294 | ||
| Total | 14,433.643 | 18 | |||
| Corrected Total | 189.664 | 17 |
| (I) Dosage | (J) Dosage | Mean Difference (I-J) | Std. Error | Sig. b | 95% Confidence Interval for Difference b | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| 0 | 1 | −3.860 * | 1.448 | 0.018 | −6.966 | −0.753 |
| 3 | −4.457 * | 1.448 | 0.008 | −7.564 | −1.351 | |
| 1 | 0 | 3.860 * | 1.448 | 0.018 | 0.753 | 6.966 |
| 3 | −0.597 | 1.448 | 0.686 | −3.704 | 2.509 | |
| 3 | 0 | 4.457 * | 1.448 | 0.008 | 1.351 | 7.564 |
| 1 | 0.597 | 1.448 | 0.686 | −2.509 | 3.704 | |
| Source | Type III Sum of Squares | df | Mean Square | F | Sig. |
|---|---|---|---|---|---|
| Corrected Model | 915.342 a | 3 | 305.114 | 15.248 | 0.000 |
| Intercept | 17,393.915 | 1 | 17,393.915 | 869.246 | 0.000 |
| Dosage | 817.234 | 2 | 408.617 | 20.420 | 0.000 |
| Conductivity | 98.108 | 1 | 98.108 | 4.903 | 0.044 |
| Error | 280.145 | 14 | 20.010 | ||
| Total | 18,589.402 | 18 | |||
| Corrected Total | 1195.487 | 17 |
| (I) Dosage | (J) Dosage | Mean Difference (I-J) | Std. Error | Sig. b | 95% Confidence Interval for Difference b | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| 0 | 1 | −16.505 * | 2.583 | 0.000 | −22.044 | −10.966 |
| 3 | −8.281 * | 2.583 | 0.006 | −13.820 | −2.741 | |
| 1 | 0 | 16.505 * | 2.583 | 0.000 | 10.966 | 22.044 |
| 3 | 8.224 * | 2.583 | 0.007 | 2.685 | 13.763 | |
| 3 | 0 | 8.281 * | 2.583 | 0.006 | 2.741 | 13.820 |
| 1 | −8.224 * | 2.583 | 0.007 | −13.763 | −2.685 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kataya, G.; El Badan, D.; Cornu, D.; Mousawi, A.A.; Bechelany, M.; Hijazi, A. Evaluating Biochar’s Role in Dye Adsorption and Wheat Performance Under Saline Conditions. Materials 2025, 18, 4678. https://doi.org/10.3390/ma18204678
Kataya G, El Badan D, Cornu D, Mousawi AA, Bechelany M, Hijazi A. Evaluating Biochar’s Role in Dye Adsorption and Wheat Performance Under Saline Conditions. Materials. 2025; 18(20):4678. https://doi.org/10.3390/ma18204678
Chicago/Turabian StyleKataya, Ghenwa, Dalia El Badan, David Cornu, Assi Al Mousawi, Mikhael Bechelany, and Akram Hijazi. 2025. "Evaluating Biochar’s Role in Dye Adsorption and Wheat Performance Under Saline Conditions" Materials 18, no. 20: 4678. https://doi.org/10.3390/ma18204678
APA StyleKataya, G., El Badan, D., Cornu, D., Mousawi, A. A., Bechelany, M., & Hijazi, A. (2025). Evaluating Biochar’s Role in Dye Adsorption and Wheat Performance Under Saline Conditions. Materials, 18(20), 4678. https://doi.org/10.3390/ma18204678








